Innovative Binocular Vision Testing for Phoria and Vergence Ranges Using Automatic Dual Rotational Risley Prisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Basic Refraction Examination
2.3. Binocular Visual Function Examination
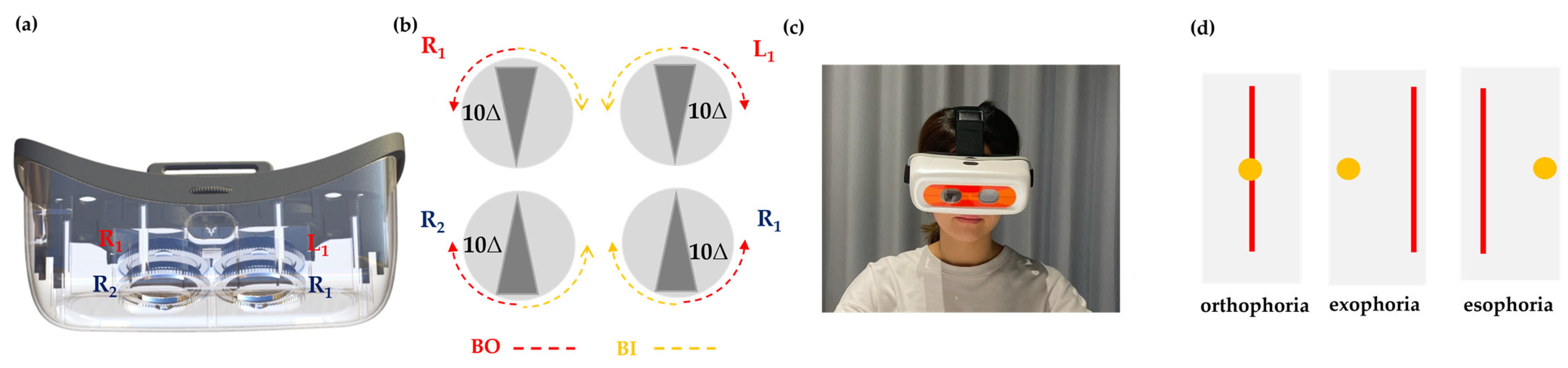
2.4. Design of the ADRRPs
2.5. Examiner’s Role in ADRRPs Testing
2.6. Phoria Testing
2.7. Vergence Ranges Testing
2.8. Statistical Analysis
3. Results
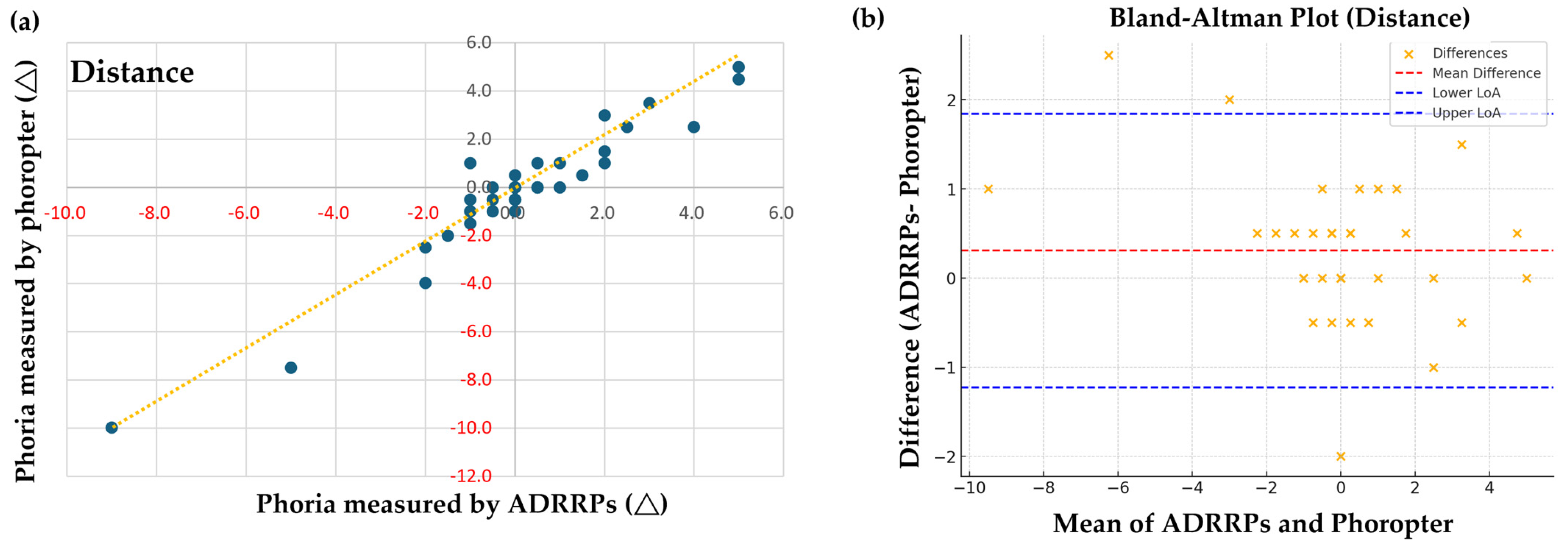
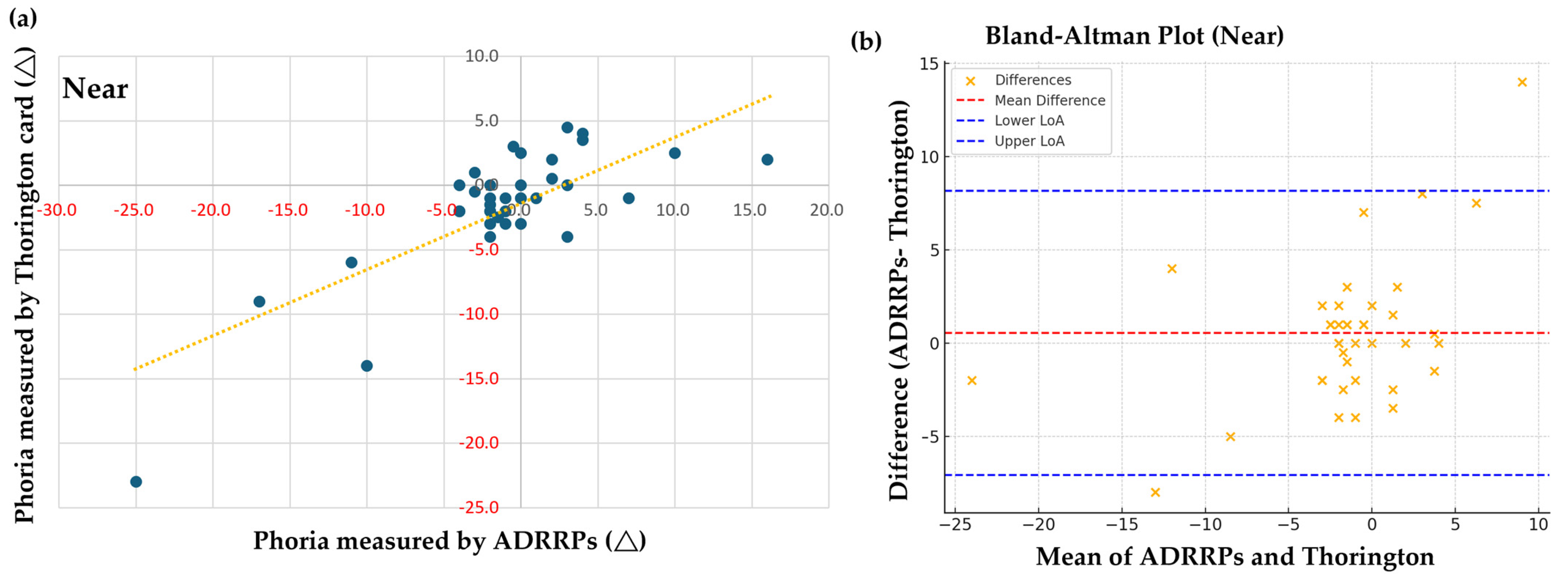
3.1. Comparison of Phoria Measurements Using ADRRPs and a Phoropter
3.2. Horizontal Fusional Vergence Testing
3.3. Positive Fusional Vergence
3.4. Negative Fusional Vergence
4. Discussion
Limitations
5. Conclusions
6. Patent
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADRRPs | automatic dual rotational Risley prisms |
| PFV | positive fusional vergence |
| NFV | negative fusional vergence |
| OD | right eye |
| OS | left eye |
| BCVA | best corrected visual acuity |
| SD | standard deviation |
References
- Hatt, S.R.; Leske, D.A.; Castañeda, Y.S.; Wernimont, S.M.; Liebermann, L.; Cheng-Patel, C.S.; Birch, E.E.; Holmes, J.M. Association of Strabismus with Functional Vision and Eye-Related Quality of Life in Children. JAMA Ophthalmol. 2020, 138, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Granet, D.B.; Gomi, C.F.; Ventura, R.; Miller-Scholte, A. The relationship between convergence insufficiency and ADHD. Strabismus 2005, 13, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Hamed, M.-M.; David, A.G.; Marzieh, E. The relationship between binocular vision symptoms and near point of convergence. Indian J. Ophthalmol. 2013, 61, 325–328. [Google Scholar] [CrossRef]
- Mena-Guevara, K.J.; de Fez, D.; Molina-Martín, A.; Piñero, D.P. Binocular vision measurements with a new online digital platform: Comparison with conventional clinical measures. Clin. Exp. Optom. 2023, 107, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, D.L.; Rosner, M.S.; Rosner, A.J. Validation of the Binocular Vision Dysfunction Questionnaire (BVDQ). Otol. Neurotol. 2021, 42, e66–e74. [Google Scholar] [CrossRef]
- Bossie, T.; Reilly, J.; Vera-Diaz, F.A. Comparison of a Novel Binocular Refraction System with Standard Digital Phoropter Refraction. Optom. Vis. Sci. 2023, 100, 451–458. [Google Scholar] [CrossRef]
- Ozgur, E.; Blanche, P.A.; Bedrick, E.J.; Conway, M.D.; Peyman, G.A.; Peyghambarian, N.N. Accuracy of an objective binocular automated phoropter for providing spectacle prescriptions. Clin. Exp. Optom. 2023, 107, 698–703. [Google Scholar] [CrossRef]
- Borish, I.M.; Benjamin, W.J. Monocular and binocular subjective refraction. In Borish’s Clinical Refraction, 2nd ed.; Benjamin, W.J., Ed.; Butterworth Heinemann: Oxford, UK; Elsevier: St. Louis, MO, USA, 2006; pp. 790–872. [Google Scholar]
- Harris, W. Subjective refraction: The mechanism underlying the routine. Ophthalmic Physiol. Opt. 2007, 27, 594–602. [Google Scholar] [CrossRef]
- Yuan, W.; Semmlow, J.L. The influence of repetitive eye movements on vergence performance. Vis. Res. 2000, 40, 3089–3098. [Google Scholar] [CrossRef]
- Alvarez, T.L.; Scheiman, M.; Santos, E.M.; Morales, C.; Yaramothu, C.; D’Antonio-Bertagnolli, J.D.; Biswal, B.B.; Gohel, S.; Li, X. The Convergence Insufficiency Neuro-mechanism in Adult Population Study (CINAPS) Randomized Clinical Trial: Design, Methods, and Clinical Data. Ophthalmic Epidemiol. 2020, 27, 52–72. [Google Scholar] [CrossRef]
- Elliott, D.B. Refraction and prescribing. In Clinical Procedures in Primary Eye Care, 5th ed.; Elliott, D.B., Ed.; Elsevier: New York, NY, USA, 2020; pp. 65–108. [Google Scholar]
- Carlson, N.B.; Kurtz, D. Refraction. In Clinical Procedures for Ocular Examination, 4th ed.; Carlson, N.B., Kurtz, D., Eds.; McGraw Hill Education: New York, NY, USA, 2016; pp. 111–205. [Google Scholar]
- Kinge, B.; Midelfart, A.; Jacobsen, G. Clinical Evaluation of the Allergan Humphrey 500 Autorefractor and the Nidek AR-1000 Autorefractor. Br. J. Ophthalmol. 1996, 80, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Bullimore, M.A.; Fusaro, R.E.; Adams, C.W. The Repeatability of Automated and Clinician Refraction. Optom. Vis. Sci. 1998, 75, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Rosenfield, M.; Chiu, N.N. Repeatability of Subjective and Objective Refraction. Optom. Vis. Sci. 1995, 72, 577–579. [Google Scholar] [CrossRef]
- Ohlendorf, A.; Leube, A.; Wahl, S. Steps Towards Smarter Solutions in Optometry and Ophthalmology—Inter-device Agreement of Subjective Methods to Assess the Refractive Errors of the Eye. Healthcare 2016, 4, 41. [Google Scholar] [CrossRef] [PubMed]
- Carracedo, G.; Carpena-Torres, C.; Serramito, M.; Batres-Valderas, L.; Gonzalez-Bergaz, A. Comparison between Aberrometry-based Binocular Refraction and Subjective Refraction. Transl. Vis. Sci. Technol. 2018, 7, 11. [Google Scholar] [CrossRef]
- Dave, T.; Fukuma, Y. Clinical Evaluation of the Topcon BV-1000 Automated Subjective Refraction System. Optom. Vis. Sci. 2004, 81, 323–333. [Google Scholar] [CrossRef]
- McCaghrey, G.E.; Matthews, F.E. Clinical Evaluation of a Range of Autorefractors. Ophthalmic Physiol. Opt. 1993, 13, 129–137. [Google Scholar] [CrossRef]
- Mallen, E.A.; Wolffsohn, J.S.; Gilmartin, B.; Tsujimura, S. Clinical Evaluation of the Shin-Nippon SRW-5000 Autorefractor in Adults. Ophthalmic Physiol. Opt. 2001, 21, 101–107. [Google Scholar] [CrossRef]
- Elliott, M.; Simpson, T.; Richter, D.; Fonn, D. Repeatability and Accuracy of Automated Refraction: A Comparison of the Nikon NRK-8000, the Nidek AR-1000, and Subjective Refraction. Optom. Vis. Sci. 1997, 74, 434–438. [Google Scholar] [CrossRef]
- Zadnik, K.; Mutti, D.O.; Adams, A.J. The Repeatability of Measurement of the Ocular Components. Investig. Ophthalmol. Vis. Sci. 1992, 33, 2325–2333. [Google Scholar]
- Chat, S.W.; Edwards, M.H. SRW-5000 Autorefractor in Children. Ophthalmic Physiol. Opt. 2001, 21, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Raasch, T.W.; Schechtman, K.B.; Davis, L.J.; Zadnik, K.; CLEK Study Group—Collaborative Longitudinal Evaluation of Keratoconus Study. Repeatability of Subjective Refraction in Myopic and Keratoconic Subjects: Results of Vector Analysis. Ophthalmic Physiol. Opt. 2001, 21, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.R.; Stalboerger, G.M.; Hodge, D.O.; Schornack, M.M. Comparison of Refractive Assessment by Wavefront Aberrometry, Autorefraction, and Subjective Refraction. J. Optom. 2015, 8, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Cowen, L. Binocular Refraction: A Simplified Clinical Routine. Br. J. Physiol. Opt. 1959, 16, 60–82. [Google Scholar]
- Amos, J.F. Binocular Refraction. In Clinical Procedures of Optometry; Eskridge, J.B., Amos, J.F., Bartlett, J.D., Eds.; J.B. Lippincott: Philadelphia, PA, USA, 1991; pp. 687–691. [Google Scholar]
- Kobashi, H.; Kamiya, K.; Handa, T.; Ando, W.; Kawamorita, T.; Igarashi, A.; Shimizu, K. Comparison of Subjective Refraction under Binocular and Monocular Conditions in Myopic Subjects. Sci. Rep. 2015, 5, 12606. [Google Scholar] [CrossRef]
- Segura, F.; Sanchez-Cano, A.; Lopez de la Fuente, C.; Fuentes-Broto, L.; Pinilla, I. Evaluation of Patient Visual Comfort and Repeatability of Refractive Values in Non-presbyopic Healthy Eyes. Int. J. Ophthalmol. 2015, 8, 1031–1036. [Google Scholar]
- Chen, Y.Y.; Hu, Y.S.; Huang, S.Y.; Tsai, T.-H. A Novel Device for Vergence Exercises: Testing Automatic Dual Rotational Risley Prisms on Asymptomatic Adults and Patients with Convergence Insufficiency. Ophthalmol. Ther. 2024, 13, 3189–3201. [Google Scholar] [CrossRef]
- Huang, S.Y.; Su, H.R.; Hu, Y.S.; Lee, C.-H.; Tsai, M.-S.; Yeh, S.-M.; Chen, Y.-Y.; Tsai, T.-H. Immediate Effects of Vergence Exercises Using Automatic Dual Rotational Risley Prisms on Accommodative Lag and Facility. Ophthalmol. Ther. 2023, 12, 3361–3372. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Agreement between methods of measurement with multiple observations per individual. J. Biopharm. Stat. 2007, 17, 571–582. [Google Scholar] [CrossRef]
- Lam, A.K.; Lam, A.; Charm, J.; Wong, K.M. Comparison of near heterophoria tests under varying conditions on an adult sample. Ophthalmic Physiol. Opt. 2005, 25, 162–167. [Google Scholar] [CrossRef]
- Oh, K.K.; Cho, H.G.; Moon, B.Y.; Kim, S.Y.; Yu, D.S. Change in lateral phoria under a phoropter and trial frame in phoria tests. J. Korean Ophthalmic Optom. Soc. 2020, 25, 395–403. [Google Scholar] [CrossRef]
- Casillas, E.; Rosenfield, M. Comparison of subjective heterophoria testing with a phoropter and trial frame. Optom. Vis. Sci. 2006, 83, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Han, S.J.; Guo, Y.; Granger-Donetti, B.; Vicci, V.R.; Alvarez, T.L. Quantification of heterophoria and phoria adaptation using an automated objective system compared to clinical methods. Ophthalmic Physiol. Opt. 2010, 30, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Mestre, C.; Otero, C.; Díaz-Doutón, F.; Gautier, J.; Pujol, J. An automated and objective cover test to measure heterophoria. PLoS ONE 2018, 13, e0206674. [Google Scholar] [CrossRef]
- Gantz, L.; Caspi, A. Synchronization of a removable optical element with an eye tracker: Test case for heterophoria measurement. Transl. Vis. Sci. Technol. 2020, 9, 40. [Google Scholar] [CrossRef]
- Rainey, B.B.; Schroeder, T.L.; Goss, D.A.; Grosvenor, T.P. Reliability of and Comparisons Among Methods of Measuring Dissociated Phoria. Optom. Vis. Sci. 1998, 75, 342–347. [Google Scholar]
- Anstice, N.S.; Davidson, B.; Field, B.; Mathan, J.; Collins, A.V.; Black, J.M. Repeatability of Phoria Measurements in Clinical Practice. J. Optom. 2021, 14, 123–129. [Google Scholar]
- Facchin, A.; Maffioletti, S. Inter-Instrument Agreement for Vergence Range Assessments. J. Optom. 2021, 14, 223–230. [Google Scholar]
- McDaniel, C.; Fogt, N. Vergence adaptation in clinical vergence testing. Optometry 2010, 81, 469–475. [Google Scholar] [CrossRef]
- Antona, B.; Barrio, A.; Barra, F.; Gonzalez, E.; Sanchez, I. Repeatability and Agreement in Fusional Vergence Measurements. Ophthalmic Physiol. Opt. 2008, 28, 475–486. [Google Scholar] [CrossRef]

| Inclusion Criteria (All of the Following Criteria Must Be Met) | Exclusion Criteria (Participants Will Be Excluded if Any of the Following Conditions Apply) |
|---|---|
| Age: 20–40 years Spherical refractive error between +0.00 and −11.00 Astigmatic refractive error ≤ −1.25 Best corrected visual acuity (BCVA) in one eye ≤0.1 logMAR Normal binocular visual function | Presence of ocular diseases Presence of systemic diseases History of ocular surgery Use of ocular-related medications History of strabismus or amblyopia |
| Participants (N = 39) | |
|---|---|
| Characteristics | Mean ± SD |
| Gender, (F/M) | 22/17 |
| Age (y) | 21.82 ± 1.10 |
| D Phoria (∆) | −0.18 ± 2.67 |
| N Phoria (∆) | −1.21 ± 6.89 |
| Spherical Equivalent (D) | |
| OD | −4.66 ± 2.92 |
| OS | −4.59 ± 2.96 |
| BCVA (logMAR) | |
| OD | 0.00 ± 0.07 |
| OS | 0.00 ± 0.06 |
| OU | −0.07 ± 0.04 |
| Measurements | ADRRPs | ||||
|---|---|---|---|---|---|
| Break Point | Recovery Point | ||||
| Phoropter | PFV | Distance | 0.758 *** | 0.452 *** | |
| Near | 0.817 *** | 0.727 *** | |||
| NFV | Distance | 0.863 *** | 0.458 *** | ||
| Near | 0.777 *** | 0.623 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, H.-R.; Chen, Y.-J.; Hu, Y.-S.; Lee, C.-H.; Yeh, S.-M.; Huang, S.-Y. Innovative Binocular Vision Testing for Phoria and Vergence Ranges Using Automatic Dual Rotational Risley Prisms. Sensors 2025, 25, 1604. https://doi.org/10.3390/s25051604
Su H-R, Chen Y-J, Hu Y-S, Lee C-H, Yeh S-M, Huang S-Y. Innovative Binocular Vision Testing for Phoria and Vergence Ranges Using Automatic Dual Rotational Risley Prisms. Sensors. 2025; 25(5):1604. https://doi.org/10.3390/s25051604
Chicago/Turabian StyleSu, Hui-Rong, Yu-Jung Chen, Yun-Shao Hu, Chi-Hung Lee, Shang-Min Yeh, and Shuan-Yu Huang. 2025. "Innovative Binocular Vision Testing for Phoria and Vergence Ranges Using Automatic Dual Rotational Risley Prisms" Sensors 25, no. 5: 1604. https://doi.org/10.3390/s25051604
APA StyleSu, H.-R., Chen, Y.-J., Hu, Y.-S., Lee, C.-H., Yeh, S.-M., & Huang, S.-Y. (2025). Innovative Binocular Vision Testing for Phoria and Vergence Ranges Using Automatic Dual Rotational Risley Prisms. Sensors, 25(5), 1604. https://doi.org/10.3390/s25051604







