Design of Wood-Based Gd (III)-Hemoporphyrin Monomethyl Ether Eco-Material for Optical Oxygen Sensing with a Wide Detection Range
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Wood-Based Gd-HMME Oxygen Sensing Material
2.3. Instruments and Characterization
3. Results and Discussion
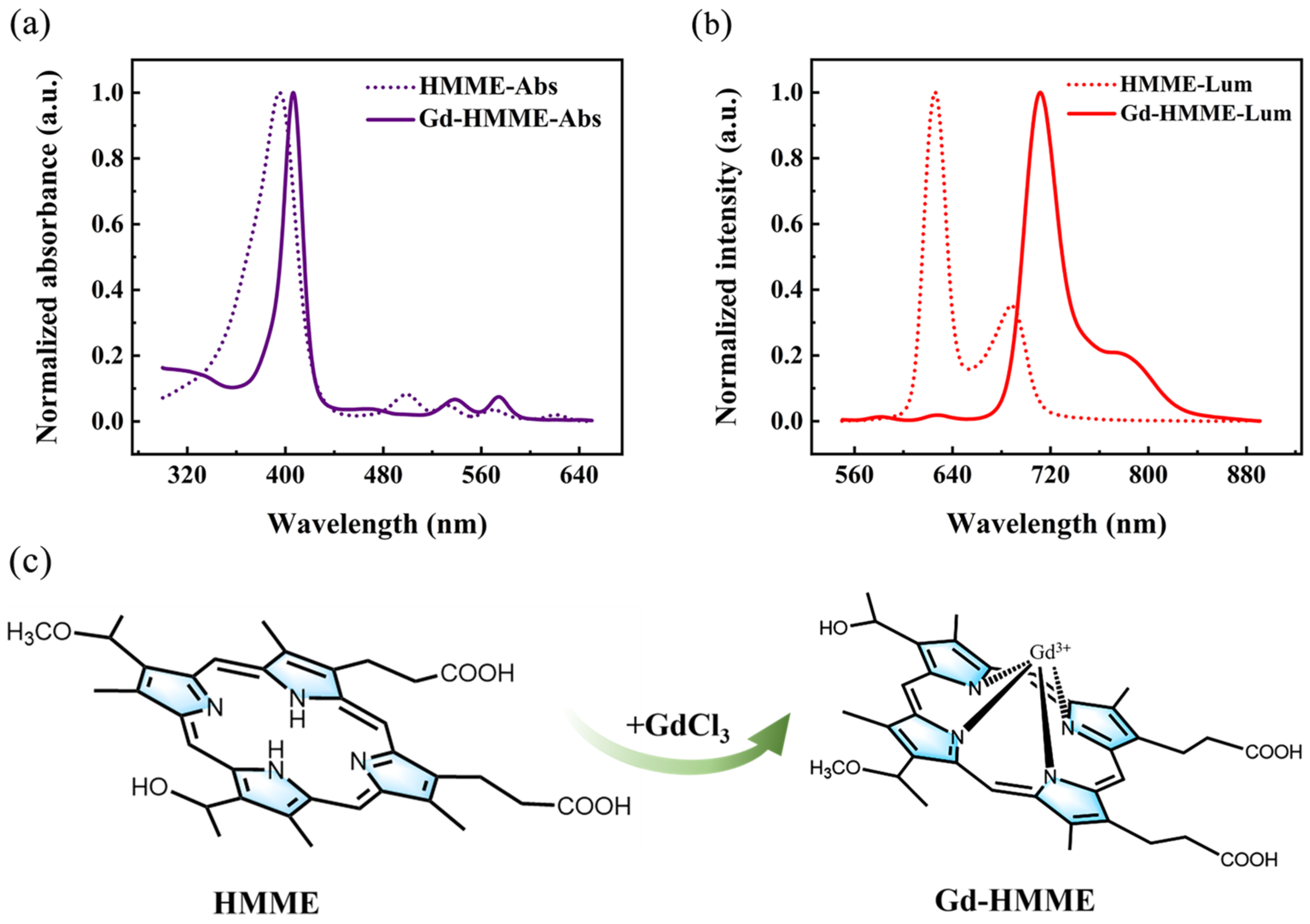
3.1. Optical Properties of Gd-HMME in Methanol Solution
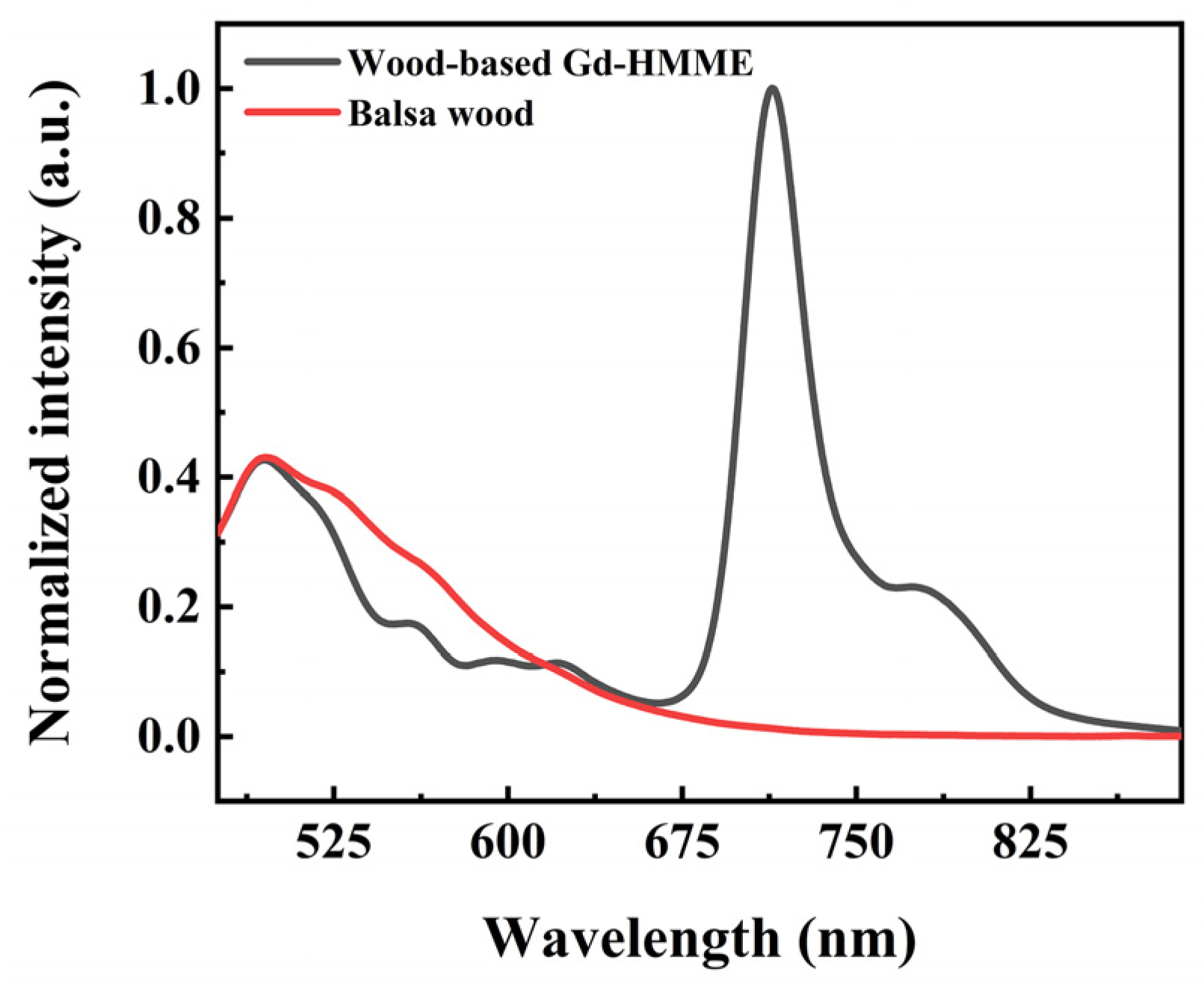
3.2. Optical Properties of Wood-Based Gd-HMME Material
3.3. Morphological Characteristics of Balsa Wood and Wood-Based Gd-HMME
3.4. The Relationship Between OP and Oxygen Partial Pressure Using Wood-Based Gd-HMME
3.5. Effect of Gd-HMME Concentration on the KSV Value of Wood-Based Gd-HMME Material
3.6. Photobleaching Behavior of Wood-Based Gd-HMME Material
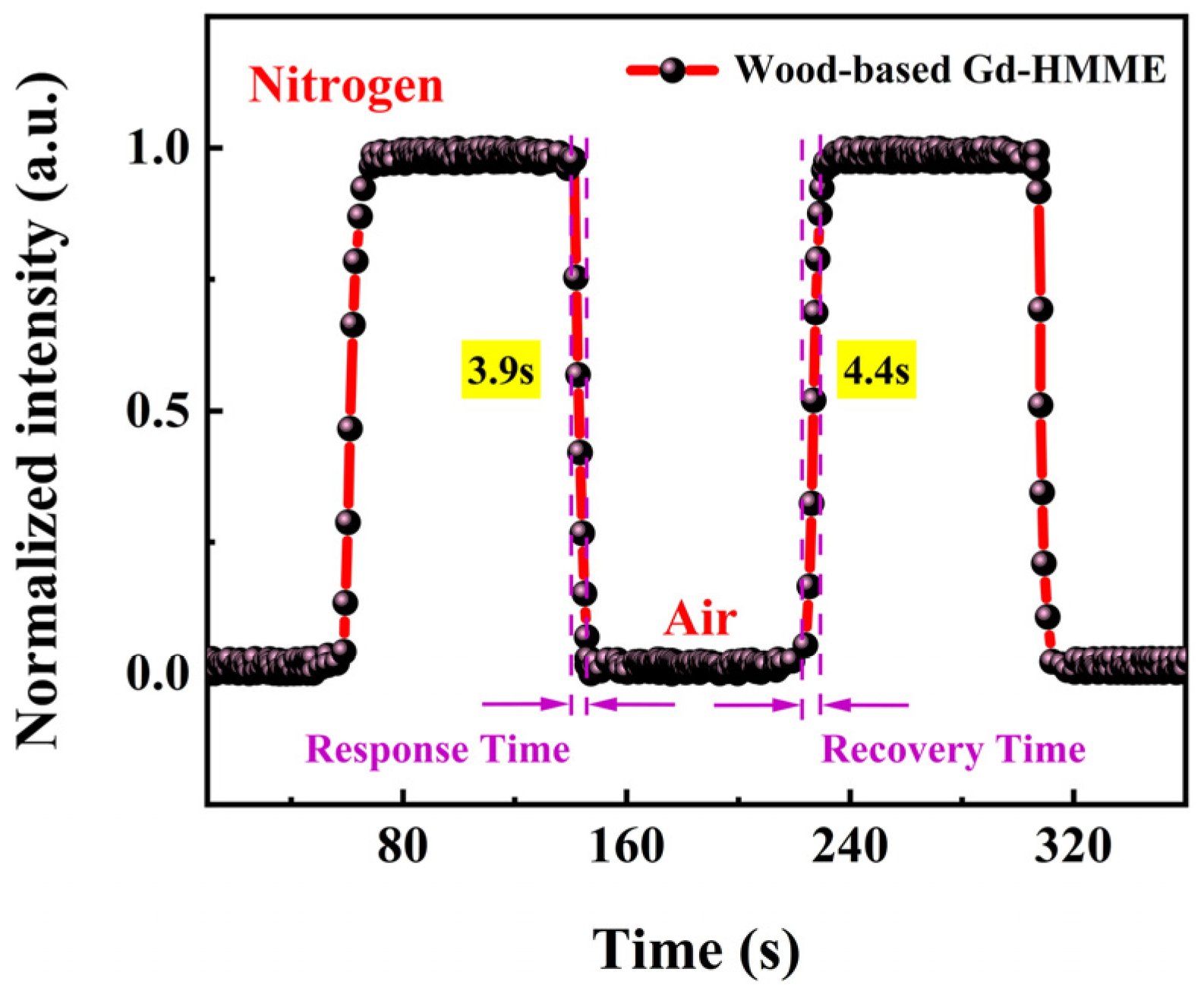
3.7. The Response Time and Reversibility of the Wood-Based Gd-HMME Material for Oxygen Sensing
3.8. Effect of Humidity and Interference Gases on Oxygen Measurement Performance of Wood-Based Gd-HMME
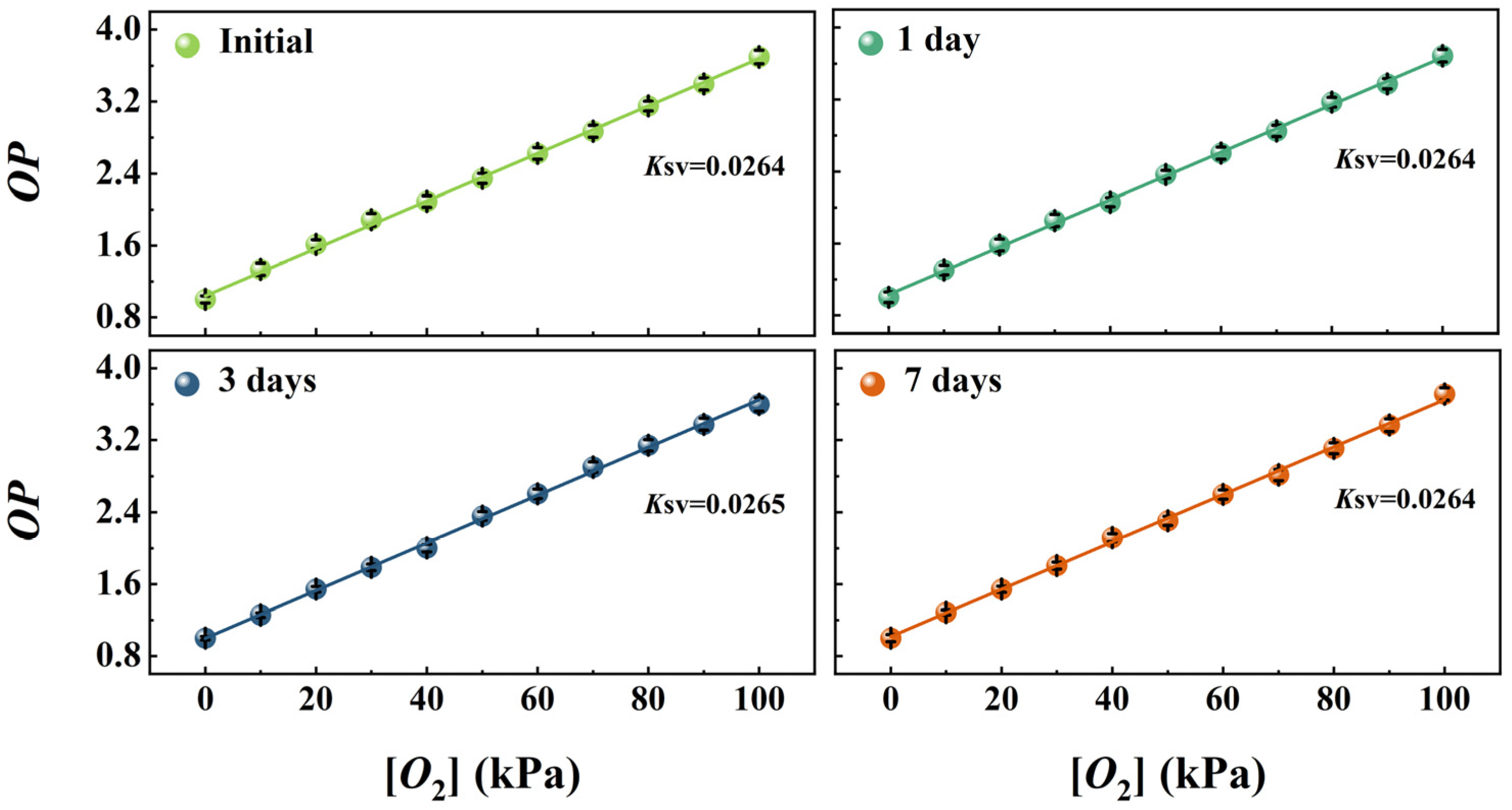
3.9. Long-Term Stability of the Wood-Based Gd-HMME
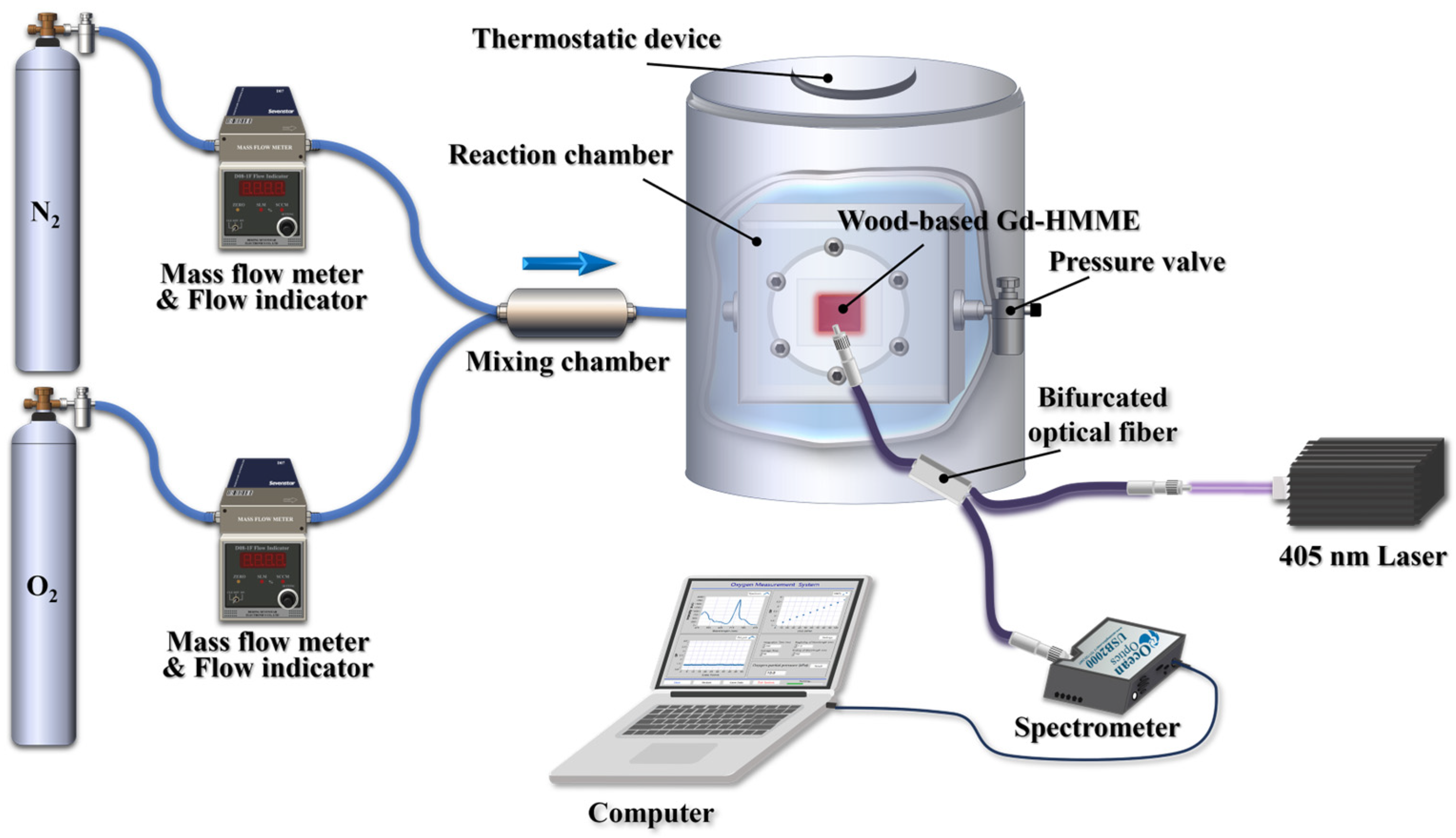
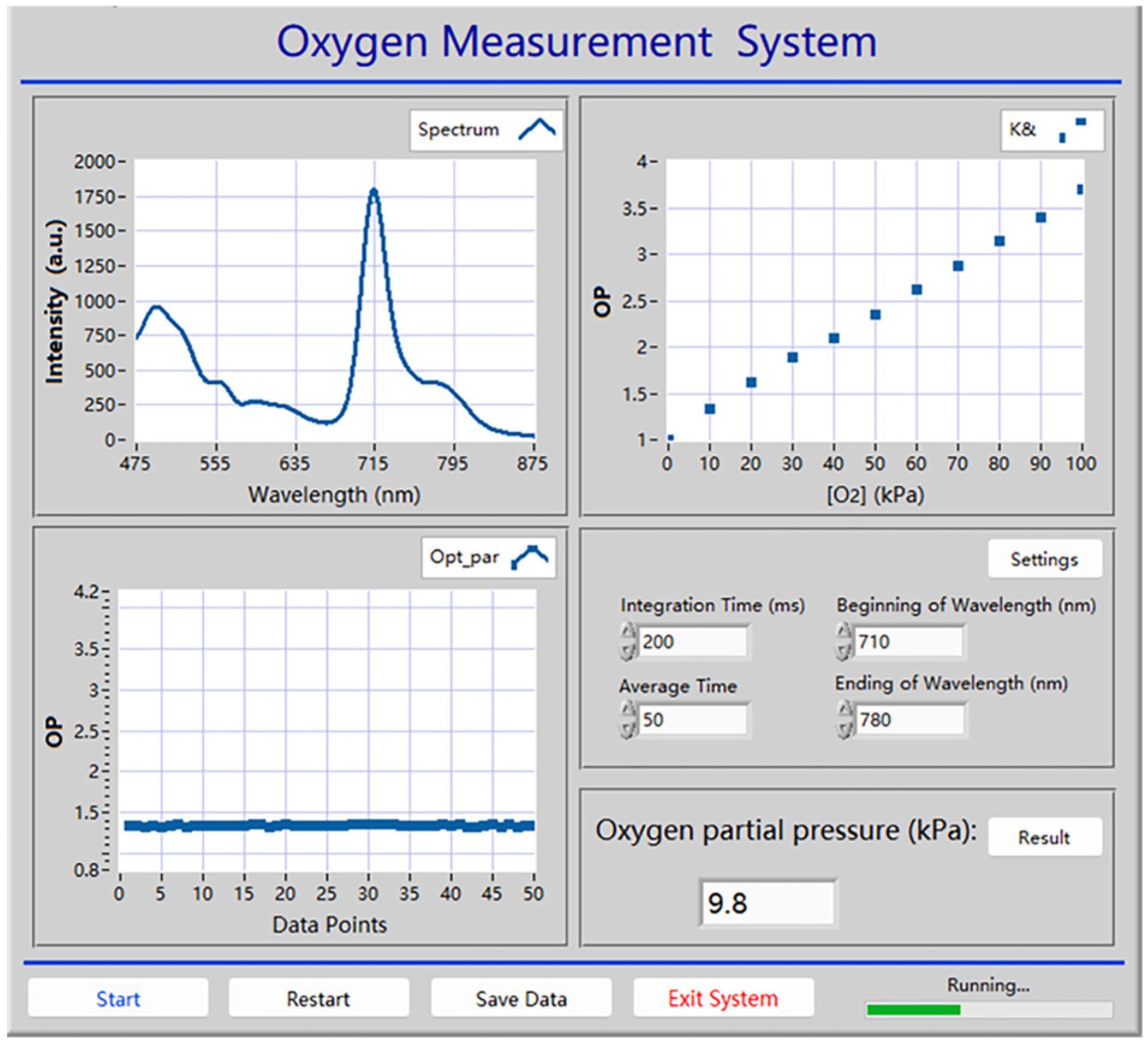
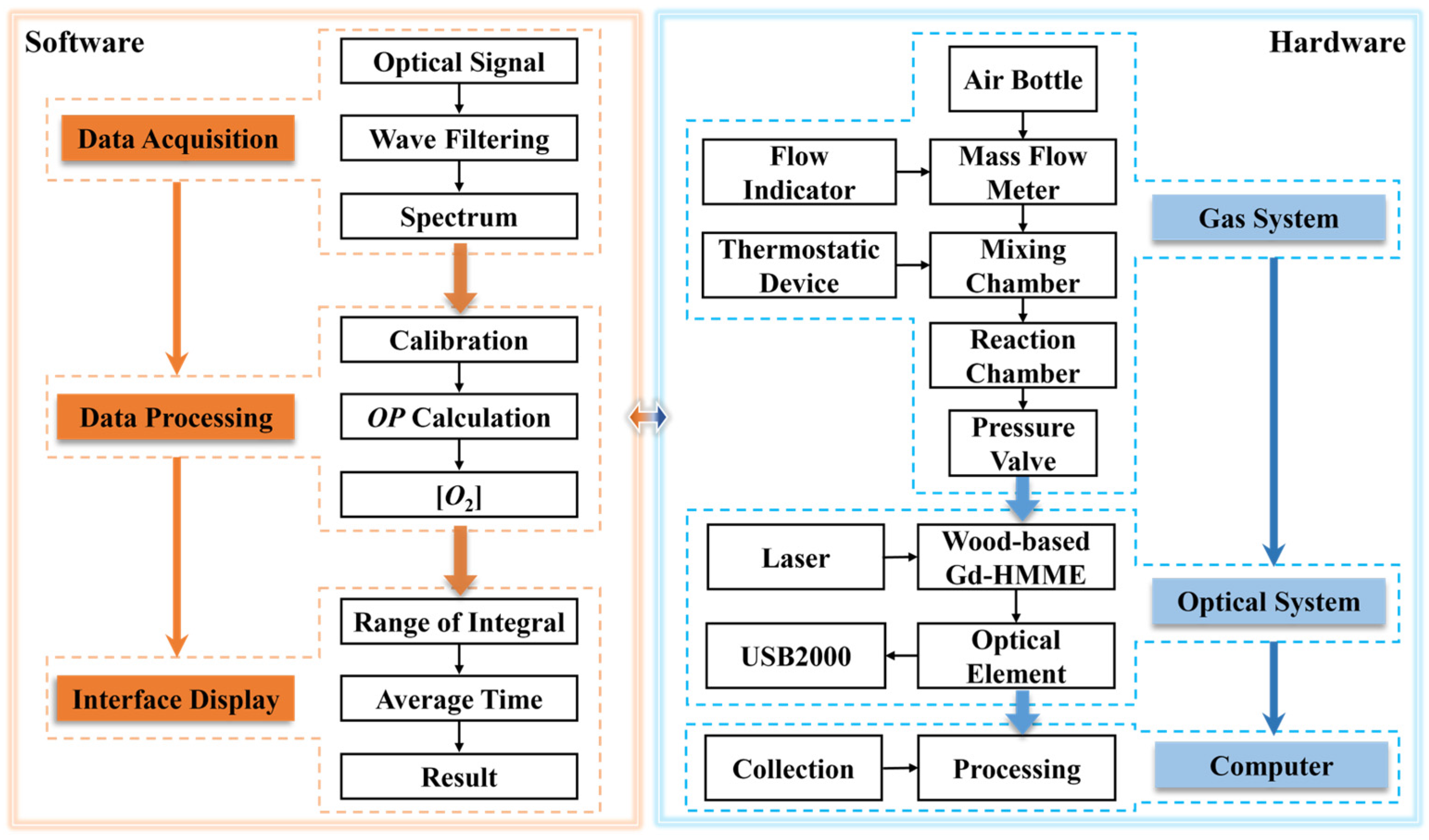
3.10. Automatic Oxygen Detection System Based on Wood-Based Gd-HMME Material
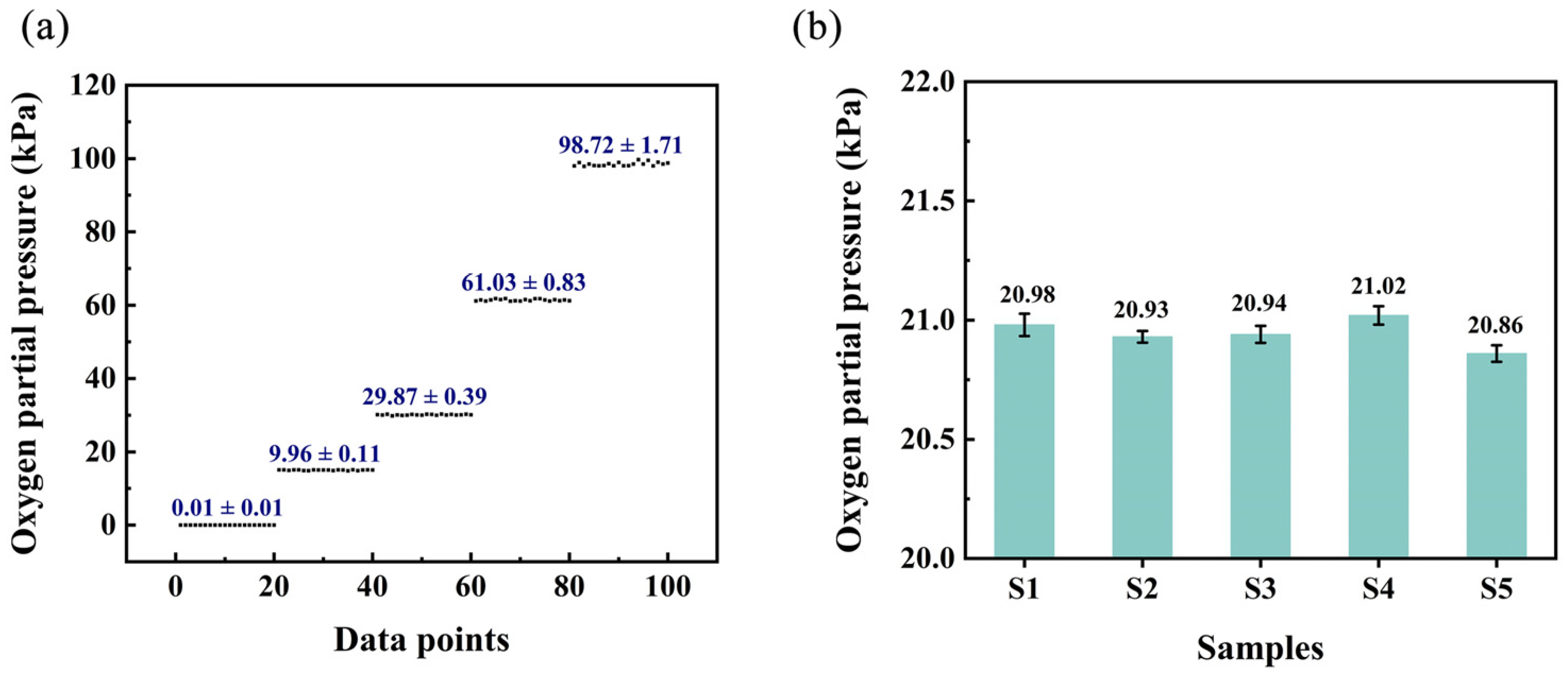
3.11. Performance of the Automatic Detection System in Practical Gaseous Oxygen Detection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Gd-HMME | Gadolinium-labeled hematoporphyrin monomethyl ether |
| HMME | Hematoporphyrin monomethyl ether |
| SEM | Scanning electron microscope |
| EDS | Energy dispersive spectrometer |
| OP | Optical parameter |
| [O2] | Oxygen partial pressure |
| KSV | Stern–Volmer constant |
| RH | Relative humidity |
References
- Piedra, F.J.; Yanos, C.M.V.; Mieles, J.M.; Macias, M.C.; Loor, A.M.A.; Vélez, M.I.Z.; Flor, F.G.I.; Intriago, S.N.G.; Vega, E.P.; Velásquez, R.M.L.; et al. Quantification of lactic acid in wines using an amperometric biosensor. Food Control 2025, 167, 110821. [Google Scholar] [CrossRef]
- Yan, L.L.; Dong, G.J.; Huang, X.J.; Zhang, Y.; Bi, Y.P. Unraveling oxygen vacancy changes of WO3 photoanodes for promoting oxygen evolution reaction. Appl. Catal. B-Environ. Energy 2024, 345, 123682. [Google Scholar] [CrossRef]
- Zhao, H.C.; Li, J.; She, X.P.; Chen, Y.; Wang, M.Q.; Wang, Y.J.; Du, A.J.; Tang, C.; Zou, C.; Zhou, Y. Oxygen Vacancy-Rich Bimetallic Au@Pt Core-Shell Nanosphere-Functionalized Electrospun ZnFe2O4ZnFe2O4 Nanofibers for Chemiresistive Breath Acetone Detection. ACS Sens. 2024, 9, 2183–2193. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.X.; Liu, G.; Hu, Q.; Huang, Y.Z.; Liu, L.M.; Dong, L.T.; Teobaldi, G.; Guo, L. Parallel Nanosheet Arrays for Industrial Oxygen Production. J. Am. Chem. Soc. 2023, 145, 25143–25149. [Google Scholar] [CrossRef]
- Zhou, Y.P.; Bastida, F.; Liu, Y.Z.; He, J.; Chen, W.J.; Wang, X.Y.; Xiao, Y.; Song, P.; Li, Y.K. Impacts and mechanisms of nanobubbles level in drip irrigation system on soil fertility, water use efficiency and crop production: The perspective of soil microbial community. J. Clean Prod. 2022, 333, 130050. [Google Scholar] [CrossRef]
- Konstantogianni, O.; Panou, T.; Zikopoulos, A.; Skentou, C.; Stavros, S.; Asimakopoulos, B. Culture of Human Embryos at High and Low Oxygen Levels. J. Clin. Med.J. Clin. Med. 2024, 13, 2222. [Google Scholar] [CrossRef]
- Zheng, G.D.; Wang, Y.W.; Wang, X.K.; Yang, J.X.; Chen, T.B. Oxygen Monitoring Equipment for Sewage-Sludge Composting and Its Application to Aeration Optimization. Sensors 2018, 18, 4017. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.L.; Wen, J.; Wang, Q.; Li, Y.J.; Zhao, Y.; Tian, Y.; Wang, X.F.; Cao, X.F.; Zhang, Y.L.; Lu, G.M.; et al. Sensitive, Real-Time, and In-Vivo Oxygen Monitoring for Photodynamic Therapy by Multifunctional Mesoporous Nanosensors. ACS Appl. Mater. Interfaces 2019, 11, 187–194. [Google Scholar] [CrossRef]
- Somesfalean, G.; Sjoholm, M.; Alnis, J.; af Klinteberg, C.; Andersson-Engels, S.; Svanberg, S. Concentration measurement of gas embedded in scattering media by employing absorption and time-resolved laser spectroscopy. Appl. Optics 2002, 41, 3538–3544. [Google Scholar] [CrossRef]
- Helm, I.; Jalukse, L.; Leito, I. A highly accurate method for determination of dissolved oxygen: Gravimetric Winkler method. Anal. Chim. Acta 2012, 741, 21–31. [Google Scholar] [CrossRef]
- Melnikov, P.V.; Alexandrovskaya, A.Y.; Naumova, A.O.; Arlyapov, V.A.; Kamanina, O.A.; Popova, N.M.; Zaitsev, N.K.; Yashtulov, N.A. Optical Oxygen Sensing and Clark Electrode: Face-to-Face in a Biosensor Case Study. Sensors 2022, 22, 7626. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.P.; Yang, Y.; Li, D.; Yu, H.; Dong, X.T.; Wang, T.Q. First polyoxometalate-modified SnS2 composite nanostructure gas sensor toward enhanced sensitivity and high selectivity for NO2 detection. Sens. Actuator B-Chem. 2024, 409, 135641. [Google Scholar] [CrossRef]
- Li, P.; Yang, Y.; Li, F.; Pei, W.Y.; Li, D.; Yu, H.; Dong, X.T.; Wang, T.Q. Effect of polyoxometalates electron acceptor decoration on NO2 sensing behavior of ZnS microspheres toward rapid and ultrahigh response. Sens. Actuator B-Chem. 2025, 426, 137111. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.Y.; Yuan, D.; Zhang, Y.Y.; Wu, B.W.; Feng, X.D. Calibration method of multi-parameter compensation for optical dissolved oxygen sensor in seawater based on machine learning algorithm. Deep-Sea Res. Part I-Oceanogr. Res. Pap. 2022, 188, 103856. [Google Scholar] [CrossRef]
- Zhang, L.W.; Li, F.; Yang, Y.; Li, D.; Yu, H.; Dong, X.T.; Wang, T.Q. Polyoxometalates/metal-organic frameworks-derived ZnO/ZnWO4 nanoparticles for highly sensitive and selective ppb-level NO2 detection. Chem. Eng. J. 2024, 499, 156604. [Google Scholar] [CrossRef]
- Herres-Pawlis, S.; Berth, G.; Wiedemeier, V.; Schmidt, L.; Zrenner, A.; Warnecke, H.J. Oxygen sensing by fluorescence quenching of Cu(btmgp)I. J. Lumines 2010, 130, 1958–1962. [Google Scholar] [CrossRef]
- Hussain, E.; Cheng, C.; Li, Y.X.; Niu, N.; Zhou, H.P.; Jin, X.; Kong, J.F.; Yu, C. Benzo ghi perylene & coronene as ratiometric reversible optical oxygen nano-sensors. Sens. Actuator B-Chem. 2019, 287, 27–34. [Google Scholar]
- Shehata, N.; Samir, E.; Gaballah, S.; Salah, M. Optical sensing of peroxide using ceria nanoparticles via fluorescence quenching technique. J. Nanophotonics 2016, 10, 036002. [Google Scholar] [CrossRef]
- Ghosh, R.N.; Askeland, P.A.; Kramer, S.; Loloee, R. Optical dissolved oxygen sensor utilizing molybdenum chloride cluster phosphorescence. Appl. Phys. Lett. 2011, 98, 221103. [Google Scholar] [CrossRef]
- Lee, J.C.M.; Li, J.W.; Cheng, K.F.; Chen, J.X.; Ciou, Y.S.; Wang, J.H.; Lu, M.C.; Chen, Y.F.; Chiu, C.W. Facile Fabrication and Analysis of Highly Sensitive PtTFPP/Carbon Black/Polystyrene Oxygen-Sensitive Composite Films for Optical Dissolved-Oxygen Sensor. ACS Appl. Electron. Mater. 2024, 6, 1617–1627. [Google Scholar] [CrossRef]
- Werner, J.; Belz, M.; Klein, K.F.; Sun, T.; Grattan, K.T. Evaluation and optimization of the performance characteristics of fast response fiber optic oxygen gas probes. Sens. Actuator A-Phys. 2024, 365, 114933. [Google Scholar] [CrossRef]
- Sun, X.J.; Liu, F.M.; Wang, C.L.; Liu, X.X.; Jian, J.; Zeng, D.C.; Liu, L.J.; Yuan, H.M.; Lu, G.Y. A dense diffusion barrier limiting current oxygen sensor for detecting full concentration range. Sens. Actuator B-Chem. 2020, 305, 127521. [Google Scholar] [CrossRef]
- Lim, C.J.; Park, J.W. Luminescent oxygen-sensing films with improved sensitivity based on light scattering by TiO2 particles. Sens. Actuator B-Chem. 2017, 253, 934–941. [Google Scholar] [CrossRef]
- Dalfen, I.; Pol, A.; Borisov, S.M. Optical Oxygen Sensors Show Reversible Cross-Talk and/or Degradation in the Presence of Nitrogen Dioxide. ACS Sens. 2022, 7, 3057–3066. [Google Scholar] [CrossRef]
- Ko, C.N.; Li, G.D.; Leung, C.H.; Ma, D.L. Dual function luminescent transition metal complexes for cancer theranostics: The combination of diagnosis and therapy. Coord. Chem. Rev. 2019, 381, 79–103. [Google Scholar] [CrossRef]
- Li, X.L.; Roussakis, E.; Cascales, J.P.; Marks, H.L.; Witthauer, L.; Evers, M.; Manstein, D.; Evans, C.L. Optimization of bright, highly flexible, and humidity insensitive porphyrin-based oxygen-sensing materials. J. Mater. Chem. C 2021, 9, 7555–7567. [Google Scholar] [CrossRef]
- Liu, Y.H.; Li, B.; Wu, X.D.; Cong, Y. Effect of organic modification on oxygen sensing properties of xerogel with a covalently linked ruthenium(II) complex. J. Optoelectron. Adv. Mater. 2009, 11, 880–886. [Google Scholar]
- Zhao, H.M.; Zang, L.X.; Zhao, H.; Zhang, Y.G.; Zheng, Y.D.; Zhang, Z.G.; Cao, W.W. Oxygen sensing properties of gadolinium labeled hematoporphyrin monomethyl ether based on filter paper. Sens. Actuator B-Chem. 2015, 206, 351–356. [Google Scholar] [CrossRef]
- Kou, M.; Qin, F.; Wang, Y.D.; Zhang, X.Y.; Hu, Z.; Zhang, Z.G. Insight into the Heavy Atom Effect Induced by Environmental Heavy Atoms for Gadolinium-Labeled Hematoporphyrin Monomethyl Ether. J. Phys. Chem. B 2023, 127, 777–782. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, X.Y.; Zhang, H.L.; Zhao, H.; Zhang, Z.G.; Tian, Y. Method for monitoring singlet oxygen quantum yield in real time by time resolved spectroscopy measurement. Opt. Express 2020, 28, 25757–25766. [Google Scholar] [CrossRef]
- Zhao, H.M.; Zang, L.X.; Liu, Q.Y.; Ma, B.J.; Kou, M.; Lv, J.H.; Guo, C.S. Enhancement of the room temperature phosphorescence of metalloporphyrins using imidazole as a triplet state protector. J. Lumines. 2018, 194, 29–32. [Google Scholar] [CrossRef]
- Zhang, H.L.; Liu, T.; Li, Q.H.; Zhang, X.Y.; Zhao, H.; Zheng, Y.D.; Qin, F.; Zhang, Z.G.; Sheng, T.Q.; Tian, Y. Large-scale sensitivity adjustment for Gd-HMME room temperature phosphorescence oxygen sensing. Spectroc. Acta Pt. A-Molec. Biomolec. Spectr. 2022, 267, 120490. [Google Scholar] [CrossRef]
- Jang, E.S.; Kang, C.W. The pore structure and sound absorption capabilities of Homalium (Homalium foetidum) and Jelutong (Dyera costulata). Wood Sci. Technol. 2022, 56, 323–344. [Google Scholar] [CrossRef]
- Galos, J.; Das, R.; Sutcliffe, M.P.; Mouritz, A.P. Review of balsa core sandwich composite structures. Mater. Des. 2022, 221, 111013. [Google Scholar] [CrossRef]
- He, S.M.; Chen, C.J.; Kuang, Y.D.; Mi, R.Y.; Liu, Y.; Pei, Y.; Kong, W.Q.; Gan, W.T.; Xie, H.; Hitz, E.; et al. Nature-inspired salt resistant bimodal porous solar evaporator for efficient and stable water desalination. Energy Environ. Sci. 2019, 12, 1558–1567. [Google Scholar] [CrossRef]
- Alqrinawi, H.; Ahmed, B.; Wu, Q.L.; Lin, H.; Kameshwar, S.; Shayan, M. Effect of partial delignification and densification on chemical, morphological, and mechanical properties of wood: Structural property evolution. Ind. Crop. Prod. 2024, 213, 118430. [Google Scholar] [CrossRef]
- Zhu, X.J.; Zhang, T.; Zhao, S.S.; Wong, W.K.; Wong, W.Y. Synthesis, Structure, and Photophysical Properties of Some Gadolinium(III) Porphyrinate Complexes. J. Inorg. Chem. 2011, 2011, 3314–3320. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Qin, F.; Li, L.P.; Liu, T.; Zhang, H.L.; Zhao, H.; Zhang, Z.G. A high sensitivity optical thermometry on the basis of the intensity ratio between the fluorescence and phosphorescence in lutecium porphyrin. J. Lumines. 2022, 246, 118823. [Google Scholar] [CrossRef]
- Zhao, H.M.; Zang, L.X.; Guo, C.S. Influence of lanthanide ion energy levels on luminescence of corresponding metalloporphyrins. Phys. Chem. Chem. Phys. 2017, 19, 7728–7732. [Google Scholar] [CrossRef]
- Gehlen, M.H. The centenary of the Stern-Volmer equation of fluorescence quenching: From the single line plot to the SV quenching map. Photochem. Photobiol. C-Photochem. Rev. 2020, 42, 100338. [Google Scholar] [CrossRef]
- Seo, D.; Lim, D.; Seo, J.; Shin, D. Quantification of auto-photobleaching effects during Raman measurements for microplastic detection. Sens. Actuator B-Chem. 2025, 423, 136702. [Google Scholar] [CrossRef]
- Ji, S.; Kim, S.; Kim, H.; Koh, H.R. Investigation on photobleaching of fluorophores: Effect of excitation power and buffer system. Bull. Korean Chem. Soc. 2022, 43, 191–195. [Google Scholar] [CrossRef]
- Akram, M.; Shi, J.Y.; Khalid, H.; Zeng, F.; Tian, Y.Q. Morphological effect of fabricated surfaces obtained from fluorinated porphyrin based copolymer for oxygen and pressure sensing applications. Eur. Polym. J. 2023, 192, 112081. [Google Scholar] [CrossRef]
- Wang, Y.D.; Sun, Z.Y.; Peng, L.X.; Kou, M.; Qin, F.; Zhang, Z.G. A wide range oxygen sensing strategy with the collaboration of multiple phosphorescence probes. Phys. Scr. 2024, 99, 075038. [Google Scholar] [CrossRef]
- Salaris, N.; Chen, W.Q.; Haigh, P.; Caciolli, L.; Giobbe, G.G.; De Coppi, P.; Papakonstantinou, I.; Tiwari, M.K. Nonwoven fiber meshes for oxygen sensing. Biosens. Bioelectron. 2024, 255, 116198. [Google Scholar] [CrossRef] [PubMed]
- Koren, K.; Hutter, L.; Enko, B.; Pein, A.; Borisov, S.M.; Klimant, I. Tuning the dynamic range and sensitivity of optical oxygen-sensors by employing differently substituted polystyrene-derivatives. Sens. Actuator B-Chem. 2013, 176, 344–350. [Google Scholar] [CrossRef]
- Zhang, K.H.; Lu, S.Y.; Qu, Z.; Feng, X. Tuning the Sensitivity and Dynamic Range of Optical Oxygen Sensing Films by Blending Various Polymer Matrices. Biosensors 2022, 12, 5. [Google Scholar] [CrossRef]
- Yang, Z.Q.; Zhao, S.Q.; Zhang, X.Y.; Liu, M.; Liu, H.C.; Yang, B. Efficient Room-Temperature Phosphorescence from Discrete Molecules Based on Thianthrene Derivatives for Oxygen Sensing and Detection. Front. Chem. 2022, 9, 810304. [Google Scholar] [CrossRef]






| Matrix and Dye | Detection Range (kPa) | Limit of Detection | KSV (kPa−1) | Response Time | Reference |
|---|---|---|---|---|---|
| PS and PtTPP | 5–20 | - | <0.85 | <60 s | [44] |
| Fiber and PtOEP | 0–60 | - | - | <164 s | [45] |
| tButPS and PtTPTBPF4 | 0–55 | - | 0.477 | <10 s | [46] |
| EC and PtOEP | 0–70 | - | 0.298 | 3 s | [47] |
| PMMA and TA2P | 0–1.61 | 0.0979 Pa | 10.22 | - | [48] |
| Methanol and Gd-HMME | 0–8 | ~0.03 kPa | 0.124 | - | [32] |
| Filter paper and Gd-HMME | 10–100 | - | ~0.016 | ~0.4 s | [28] |
| Balsa wood and Gd-HMME | 0–100 | 0.01 kPa | <0.0264 | ~3.9 s | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, Y.; Wang, J.; Zhang, Z.; Liu, T. Design of Wood-Based Gd (III)-Hemoporphyrin Monomethyl Ether Eco-Material for Optical Oxygen Sensing with a Wide Detection Range. Sensors 2025, 25, 1670. https://doi.org/10.3390/s25061670
Niu Y, Wang J, Zhang Z, Liu T. Design of Wood-Based Gd (III)-Hemoporphyrin Monomethyl Ether Eco-Material for Optical Oxygen Sensing with a Wide Detection Range. Sensors. 2025; 25(6):1670. https://doi.org/10.3390/s25061670
Chicago/Turabian StyleNiu, Yujie, Jinxin Wang, Zhongxing Zhang, and Ting Liu. 2025. "Design of Wood-Based Gd (III)-Hemoporphyrin Monomethyl Ether Eco-Material for Optical Oxygen Sensing with a Wide Detection Range" Sensors 25, no. 6: 1670. https://doi.org/10.3390/s25061670
APA StyleNiu, Y., Wang, J., Zhang, Z., & Liu, T. (2025). Design of Wood-Based Gd (III)-Hemoporphyrin Monomethyl Ether Eco-Material for Optical Oxygen Sensing with a Wide Detection Range. Sensors, 25(6), 1670. https://doi.org/10.3390/s25061670






