Non-Invasive Monitoring and Differentiation of Aging Mice Treated with Goat Whey Powder by an Electronic Nose Coupled with Chemometric Methods †
Abstract
1. Introduction
2. Materials and Methods
2.1. Aging Animal Model
2.2. Test Methodology
2.2.1. Weight, Antioxidant Ability, Serum Antibody, and Cytokine Determination
2.2.2. Intestinal Bacteria
2.2.3. Collection of Fecal Samples
2.2.4. Electronic Nose (E-Nose) and the Detection Procedure
2.3. Statistical Analysis
3. Results and Discussion
3.1. Typical Response of E-Nose Sensor to Fecal Samples
3.2. Correlation Between Sensor Responses and Physiological Indexes
3.2.1. Correlation Between Sensor Responses and Antioxidant Indexes
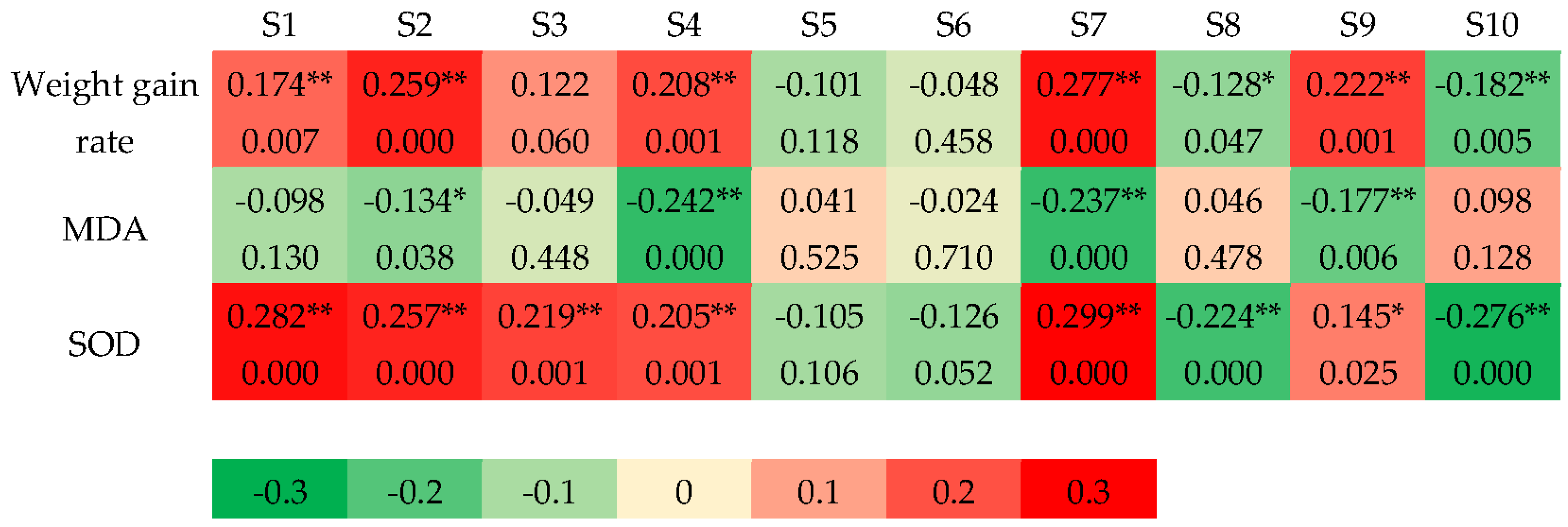
3.2.2. Correlation Between Sensor Responses and Serum Antibodies and Cytokines
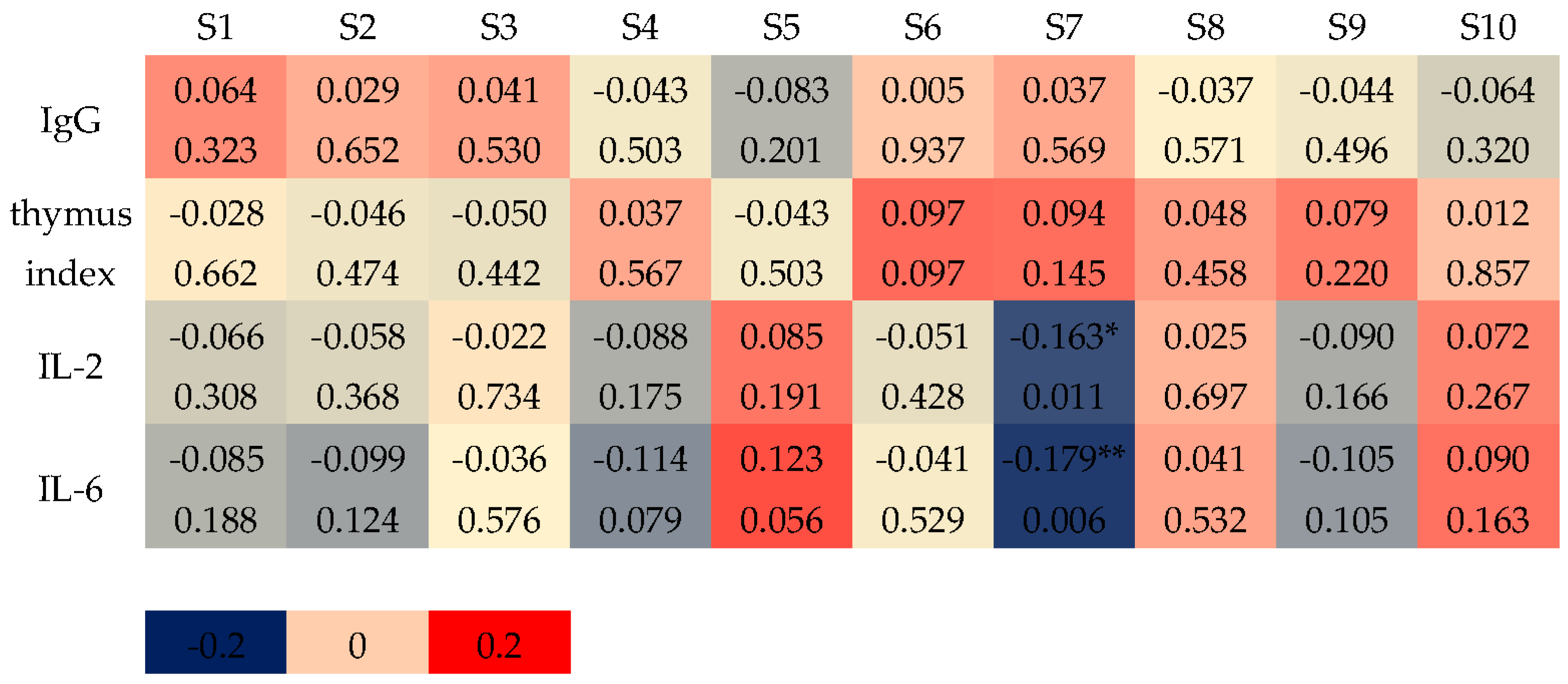
3.2.3. Correlation Between Sensor Responses and Intestinal Bacteria
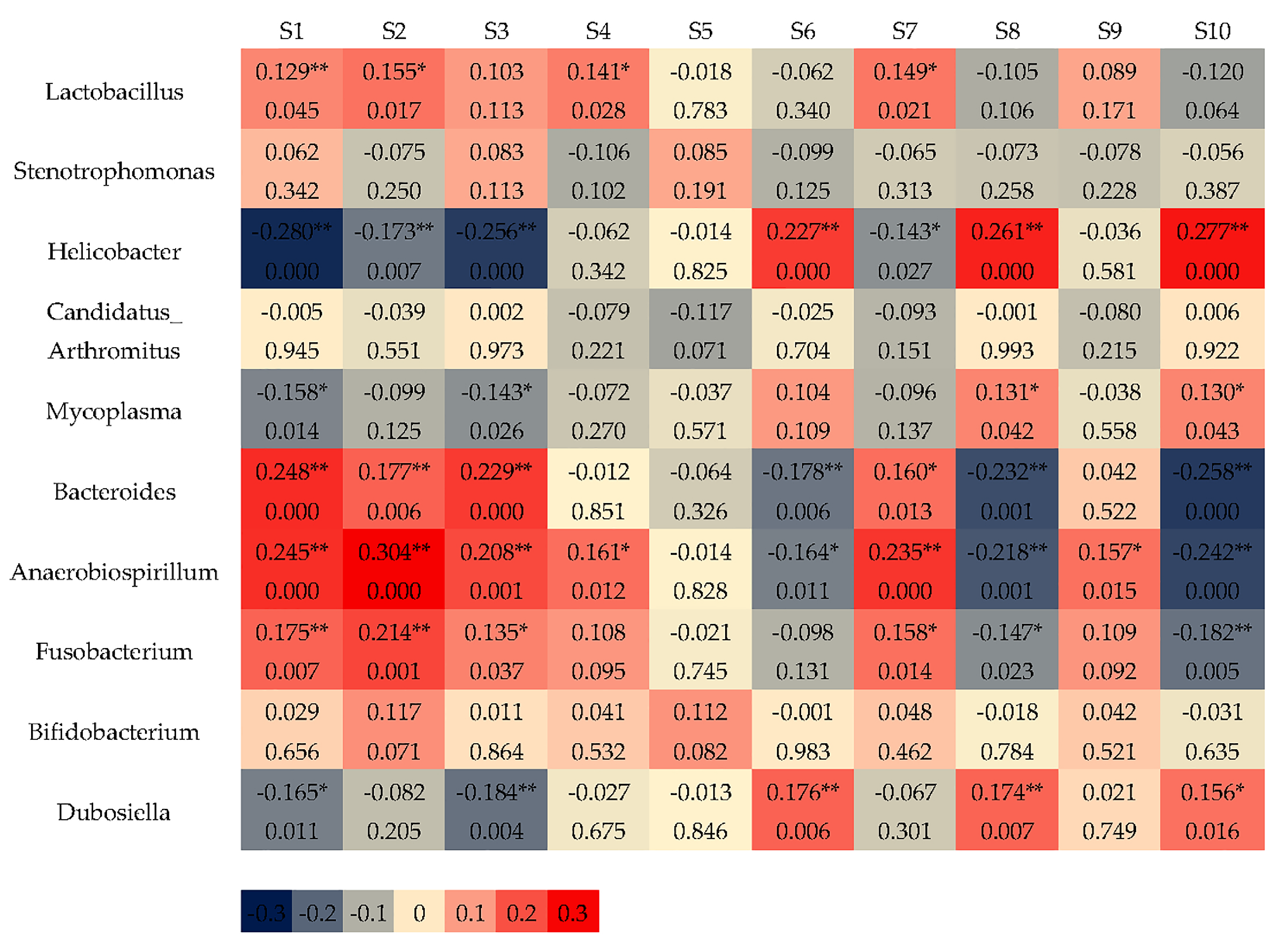
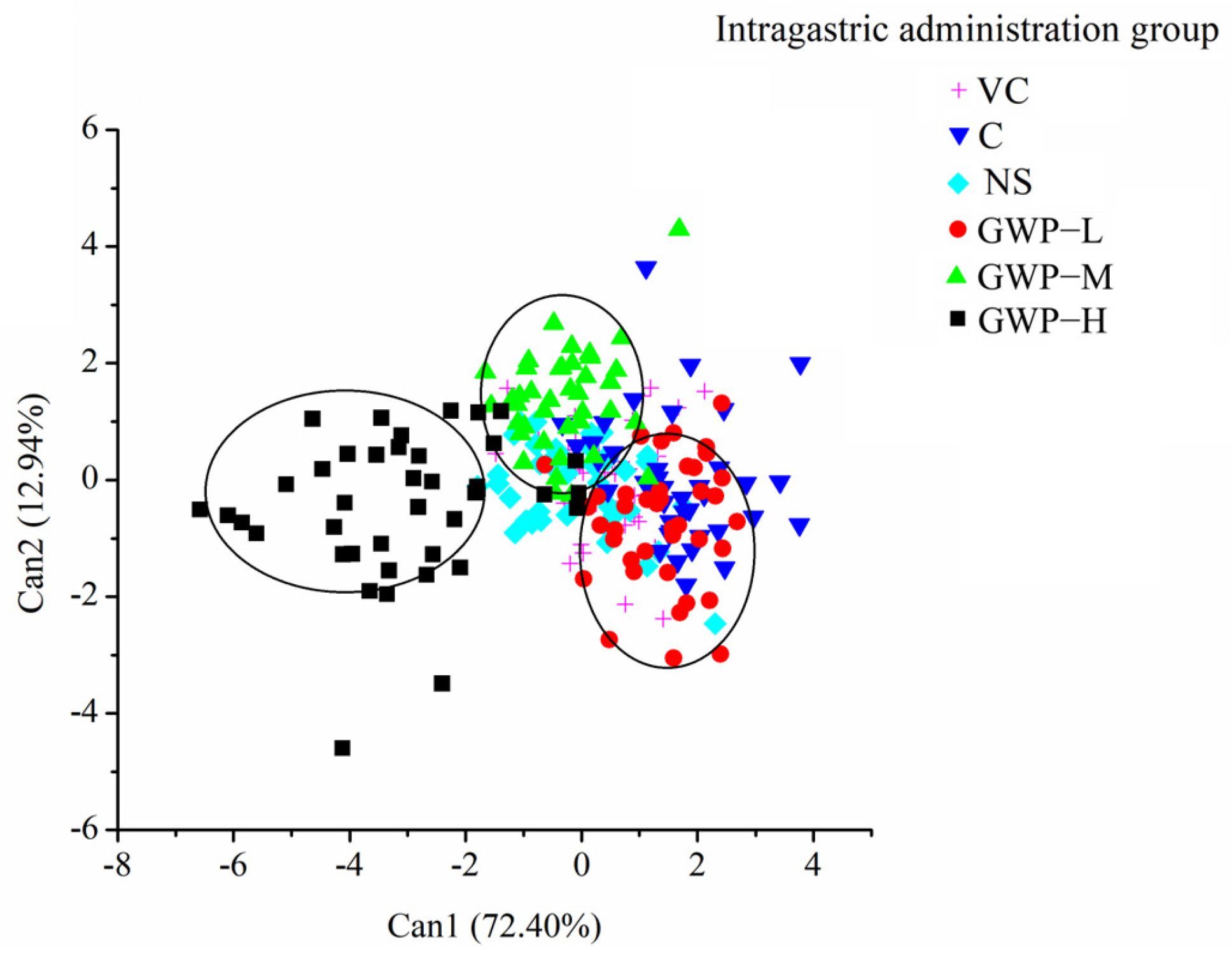
3.3. Monitoring the GWP Intervention Process by E-Nose
3.4. Multilayer Perceptron Neural Network (MLP) Results
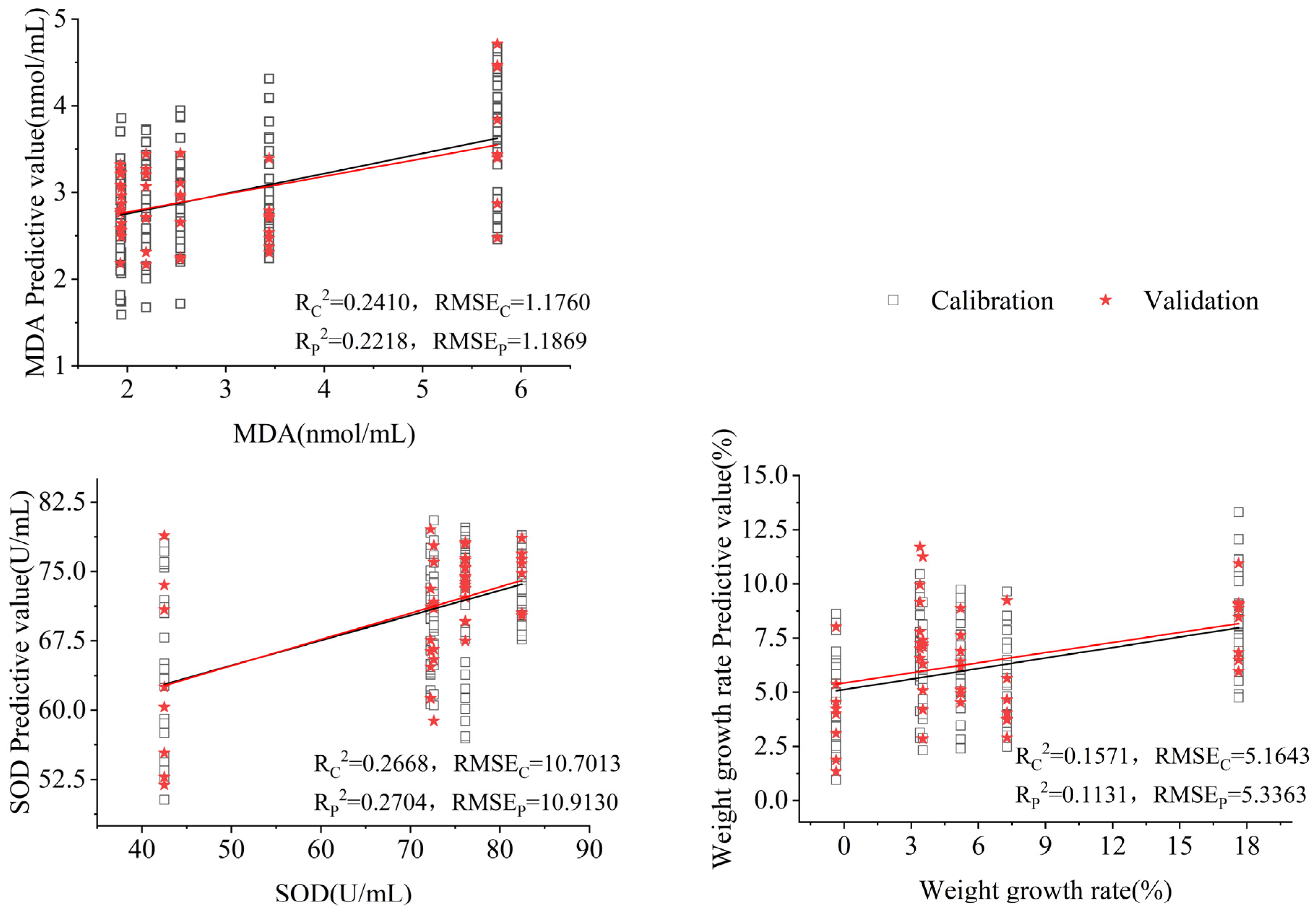
3.5. Rapid Characterization of Antioxidant Indicators for Aging Mice with Different Interventions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hejtmánková, A.; Pivec, V.; Trnková, E.; Dragounová, H. Differences in the composition of total and whey proteins in goat and ewe milk and their changes throughout the lactation period. Czech J. Anim. Sci. 2012, 57, 323–331. [Google Scholar] [CrossRef]
- Wit, J.N.D. Nutritional and Functional Characteristics of Whey Proteins in Food Products. J. Dairy Sci. 1998, 81, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Tipton, K.D.; Elliott, T.A.; Cree, M.G.; Aarsland, A.A.; Sanford, A.P.; Wolfe, R.R. Stimulation of net muscle protein synthesis by whey protein ingestion before and after exercise. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E71–E76. [Google Scholar] [CrossRef] [PubMed]
- Hati, S.; Patel, N.; Skaure, A.; Mandal, S. Influence of Whey Protein Concentrate on the Production of Antibacterial Peptides Derived from Fermented Milk by Lactic Acid Bacteria. Int. J. Pept. Res. Ther. 2018, 24, 87–98. [Google Scholar] [CrossRef]
- Garg, G.; Singh, S.; Singh, A.K.; Rizvi, S.I. Whey protein concentrate supplementation protects rat brain against aging-induced oxidative stress and neurodegeneration. Appl. Physiol. Nutr. Metab. 2018, 43, 437–444. [Google Scholar] [CrossRef]
- Corrochano, A.R.; Buckin, V.; Kelly, P.M.; Giblin, L. Invited review: Whey proteins as antioxidants and promoters of cellular antioxidant pathways. J. Dairy Sci. 2018, 101, 4747–4761. [Google Scholar] [CrossRef]
- Bounous, G.; Gervais, F.; Amer, V.; Batist, G.; Gold, P. The influence of dietary whey protein on tissue glutathione and the diseases of aging. Clin. Investig. Med. 1989, 12, 343–349. [Google Scholar]
- Solak, B.B.; Akin, N. Health Benefits of Whey Protein: A Review. J. Food Eng. 2012, 2, 129–137. [Google Scholar]
- Patel, S. Functional food relevance of whey protein: A review of recent findings and scopes ahead. J. Funct. Foods 2015, 19, 308–319. [Google Scholar] [CrossRef]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Scientific Opinion on the essential composition of infant and follow-on formulae. EFSA J. 2014, 12, 3760. [Google Scholar] [CrossRef]
- Bido, R.D.C.D.A.; Pereira, D.E.; Alves, M.D.C.; Dutra, L.M.G.; Coata, A.C.D.S.; Viera, V.B.; Araújo, W.J.D.; Leite, E.L.; Oliveira, C.J.B.D.; Alves, A.F.; et al. Mix of almond baru (Dipteryx alata Vog.) and goat whey modulated intestinal microbiota, improved memory and induced anxiolytic like behavior in aged rats. J. Psychiatr. Res. 2023, 164, 98–117. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zhang, F.; Ma, H.; Chen, X.; Yang, J.; Yang, Y.; Yang, X.; Tian, X.; Yu, Q.; Ma, Z.; et al. Effects of different types and doses of whey protein on the physiological and intestinal flora in D-galactose induced aging mice. PLoS ONE 2021, 16, e0248329. [Google Scholar] [CrossRef]
- Rossi, M.; Aggio, R.; Staudacher, H.M.; Lomer, M.C.; Whelan, K. Volatile Organic Compounds in Feces Associate with Response to Dietary Intervention in Patients With Irritable Bowel Syndrome. Clin. Gastroenterol. Hepatol. 2018, 16, 385–391.e1. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gong, W.; He, Z.; Li, G. Investigation on the Current Situation of Laboratory Animals Units, Personnel and Facilities in Mainland China. Lab. Anim. Sci. 2017, 34, 43–54, (In Chinese, with an English abstract). [Google Scholar]
- Leschelle, X.; Goubern, M.; Andriamihaja, M.; Blottière, H.M.; Couplan, E.; Gonzalez-Barroso, M.D.M.; Petit, C.; Pagniez, A.; Chaumontet, C.; Mignotte, B. Adaptative metabolic response of human colonic epithelial cells to the adverse effects of the luminal compound sulfifide. Biochim. Biophys. Acta BBA-Gen. Subj. 2005, 1725, 201–212. [Google Scholar] [CrossRef]
- Hamer, H.M.; Jonkers, D.M.A.E.; Bast, A.; Vanhoutvin, S.A.L.W.; Fischer, M.A.J.G.; Kodde, A.; Troost, F.J.; Venema, K.; Brummer, R.J.M. Butyrate modulates oxidative stress in the colonic mucosa of healthy humans. Clin. Nutr. 2009, 28, 88–93. [Google Scholar] [CrossRef]
- Li, J.; Gao, L.; Wang, K.; Zhou, Y.; Qin, X.; Du, G. Fecal metabolome and gut microbiome alterations in a mouse model of senescence accelerated mouse prone 8 (SAMP8). Chin. Tradit. Herb. Drugs 2018, 621, 50–58, (In Chinese, with an English abstract). [Google Scholar]
- Swanson, B.; Fogg, L.; Julion, W.; Arrieta, M.T. Electronic Nose Analysis of Exhaled Breath Volatiles to Identify Lung Cancer Cases: A Systematic Review. J. Assoc. Nurses AIDS Care 2020, 31, 71–79. [Google Scholar] [CrossRef]
- Tan, J.; Xu, J. Applications of electronic nose (e-nose) and electronic tongue (e-tongue) in food quality-related properties determination: A review. AIIA 2020, 4, 104–115. [Google Scholar] [CrossRef]
- Dragonieri, S.; Quaranta, V.N.; Carratu, P.; Ranieri, T.; Resta, O. Influence of age and gender on the profile of exhaled volatile organic compounds analyzed by an electronic nose. J. Bras. Pneumol. 2016, 42, 143–145. [Google Scholar] [CrossRef]
- Sofia, E.; Niemarkt, H.; Said, H.; Berkhout, D.; Kaam, A.V.; Lingen, R.V.; Benninga, M.; Bore, N.D.; Meij, T.D. Fecal Volatile Organic Compounds in Preterm Infants Are Influenced by Enteral Feeding Composition. Sensors 2018, 18, 3037. [Google Scholar] [CrossRef] [PubMed]
- Hosfield, B.D.; Pecoraro, A.R.; Baxter, N.T.; Markel, T.A. The Assessment of Fecal Volatile Organic Compounds in Healthy Infants: Electronic Nose Device Predicts Patient Demographics and Microbial Enterotype. J. Surg. Res. 2020, 254, 340–347. [Google Scholar] [CrossRef]
- Berkhout, D.J.C.; Benninga, M.A.; Stein, R.M.V.; Brinkman, P.; Meij, T.G.J.D. Effects of sampling conditions and environmental factors on fecal volatile organic compound analysis by an electronic nose device. Sensors 2016, 16, 1967. [Google Scholar] [CrossRef]
- Bosch, S.; Lemmen, J.P.M.; Menezes, R.; Hulst, R.V.D.; Boer, N.K.D. The influence of lifestyle factors on fecal volatile organic compound composition as measured by an electronic nose. J. Breath Res. 2019, 13, 046001. [Google Scholar] [CrossRef]
- Li, S.; Wu, Q.; Wang, R.; Sun, Z.D.; Xie, B.J. Effect of Litchi Pericarp Procyanidins as a Dietary Supplement on Excreta of Rats. Food Sci. 2013, 34, 274–278, (In Chinese, with an English abstract). [Google Scholar]
- Ou, J.Z.; Cottrell, J.J.; Ha, N.; Pillai, N.; Yao, C.K.; Berean, K.J.; Ward, S.A.; Grando, D.; Muir, J.G.; Harrison, C.J. Potential of in vivo real-time gastric gas profiling: A pilot evaluation of heat-stress and modulating dietary cinnamon effect in an animal model. Sci. Rep. 2016, 6, 33387. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, L.; Wei, J.; Tian, X. Non-Invasive Blood. Pressure Tracking of Spontaneous Hypertension Rats Using an Electronic Nose. Sensors 2024, 24, 238. [Google Scholar] [CrossRef]
- Long, M.; Zhang, P.; Liu, Y.; Bai, X.; Zhang, F.; Tian, X.; Zhou, X.; Ma, Z.; Luo, L.; Song, L.; et al. Identification of functional effects of bovine whey protein based on odor information of mouse feces. Acta Agric. Zhejiangensis 2021, 33, 714–723. [Google Scholar]
- Ge, Y.; Bao, X.; Liu, S.; Xiang, S.; Zhang, Y.; Zhu, X. Study on the method of rapidly predicting the structure of gut microbiota by smart nose. In Proceedings of the Abstract of Food Summit in China & 16th Annual meeting of CIFST, Wuhan, China, 13–14 November 2019; pp. 385–386, (In Chinese, with an English abstract). [Google Scholar]
- Fardin, A.; Esmaeil, M.-G.; Hekmat, R.; Kobra, H. Using an E-nose machine for detection the adulteration of margarine in cow ghee. J. Food Process Eng. 2018, 41, e12806. [Google Scholar]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Chan, D.K.; Leggett, C.L.; Wang, K.K. Diagnosing gastrointestinal illnesses using fecal headspace volatile organic compounds. World J. Gastroenterol. 2016, 22, 1639–1649. [Google Scholar] [CrossRef] [PubMed]
- Chavez, C.; Coufal, C.D.; Carey, J.B.; Lacey, R.E.; Beier, R.C.; Zahn, J.A. The Impact of Supplemental Dietary Methionine Sources on Volatile Compound Concentrations in Broiler Excreta. Poult. Sci. 2004, 83, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Paleczek, A.; Grochala, J.; Grochala, D.; Słowik, J.; Pihut, M.; Loster, J.E.; Rydosz, A. Noninvasive Total Cholesterol Level Measurement Using an E-Nose System and Machine Learning on Exhaled Breath Samples. ACS Sens. 2024, 9, 6630–6637. [Google Scholar] [CrossRef] [PubMed]



| Short Name | Sensor Name | General Description | Reference |
|---|---|---|---|
| S1 | W1C-aromatic | Aromatic compounds | Toluene, 10 ppm |
| S2 | W5S-broadrange | Very sensitive, broad range sensitivity, reacts with nitrogen oxides, very sensitive with negative signal | NO2, 1 ppm |
| S3 | W3C-aromatic | Ammonia, used as sensor for aromatic compounds | Benzene, 1 ppm |
| S4 | W6S-hydrogen | Mainly hydrogen, selectively (breath gases) | H2, 100 ppb |
| S5 | W5C-arom-aliph | Alkanes, aromatic compounds, less polar compounds | Propane, 1 ppm |
| S6 | W1S-broad-methane | Sensitive to methane (environment) ca, 10 ppm, broad range, similar to No. 8 | CH4, 100 ppm |
| S7 | W1W-sulphur-oganic | Reacts with sulfur compounds, H2S 0.1 ppm. Otherwise, sensitive to many terpenes and sulfur organic compounds, which are important for smell, limonene, pyrazine | H2S, 1 ppm |
| S8 | W2S-broad-alcohol | Detects alcohols, partially aromatic compounds, broad range | CO, 100 ppm |
| S9 | W2W-sulph-chlor | Aromatic compounds, sulfur organic compounds | H2S, 1 ppm |
| S10 | W3S methane-aliph | Reacts with high concentrations > 100 ppm, sometimes very selective (methane) | CH4, 100 ppm |
| Intervention Groups | Sensor Response Value (G/G0) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | |
| C | 0.887 a | 1.685 a | 0.944 a | 1.186 a | 1.003 ab | 2.885 bc | 5.241 a | 1.667 d | 1.714 a | 1.277 d |
| NS | 0.816 c | 1.157 c | 0.912 c | 1.151 bc | 0.999 b | 3.462 b | 3.299 c | 2.069 b | 1.567 b | 1.461 b |
| Vc | 0.872 ab | 1.434 b | 0.937 a | 1.156 bc | 1.007 ab | 2.959 c | 4.105 b | 1.744 cd | 1.562 b | 1.317 cd |
| GWP-L | 0.876 a | 1.501 b | 0.940 ab | 1.186 a | 1.008 a | 2.879 c | 4.674 ab | 1.728 cd | 1.633 ab | 1.318 cd |
| GWP-M | 0.852 b | 1.192 c | 0.930 b | 1.150 c | 1.004 ab | 3.181 bc | 4.332 b | 1.852 c | 1.622 ab | 1.356 c |
| GWP-H | 0.742 d | 0.932 d | 0.864 d | 1.169 b | 1.003 ab | 4.996 a | 3.399 c | 2.643 a | 1.654 ab | 1.644 a |
| Types | Samples | C | Ns | Vc | GWP-L | GWP-M | GWP-H |
|---|---|---|---|---|---|---|---|
| Effect evaluation | C | 25 | 5 | 6 | 1 | 3 | 0 |
| Ns | 1 | 23 | 3 | 12 | 1 | 0 | |
| Vc | 5 | 8 | 20 | 4 | 2 | 1 | |
| GWP-L | 4 | 13 | 3 | 20 | 0 | 0 | |
| GWP-M | 5 | 1 | 3 | 0 | 31 | 0 | |
| GWP-H | 0 | 0 | 0 | 0 | 0 | 40 | |
| Correct classification rate | 66.30% | ||||||
| Types | Samples | Accuracy/% | Precision/% | Sensitivity/% | Specificity/% | AUC/% |
|---|---|---|---|---|---|---|
| Intervention groups | C | 87.50 (83.32, 91.68) | 62.50 | 62.50 | 92.50 | 90.80 |
| Ns | 81.67 (76.79, 86.55) | 46.00 | 57.50 | 86.50 | 100 | |
| Vc | 85.42 (80.95, 89.89) | 57.14 | 50.00 | 92.50 | 87.60 | |
| GWP-L | 84.58 (79.93, 89.23) | 54.05 | 50.00 | 91.50 | 97.70 | |
| GWP-M | 93.75 (90.69, 96.81) | 83.78 | 77.50 | 97.00 | 88.10 | |
| GWP-H | 99.58 (98.78, 100.38) | 100 | 100 | 99.50 | 86.40 | |
| Average | 88.75 (85.08, 92.42) | 67.25 | 66.25 | 93.25 | 91.77 |
| Prediction Methods | Antioxidant Index | Calibration | Validation | ||
|---|---|---|---|---|---|
| R2 | RMESC | R2 | RMESP | ||
| MLP | SOD | 0.5352 | 0.5376 | 0.5092 | 0.5196 |
| MDA | 0.6361 | 0.638 | 0.6242 | 0.6322 | |
| Weight gain rate | 0.5475 | 0.5499 | 0.5257 | 0.5358 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, G.; Yang, Y.; Zhang, F.; Wei, J.; Tian, X.; Liu, L.; Ma, Z.; Zhang, G. Non-Invasive Monitoring and Differentiation of Aging Mice Treated with Goat Whey Powder by an Electronic Nose Coupled with Chemometric Methods. Sensors 2025, 25, 1496. https://doi.org/10.3390/s25051496
Zhu G, Yang Y, Zhang F, Wei J, Tian X, Liu L, Ma Z, Zhang G. Non-Invasive Monitoring and Differentiation of Aging Mice Treated with Goat Whey Powder by an Electronic Nose Coupled with Chemometric Methods. Sensors. 2025; 25(5):1496. https://doi.org/10.3390/s25051496
Chicago/Turabian StyleZhu, Guilong, Yahe Yang, Fumei Zhang, Jia Wei, Xiaojing Tian, Lixia Liu, Zuolin Ma, and Guoheng Zhang. 2025. "Non-Invasive Monitoring and Differentiation of Aging Mice Treated with Goat Whey Powder by an Electronic Nose Coupled with Chemometric Methods" Sensors 25, no. 5: 1496. https://doi.org/10.3390/s25051496
APA StyleZhu, G., Yang, Y., Zhang, F., Wei, J., Tian, X., Liu, L., Ma, Z., & Zhang, G. (2025). Non-Invasive Monitoring and Differentiation of Aging Mice Treated with Goat Whey Powder by an Electronic Nose Coupled with Chemometric Methods. Sensors, 25(5), 1496. https://doi.org/10.3390/s25051496





