Molecular Shape-Preserving Au Electrode for Progesterone Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instrumentation
2.2. Apparatus and Measurements
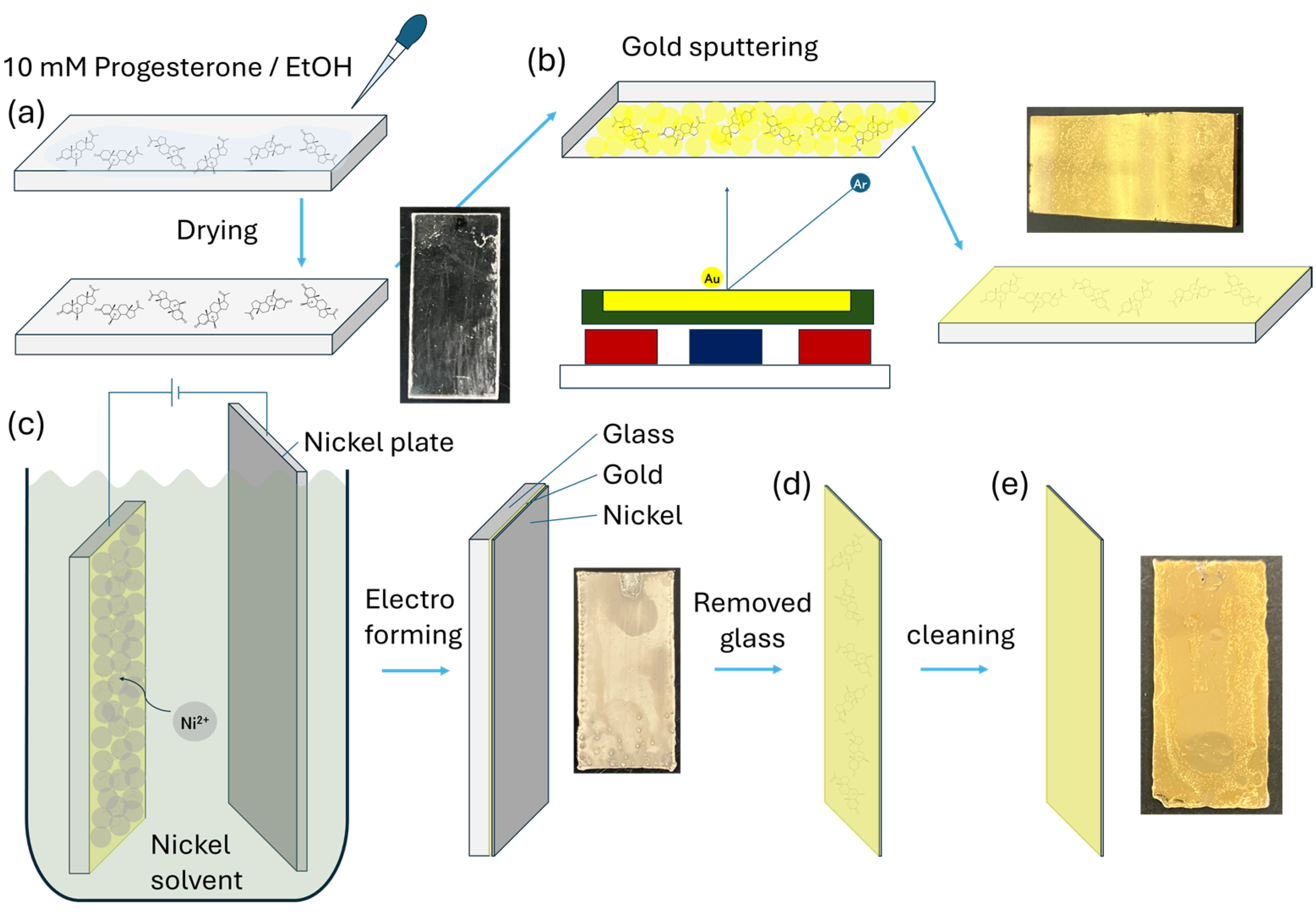
2.3. Au Sputtering on Progesterone
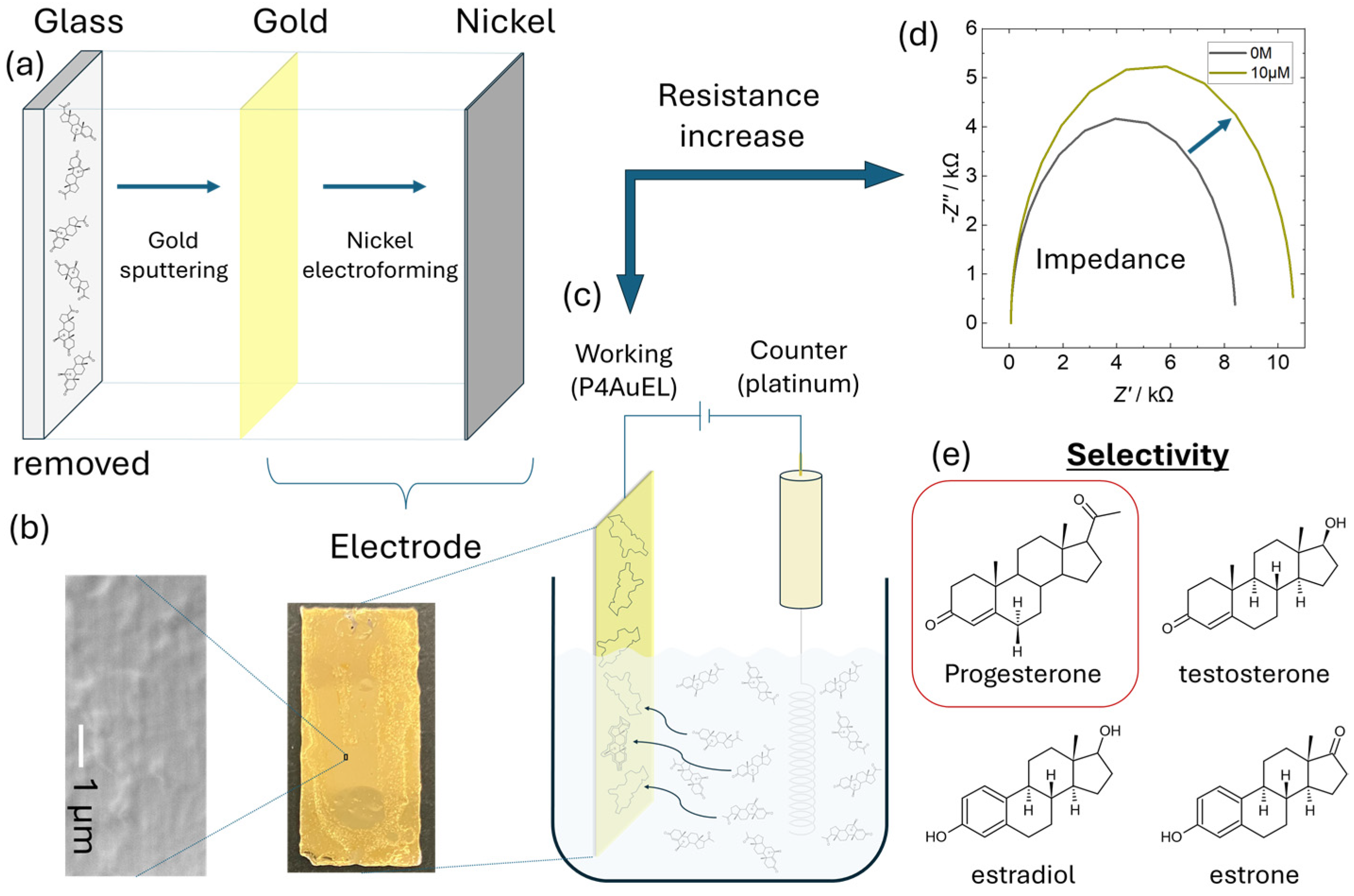
2.3.1. Progesterone Au Electrode (P4AuEL) Fabrication
2.3.2. Electrochemical Progesterone Detection
3. Results and Discussion
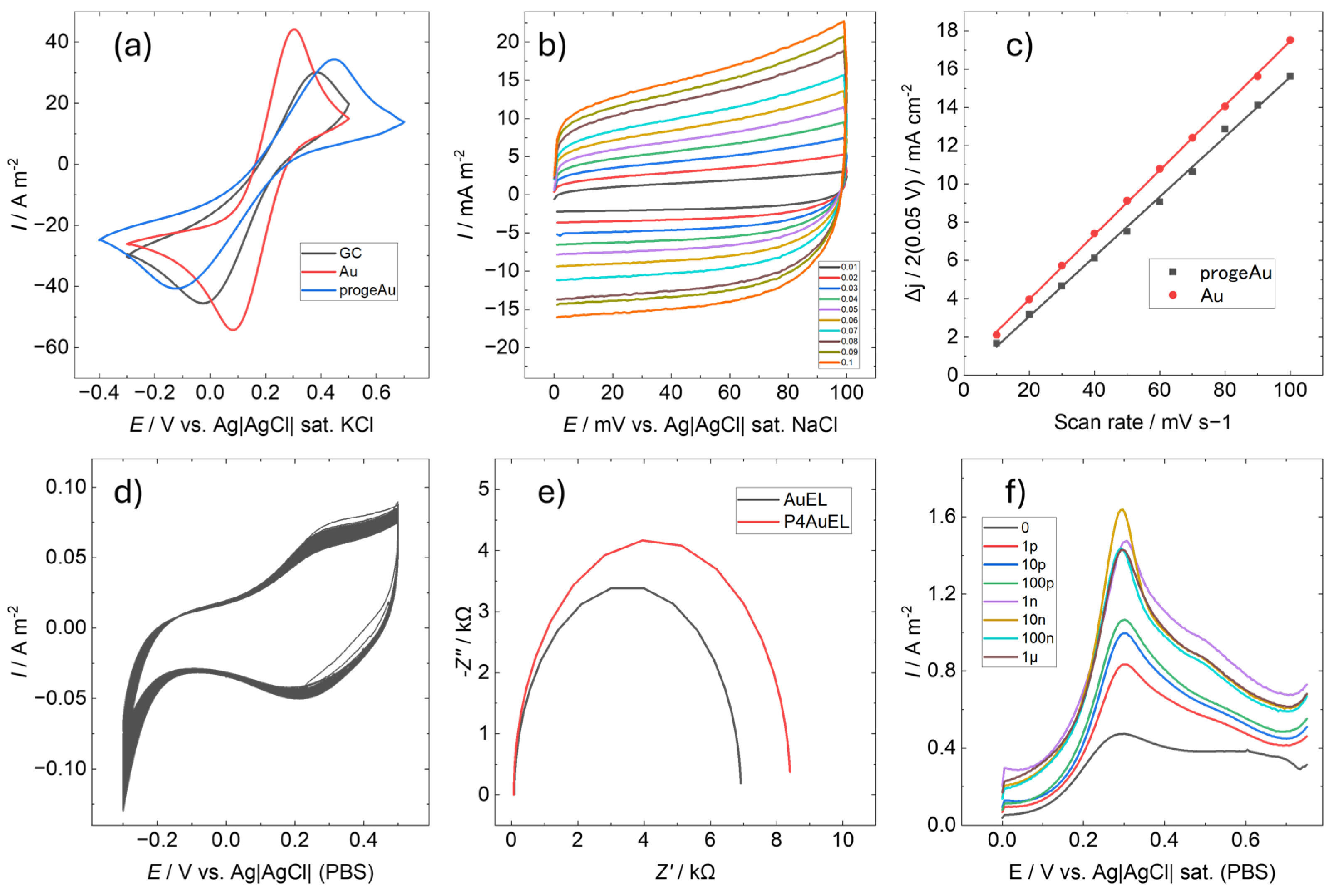
3.1. Structural Properties of P4Au
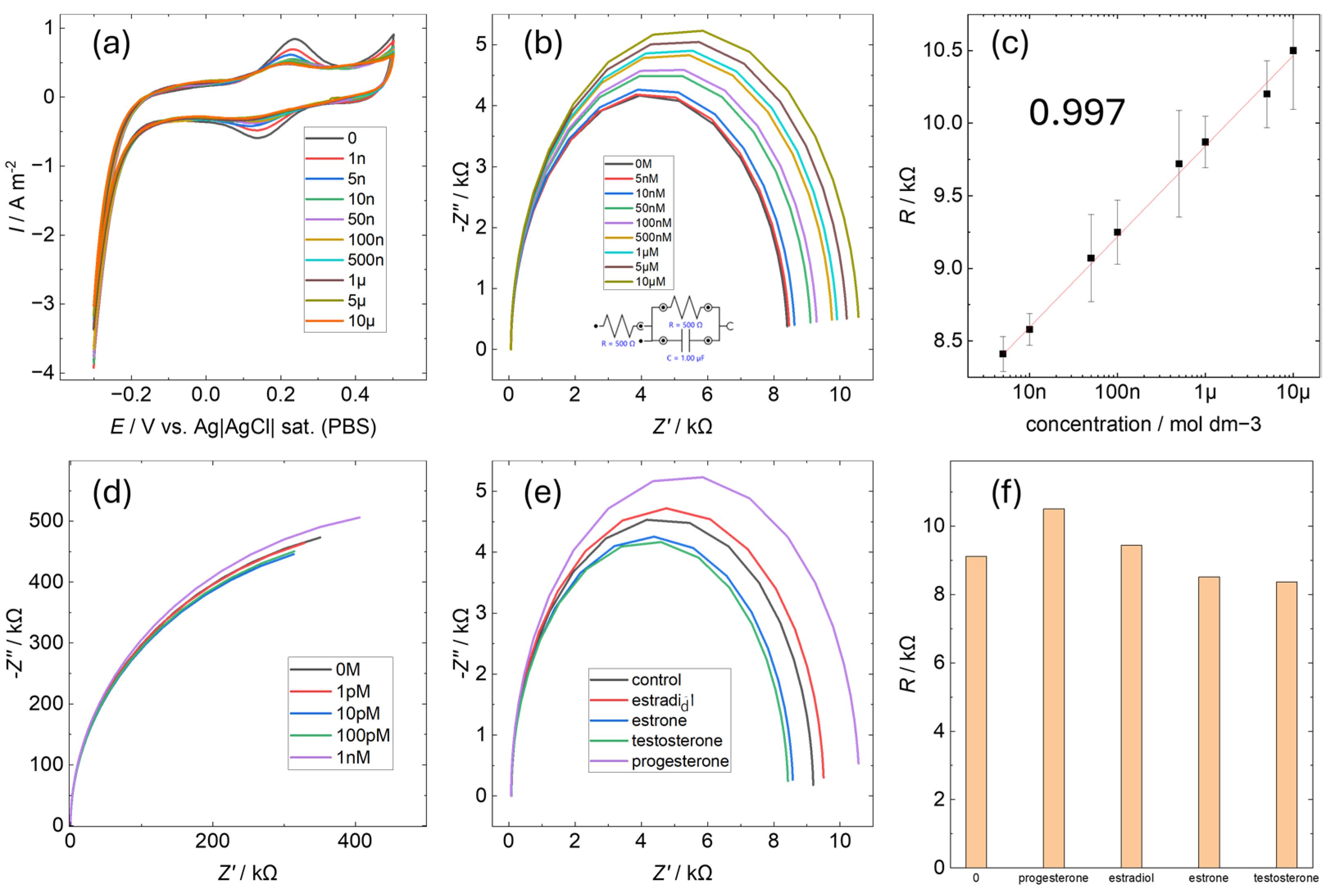
3.2. Electrochemical Properties of P4AuEL
3.3. Electrochemical Selectivity of P4AuEL for Progesterone
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFM | atomic force microscopy |
| CV | cyclic voltammetry |
| ECSA | electrochemical surface area |
| ELISA | enzyme-linked immunosorbent assay |
| FIA | fluorescence immunoassay |
| FRET | fluorescence resonance energy transfer |
| MIP | molecularly imprinted polymer |
| RLS | resonance light scattering |
| SPR | surface plasmon resonance |
| SWV | square wave voltammetry |
References
- Karsenty, G. The facts of the matter: What is a hormone? PLoS Genet. 2020, 16, e1008938. [Google Scholar] [CrossRef] [PubMed]
- Emanuel, R.H.K.; Roberts, J.; Docherty, P.D.; Lunt, H.; Campbell, R.E.; Möller, K. A review of the hormones involved in the endocrine dysfunctions of polycystic ovary syndrome and their interactions. Front. Endocrinol. 2022, 13, 1017468. [Google Scholar] [CrossRef] [PubMed]
- Hiller-Sturmhöfel, S.; Bartke, A. The endocrine system: An overview. Alcohol Health Res. World 1998, 22, 153–164. [Google Scholar] [PubMed]
- Bouman, A.; Heineman, M.J.; Faas, M.M. Sex hormones and the immune response in humans. Hum. Reprod. Update 2005, 11, 411–423. [Google Scholar] [CrossRef]
- Taraborrelli, S. Physiology, production and action of progesterone. Acta Obstet. Gynecol. Scand. 2015, 94 (Suppl. S161), 8–16. [Google Scholar] [CrossRef]
- Konno, T.; Graham, A.R.; Rempel, L.A.; Ho-Chen, J.K.; Alam, S.M.K.; Bu, P.; Rumi, M.A.K.; Soares, M.J. Subfertility linked to combined luteal insufficiency and uterine progesterone resistance. Endocrinology 2010, 151, 4537–4550. [Google Scholar] [CrossRef]
- Li, X.; Feng, Y.; Lin, J.-F.; Billig, H.; Shao, R. Endometrial progesterone resistance and PCOS. J. Biomed. Sci. 2014, 21, 2. [Google Scholar] [CrossRef]
- Yonkers, K.A.; O’Brien, P.M.S.; Eriksson, E. Premenstrual syndrome. Lancet 2008, 371, 1200–1210. [Google Scholar] [CrossRef]
- Hughes, G.C. Progesterone and autoimmune disease. Autoimmun. Rev. 2012, 11, A502–A514. [Google Scholar] [CrossRef]
- Adriaens, I.; Saeys, W.; Huybrechts, T.; Lamberigts, C.; François, L.; Geerinckx, K.; Leroy, J.; De Ketelaere, B.; Aernouts, B. A novel system for on-farm fertility monitoring based on milk progesterone. J. Dairy Sci. 2018, 101, 8369–8382. [Google Scholar] [CrossRef]
- Yazdan, M.M.S.; Kumar, R.; Leung, S.W. The environmental and health impacts of steroids and hormones in wastewater effluent, as well as existing removal technologies: A review. Ecologies 2022, 3, 206–224. [Google Scholar] [CrossRef]
- Posthuma-Trumpie, G.A.; van Amerongen, A.; Korf, J.; van Berkel, W.J.H. Perspectives for on-site monitoring of progesterone. Trends Biotechnol. 2009, 27, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Nezhadali, A.; Es’Haghi, Z.; Khatibi, A. Selective extraction of progesterone hormones from environmental and biological samples using a polypyrrole molecularly imprinted polymer and determination by gas chromatography. Anal. Methods 2016, 8, 1813–1827. [Google Scholar] [CrossRef]
- Bienboire-Frosini, C.; Chabaud, C.; Esposito, R.; Mathieu, M. Application Note Validation of Two Steroid Hormone ELISA Kits with Ovine Blood Samples. 2018. Available online: https://www.researchgate.net/publication/331320404_APPLICATION_NOTE_Validation_of_Two_Steroid_Hormone_ELISA_Kits_with_Ovine_Blood_Samples?channel=doi&linkId=5c73c3df299bf1268d23200c&showFulltext=true (accessed on 10 December 2018).
- Nagy, B.; Poto, L.; Farkas, N.; Koppan, M.; Varnagy, A.; Kovacs, K.; Papp, S.; Bohonyi, N.; Bodis, J. Follicular fluid progesterone concentration is associated with fertilization outcome after IVF: A systematic review and meta-analysis. Reprod. Biomed. Online 2019, 38, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Xu, Q.; Sun, J.; Lin, X.; Pan, H. Design and development of biosensors for progesterone detection. J. Sens. 2023, 2023, 4803143. [Google Scholar] [CrossRef]
- Yu, J.Y.; Jiao, S.Q.; Nawaz, T.; Wang, S.Q.; Wei, T.X. Surface plasmone resonance sensor for biomimetic detection of progesterone with macroporous molecularly imprinted polymers prepared by visible light. IOP Conf. Ser. Mater. Sci. Eng. 2019, 688, 033032. [Google Scholar] [CrossRef]
- Disha, P.; Kumari, P.; Patel, M.K.; Kumar, P.; Nayak, M.K. Carbon Dots Conjugated Antibody as an Effective FRET-Based Biosensor for Progesterone Hormone Screening. Biosensors 2022, 12, 993. [Google Scholar] [CrossRef]
- Kohek, M.B.F.; Leme, C.R.M.; Nakamura, I.T.; De Oliveira, S.A.; Lando, V.; Mendonca, B.B. Effects of EDTA and sodium citrate on hormone measurements by fluorometric (FIA) and immunofluorometric (IFMA) methods. BMC Clin. Pathol. 2002, 2, 2. [Google Scholar] [CrossRef]
- Elbadawi, M.; Ong, J.J.; Pollard, T.D.; Gaisford, S.; Basit, A.W. Additive manufacturable materials for electrochemical biosensor electrodes. Adv. Funct. Mater. 2021, 31, 2006407. [Google Scholar] [CrossRef]
- Mohammad Mayet, A.; Ebrahimi, S.; Shukhratovich Abdullaev, S.; Alsaab, H.O.; Mansouri, S.; Malviya, J.; Hussien Alawadi, A.; Alsaalamy, A.; Kadhem Abid, M.; Thakur, G. Molecularly imprinted polymers for biosensing of hormones in food safety and biomedical analysis: Progress and perspectives. Mater. Today Chem. 2024, 35, 101899. [Google Scholar] [CrossRef]
- Mariappan, S.A.; Manickam, P. Electro-responsive molecularly imprinted polymers (E-MIPs) for rapid detection of progesterone in human serum samples. Anal. Chem. 2024, 96, 15222–15230. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-H.; Thomas, J.L.; Liu, W.-C.; Zhang, Z.-X.; Liu, B.-D.; Yang, C.-H.; Lin, H.-Y. A multichannel system integrating molecularly imprinted conductive polymers for ultrasensitive voltammetric determination of four steroid hormones in urine. Mikrochim. Acta 2019, 186, 695. [Google Scholar] [CrossRef] [PubMed]
- Qasim, S.; Hsu, S.-Y.; Rossi, E.; Salahshoor, Z.; Lin, C.-H.; Parada, L.P.; Fidalgo, M. Detection of progesterone in aqueous samples by molecularly imprinted photonic polymers. Mikrochim. Acta 2022, 189, 174. [Google Scholar] [CrossRef] [PubMed]
- Takemura, K.; Motomura, T.; Iwasaki, W.; Matsuda, N. Metal deposition and shape reproduction at biological temperatures on cell-level samples. Sci. Rep. 2022, 12, 13328. [Google Scholar] [CrossRef]
- Li, R.; Chen, Z.; Datye, A.; Simon, G.H.; Ketkaew, J.; Kinser, E.; Liu, Z.; Zhou, C.; Dagdeviren, O.E.; Sohn, S.; et al. Atomic imprinting into metallic glasses. Commun. Phys. 2018, 1, 75. [Google Scholar] [CrossRef]
- Zhou, C.; Datye, A.; Chen, Z.; Simon, G.H.; Wang, X.; Schroers, J.; Schwarz, U.D. Atomic imprinting in the absence of an intrinsic length scale. APL Mater. 2020, 8, 111104. [Google Scholar] [CrossRef]
- Chen, Z.; Datye, A.; Simon, G.H.; Zhou, C.; Kube, S.A.; Liu, N.; Liu, J.; Schroers, J.; Schwarz, U.D. Atomic-Scale Imprinting by Sputter Deposition of Amorphous Metallic Films. ACS Appl. Mater. Interfaces 2020, 12, 52908–52914. [Google Scholar] [CrossRef]
- Schwartzkopf, M.; Buffet, A.; Körstgens, V.; Metwalli, E.; Schlage, K.; Benecke, G.; Perlich, J.; Rawolle, M.; Rothkirch, A.; Heidmann, B.; et al. From atoms to layers: In situ gold cluster growth kinetics during sputter deposition. Nanoscale 2013, 11, 5053–5062. [Google Scholar] [CrossRef]
- Siegel, J.; Lyutakov, O.; Rybka, V.; Kolská, Z.; Švorčík, V. Properties of gold nanostructures sputtered on glass. Nanoscale Res. Lett. 2011, 1, 96. [Google Scholar] [CrossRef]
- de Sá, M.H.; Pereira, C.M. The relevance of the initial conditions in glassy carbon electrode sensing applications: The ferri/ferrocyanide redox reaction model system in aqueous solution. Electrochim. Acta 2024, 489, 144158. [Google Scholar] [CrossRef]
- Libansky, M.; Zima, J.; Barek, J.; Reznickova, A.; Svorcik, V.; Dejmkova, H. Basic electrochemical properties of sputtered gold film electrodes. Electrochim. Acta 2017, 251, 452–460. [Google Scholar] [CrossRef]
- Chinnaiah, J.; Kasian, O.; Dekshinamoorthy, A.; Vijayaraghavan, S.; Mayrhofer, K.J.J.; Cherevko, S.; Scholz, F. Tuning the Anodic and Cathodic Dissolution of Gold by Varying the Surface Roughness. ChemElectroChem 2021, 8, 1524–1530. [Google Scholar] [CrossRef]
- Laza, A.; Godoy, A.; Pereira, S.; Aranda, P.R.; Messina, G.A.; Garcia, C.D.; Raba, J.; Bertolino, F.A. Electrochemical determination of progesterone in calf serum samples using a molecularly imprinted polymer sensor. Microchem. J. 2022, 183, 108113. [Google Scholar] [CrossRef]
- Disha; Kumari, P.; Nayak, M.K.; Kumar, P. An electrochemical biosensing platform for progesterone hormone detection using magnetic graphene oxide. J. Mat. Chem. B 2021, 26, 5264–5271. [Google Scholar] [CrossRef]
- Pali, M.; Garvey, J.E.; Small, B.; Suni, I.I. Detection of Fish Hormones by Electrochemical Impedance Spectroscopy and Quartz Crystal Microbalance. Sens. Bio-Sens. Res. 2017, 13, 1–8. [Google Scholar] [CrossRef]
- Liu, W.; Ma, Y.; Sun, G.; Wang, S.; Deng, J.; Wei, H. Molecularly imprinted polymers on graphene oxide surface for EIS sensing of testosterone. Biosens. Bioelectron. 2017, 92, 305–312. [Google Scholar] [CrossRef]
- Ričanyová, J.; Gadzała-Kopciuch, R.; Reiffova, K.; Bazel, Y.; Buszewski, B. Molecularly imprinted adsorbents for preconcentration and isolation of progesterone and testosterone by solid phase extraction combined with HPLC. Adsorption 2010, 16, 473–483. [Google Scholar] [CrossRef]
- Contreras Jiménez, G.; Eissa, S.; Ng, A.; Alhadrami, H.; Zourob, M.; Siaj, M. Aptamer-Based Label-Free Impedimetric Biosensor for Detection of Progesterone. Anal. Chem. 2015, 87, 1075–1082. [Google Scholar] [CrossRef]
- Zeidan, E.; Shivaji, R.; Henrich, V.C.; Sandros, M.G. Nano-SPRi Aptasensor for the Detection of Progesterone in Buffer. Sci. Rep. 2016, 6, 26714. [Google Scholar] [CrossRef]
- Gevaerd, A.; Blaskievicz, S.F.; Zarbin, A.J.G.; Orth, E.S.; Bergamini, M.F.; Marcolino-Junior, L.H. Nonenzymatic electrochemical sensor based on imidazole-functionalized graphene oxide for progesterone detection. Biosens. Bioelectron. 2018, 112, 108–113. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soyama, F.; Motomura, T.; Takemura, K. Molecular Shape-Preserving Au Electrode for Progesterone Detection. Sensors 2025, 25, 1620. https://doi.org/10.3390/s25051620
Soyama F, Motomura T, Takemura K. Molecular Shape-Preserving Au Electrode for Progesterone Detection. Sensors. 2025; 25(5):1620. https://doi.org/10.3390/s25051620
Chicago/Turabian StyleSoyama, Fukuto, Taisei Motomura, and Kenshin Takemura. 2025. "Molecular Shape-Preserving Au Electrode for Progesterone Detection" Sensors 25, no. 5: 1620. https://doi.org/10.3390/s25051620
APA StyleSoyama, F., Motomura, T., & Takemura, K. (2025). Molecular Shape-Preserving Au Electrode for Progesterone Detection. Sensors, 25(5), 1620. https://doi.org/10.3390/s25051620






