Test-Retest Reliability and Minimal Detectable Changes for Wearable Sensor-Derived Gait Stability, Symmetry, and Smoothness in Individuals with Severe Traumatic Brain Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
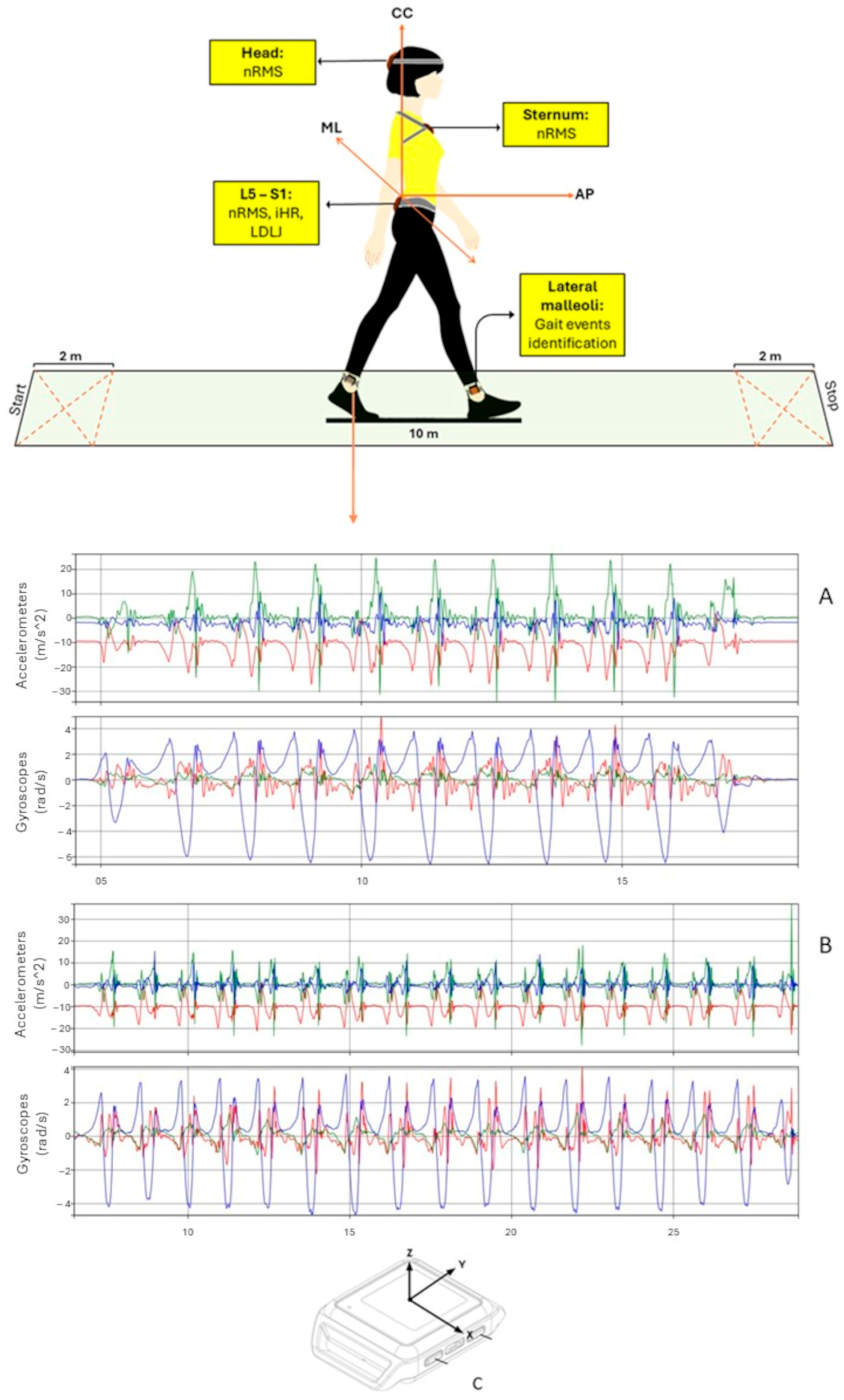
2.2. Data Collection
2.3. Sensor-Derived Indexes
2.4. Clinical Assessment
2.5. Statistical Analysis
3. Results
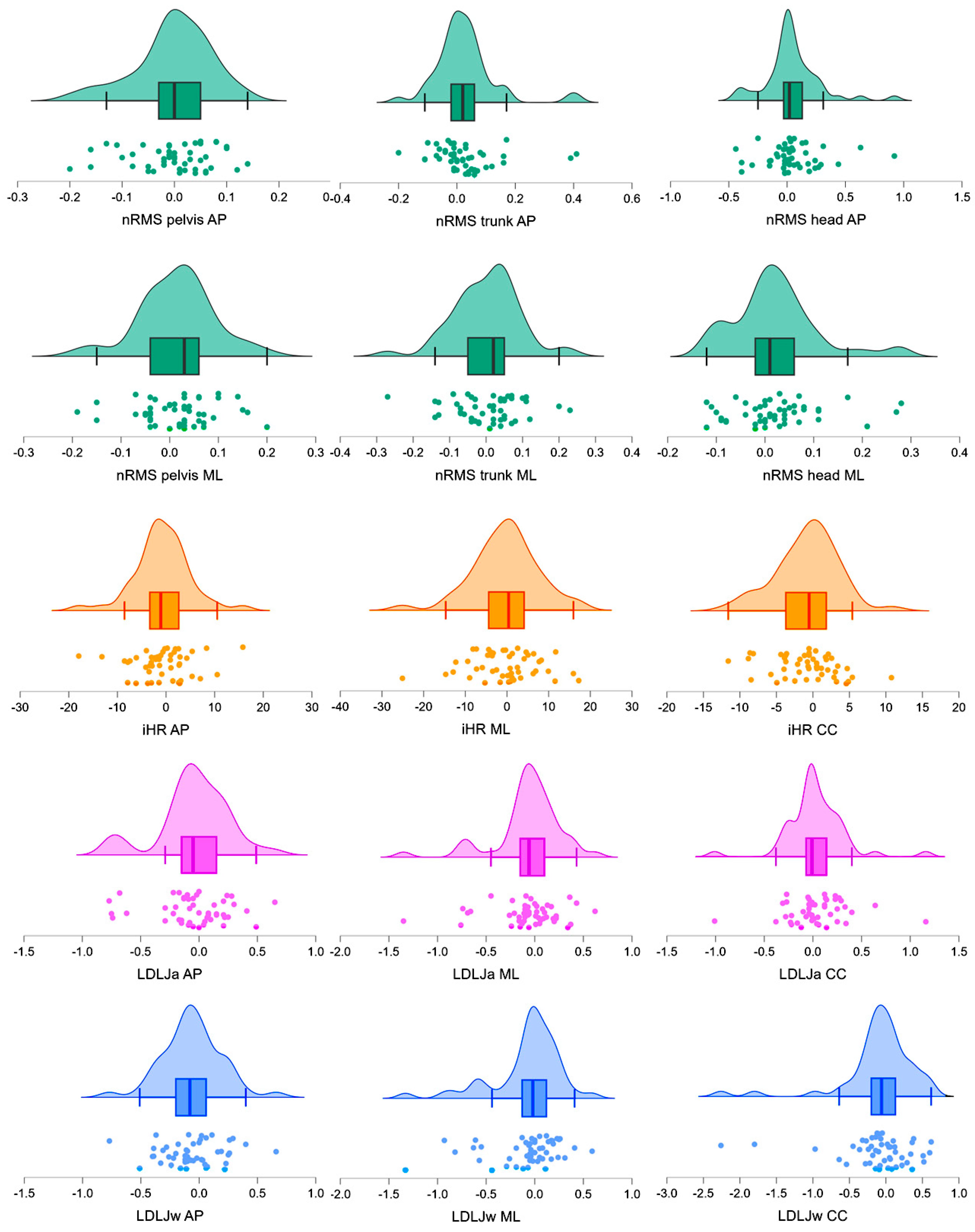
3.1. Test-Retest Reliability
3.2. Standard Error of Measurement (SEM) and Minimal Detectable Change (MDC)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the Global Incidence of Traumatic Brain Injury. J. Neurosurg. 2019, 130, 1080–1097. [Google Scholar] [CrossRef]
- Khan, A.; Prince, M.; Brayne, C.; Prina, A.M. Lifetime Prevalence and Factors Associated with Head Injury among Older People in Low and Middle Income Countries: A 10/66 Study. PLoS ONE 2015, 10, e0132229. [Google Scholar] [CrossRef] [PubMed]
- Lecky, F.E.; Otesile, O.; Marincowitz, C.; Majdan, M.; Nieboer, D.; Lingsma, H.F.; Maegele, M.; Citerio, G.; Stocchetti, N.; Steyerberg, E.W.; et al. The burden of traumatic brain injury from low-energy falls among patients from 18 countries in the CENTER-TBI Registry: A comparative cohort study. PLoS Med. 2021, 18, e1003761. [Google Scholar] [CrossRef]
- Capizzi, A.; Woo, J.; Verduzco-Gutierrez, M. Traumatic Brain Injury: An Overview of Epidemiology, Pathophysiology, and Medical Management. Med. Clin. N. Am. 2020, 104, 213–238. [Google Scholar] [CrossRef] [PubMed]
- Formisano, R. Severe acquired brain injury and high specialty neurorehabilitation needs. Neurol. Sci. 2020, 42, 347–348. [Google Scholar] [CrossRef]
- Lee, S.Y.; Amatya, B.; Judson, R.; Truesdale, M.; Reinhardt, J.D.; Uddin, T.; Xiong, X.H.; Khan, F. Clinical practice guidelines for rehabilitation in traumatic brain injury: A critical appraisal. Brain Inj. 2019, 33, 1263–1271. [Google Scholar] [CrossRef]
- Alashram, A.R.; Padua, E.; Annino, G. Virtual reality for balance and mobility rehabilitation following traumatic brain injury: A systematic review of randomized controlled trials. J. Clin. Neurosci. 2022, 105, 115–121. [Google Scholar] [CrossRef]
- Tefertiller, C.; Ketchum, J.M.; Bartelt, P.; Peckham, M.; Hays, K. Feasibility of virtual reality and treadmill training in traumatic brain injury: A randomized controlled pilot trial. Brain Inj. 2022, 36, 898–908. [Google Scholar] [CrossRef]
- Belluscio, V.; Bergamini, E.; Tramontano, M.; Orejel Bustos, A.; Allevi, G.; Formisano, R.; Vannozzi, G.; Buzzi, M.G. Gait Quality Assessment in Survivors from Severe Traumatic Brain Injury: An Instrumented Approach Based on Inertial Sensors. Sensors 2019, 19, 5315. [Google Scholar] [CrossRef]
- Kunker, K.; Peters, D.M.; Mohapatra, S. Long-term impact of mild traumatic brain injury on postural stability and executive function. Neurol. Sci. 2020, 41, 1899–1907. [Google Scholar] [CrossRef]
- Pan, T.; Liao, K.; Roenigk, K.; Daly, J.J.; Walker, M.F. Static and dynamic postural stability in veterans with combat-related mild traumatic brain injury. Gait Posture 2015, 42, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Zengin-Metli, D.; Özbudak-Demir, S.; Eraktaş, İ.; Safer, V.B.; Ekiz, T. Effects of robot assistive upper extremity rehabilitation on motor and cognitive recovery, the quality of life, and activities of daily living in stroke patients. J. Back Musculoskelet. Rehabil. 2018, 31, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Proulx, C.E.; Jean, M.T.L.; Higgins, J.; Gagnon, D.H.; Dancause, N. Somesthetic, visual, and auditory feedback and their interactions applied to upper limb neurorehabilitation technology: A narrative review to facilitate contextualization of knowledge. Front. Rehabil. Sci. 2022, 3, 789479. [Google Scholar] [CrossRef]
- Dever, A.; Powell, D.; Graham, L.; Mason, R.; Das, J.; Marshall, S.J.; Vitorio, R.; Godfrey, A.; Stuart, S. Gait Impairment in Traumatic Brain Injury: A Systematic Review. Sensors 2022, 22, 1480. [Google Scholar] [CrossRef]
- Corrigan, F.; Wee, I.C.; Collins-Praino, L.E. Chronic motor performance following different traumatic brain injury severity-A systematic review. Front. Neurol. 2023, 14, 1180353. [Google Scholar] [CrossRef] [PubMed]
- Vagnini, A.; Furone, R.; Zanotti, G.; Adamo, P.; Temporiti, F.; Gatti, R. Agreement between inertial measurement unit and optoelectronic system to measure postural sway. Technol. Health Care 2022, 30, 757–762. [Google Scholar] [CrossRef]
- Prasanth, H.; Caban, M.; Keller, U.; Courtine, G.; Ijspeert, A.; Vallery, H.; Von Zitzewitz, J. Wearable Sensor-Based Real-Time Gait Detection: A Systematic Review. Sensors 2021, 21, 2727. [Google Scholar] [CrossRef]
- Tramontano, M.; Belluscio, V.; Bergamini, E.; Allevi, G.; De Angelis, S.; Verdecchia, G.; Formisano, R.; Vannozzi, G.; Buzzi, M.G. Vestibular Rehabilitation Improves Gait Quality and Activities of Daily Living in People with Severe Traumatic Brain Injury: A Randomized Clinical Trial. Sensors 2022, 22, 8553. [Google Scholar] [CrossRef]
- Trabassi, D.; Serrao, M.; Varrecchia, T.; Ranavolo, A.; Coppola, G.; De Icco, R.; Tassorelli, C.; Castiglia, S.F. Machine Learning Approach to Support the Detection of Parkinson's Disease in IMU-Based Gait Analysis. Sensors 2022, 22, 3700. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Trabassi, D.; Castiglia, S.F.; Bini, F.; Marinozzi, F.; Ajoudani, A.; Lorenzini, M.; Chini, G.; Varrecchia, T.; Ranavolo, A.; De Icco, R.; et al. Optimizing Rare Disease Gait Classification through Data Balancing and Generative AI: Insights from Hereditary Cerebellar Ataxia. Sensors 2024, 24, 3613. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marimon, X.; Mengual, I.; López-de-Celis, C.; Portela, A.; Rodríguez-Sanz, J.; Herráez, I.A.; Pérez-Bellmunt, A. Kinematic Analysis of Human Gait in Healthy Young Adults Using IMU Sensors: Exploring Relevant Machine Learning Features for Clinical Applications. Bioengineering 2024, 11, 105. [Google Scholar] [CrossRef] [PubMed]
- Seamon, B.A.; Kautz, S.A.; Bowden, M.G.; Velozo, C.A. Revisiting the Concept of Minimal Detectable Change for Patient-Reported Outcome Measures. Phys. Ther. 2022, 102, pzac068. [Google Scholar] [CrossRef]
- Cesar, G.M.; Buster, T.W.; Burnfield, J.M. Test-retest reliability and minimal detectable change of the computerized dynamic posturography PROPRIO for adults with chronic traumatic brain injury. Disabil. Rehabil. 2021, 43, 2038–2044. [Google Scholar] [CrossRef] [PubMed]
- Tramontano, M.; Argento, O.; Bustos, A.S.O.; De Angelis, S.; Montemurro, R.; Bossa, M.; Belluscio, V.; Bergamini, E.; Vannozzi, G.; Nocentini, U. Cognitive-motor dual-task training improves dynamic stability during straight and curved gait in patients with multiple sclerosis: A randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2024, 60, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Pinto, C.; Schuch, C.P.; Balbinot, G.; Salazar, A.P.; Hennig, E.M.; Kleiner, A.F.R.; Pagnussat, A.S. Movement smoothness during a functional mobility task in subjects with Parkinson's disease and freezing of gait-an analysis using inertial measurement units. J. Neuroeng. Rehabil. 2019, 16, 110. [Google Scholar] [CrossRef]
- Castiglia, S.F.; Trabassi, D.; Tatarelli, A.; Ranavolo, A.; Varrecchia, T.; Fiori, L.; Di Lenola, D.; Cioffi, E.; Raju, M.; Coppola, G.; et al. Identification of Gait Unbalance and Fallers Among Subjects with Cerebellar Ataxia by a Set of Trunk Acceleration-Derived Indices of Gait. Cerebellum (Lond. Engl.) 2023, 22, 46–58. [Google Scholar] [CrossRef]
- Kobsar, D.; Charlton, J.M.; Tse, C.T.F.; Esculier, J.F.; Graffos, A.; Krowchuk, N.M.; Thatcher, D.; Hunt, M.A. Validity and reliability of wearable inertial sensors in healthy adult walking: A systematic review and meta-analysis. J. Neuroeng. Rehabil. 2020, 17, 62. [Google Scholar] [CrossRef]
- Walker, W.C.; Pickett, T.C. Motor impairment after severe traumatic brain injury: A longitudinal multicenter study. J. Rehabil. Res. Dev. 2007, 44, 975–982. [Google Scholar] [CrossRef]
- Riva, F.; Bisi, M.C.; Stagni, R. Gait variability and stability measures: Minimum number of strides and within-session reliability. Comput. Biol. Med. 2014, 50, 9–13. [Google Scholar] [CrossRef]
- Kroneberg, D.; Elshehabi, M.; Meyer, A.C.; Otte, K.; Doss, S.; Paul, F.; Nussbaum, S.; Berg, D.; Kühn, A.A.; Maetzler, W.; et al. Less Is More-Estimation of the Number of Strides Required to Assess Gait Variability in Spatially Confined Settings. Front. Aging Neurosci. 2019, 10, 435. [Google Scholar] [CrossRef]
- Ciurli, P.; Bivona, U.; Barba, C.; Onder, G.; Silvestro, D.; Azicnuda, E.; Rigon, J.; Formisano, R. Metacognitive unawareness correlates with executive function impairment after severe traumatic brain injury. J. Int. Neuropsychol. Soc. 2010, 16, 360–368. [Google Scholar] [CrossRef]
- Gouvier, W.D.; Blanton, P.D.; LaPorte, K.K.; Nepomuceno, C. Reliability and validity of the Disability Rating Scale and the Levels of Cognitive Functioning Scale in monitoring recovery from severe head injury. Arch. Phys. Med. Rehabil. 1987, 68, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Ilg, W.; Milne, S.; Schmitz-Hübsch, T.; Alcock, L.; Beichert, L.; Bertini, E.; Mohamed Ibrahim, N.; Dawes, H.; Gomez, C.M.; Hanagasi, H.; et al. Quantitative Gait and Balance Outcomes for Ataxia Trials: Consensus Recommendations by the Ataxia Global Initiative Working Group on Digital-Motor Biomarkers. Cerebellum (Lond. Engl.) 2024, 23, 1566–1592. [Google Scholar] [CrossRef] [PubMed]
- Trojaniello, A.; Cereatti, E.; Pelosin, L.; Avanzino, A.; Mirelman, J.M.; Hausdorff, U. Della Croce, Estimation of step-by-step spatio-temporal parameters of normal and impaired gait using shank-mounted magneto-inertial sensors: Application to elderly, hemiparetic, parkinsonian and choreic gait. J. Neuroeng. Rehabil. 2014, 11, 152. [Google Scholar] [CrossRef] [PubMed]
- Bergamini, E.; Ligorio, G.; Summa, A.; Vannozzi, G.; Cappozzo, A.; Sabatini, A.M. Estimating orientation using magnetic and inertial sensors and different sensor fusion approaches: Accuracy assessment in manual and locomotion tasks. Sensors 2014, 14, 18625–18649. [Google Scholar] [CrossRef]
- Tramontano, M.; Orejel Bustos, A.S.; Montemurro, R.; Vasta, S.; Marangon, G.; Belluscio, V.; Morone, G.; Modugno, N.; Buzzi, M.G.; Formisano, R.; et al. Dynamic Stability, Symmetry, and Smoothness of Gait in People with Neurological Health Conditions. Sensors 2024, 24, 2451. [Google Scholar] [CrossRef]
- Sekine, M.; Tamura, T.; Yoshida, M.; Suda, Y.; Kimura, Y.; Miyoshi, H.; Kijima, Y.; Higashi, Y.; Fujimoto, T. A gait abnormality measure based on root mean square of trunk acceleration. J. Neuroeng. Rehabil. 2013, 10, 118. [Google Scholar] [CrossRef]
- Iosa, M.; Fusco, A.; Morone, G.; Pratesi, L.; Coiro, P.; Venturiero, V.; De Angelis, D.; Bragoni, M.; Paolucci, S. Assessment of upper-body dynamic stability during walking in patients with subacute stroke. J. Rehabil. Res. Dev. 2012, 49, 439–450. [Google Scholar] [CrossRef]
- Pasciuto, I.; Bergamini, E.; Iosa, M.; Vannozzi, G.; Cappozzo, A. Overcoming the limitations of the Harmonic Ratio for the reliable assessment of gait symmetry. J. Biomech. 2017, 53, 84–89. [Google Scholar] [CrossRef]
- Melendez-Calderon, A.; Shirota, C.; Balasubramanian, S. Estimating Movement Smoothness From Inertial Measurement Units. Front. Bioeng. Biotechnol. 2021, 8, 558771. [Google Scholar] [CrossRef] [PubMed]
- Shumway-Cook, A.; Baldwin, M.; Polissar, N.L.; Gruber, W. Predicting the probability for falls in community-dwelling older adults. Phys. Ther. 1997, 77, 812–819. [Google Scholar] [CrossRef]
- Herman, T.; Inbar-borovsky, N.; Brozgol, M.; Giladi, N.; Hausdorff, J.M. The Dynamic Gait Index in Healthy Older Adults: The Role of StairClimbing, Fear of Falling and Gender. Gait Posture 2009, 29, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.O.; Wood-Dauphinee, S.L.; Williams, J.I.; Maki, B. Measuring balance in the elderly: Validation of an instrument. Can. J. Public Health 1992, 83 (Suppl. S2), S7–S11. [Google Scholar] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: London, UK, 1988. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Weir, J.P. Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. J. Strength Cond. Res. 2005, 19, 231–240. [Google Scholar] [CrossRef]
- Henriksen, M.; Lund, H.; Moe-Nilssen, R.; Bliddal, H.; Danneskiod-Samsøe, B. Test-retest reliability of trunk accelerometric gait analysis. Gait Posture 2004, 19, 288–297. [Google Scholar] [CrossRef]
- Iosa, M.; Bini, F.; Marinozzi, F.; Fusco, A.; Morone, G.; Koch, G.; Martino Cinnera, A.; Bonnì, S.; Paolucci, S. Stability and Harmony of Gait in Patients with Subacute Stroke. J. Med. Biol. Eng. 2016, 36, 635–643. [Google Scholar] [CrossRef]
- Loyd, B.J.; Dibble, L.E.; Weightman, M.M.; Pelo, R.; Hoppes, C.W.; Lester, M.; King, L.A.; Fino, P.C. Volitional Head Movement Deficits and Alterations in Gait Speed Following Mild Traumatic Brain Injury. J. Head Trauma Rehabil. 2023, 38, E223–E232. [Google Scholar] [CrossRef]
- Shiozaki, T.; Okada, Y.; Nakamura, J.; Ueta, K.; Tanaka, H.; Moritani, M.; Kitahara, T. Relationships between changes in lateral vestibulospinal tract excitability and postural control by dynamic balance intervention in healthy individuals: A preliminary study. Front. Hum. Neurosci. 2023, 17, 1109690. [Google Scholar] [CrossRef] [PubMed]
- Bellanca, J.; Lowry, K.; VanSwearingen, J.; Brach, J.; Redfern, M. Harmonic ratios: A quantification of step to step symmetry. J. Biomech. 2013, 46, 828–831. [Google Scholar] [CrossRef] [PubMed]
- Bergamini, E.; Cereatti, A.; Pavei, G. Walking symmetry is speed and index dependent. Sci. Rep. 2024, 14, 19548. [Google Scholar] [CrossRef] [PubMed]
- Pavei, G.; Seminati, E.; Cazzola, D.; Minetti, A.E. On the Estimation Accuracy of the 3D Body Center of Mass Trajectory during Human Locomotion: Inverse vs. Forward Dynamics. Front. Physiol. 2017, 8, 129. [Google Scholar] [CrossRef]
- Bergamini, E.; Cereatti, A.; Pavei, G. Just a matter of indexes: Symmetry in walking. Gait Posture 2022, 97 (Suppl. S2), 11–12. [Google Scholar] [CrossRef]
| Mean (SD) | Min–Max | |
|---|---|---|
| Age (years) | 36.7 (13.2) | 17–67 |
| Sample (F/M) | 49 (17/32) | NA |
| Time since trauma (months) | 9 (6.75) * | 3–26 |
| Leg length (cm) | 76.6 (5.08) | 66–85 |
| BMI (kg/m2) | 23.6 (3.8) | 20.7–30.4 |
| Walking speed | 0.79 (0.16) | 0.5–1.1 |
| Berg Balance Scale (score) | 48.4 (7.78) | 40–56 |
| Dynamic gait Index (score) | 18.96 (4.76) | 13–24 |
| Index | Test (95% CI) | Re-Test (95% CI) | Test-Retest Difference (95% CI) | p | d | |
|---|---|---|---|---|---|---|
| nRMS | AP (pelvis) | 0.82 (0.76, 0.89) | 0.83 (0.76, 0.90) | 0.05 (0.04, 0.07) | 0.85 | 0.03 |
| ML (pelvis) | 0.85 (0.76, 0.93) | 0.83 (0.75, 0.91) | 0.06 (0.05, 0.07) | 0.17 | 0.20 | |
| AP (trunk) | 0.61 (0.53, 0.69) | 0.58 (0.51, 0.65) | 0.07 (0.05, 0.09) | 0.10 | 0.27 | |
| ML (trunk) | 0.71 (0.62, 0.80) | 0.71 (0.62, 0.80) | 0.07 (0.05, 0.09) | 0.81 | 0.02 | |
| AP (head) | 0.68 (0.57, 0.79) | 0.64 (0.55, 0.73) | 0.15 (0.10, 0.21) | 0.18 | 0.19 | |
| ML (head) | 0.68 (0.59, 0.78) | 0.66 (0.57, 0.75) | 0.06 (0.05, 0.08) | 0.13 | 0.25 | |
| iHR | AP | 75.00 (70.07, 79.93) | 75.74 (70.77, 80.72) | 4.18 (3.07, 5.29) | 0.37 | 0.13 |
| ML | 71.26 (67.75, 74.78) | 71.60 (67.96, 75.23) | 5.83 (4.34, 7.33) | 0.76 | 0.04 | |
| CC | 78.20 (74.07, 82.32) | 78.99 (74.96. 83.01) | 3.18 (2.37, 4.00) | 0.20 | 0.19 | |
| LDLJa | AP | −5.21 (−5.31, −5.11) | −5.17 (−5.26, −5.08) | 0.22 (0.16, 0.28) | 0.54 | 0.14 |
| ML | −5.43 (−5.53, −5.33) | −5.37 (−5.49, −5.24) | 0.22 (0.15, 0.30) | 0.30 | 0.20 | |
| CC | −5.06 (−5.15, −4.97) | −5.09 (−5.17, −5.00) | 0.19 (0.13, 0.26) | 0.50 | 0.10 | |
| LDLJw | AP | −4.57 (−4.72, −4.43) | −4.52 (−4.66, −4.37) | 0.20 (0.15, 0.24) | 0.11 | 0.24 |
| ML | −4.73 (−4.88, −4.58) | −4.66 (−4.81, −4.50) | 0.23 (0.16, 0.31) | 0.52 | 0.22 | |
| CC | −4.39 (−4.59, −4.18) | −4.29 (−4.48, −4.10) | 0.31 (0.19, 0.43) | 0.38 | 0.19 | |
| Index | ICC (95% CI) | SEM (95% CI) | MDC (95% CI) | |
|---|---|---|---|---|
| nRMS | AP (pelvis) | 0.95 (0.91–0.97) | 0.01 (−0.01, 0.03) | 0.04 (−0.04, 0.13) |
| ML (pelvis) | 0.96 (0.92–0.97) | 0.01 (−0.01, 0.03) | 0.05 (−0.05, 0.14) | |
| AP (trunk) | 0.92 (0.86–0.95) | 0.02 (−0.02, 0.06) | 0.09 (−0.09, 0.27) | |
| ML (trunk) | 0.96 (0.93, 0.98) | 0.01 (−0.01, 0.03) | 0.05 (−0.05, 0.14) | |
| AP (head) | 0.78 (0.65, 0.86) | 0.08 (−0.08, 0.24) | 0.30 (−0.29, 0.88) | |
| ML (head) | 0.96 (0.93, 0.98) | 0.01 (−0.01, 0.03) | 0.05 (−0.05, 0.14) | |
| iHR | AP | 0.94 (0.90, 0.97) | 0.95 (−0.91, 2.81) | 3.69 (−3.54, 10.92) |
| ML | 0.80 (0.68, 0.88) | 3.51 (−3.37, 10.39) | 9.74 (−9.36, 28.85) | |
| CC | 0.95 (0.92, 0.97) | 0.64 (−0.61, 1.89) | 2.51 (−2.41, 7.43) | |
| LDLJa | AP | 0.57 (0.35, 0.74) | 0.19 (−0.18, 0.56) | 0.55 (−0.53, 1.62) |
| ML | 0.63 (0.43, 0.78) | 0.20 (−0.19, 0.59) | 0.57 (−0.55, 1.7) | |
| CC | 0.61 (0.40, 0.76) | 0.27 (−0.26, 0.8) | 0.75 (−0.72, 2.23) | |
| LDLJw | AP | 0.69 (0.52, 0.81) | 0.31 (−0.3, 0.92) | 0.68 (−0.65, 2.01) |
| ML | 0.77 (0.62, 0.86) | 0.30 (−0.29, 0.89) | 0.57 (−0.55, 1.7) | |
| CC | 0.72 (0.56, 0.83) | 0.32 (−0.31, 0.95) | 0.65 (−0.62, 1.92) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dal Farra, F.; Castiglia, S.F.; Buzzi, M.G.; Brasiliano, P.; De Angelis, S.; Paolocci, G.; Vasta, S.; Marangon, G.; Orejel Bustos, A.S.; Bergamini, E.; et al. Test-Retest Reliability and Minimal Detectable Changes for Wearable Sensor-Derived Gait Stability, Symmetry, and Smoothness in Individuals with Severe Traumatic Brain Injury. Sensors 2025, 25, 1764. https://doi.org/10.3390/s25061764
Dal Farra F, Castiglia SF, Buzzi MG, Brasiliano P, De Angelis S, Paolocci G, Vasta S, Marangon G, Orejel Bustos AS, Bergamini E, et al. Test-Retest Reliability and Minimal Detectable Changes for Wearable Sensor-Derived Gait Stability, Symmetry, and Smoothness in Individuals with Severe Traumatic Brain Injury. Sensors. 2025; 25(6):1764. https://doi.org/10.3390/s25061764
Chicago/Turabian StyleDal Farra, Fulvio, Stefano Filippo Castiglia, Maria Gabriella Buzzi, Paolo Brasiliano, Sara De Angelis, Gianluca Paolocci, Simona Vasta, Gabriele Marangon, Amaranta Soledad Orejel Bustos, Elena Bergamini, and et al. 2025. "Test-Retest Reliability and Minimal Detectable Changes for Wearable Sensor-Derived Gait Stability, Symmetry, and Smoothness in Individuals with Severe Traumatic Brain Injury" Sensors 25, no. 6: 1764. https://doi.org/10.3390/s25061764
APA StyleDal Farra, F., Castiglia, S. F., Buzzi, M. G., Brasiliano, P., De Angelis, S., Paolocci, G., Vasta, S., Marangon, G., Orejel Bustos, A. S., Bergamini, E., Betti, V., & Tramontano, M. (2025). Test-Retest Reliability and Minimal Detectable Changes for Wearable Sensor-Derived Gait Stability, Symmetry, and Smoothness in Individuals with Severe Traumatic Brain Injury. Sensors, 25(6), 1764. https://doi.org/10.3390/s25061764








