Dynamic Response of Heart Rate Variability to Active Standing in Aortic Valve Disease: Insights from Recurrence Quantification Analysis
Abstract
1. Introduction
1.1. Epidemiology of AVD
1.2. Pathophysiology of AVD
1.3. Heart Rate Variability (HRV)
1.3.1. Linear Analysis of HRV
1.3.2. Nonlinear Analysis of HRV
2. Materials and Methods
2.1. Study Design and Patients
2.2. Study Protocol
2.3. Electrocardiogram Recording and Beat Identification
2.4. Heart Rate Variability Analysis
2.4.1. Time Domain and Frequency Domain
2.4.2. Recurrence Plot Analysis
2.5. Statistical Analysis
3. Results
3.1. Demographic Data
3.2. HRV Linear Indices
3.3. HRV Nonlinear Indices
3.4. Correlations Between meanNN and HRV Indices
3.5. Multiple Regression Analyses and HRV Indices
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.G.; Enriquez-Sarano, M. Burden of Valvular Heart Diseases: A Population-Based Study. Lancet 2006, 368, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Eveborn, G.W.; Schirmer, H.; Heggelund, G.; Lunde, P.; Rasmussen, K. The Evolving Epidemiology of Valvular Aortic Stenosis. The Tromsø Study. Heart 2013, 99, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, Z.; Bao, Q.; Lei, S.; You, Y.; Yin, Z.; Xie, X. Global Burden of Calcific Aortic Valve Disease and Attributable Risk Factors from 1990 to 2019. Front. Cardiovasc. Med. 2022, 9, 1003233. [Google Scholar] [CrossRef]
- Yan, A.T.; Koh, M.; Chan, K.K.; Guo, H.; Alter, D.A.; Austin, P.C.; Tu, J.V.; Wijeysundera, H.C.; Ko, D.T. Association Between Cardiovascular Risk Factors and Aortic Stenosis: The CANHEART Aortic Stenosis Study. J. Am. Coll. Cardiol. 2017, 69, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.D.; Probstfield, J.L.; Caulfield, M.T.; Nasir, K.; Takasu, J.; Shavelle, D.M.; Wu, A.H.; Zhao, X.-Q.; Budoff, M.J. Angiotensin-Converting Enzyme Inhibitors and Change in Aortic Valve Calcium. Arch. Intern. Med. 2005, 165, 858–862. [Google Scholar] [CrossRef]
- Stewart, B.F.; Siscovick, D.; Lind, B.K.; Gardin, J.M.; Gottdiener, J.S.; Smith, V.E.; Kitzman, D.W.; Otto, C.M. Clinical Factors Associated with Calcific Aortic Valve Disease Fn1. J. Am. Coll. Cardiol. 1997, 29, 630–634. [Google Scholar] [CrossRef]
- Owens, D.S.; Katz, R.; Takasu, J.; Kronmal, R.; Budoff, M.J.; O’Brien, K.D. Incidence and Progression of Aortic Valve Calcium in the Multi-Ethnic Study of Atherosclerosis (MESA). Am. J. Cardiol. 2010, 105, 701–708. [Google Scholar] [CrossRef]
- Chan, K.L.; Teo, K.; Dumesnil, J.G.; Ni, A.; Tam, J.; ASTRONOMER Investigators. Effect of Lipid lowering with rosuvastatin on progression of aortic stenosis: Results of the aortic stenosis progression observation: Measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation 2010, 121, 306–314. [Google Scholar] [CrossRef]
- Rosenhek, R.; Binder, T.; Porenta, G.; Lang, I.; Christ, G.; Schemper, M.; Maurer, G.; Baumgartner, H. Predictors of outcome in severe, asymptomatic aortic stenosis. N. Engl. J. Med. 2000, 343, 611–617. [Google Scholar] [CrossRef]
- Rossebø, A.B.; Pedersen, T.R.; Boman, K.; Brudi, P.; Chambers, J.B.; Egstrup, K.; Gerdts, E.; Gohlke-Bärwolf, C.; Holme, I.; Kesäniemi, Y.A.; et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N. Engl. J. Med. 2008, 359, 1343–1356. [Google Scholar] [CrossRef]
- Praz, F.; Beyersdorf, F.; Haugaa, K.; Prendergast, B. Valvular Heart Disease: From Mechanisms to Management. Lancet 2024, 403, 1576–1589. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.; Thyberg, J.; Nilsson, J. Presence of Oxidized Low Density Lipoprotein in Nonrheumatic Stenotic Aortic Valves. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 1218–1222. [Google Scholar] [CrossRef]
- Marquis-Gravel, G.; Redfors, B.; Leon, M.B.; Généreux, P. Medical Treatment of Aortic Stenosis. Circulation 2016, 134, 1766–1784. [Google Scholar] [CrossRef]
- González, H.; Infante, O.; Pérez-Grovas, H.; Jose, M.V.; Lerma, C. Nonlinear Dynamics of Heart Rate Variability in Response to Orthostatism and Hemodialysis in Chronic Renal Failure Patients: Recurrence Analysis Approach. Med. Eng. Phys. 2013, 35, 178–187. [Google Scholar] [CrossRef]
- Torres-Arellano, J.M.; Echeverría, J.C.; Ávila-Vanzzini, N.; Springall, R.; Toledo, A.; Infante, O.; Bojalil, R.; Cossío-Aranda, J.E.; Fajardo, E.; Lerma, C. Cardiac Autonomic Response to Active Standing in Calcific Aortic Valve Stenosis. J. Clin. Med. 2021, 10, 2004. [Google Scholar] [CrossRef] [PubMed]
- Henriques, T.; Ribeiro, M.; Teixeira, A.; Castro, L.; Antunes, L.; Costa-Santos, C. Nonlinear Methods Most Applied to Heart-Rate Time Series: A Review. Entropy 2020, 22, 309. [Google Scholar] [CrossRef]
- Papaioannou, V.E.; Pneumatikos, I.; Maglaveras, N. Association of Heart Rate Variability and Inflammatory Response in Patients with Cardiovascular Diseases: Current Strengths and Limitations. Front. Physiol. 2013, 4, 174. [Google Scholar] [CrossRef]
- Vukasovic, J.L.; Florenzano, F.; Adriazola, P.; Escobar, E. Heart Rate Variability in Severe Aortic Stenosis. J. Heart Valve Dis. 1999, 8, 143–148. [Google Scholar] [PubMed]
- Thayer, J.F.; Yamamoto, S.S.; Brosschot, J.F. The Relationship of Autonomic Imbalance, Heart Rate Variability and Cardiovascular Disease Risk Factors. Int. J. Cardiol. 2010, 141, 122–131. [Google Scholar] [CrossRef]
- Rodríguez-Carbó, J.; Torres-Arellano, J.M.; Ávila-Vanzzini, N.; Springall, R.; Bojalil, R.; Infante, O.; Lerma, C.; Echeverría, J.C. Association of the Heart Rate Variability Response to Active Standing with the Severity of Calcific Aortic Valve Disease: Novel Insights of a Neurocardiovascular Pathology. J. Clin. Med. 2022, 11, 4771. [Google Scholar] [CrossRef]
- Abboud, F.M.; Singh, M.V. Autonomic Regulation of the Immune System in Cardiovascular Diseases. Adv. Physiol. Educ. 2017, 41, 578–593. [Google Scholar] [CrossRef]
- Calderón-Juárez, M.; González-Gómez, G.H.; Echeverría, J.C.; Pérez-Grovas, H.; Lerma, C. Association between Mean Heart Rate and Recurrence Quantification Analysis of Heart Rate Variability in End-Stage Renal Disease. Entropy 2020, 22, 114. [Google Scholar] [CrossRef]
- Monfredi, O.; Lyashkov, A.E.; Johnsen, A.-B.; Inada, S.; Schneider, H.; Wang, R.; Nirmalan, M.; Wisloff, U.; Maltsev, V.A.; Lakatta, E.G.; et al. Biophysical Characterization of the Underappreciated and Important Relationship Between Heart Rate Variability and Heart Rate. Hypertension 2014, 64, 1334–1343. [Google Scholar] [CrossRef] [PubMed]
- Ackun, P. Association Between Heart Rate Resting State Entropy and Heart Rate Dynamics Among Healthy Adults and Patients With Aortic Stenosis. Master’s Thesis, The University of Arizona, Tucson, AZ, USA, 2024. [Google Scholar]
- Marwan, N.; Carmenromano, M.; Thiel, M.; Kurths, J. Recurrence Plots for the Analysis of Complex Systems. Phys. Rep. 2007, 438, 237–329. [Google Scholar] [CrossRef]
- Javorka, M.; Turianikova, Z.; Tonhajzerova, I.; Javorka, K.; Baumert, M. The Effect of Orthostasis on Recurrence Quantification Analysis of Heart Rate and Blood Pressure Dynamics. Physiol. Meas. 2008, 30, 29. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the Management of Valvular Heart Disease: Developed by the Task Force for the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Task Force of the European Society of Cardiology the North American Society of Pacing Electrophysiology; Camm, A.J.; Malik, M.; Bigger, J.T.; Breithardt, G.; Cerutti, S.; Cohen, R.J.; Coumel, P.; Fallen, E.L.; Kennedy, H.L.; et al. Heart Rate Variability: Standards of Measurement, Physiological Interpretation, and Clinical Use. Eur. Heart J. 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Infante, O.; Valenzuela, F.; Polo, S. Algoritmo Que Utiliza La Segunda Derivada Para Identificar El Complejo QRS En Tiempo Real. Rev. Mex. Ing. 1992, 13, 6. [Google Scholar]
- Wessel, N.; Voss, A.; Malberg, H.; Ziehmann, C.; Voss, H.U.; Schirdewan, A.; Meyerfeldt, U.; Kurths, J. Nonlinear Analysis of Complex Phenomena in Cardiological Data. Herzschrittmachertherapie Elektrophysiolog. 2000, 11, 159–173. [Google Scholar] [CrossRef]
- Peng, C.-K.; Havlin, S.; Stanley, H.E.; Goldberger, A.L. Quantification of Scaling Exponents and Crossover Phenomena in Nonstationary Heartbeat Time Series. Chaos Interdiscip. J. Nonlinear Sci. 1995, 5, 82–87. [Google Scholar] [CrossRef]
- Webber, C.L.; Zbilut, J.P. Dynamical Assessment of Physiological Systems and States Using Recurrence Plot Strategies. J. Appl. Physiol. 1994, 76, 965–973. [Google Scholar] [CrossRef]
- Zbilut, J.P.; Webber, C.L., Jr. Recurrence Quantification Analysis. In Wiley Encyclopedia of Biomedical Engineering; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006. [Google Scholar] [CrossRef]
- Eckmann, J.-P.; Kamphorst, S.O.; Ruelle, D. Recurrence Plots of Dynamical Systems. Europhys. Lett. 1987, 4, 973. [Google Scholar] [CrossRef]
- Marwan, N.; Wessel, N.; Meyerfeldt, U.; Schirdewan, A.; Kurths, J. Recurrence-plot-based measures of complexity and their application to heart-rate-variability data. Phys. Rev. E 2002, 66 Pt 2, 026702. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.M.; Bae, B.H.; Kim, S.Y. Divergence in Perpendicular Recurrence Plot; Quantification of Dynamical Divergence from Short Chaotic Time Series. Phys. Lett. A 1999, 263, 299–306. [Google Scholar] [CrossRef]
- Calderón-Juárez, M.; González-Gómez, G.H.; Echeverría, J.C.; Lerma, C. Revisiting Nonlinearity of Heart Rate Variability in Healthy Aging. Sci. Rep. 2023, 13, 13185. [Google Scholar] [CrossRef]
- Calderón-Juárez, M.; Cruz-Vega, I.B.; González-Gómez, G.H.; Lerma, C. Nonlinear Dynamics of Heart Rate Variability after Acutely Induced Myocardial Ischemia by Percutaneous Transluminal Coronary Angioplasty. Entropy 2023, 25, 469. [Google Scholar] [CrossRef]
- Acharya, U.R.; Faust, O.; Sree, V.; Swapna, G.; Martis, R.J.; Kadri, N.A.; Suri, J.S. Linear and Nonlinear Analysis of Normal and CAD-Affected Heart Rate Signals. Comput. Methods Programs Biomed. 2014, 113, 55–68. [Google Scholar] [CrossRef]
- Fielitz, J.; Hein, S.; Mitrovic, V.; Pregla, R.; Zurbrügg, H.R.; Warnecke, C.; Schaper, J.; Fleck, E.; Regitz-Zagrosek, V. Activation of the Cardiac Renin-Angiotensin System and Increased Myocardial Collagen Expression in Human Aortic Valve Disease. J. Am. Coll. Cardiol. 2001, 37, 1443–1449. [Google Scholar] [CrossRef]
- Tracey, K.J. The Inflammatory Reflex. Nature 2002, 420, 853–859. [Google Scholar] [CrossRef]
- Bojalil-Parra, R.; Massó-Rojas, F.A.; Amezcua-Guerra, L.M. Inflamación. In Inmunología Molecular, Celular y Traslacional, 1st ed.; Pavon, L., Jiménez, M.C., Garcés, M.E., Eds.; Wolters-Kluwer: Alphen aan den Rijn, The Netherlands, 2016; p. 106. [Google Scholar]





| Variables | Healthy Valve (N = 22) | Aortic Valve Sclerosis (N = 73) | Aortic Valve Stenosis (N = 32) | p |
|---|---|---|---|---|
| Age (years) | 41.3 ± 7.9 | 45.3 ± 9.3 | 63.3 ± 6.6 | <0.001 |
| Gender (Men) | 12 (54.5) | 33 (45.2) | 21 (65.6) | 0.151 |
| Metabolic variables | ||||
| BMI (kg/m2) | 27.35 ± 3.69 | 28.16 ± 4.72 | 29.50 ± 4.94 | 0.210 |
| Hypertension | 2 (3.8) | 4 (12.6) | 16 (50) | <0.001 |
| Diabetes type II | 0 | 2 (2.7) | 7 (21.9) | 0.002 |
| Dyslipidemia | 0 | 5 (6.8) | 10 (31.3) | 0.001 |
| Alcoholism | 10 (45.5) | 32 (43.8) | 17 (53.1) | 0.676 |
| Smoking | 6 (27.3) | 26 (35.6) | 12 (37.5) | 0.714 |
| Statins | 0 | 0 | 7 (5.5) | <0.001 |
| Acetylsalicylic acid | 0 | 0 | 10 (31.3) | <0.001 |
| Cholesterol total (mg/dL) | 192 ± 34 | 192 ± 38 | 177 ± 37 | 0.150 |
| C-HDL (mg/dL) | 41.59 ± 10.54 | 44.31 ± 8.55 | 43.17 ± 10.75 | 0.485 |
| C-LDL (mg/dL) | 125 ± 32 | 124 ± 33 | 103 ± 33 | 0.009 |
| Triglycerides (mg/dL) | 139 (113–163) | 133 (94–188) | 144 (102–188) | 0.927 |
| Atherogenic index | 3.21 ± 1.19 | 2.88 ± 0.86 | 2.58 ± 1.23 | 0.082 |
| Echocardiographic parameters | ||||
| Vmax (m/s) | 1.2 (1.0–1.3) | 1.3 (1.1–1.4) | 4.23 (3.2–5.5) | <0.001 |
| Mean gradient (mmHg) | 3.0 (2.0–3.0) | 3.0 (2.0–4.0) | 41.0 (23.0–71.0) | <0.001 |
| LVEF (%) | 61.9 ± 6.4 | 62.3 ± 6.6 | 54.7 ± 8.6 | <0.001 |
| Heart rate (beats per minute) | 60.8 ± 9.7 | 60.9 ± 8.7 | 63.0 ± 10.8 | 0.549 |
| AVA (cm2) | 4.2 (4.03–4.2) | 4.1 (3.96–4.26) | 0.6 (0.41–1.26) | <0.001 |
| SBP (mmHg) | 110 (110–118) | 116 (110–122) | 130 (120–160) | <0.001 |
| DBP (mmHg) | 78.0 (70.0–80.0) | 78.0 (70.0–80.0) | 80.0 (70.0–80.0) | 0.478 |
| Healthy Valve (N = 22) | Aortic Valve Sclerosis (N = 73) | Aortic Valve Stenosis (N = 32) | |
|---|---|---|---|
| Supine position | |||
| Mean NN (s) | 0.995 ± 0.180 ** | 0.991 ± 0.137 ** | 0.988 ± 0.159 ** |
| SDNN (s) | 0.051 ± 0.019 | 0.054 ± 0.025 * | 0.045 ± 0.024 |
| pNN20 (%) | 57.4 ± 17.5 ** | 55.35 ± 19.39 ** | 40.37 ± 24.89 * b,c |
| SDSD (s) | 0.041 ± 0.021 * | 0.038 ± 0.020 ** | 0.029 ± 0.017 * |
| LF n.u | 58.51 ± 15.66 ** | 65.14 ± 19.36 ** | 73.54 ± 28.17 b |
| HF n.u | 44.06 ± 14.46 ** | 37.90 ± 17.96 ** | 31.42 ± 20.09 * b |
| LF/HF | 1.57 ± 0.870 * | 2.91 ± 4.03 ** | 4.92 ± 7.08 b |
| Active Standing | |||
| Mean NN (s) | 0.823 ± 0.153 | 0.835 ± 0.102 | 0.887 ± 0.121 |
| SDNN (s) | 0.053 ± 0.030 | 0.045 ± 0.013 | 0.039 ± 0.016 b |
| pNN20 (%) | 39.28 ± 21.73 | 34.90 ± 18.07 | 30.10 ± 18.66 |
| SDSD (s) | 0.031 ± 0.024 | 0.023 ± 0.010 | 0.021 ± 0.009 b |
| LF n.u | 80.74 ± 10.27 | 81.28 ± 11.96 | 75.79 ± 15.71 |
| HF n.u | 19.25 ± 10.27 | 18.71 ± 11.96 | 24.20 ± 15.71 |
| LF/HF | 5.61 ± 3.30 | 6.76 ± 5.25 | 4.59 ± 2.99 |
| Magnitude of change | |||
| Δ Mean NN (s) | 0.171 ± 0.068 | 0.156 ± 0.081 | 0.100 ± 0.097 b,c |
| Δ SDNN (s) | −0.001 ± 0.024 | 0.009 ± 0.025 | 0.006 ± 0.020 |
| Δ pNN20 (%) | 18.128 ± 13.401 | 20.445 ± 16.450 | 10.274 ± 19.377 c |
| Δ SDSD | 0.010 ± 0.011 | 0.015 ± 0.017 | 0.008 ± 0.015 |
| Δ LF (n.u) | −22.235 ± 19.017 | −16.144 ± 17.092 | −2.256 ± 24.431 b,c |
| Δ HF (n.u) | 24.816 ± 17.708 | 19.190 ± 15.908 | 7.223 ± 17.296 b,c |
| Δ LF/HF | −4.038 ± 3.343 | −3.854 ± 5.929 | 0.337 ± 5.201 b,c |
| Healthy Valve (N = 22) | Aortic Valve Sclerosis (N = 73) | Aortic Valve Stenosis (N = 32) | Total (N = 127) | |
|---|---|---|---|---|
| Statistical indices | ||||
| SDNN (s) | 0.611 ** | 0.394 ** | 0.292 * | 0.404 ** |
| pNN20 (%) | 0.830 ** | 0.602 ** | 0.686 ** | 0.635 ** |
| SDSD (s) | 0.832 ** | 0.595 ** | 0.643 ** | 0.638 ** |
| Spectral indices | ||||
| LF n.u | −0.487 ** | −0.312 ** | −0.362 * | −0.349 ** |
| HF n.u | 0.486 ** | 0.363 ** | 0.396 * | 0.389 ** |
| LF/HF | −0.490 ** | −0.264 ** | −0.167 | −0.255 ** |
| Healthy Valve (N = 22) | Aortic Valve Sclerosis (N = 73) | Aortic Valve Stenosis (N = 32) | Total (N = 127) | |
|---|---|---|---|---|
| Determinism | −0.742 ** | −0.562 ** | −0.426 ** | −0.554 ** |
| Mean diagonal length | −0.641 ** | −0.354 ** | −0.304 * | −0.329 ** |
| Maximum diagonal length | −0.550 ** | −0.507 ** | −0.341 * | −0.446 ** |
| Shannon entropy | −0.716 ** | −0.446 ** | −0.412 ** | −0.450 ** |
| Laminarity | −0.765 ** | −0.554 ** | −0.463 ** | −0.564 ** |
| Trapping Time | −0.660 ** | −0.443 ** | −0.449 ** | −0.448 ** |
| Maximum vertical length | −0.648 ** | −0.615 ** | −0.607 ** | −0.600 ** |
| TT 1 | −0.029 | 0.112 | 0.072 | 0.073 |
| TT 2 | −0.703 ** | −0.436 ** | −0.370 * | −0.449 ** |
| Variables | Standardized β | β (C.I.95%) | p-Value | Corrected R2 |
|---|---|---|---|---|
| Predicted HRV index: SDNN | 0.157 | |||
| Mean NN | 0.353 | 0.059 (0.029–0.089) | <0.001 | |
| AVS condition | Excluded variable | |||
| Age | Excluded variable | |||
| SBP | Excluded variable | |||
| Statins | Excluded variable | |||
| Predicted HRV index: pNN20 | 0.509 | |||
| Mean NN | 0.571 | 81.213 (61.857–100.569) | <0.001 | |
| AVS condition | Excluded variable | |||
| Age | −0.351 | −0.642 [−1.014–(−0.270)] | 0.001 | |
| SBP | Excluded variable | |||
| Statins | Excluded variable | |||
| Predicted HRV index: SDSD | 0.439 | |||
| Mean NN | 0.586 | 0.081 (0.061–0.101) | <0.001 | |
| AVS condition | Excluded variable | |||
| Age | −0.294 | −0.001 (−0.001–0.000) | 0.008 | |
| SBP | Excluded variable | |||
| Statins | Excluded variable | |||
| Predicted HRV index: LFnu. | 0.148 | |||
| Mean NN | −0.210 | −30.794 [−57.072–(−4.517)] | 0.022 | |
| AVS condition | Excluded variable | |||
| Age | Excluded variable | |||
| SBP | 0.348 | 0.409 (0.165–0.652) | 0.001 | |
| Statins | Excluded variable | |||
| Predicted HRV index: HFnu. | 0.148 | |||
| Mean NN | 0.206 | 24.591 (3.146–46.036) | 0.025 | |
| AVS condition | Excluded variable | |||
| Age | Excluded variable | |||
| SBP | −0.308 | −0.294 [−0.493–(−0.096)] | 0.004 | |
| Statins | Excluded variable | |||
| Predicted HRV index: LF/HF | 0.099 | |||
| Mean NN | Excluded variable | |||
| AVS condition | Excluded variable | |||
| Age | Excluded variable | |||
| SBP | 0.243 | 0.066 (0.008–0.124) | 0.026 | |
| Statins | 0.246 | 5.455 (1.016–9.893) | 0.017 | |
| Variables | Standardized β | β (C.I.95%) | p-Value | Corrected R2 |
|---|---|---|---|---|
| Predicted HRV index: Determinism | 0.313 | |||
| Mean NN | −0.409 | −0.462 [−0.644–(−0.280)] | <0.001 | |
| AVS condition | Excluded variable | |||
| Age | Excluded variable | |||
| SBP | 0.303 | 0.003 (0.001–0.004) | 0.002 | |
| Statins | 0.245 | 0.180 (0.051–0.308) | 0.006 | |
| Predicted HRV index: Mean diagonal length | 0.187 | |||
| Mean NN | −0.236 | −0.873 [−1.521–(−0.226)] | 0.009 | |
| AVS condition | Excluded variable | |||
| Age | Excluded variable | |||
| SBP | 0.326 | 0.010 (0.004–0.016) | 0.002 | |
| Statins | Excluded variable | |||
| Predicted HRV index: Maximum diagonal length | 0.283 | |||
| Mean NN | −0.315 | −24.024 [−36.549–(−11.500)] | <0.001 | |
| AVS condition | Excluded variable | |||
| Age | Excluded variable | |||
| SBP | 0.378 | 0.230 (0.114–0.346) | <0.001 | |
| Statins | 0.185 | 9.172 (0.329–18.016) | 0.042 | |
| Predicted HRV index: Shannon entropy | 0.278 | |||
| Mean NN | −0.344 | −0.762 [−1.128–(−0.397)] | <0.001 | |
| AVS condition | Excluded variable | |||
| Age | Excluded variable | |||
| SBP | 0.295 | 0.005 (0.002–0.009) | 0.003 | |
| Statins | Excluded variable | |||
| Predicted HRV index: Laminarity | 0.293 | |||
| Mean NN | −0.434 | −0.583 [−0.802–(−0.364)] | <0.001 | |
| AVS condition | Excluded variable | |||
| Age | Excluded variable | |||
| SBP | 0.226 | 0.002 (0.000–0.004) | 0.019 | |
| Statins | 0.247 | 0.216 (0.061–0.370) | 0.007 | |
| Predicted HRV index: Trapping Time | 0.302 | |||
| Mean NN | −0.321 | −0.968 [−1.457–(−0.479)] | <0.001 | |
| AVS condition | Excluded variable | |||
| Age | Excluded variable | |||
| SBP | 0.291 | 0.007 (0.002–0.012) | 0.003 | |
| Statins | Excluded variable | |||
| Predicted HRV index: Maximum vertical length | 0.391 | |||
| Mean NN | −0.566 | −12.133 [−15.379–(−8.886)] | <0.001 | |
| AVS condition | Excluded variable | |||
| Age | Excluded variable | |||
| SBP | 0.226 | 0.039 (0.009–0.069) | 0.012 | |
| Statins | Excluded variable | |||
| Predicted HRV index: TT 1 | 0.047 | |||
| Mean NN | Excluded variable | |||
| AVS condition | Excluded variable | |||
| Age | Excluded variable | |||
| SBP | −0.238 | −0.021 [−0.040–(−0.002)] | 0.034 | |
| Statins | Excluded variable | |||
| Predicted HRV index: TT 2 | 0.157 | |||
| Mean NN | −0.263 | −12.750 [−21.388–(−4.113)] | 0.004 | |
| AVS condition | Excluded variable | |||
| Age | Excluded variable | |||
| SBP | Excluded variable | |||
| Statins | 0.247 | 7.768 (1.668–13.867) | 0.013 | |
| Supine Position vs. Active Standing (Within Same Group) | Comparisons vs. Healthy Valve (During Supine Position) | ||||
|---|---|---|---|---|---|
| HRV Nonlinear Indices | Healthy Valve | Aortic Valve Sclerosis | Aortic Valve Stenosis | Aortic Valve Sclerosis | Aortic Valve Stenosis |
| Determinism |  |  |  |  |  |
| Mean diagonal length |  |  |  |  |  |
| Maximum diagonal length |  |  |  |  |  |
| Shannon entropy |  |  |  |  |  |
| Laminarity |  |  |  |  |  |
| Trapping Time |  |  |  |  |  |
| Maximum vertical length |  |  |  |  |  |
| Trapping Time 1 |  |  |  |  |  |
| Trapping Time 2 |  |  |  |  |  |
| Δ Different from Zero | Comparisons vs. Healthy Valve (During Supine Position) | ||||
|---|---|---|---|---|---|
| Δ HRV Nonlinear Indices | Healthy Valve | Aortic Valve Sclerosis | Aortic Valve Stenosis | Aortic Valve Sclerosis | Aortic Valve Stenosis |
| Δ Determinism | * | * | - | 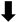 | 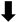 |
| Δ Mean diagonal length | * | * | - | 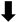 | 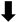 |
| Δ Maximum diagonal length | * | * | - |  |  |
| Δ Shannon entropy | * | * | - |  |  |
| Δ Laminarity | * | * | - |  |  |
| Δ Trapping Time | * | * | - |  |  |
| Δ Maximum vertical length | * | * | * |  |  |
| Δ Trapping Time 1 | - | - | - |  |  |
| Δ Trapping Time 2 | * | * | - |  |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz-Vega, I.B.; Ávila-Vanzzini, N.; González-Gómez, G.H.; Springall, R.; Echeverría, J.C.; Lerma, C. Dynamic Response of Heart Rate Variability to Active Standing in Aortic Valve Disease: Insights from Recurrence Quantification Analysis. Sensors 2025, 25, 1535. https://doi.org/10.3390/s25051535
Cruz-Vega IB, Ávila-Vanzzini N, González-Gómez GH, Springall R, Echeverría JC, Lerma C. Dynamic Response of Heart Rate Variability to Active Standing in Aortic Valve Disease: Insights from Recurrence Quantification Analysis. Sensors. 2025; 25(5):1535. https://doi.org/10.3390/s25051535
Chicago/Turabian StyleCruz-Vega, Itayetzin Beurini, Nydia Ávila-Vanzzini, Gertrudis Hortensia González-Gómez, Rashidi Springall, Juan C. Echeverría, and Claudia Lerma. 2025. "Dynamic Response of Heart Rate Variability to Active Standing in Aortic Valve Disease: Insights from Recurrence Quantification Analysis" Sensors 25, no. 5: 1535. https://doi.org/10.3390/s25051535
APA StyleCruz-Vega, I. B., Ávila-Vanzzini, N., González-Gómez, G. H., Springall, R., Echeverría, J. C., & Lerma, C. (2025). Dynamic Response of Heart Rate Variability to Active Standing in Aortic Valve Disease: Insights from Recurrence Quantification Analysis. Sensors, 25(5), 1535. https://doi.org/10.3390/s25051535









