Is the Assessment of the Non-Paretic Lower Limb in Patients After Stroke Important When Planning Rehabilitation?
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Testing Procedures
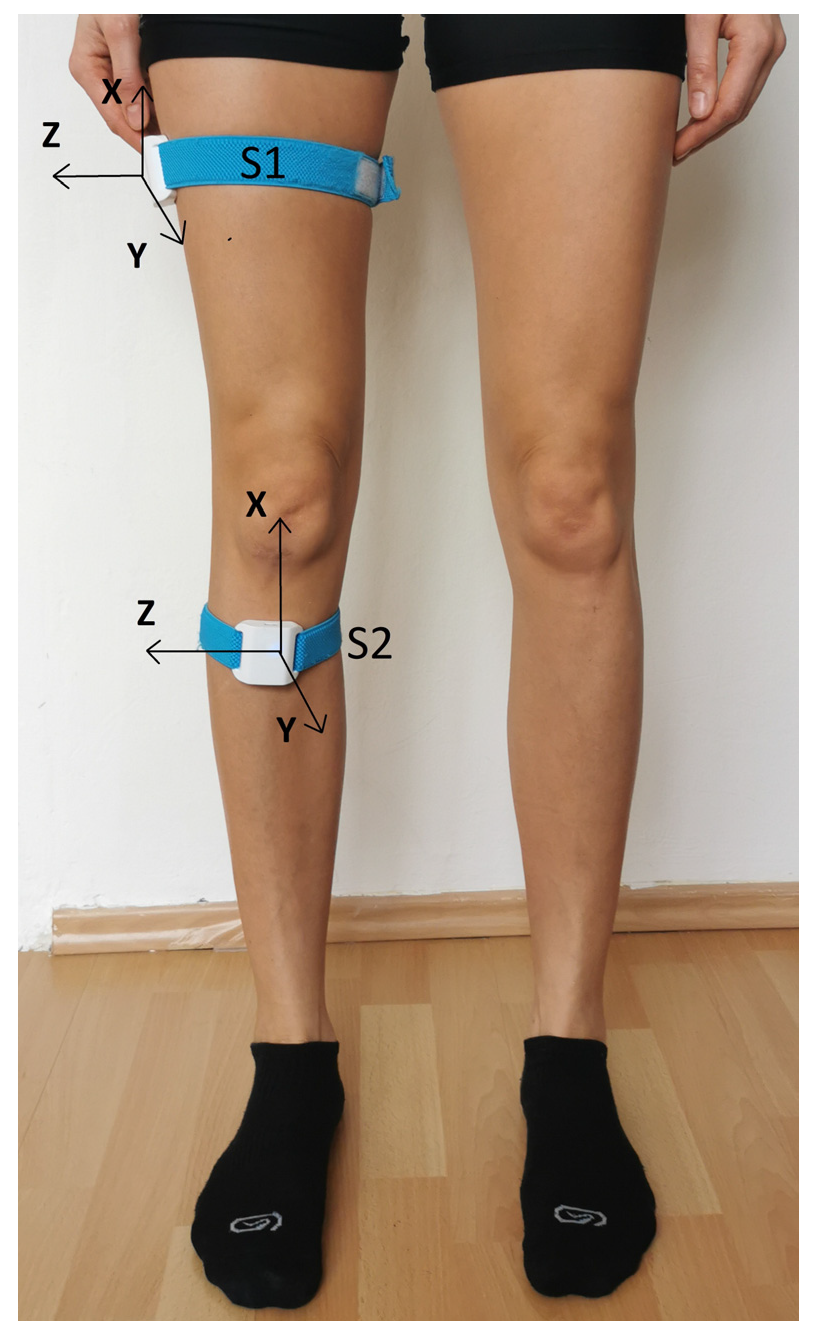
2.2.1. Assessment Using Wireless Motion Sensors
- Range of motion (degrees; °);
- Average angular velocity in the knee joint during the diagnostic examination (AVG speed, °/s);
- Maximum angular velocity during the test (MAX speed, °/s).
Passive Knee Range of Motion (PROM)
Active Knee Range of Motion (AROM)
Fast Active Knee Range of Motion (FROM)
Proprioception of the Knee Joint (Joint Position Sense, JPS)
2.2.2. Force Measurement
- Maximal voluntary isometric contraction values—MVIC [Nm];
- Average values—AVG [Nm];
- Max moment—MM [Nm/s].
2.2.3. Balance Assessment
The Step Test (ST)
Static Balance Assessment
- Standing on a firm surface with the eyes open (EO)—the “standard” test condition where all three sensory systems (i.e., proprioception, vision, and vestibular) are available to assist in maintaining balance.
- Standing on a firm surface with the eyes closed (EC)—eliminates the visual input to evaluate vestibular and somatosensory inputs.
- Standing on a foam surface with the eyes open (EOF)—the visual and vestibular systems are available, but the proprioceptive system is compromised when the subject stands on a foam surface.
- Standing on a foam surface with the eyes closed (ECF)—the visual and proprioceptive systems are compromised, which allows the singular vestibular inputs to be evaluated.
- The total path length (P; cm);
- The average velocity (AV, cm/s).
2.3. Statistical Analysis
3. Results
3.1. Assessment Using Wireless Sensors
- Passive Knee Range of Motion (PROM)
- Active Knee Range of Motion (AROM)
- Fast Active Knee Range of Motion (FROM)
- Proprioception of the Knee Joint (joint position sense, JPS)
3.2. Force Measurement
3.2.1. Extensors
3.2.2. Flexors
3.3. Balance Assessment
3.3.1. The Step Test (ST)
3.3.2. Static Balance Assessment
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pandian, S.; Arya, K.N.; Kumar, D. Does motor training of the nonparetic side influences balance and function in chronic stroke? A pilot RCT. Sci. World J. 2014, 2014, 769726. [Google Scholar] [CrossRef] [PubMed]
- Pandian, S.; Arya, K.N. Motor impairment of the ipsilesional body side in poststroke subjects. J. Bodyw. Mov. Ther. 2013, 17, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Chlebuś, E.; Wareńczak, A.; Miedzyblocki, M.; Lisiński, P. The usefulness of isometric protocol for foot flexors and extensors in assessing the effects of 16-week rehabilitation regiment in poststroke patients. Biomed. Eng. Online 2019, 18, 57. [Google Scholar] [CrossRef]
- Scano, A.; Guanziroli, E.; Mira, R.M.; Brambilla, C.; Molinari Tosatti, L.; Molteni, F. Biomechanical assessment of the ipsilesional upper limb in post-stroke patients during multi-joint reaching tasks: A quantitative study. Front. Rehabil. Sci. 2022, 3, 943397. [Google Scholar] [CrossRef]
- Jankowska, E.; Edgley, S.A. How can corticospinal tract neurons contribute to ipsilateral movements? A question with implications for recovery of motor functions. Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2006, 12, 67–79. [Google Scholar] [CrossRef]
- Alagona, G.; Delvaux, V.; Gérard, P.; De Pasqua, V.; Pennisi, G.; Delwaide, P.J.; Nicoletti, F.; Maertens de Noordhout, A. Ipsilateral Motor Responses to Focal Transcranial Magnetic Stimulation in Healthy Subjects and Acute-Stroke Patients. Stroke 2001, 32, 1304–1309. [Google Scholar] [CrossRef]
- Bundy, D.T.; Leuthardt, E.C. The Cortical Physiology of Ipsilateral Limb Movements. Trends Neurosci. 2019, 42, 825–839. [Google Scholar] [CrossRef]
- Bagnato, S.; Boccagni, C.; Boniforti, F.; Trinchera, A.; Guercio, G.; Letizia, G.; Galardi, G. Motor dysfunction of the “non-affected” lower limb: A kinematic comparative study between hemiparetic stroke and total knee prosthesized patients. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2009, 30, 107–113. [Google Scholar] [CrossRef][Green Version]
- Cramer, S.C. Stroke recovery. Lessons from functional MR imaging and other methods of human brain mapping. Phys. Med. Rehabil. Clin. N. Am. 1999, 10, 875–886, ix. [Google Scholar] [CrossRef]
- Liepert, J.; Dettmers, C.; Terborg, C.; Weiller, C. Inhibition of ipsilateral motor cortex during phasic generation of low force. Clin. Neurophysiol. 2001, 112, 114–121. [Google Scholar] [CrossRef]
- Jeon, H.J.; Hwang, B.Y. Effect of bilateral lower limb strengthening exercise on balance and walking in hemiparetic patients after stroke: A randomized controlled trial. J. Phys. Ther. Sci. 2018, 30, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Kitsos, G.H.; Hubbard, I.J.; Kitsos, A.R.; Parsons, M.W. The ipsilesional upper limb can be affected following stroke. Sci. World J. 2013, 2013, 684860. [Google Scholar] [CrossRef] [PubMed]
- Raja, B.; Neptune, R.R.; Kautz, S.A. Coordination of the non-paretic leg during hemiparetic gait: Expected and novel compensatory patterns. Clin. Biomech. 2012, 27, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.Y.; Chen, C.H.; Tseng, H.Y.; Lin, S.I. Ipsilateral lower limb motor performance and its association with gait after stroke. PLoS ONE 2024, 19, e0297074. [Google Scholar] [CrossRef]
- Hsiao, H.Y.; Gray, V.L.; Borrelli, J.; Rogers, M.W. Biomechanical control of paretic lower limb during imposed weight transfer in individuals post-stroke. J. Neuroeng. Rehabil. 2020, 17, 140. [Google Scholar] [CrossRef]
- Higgins, M.J.; Perrin, D.H. Comparison of Weight-Bearing and Non-Weight-Bearing Conditions on Knee Joint Reposition Sense. J. Sport. Rehabil. 1997, 6, 327–334. [Google Scholar] [CrossRef]
- Thilarajah, S.; Bower, K.J.; Williams, G.; Clark, R.A.; Tan, D.; Pua, Y.H. Paretic and Nonparetic Step Tests Are Noninterchangeable in Stroke: A Prospective Cohort Study. Phys. Ther. 2021, 101, pzab060. [Google Scholar] [CrossRef]
- Nowak, J.K.; Walkowiak, J. Study designs in medical research and their key characteristics. J. Med. Sci. 2024, 92, e928. [Google Scholar] [CrossRef]
- Wareńczak-Pawlicka, A.; Lisiński, P. Can We Target Close Therapeutic Goals in the Gait Re-Education Algorithm for Stroke Patients at the Beginning of the Rehabilitation Process? Sensors 2024, 24, 3416. [Google Scholar] [CrossRef]
- Goślińska, J.; Wareńczak, A.; Miedzyblocki, M.; Hejdysz, K.; Adamczyk, E.; Sip, P.; Chlebuś, E.; Gośliński, J.; Owczarek, P.; Woźniak, A.; et al. Wireless Motion Sensors—Useful in Assessing the Effectiveness of Physiotherapeutic Methods Used in Patients with Knee Osteoarthritis—Preliminary Report. Sensors 2020, 20, 2268. [Google Scholar] [CrossRef]
- Newham, D.J.; Hsiao, S.F. Knee muscle isometric strength, voluntary activation and antagonist co-contraction in the first six months after stroke. Disabil. Rehabil. 2001, 23, 379–386. [Google Scholar] [PubMed]
- Wareńczak, A.; Lisiński, P. Body balance a few years after total hip replacement. Acta Bioeng. Biomech. 2020, 22, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Antoniadou, E.; Kalivioti, X.; Stolakis, K.; Koloniari, A.; Megas, P.; Tyllianakis, M.; Panagiotopoulos, E. Reliability and validity of the mCTSIB dynamic platform test to assess balance in a population of older women living in the community. J. Musculoskelet. Neuronal Interact. 2020, 20, 185–193. [Google Scholar]
- Goble, D.J.; Brar, H.; Brown, E.C.; Marks, C.R.; Baweja, H.S. Normative data for the Balance Tracking System modified Clinical Test of Sensory Integration and Balance protocol. Med. Devices 2019, 12, 183–191. [Google Scholar] [CrossRef]
- Morone, G.; Matamala-Gomez, M.; Sanchez-Vives, M.V.; Paolucci, S.; Iosa, M. Watch your step! Who can recover stair climbing independence after stroke? Eur. J. Phys. Rehabil. Med. 2018, 54, 811–818. [Google Scholar] [CrossRef]
- Sjattar, E.L.; Megawati, I.; Irwan, A.M.; Majid, S. Development of Supportive-Educative Range of Motion Exercise for Post-stroke Patients: A Pilot Study. Home Health Care Manag. Pract. 2022, 34, 92–100. [Google Scholar] [CrossRef]
- Protopapadaki, A.; Drechsler, W.I.; Cramp, M.C.; Coutts, F.J.; Scott, O.M. Hip, knee, ankle kinematics and kinetics during stair ascent and descent in healthy young individuals. Clin. Biomech. 2007, 22, 203–210. [Google Scholar] [CrossRef]
- Rowe, P.J.; Myles, C.M.; Walker, C.; Nutton, R. Knee joint kinematics in gait and other functional activities measured using flexible electrogoniometry: How much knee motion is sufficient for normal daily life? Gait Posture 2000, 12, 143–155. [Google Scholar] [CrossRef]
- Pfeiffer, J.L.; Zhang, S.; Milner, C.E. Knee biomechanics during popular recreational and daily activities in older men. Knee 2014, 2, 683–687. [Google Scholar] [CrossRef]
- Hyodo, K.; Masuda, T.; Aizawa, J.; Jinno, T.; Morita, S. Hip, knee, and ankle kinematics during activities of daily living: A cross-sectional study. Braz. J. Phys. Ther. 2017, 21, 159–166. [Google Scholar] [CrossRef]
- Mandon, L.; Boudarham, J.; Robertson, J.; Bensmail, D.; Roche, N.; Roby-Brami, A. Faster Reaching in Chronic Spastic Stroke Patients Comes at the Expense of Arm-Trunk Coordination. Neurorehabil. Neural Repair. 2016, 30, 209–220. [Google Scholar] [CrossRef] [PubMed]
- DeJong, S.L.; Schaefer, S.Y.; Lang, C.E. Need for speed: Better movement quality during faster task performance after stroke. Neurorehabil. Neural Repair 2012, 26, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Hammerbeck, U.; Yousif, N.; Hoad, D.; Greenwood, R.; Diedrichsen, J.; Rothwell, J.C. Chronic Stroke Survivors Improve Reaching Accuracy by Reducing Movement Variability at the Trained Movement Speed. Neurorehabil. Neural Repair 2017, 31, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.N.; Ho, K.Y.; Lee, Y.J.; Ackley, C.; Aki, K.; Arias, J.; Trinh, J. Slow Walking in Individuals with Chronic Post-Stroke Hemiparesis: Speed Mediated Effects of Gait Kinetics and Ankle Kinematics. Brain Sci. 2021, 11, 365. [Google Scholar] [CrossRef]
- Yang, J.M.; Kim, S.Y. Correlation of knee proprioception with muscle strength and spasticity in stroke patients. J. Phys. Ther. Sci. 2015, 27, 2705–2708. [Google Scholar] [CrossRef]
- Hwang, J.S.; Lee, D.S.; Cho, Y.J.; Han, N.M.; Kim, H.D. Measurement of Proprioception of the Knee in Hemiplegic Patients Using an Isokinetic Dynamometer. J. Korean Acad. Rehabil. Med. 2010, 34, 27–33. [Google Scholar]
- Irisawa, H.; Mizushima, T. Assessment of changes in muscle mass, strength, and quality and activities of daily living in elderly stroke patients. Int. J. Rehabil. Res. Int. Zeitschrift fur Rehabil. Rev. Int. Rech. Readapt. 2022, 45, 161–167. [Google Scholar] [CrossRef]
- Wang, W.T.; Huang, L.T.; Chou, Y.H.; Wei, T.S.; Lin, C.C. Nonparetic knee extensor strength is the determinant of exercise capacity of community-dwelling stroke survivors. Sci. World J. 2014, 2014, 769875. [Google Scholar] [CrossRef][Green Version]
- Bohannon, R.W. Muscle strength and muscle training after stroke. J. Rehabil. Med. 2007, 39, 14–20. [Google Scholar] [CrossRef]
- Dengiz, A.; Baskan, E. Does muscle strength decrease on the unaffected side in stroke patients? The unaffected side in stroke patients. Ann. Clin. Anal. Med. 2021, 12, 1011–1015. [Google Scholar] [CrossRef]
- Kim, S.H.; Pohl, P.S. Ipsilateral Impairments in the Lower Extremity After Stroke. Phys. Ther. Rev. 2000, 5, 171–174. [Google Scholar] [CrossRef]
- Dorsch, S.; Ada, L.; Canning, C.G. Lower Limb Strength Is Significantly Impaired in All Muscle Groups in Ambulatory People with Chronic Stroke: A Cross-Sectional Study. Arch. Phys. Med. Rehabil. 2016, 97, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhong, D.; Ye, J.; He, M.; Liu, X.; Zheng, H.; Jin, R.; Zhang, S.L. Rehabilitation for balance impairment in patients after stroke: A protocol of a systematic review and network meta-analysis. BMJ Open 2019, 9, e026844. [Google Scholar] [CrossRef]
- Hugues, A.; Di Marco, J.; Ribault, S.; Ardaillon, H.; Janiaud, P.; Xue, Y.; Zhu, J.; Pires, J.; Khademi, H.; Rubio, L.; et al. Limited evidence of physical therapy on balance after stroke: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0221700. [Google Scholar] [CrossRef] [PubMed]
- Awosika, O.O.; Garver, A.; Drury, C.; Sucharew, H.J.; Boyne, P.; Schwab, S.M.; Wasik, E.; Earnest, M.; Dunning, K.; Bhattacharya, A.; et al. Insufficiencies in sensory systems reweighting is associated with walking impairment severity in chronic stroke: An observational cohort study. Front. Neurol. 2023, 14, 1244657. [Google Scholar] [CrossRef]
- Peurala, S.H.; Könönen, P.; Pitkänen, K.; Sivenius, J.; Tarkka, I.M. Postural instability in patients with chronic stroke. Restor. Neurol. Neurosci. 2007, 25, 101–108. [Google Scholar] [CrossRef]
- Wang, W.; Li, K.; Wei, N.; Yin, C.; Yue, S. Evaluation of postural instability in stroke patient during quiet standing. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Jeju, Republic of Korea, 11–15 July 2017; Volume 2017, pp. 2522–2525. [Google Scholar]
- Mercer, V.S.; Freburger, J.K.; Chang, S.H.; Purser, J.L. Step test scores are related to measures of activity and participation in the first 6 months after stroke. Phys. Ther. 2009, 89, 1061–1071. [Google Scholar] [CrossRef]
- Hong, S.J.; Goh, E.Y.; Chua, S.Y.; Ng, S.S. Reliability and validity of step test scores in subjects with chronic stroke. Arch. Phys. Med. Rehabil. 2012, 93, 1065–1071. [Google Scholar] [CrossRef]

| Variable | Stroke Group | Control Group | p | |
|---|---|---|---|---|
| age [year] | mean ± SD median min–max | 52.9 ± 7.8 53.0 39.0–64.0 | 50.9 ± 7.4 51.0 37.0–65.0 | 0.315 |
| body mass [kg] | mean ± SD median min–max | 83.6 ± 14.3 81.0 58.0–112.0 | 81.6 ± 15.7 82.0 60.0–120.0 | 0.608 |
| height [kg] | mean ± SD median min–max | 171.6 ± 8.7 173.0 152.0–186.0 | 173.8 ± 9.9 175.0 159.0–195.0 | 0.386 |
| BMI | mean ± SD median min–max | 28.5 ± 5.5 28.1 19.4–43.8 | 27.0 ± 4.4 26.5 19.2–36.0 | 0.236 |
| Variable | Paretic | Non-Paretic | Control | p 1 | P vs. N | P vs. C | N vs. C | |
|---|---|---|---|---|---|---|---|---|
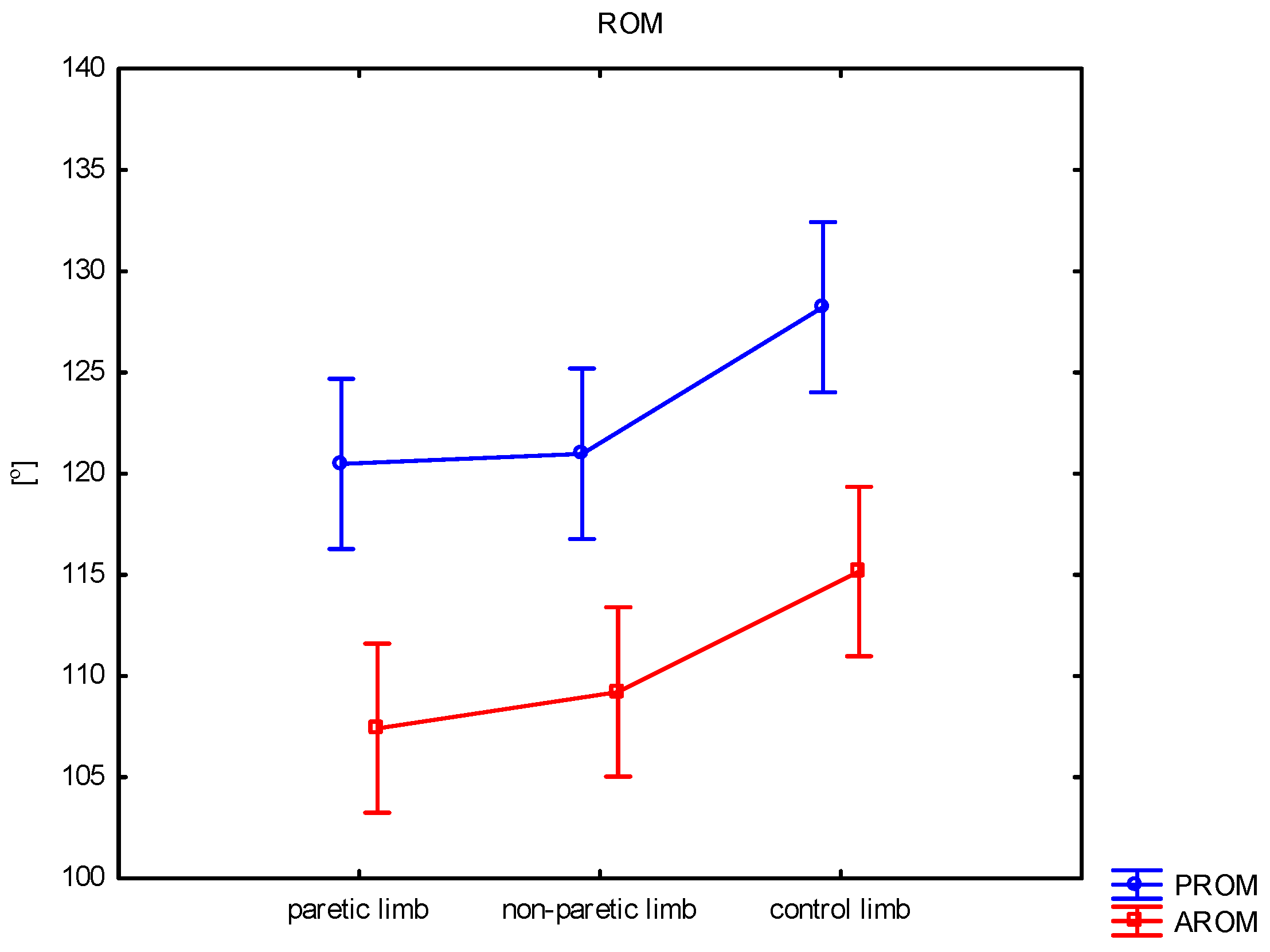
| PROM (°) | mean ± SD median min–max | 120.5 ± 11.5 119.5 96.0–146.2 | 121.0 ± 13.0 121.3 95.7–147.4 | 128.2 ± 9.4 129.2 112.4–143.9 | 0.018 | 0.866 | 0.011 | 0.018 |
| AROM (°) | mean ± SD median min–max | 107.4 ± 13.3 109.0 83.5–138.9 | 109.2 ± 12.4 109.4 86.7–133.5 | 115.2 ± 7.2 117.5 103.2–126.4 | 0.028 | 0.548 | 0.011 | 0.048 |
| FROM (°) | mean ± SD median min–max | 107.8 ± 13.1 110.5 83.2–130.8 | 110.2 ± 12.3 110.1 79.2–132.9 | 111.5 ± 8.4 112.2 95.5–128.3 | 0.456 | - | - | - |
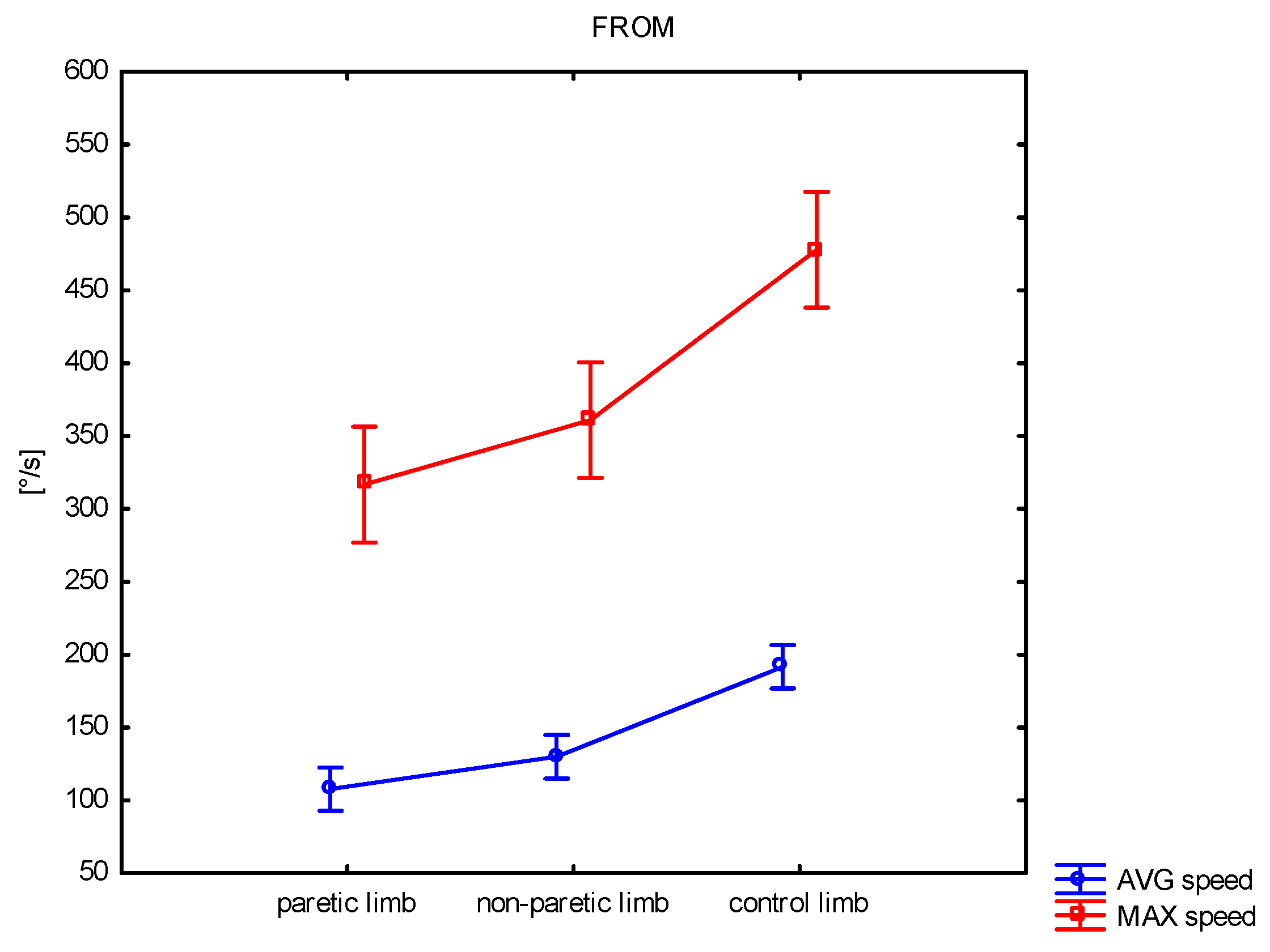
| FROM AVG speed | mean ± SD median min–max | 107.6 ± 40.8 103.4 15.5–213.1 | 129.8 ± 37.0 117.2 62.5–214.8 | 191.7 ± 43.1 178.3 133.0–282.1 | <0.001 | 0.039 | <0.001 | <0.001 |
| FROM MAX speed | mean ± SD median min–max | 316.7 ± 123.2 293.4 49.9–540.1 | 360.9 ± 104.1 346.3 109.0–588.0 | 477.9 ± 93.8 482.6 318.1–701.0 | <0.001 | 0.122 | <0.001 | <0.001 |
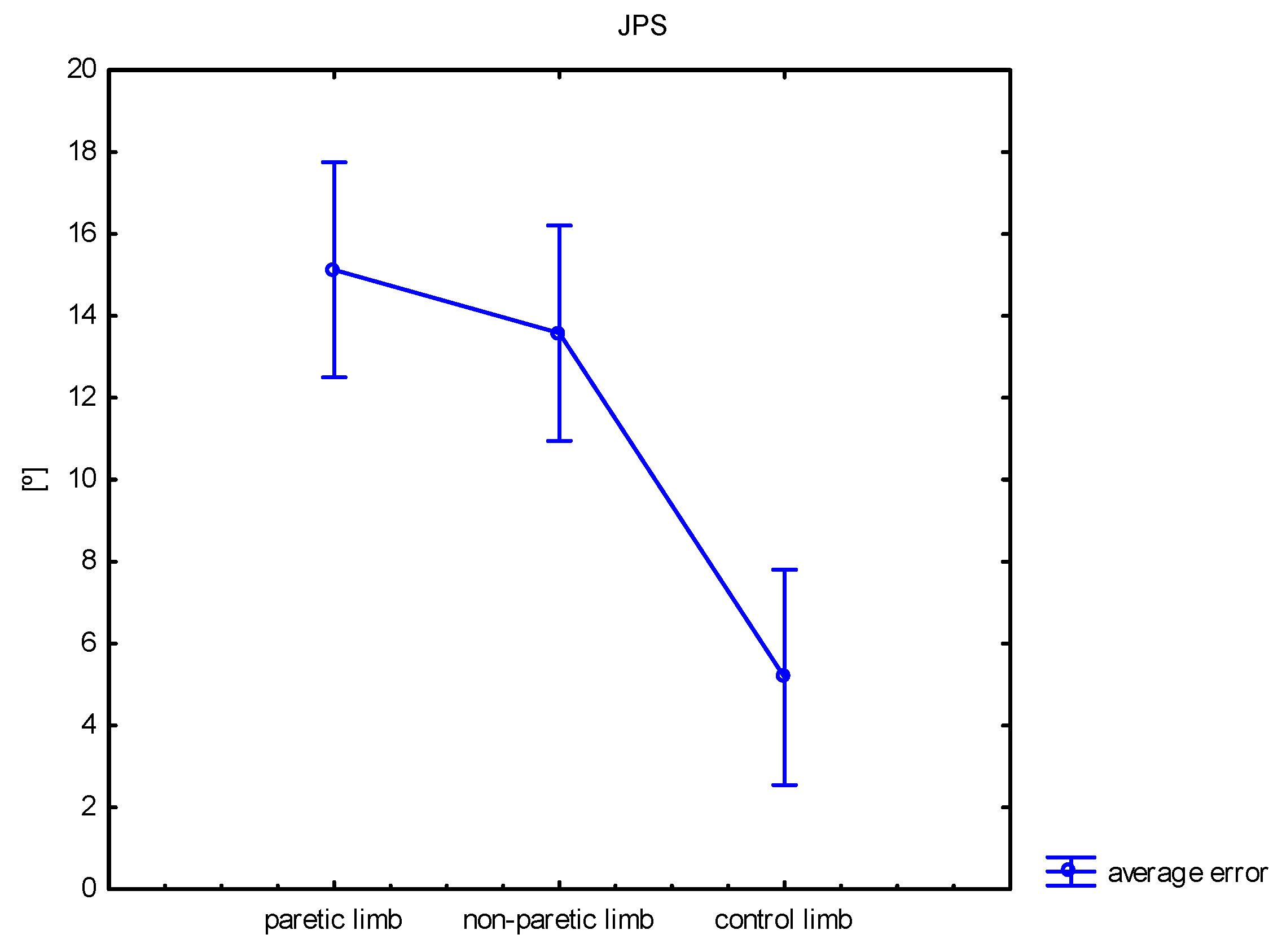
| JPS 80° | mean ± SD median min–max | 15.1 ± 9.5 13.5 2.2–42.6 | 13.6 ± 7.4 12.3 0.0–32.0 | 5.2 ± 2.6 5.2 1.1–9.8 | <0.001 | 0.411 | <0.001 | <0.001 |
| Variable | Paretic | Non-Paretic | Control | p 1 | P vs. N | P vs. C | N vs. C | |
|---|---|---|---|---|---|---|---|---|
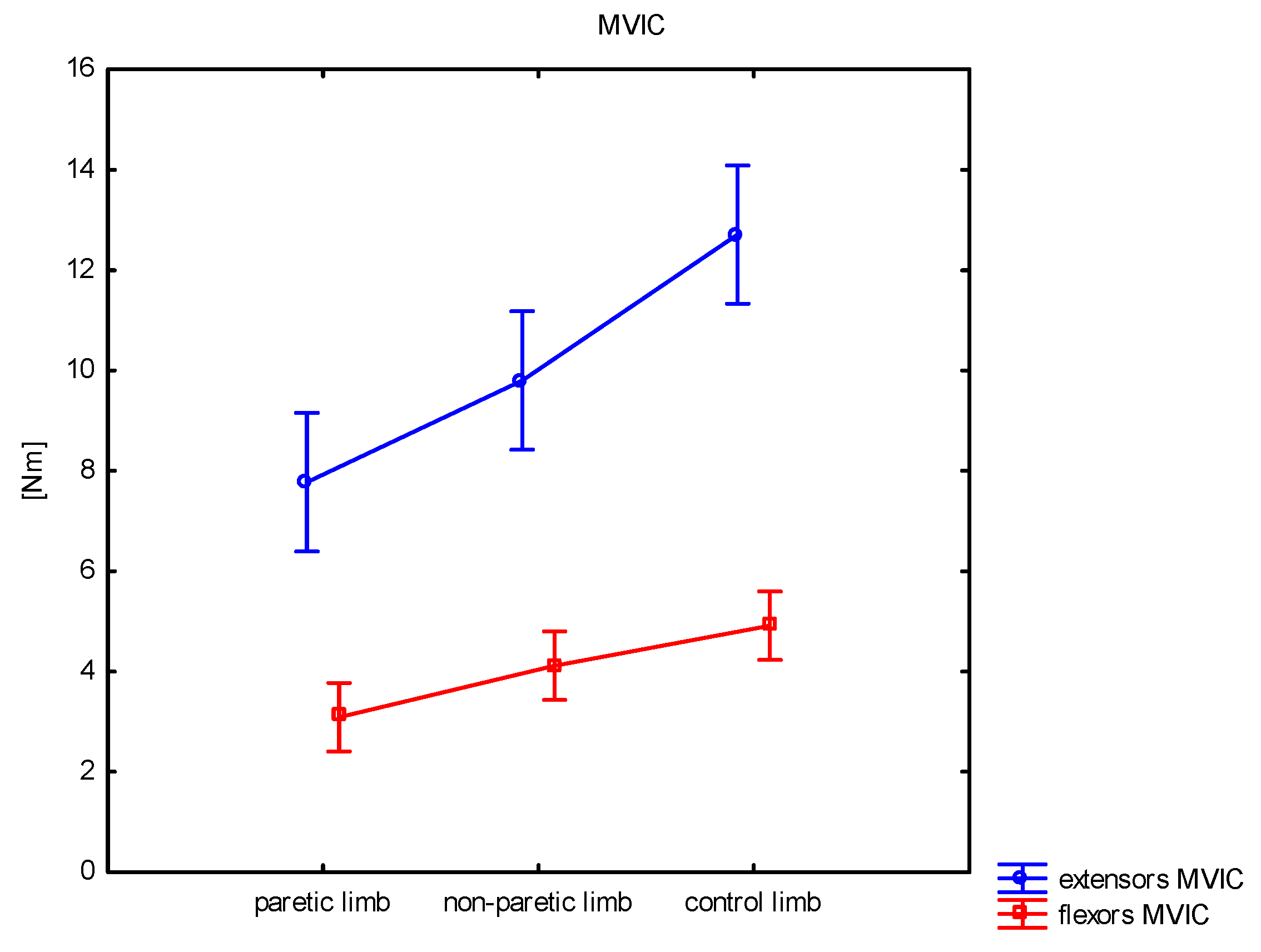
| MVIC [Nm] | mean ± SD median min–max | 7.8 ± 3.9 6.6 0.4–15.2 | 9.8 ± 3.1 9.5 5.3–17.6 | 12.7 ± 4.1 12.1 6.0–24.7 | <0.001 | 0.042 | <0.001 | 0.004 |
| AVG [Nm] | mean ± SD median min–max | 6.3 ± 3.2 5.3 0.4–12.2 | 8.0 ± 2.6 8.2 3.4–12.7 | 11.1 ± 3.6 10.2 5.4–20.7 | <0.001 | 0.042 | <0.001 | <0.001 |
| MM [Nm/s] | mean ± SD median min–max | 65.9 ± 33.8 56.2 3.6–127.9 | 84.1 ± 26.8 86.0 36.0–133.6 | 116.8 ± 37.9 107.3 56.6–218.2 | <0.001 | 0.039 | <0.001 | <0.001 |
| Variable | Paretic | Non-Paretic | Control | p 1 | P vs. N | P vs. C | N vs. C | |
|---|---|---|---|---|---|---|---|---|
| MVIC [Nm] | mean ± SD median min–max | 3.1 ± 2.0 3.0 0.0–7.2 | 4.1 ± 1.6 3.9 1.7–7.5 | 4.9 ± 1.9 4.4 2.0–9.7 | 0.001 | 0.037 | <0.001 | 0.104 |
| AVG [Nm] | mean ± SD median min–max | 2.4 ± 1.7 2.1 0.0–5.9 | 3.3 ± 1.3 3.1 1.5–5.9 | 4.2 ± 1.6 4.0 1.7–7.9 | <0.001 | 0.029 | <0.001 | 0.040 |
| MM [Nm/s] | mean ± SD median min–max | 25.5 ± 17.5 21.1 0.0–62.2 | 35.1 ± 14.2 32.5 15.4–62.2 | 43.4 ± 17.5 39.8 18.0–83.5 | <0.001 | 0.030 | <0.001 | 0.060 |
| Variable | Paretic | Non-Paretic | Control | p 1 | P vs. N | P vs. C | N vs. C | |
|---|---|---|---|---|---|---|---|---|
| Step test | mean ± SD median min–max | 11.4 ± 3.0 11.3 5.0–16.8 | 12.0 ± 2.8 12.3 5.8–16.3 | 19.8 ± 2.7 20.3 14.5–24.5 | <0.001 | 0.434 | <0.001 | <0.001 |
| Variable | Study Group | Control Group | p 1 | ||
|---|---|---|---|---|---|
| EO | (cm/s) | mean ± SD median min–max | 29.7 ± 18.9 26.0 13.2–111.9 | 16.6 ± 6.5 14.3 8.8–37.8 | <0.001 |
| (cm) | mean ± SD median min–max | 1.0 ± 0.6 0.9 0.4–3.7 | 0.5 ± 0.2 0.5 0.3–1.3 | <0.001 | |
| EC | (cm/s) | mean ± SD median min–max | 54.3 ± 37.4 42.4 16.0–206.2 | 29.1 ± 17.3 24.2 13.0–101.0 | <0.001 |
| (cm) | mean ± SD median min–max | 1.8 ± 1.2 1.4 0.5–6.9 | 1.3 ± 1.7 0.8 0.4–9.8 | <0.001 | |
| EOF | (cm/s) | mean ± SD median min–max | 68.6 ± 41.5 60.0 31.0–188.2 | 38.2 ± 13.4 34.7 19.1–75.0 | <0.001 |
| (cm) | mean ± SD median min–max | 2.3 ± 1.4 2.0 1.0–6.3 | 1.3 ± 0.4 1.2 0.6–2.5 | <0.001 | |
| ECF | (cm/s) | mean ± SD median min–max | 187.4 ± 121.0 139.0 84.0–662.1 | 106.9 ± 40.2 105.7 55.6–209.2 | <0.001 |
| (cm) | mean ± SD median min–max | 6.2 ± 4.0 4.7 2.8–22.1 | 3.6 ± 1.4 3.5 1.9–7.0 | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wareńczak-Pawlicka, A.; Lisiński, P. Is the Assessment of the Non-Paretic Lower Limb in Patients After Stroke Important When Planning Rehabilitation? Sensors 2025, 25, 1082. https://doi.org/10.3390/s25041082
Wareńczak-Pawlicka A, Lisiński P. Is the Assessment of the Non-Paretic Lower Limb in Patients After Stroke Important When Planning Rehabilitation? Sensors. 2025; 25(4):1082. https://doi.org/10.3390/s25041082
Chicago/Turabian StyleWareńczak-Pawlicka, Agnieszka, and Przemysław Lisiński. 2025. "Is the Assessment of the Non-Paretic Lower Limb in Patients After Stroke Important When Planning Rehabilitation?" Sensors 25, no. 4: 1082. https://doi.org/10.3390/s25041082
APA StyleWareńczak-Pawlicka, A., & Lisiński, P. (2025). Is the Assessment of the Non-Paretic Lower Limb in Patients After Stroke Important When Planning Rehabilitation? Sensors, 25(4), 1082. https://doi.org/10.3390/s25041082






