Neonatal Electroencephalogram Recording with a Dry Electrode Cap: A Feasibility Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview
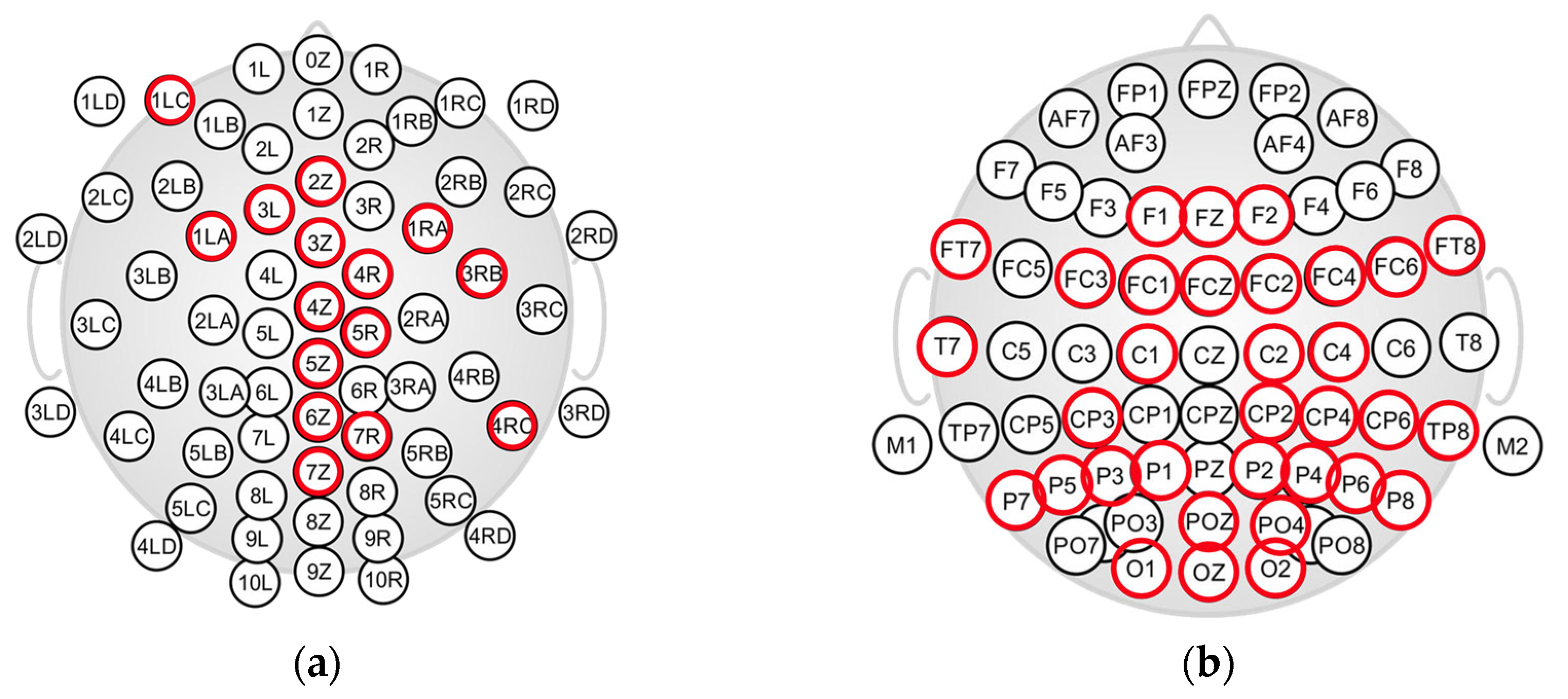
2.2. EEG Caps and EEG Recordings
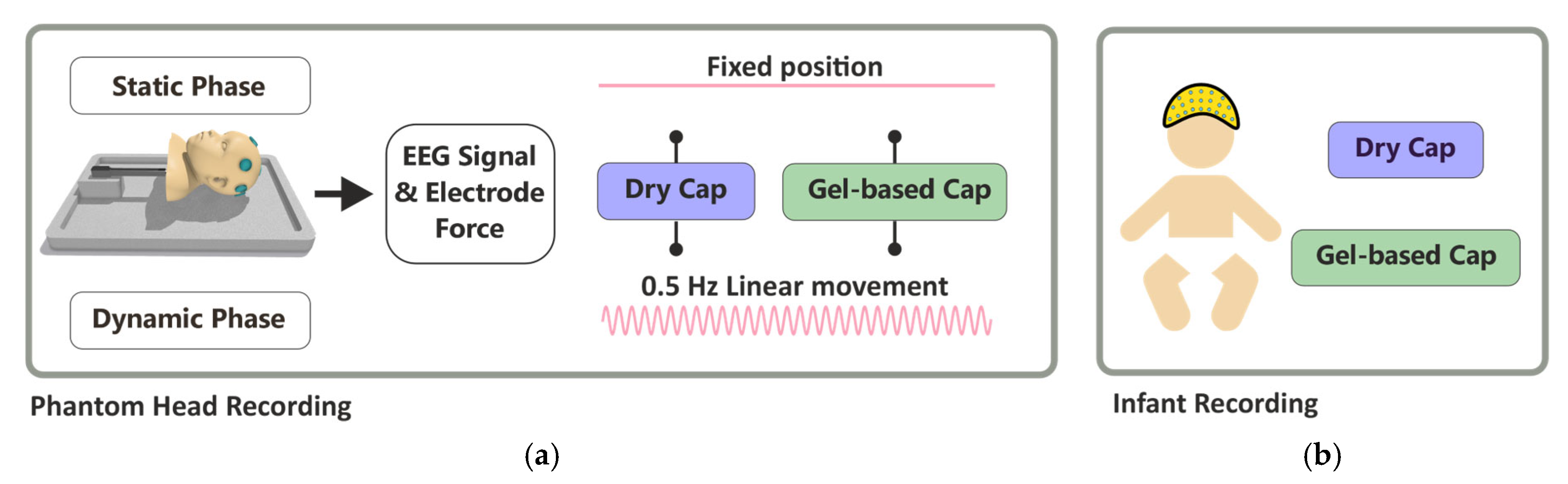
2.3. Phantom Head Recordings
2.4. Infant EEG Recording
2.5. Data Analysis
2.5.1. Preprocessing
2.5.2. Force Measurements
2.5.3. EEG Signal Analysis
2.6. Statistical Analysis
3. Results
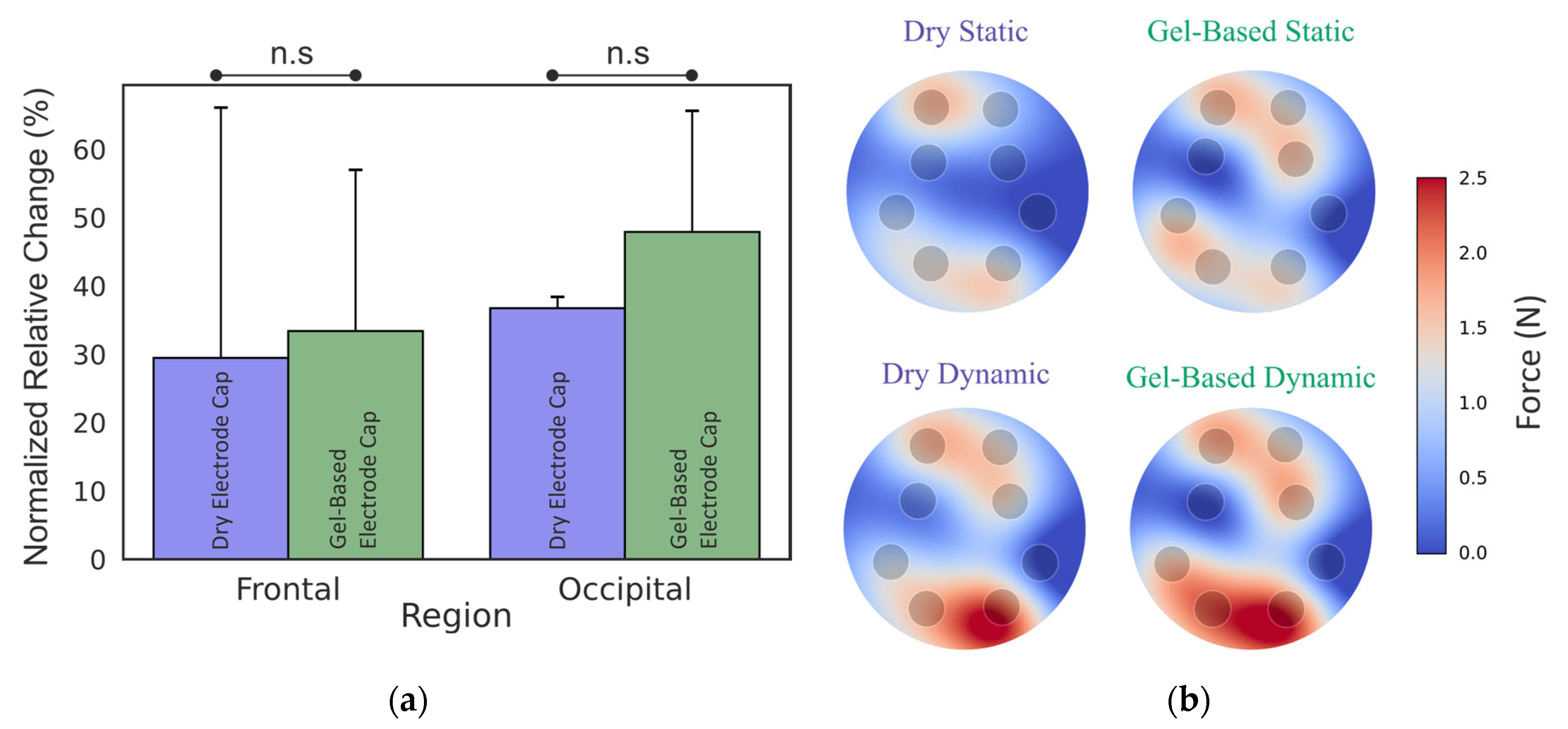
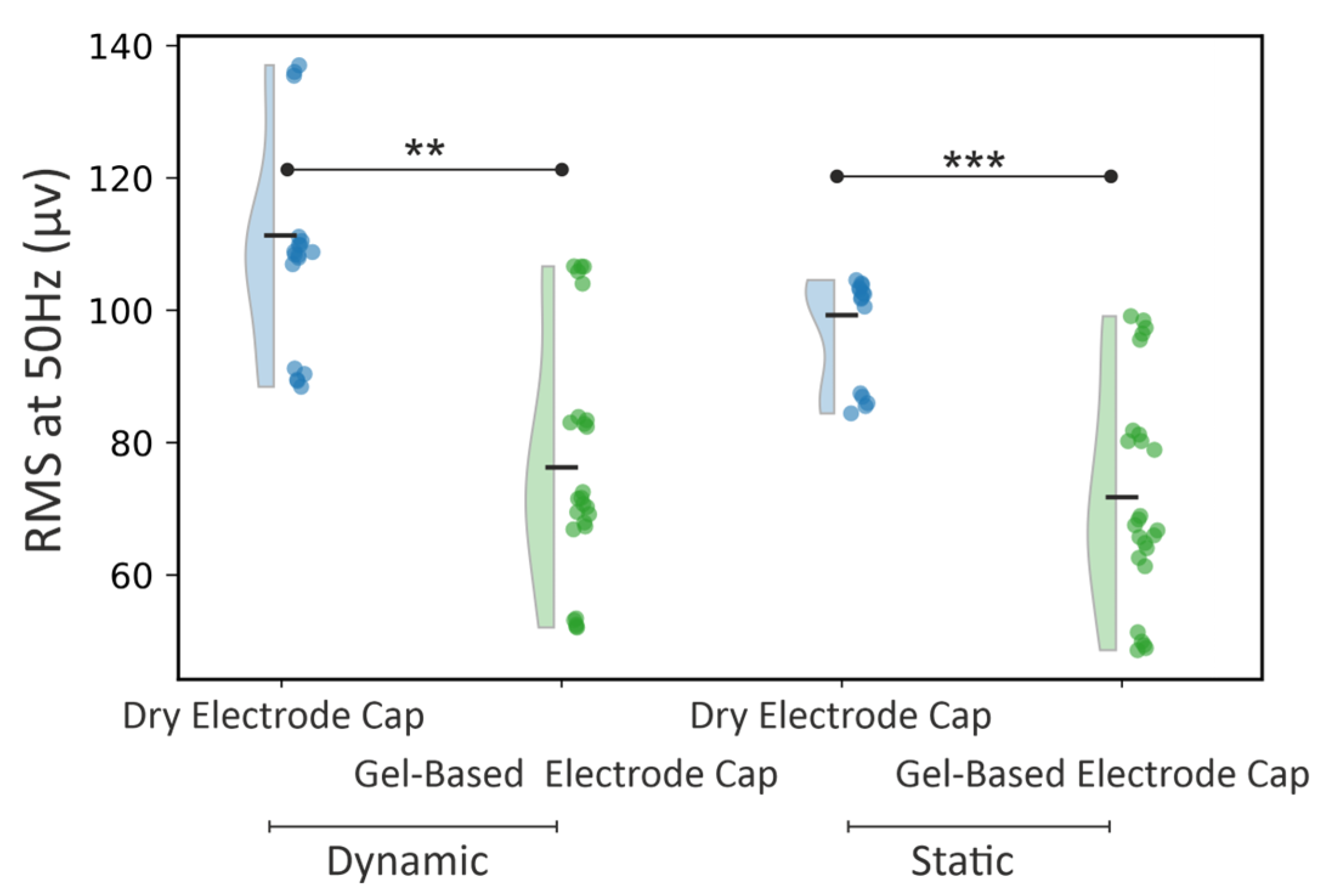
3.1. Phantom Head Recording, Force
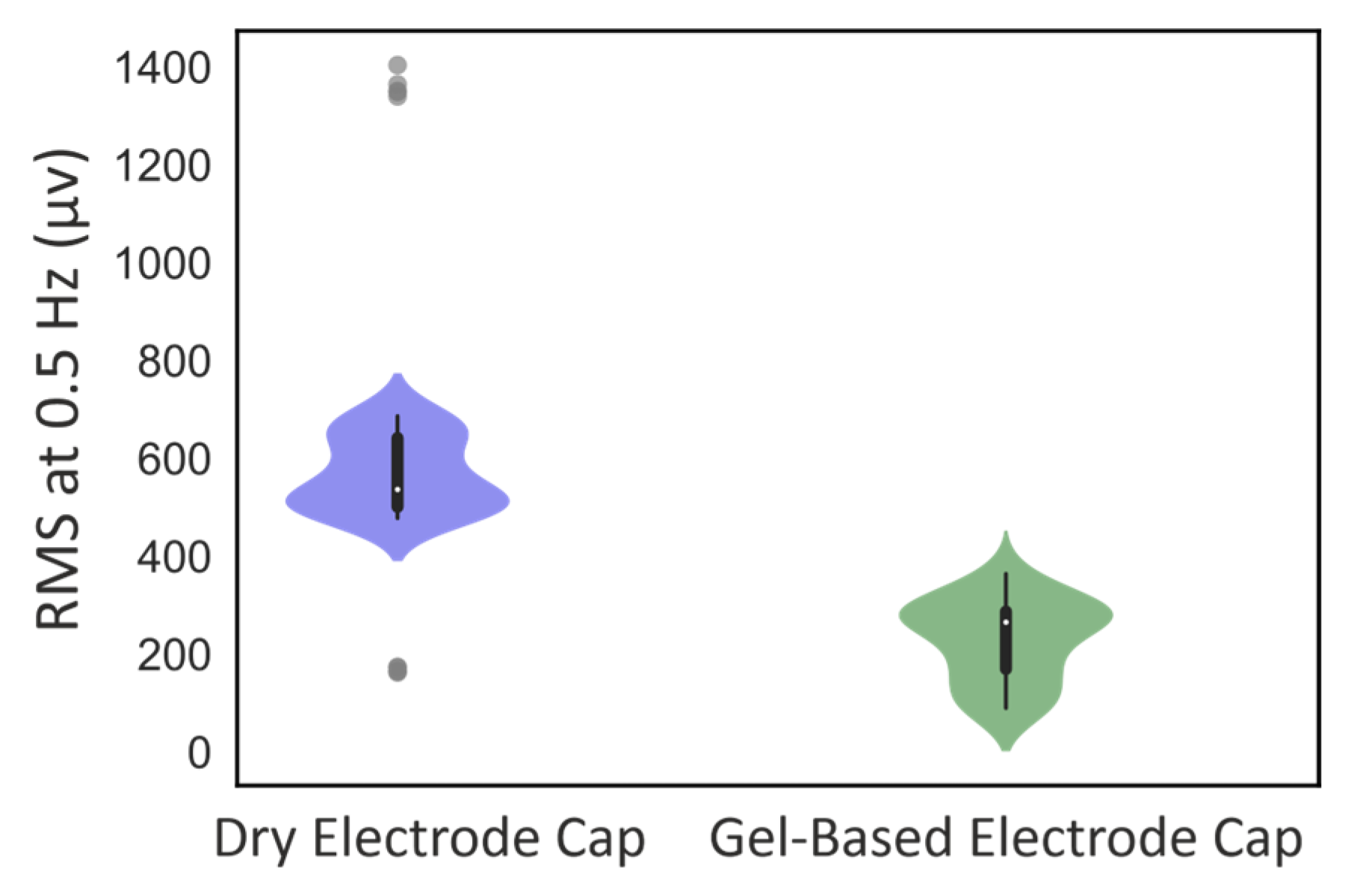
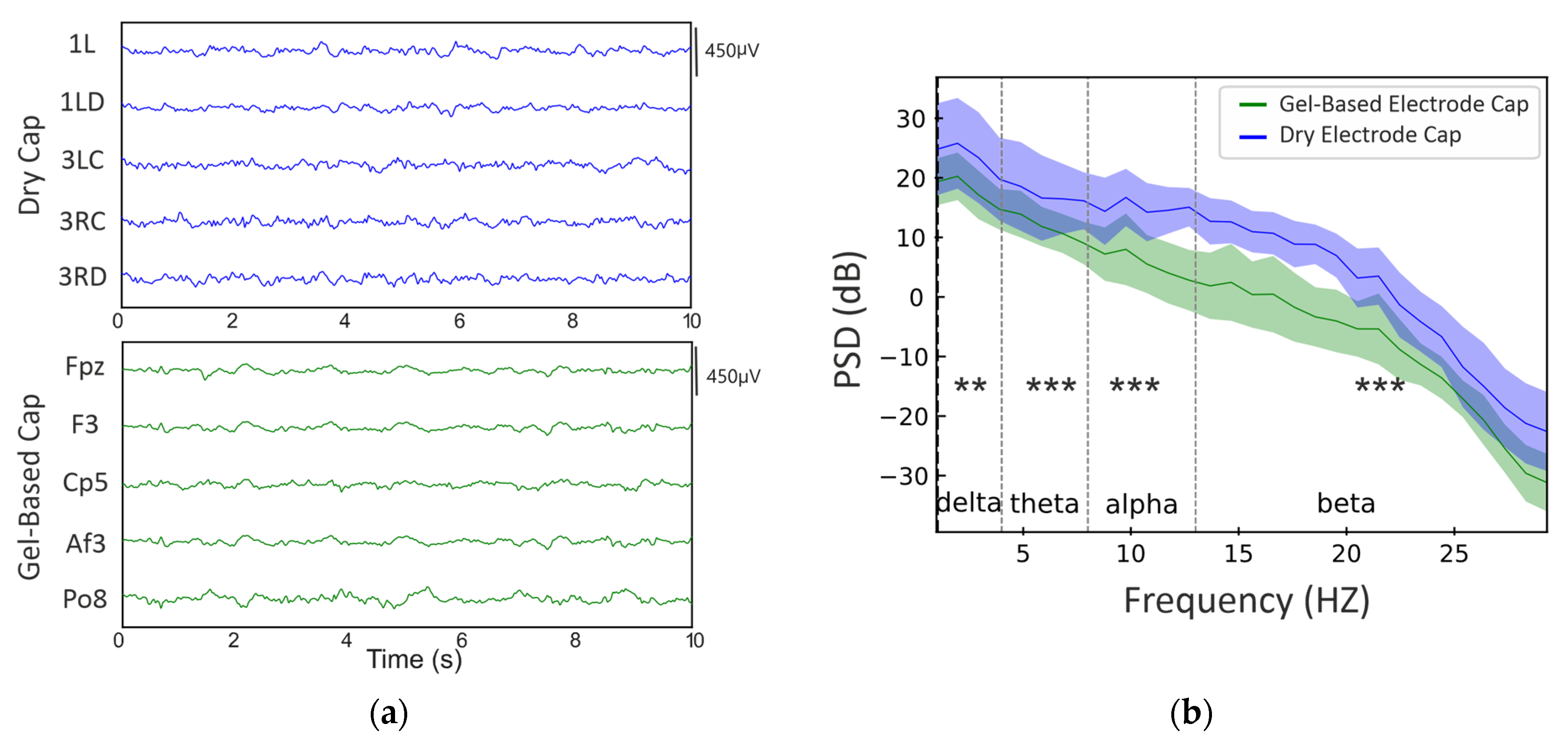
3.2. Phantom Head Recording, EEG Signal
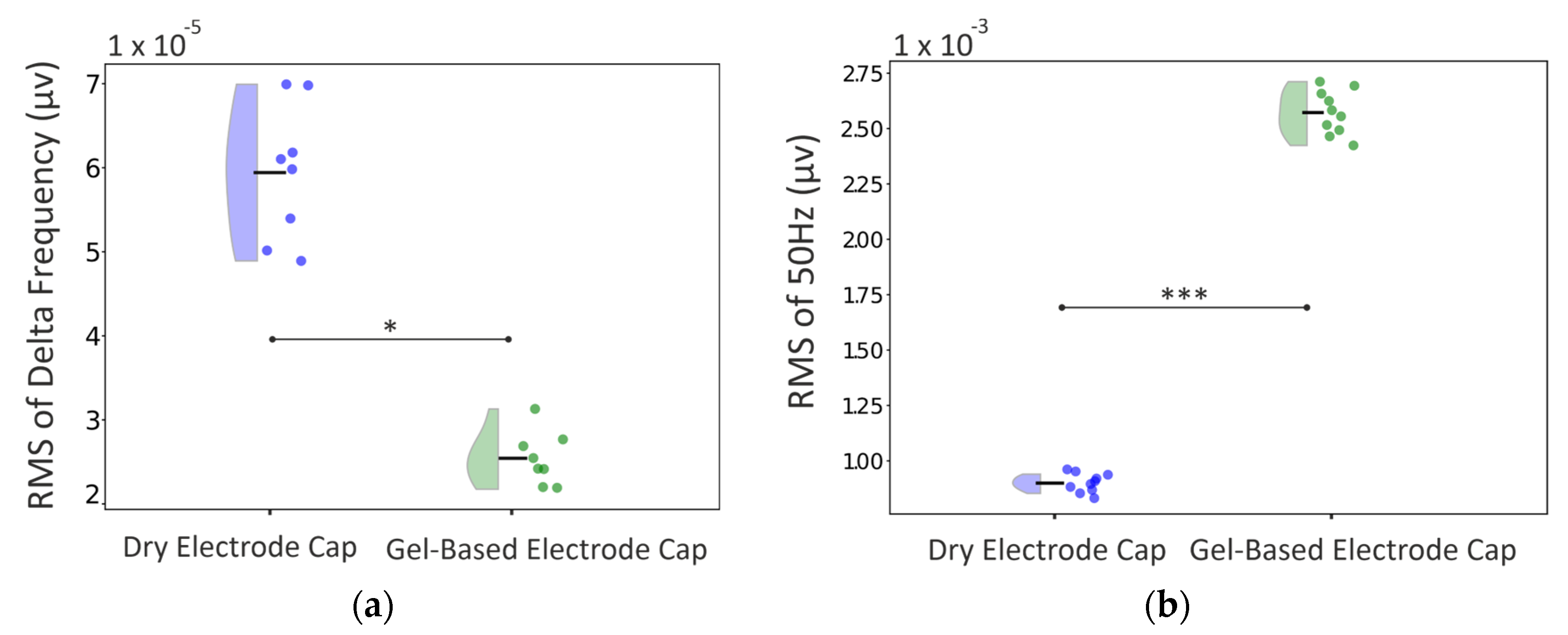
3.3. Real Infant Signal Analysis
3.4. Practical Observations of Using the Dry Electrode Cap
4. Discussion
4.1. Force Distributions
4.2. EEG Signal Quality
4.3. Practical Implications for Neonatal Applications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Acronym/Term | Full Name |
|---|---|
| EEG | Electroencephalogram |
| FSR | Force Sensing Resistor |
| Ag/AgCl | Silver/Silver Chloride |
| RMS | Root Mean Square |
| PSD | Power Spectral Density |
| FDR | False Discovery Rate |
| I2C | Inter-Integrated Circuit |
| Powerline Interference | Electrical Noise at 50 Hz |
References
- Lamblin, M.D.; André, M.; Challamel, M.J.; Curzi-Dascalova, L.; d’Allest, A.M.; De Giovanni, E.; Moussalli-Salefranque, F.; Navelet, Y.; Plouin, P.; Radvanyi-Bouvet, M.F.; et al. Electroencephalography of the premature and term newborn. Maturational aspects and glossary. Neurophysiol. Clin. 1999, 29, 123–219. [Google Scholar] [CrossRef] [PubMed]
- Shellhaas, R.A. Continuous Long-Term Electroencephalography: The Gold Standard for Neonatal Seizure Diagnosis. In Seminars in Fetal and Neonatal Medicine; Elsevier: Amsterdam, The Netherlands, 2015; pp. 149–153. [Google Scholar]
- Tseghai, G.B.; Malengier, B.; Fante, K.A.; Van Langenhove, L. The status of textile-based dry EEG electrodes. Autex Res. J. 2021, 21, 63–70. [Google Scholar] [CrossRef]
- Teplan, M. Fundamentals of EEG measurement. Meas. Sci. Rev. 2002, 2, 1–11. [Google Scholar]
- Nathalie, M.; Mathur, A.M.; Jain, S.; Vesoulis, Z.A.; Zempel, J.M. Long term electroencephalography in preterm neonates: Safety and quality of electrode types. Clin. Neurophysiol. 2018, 129, 1366–1371. [Google Scholar]
- Ferree, T.C.; Luu, P.; Russell, G.S.; Tucker, D.M. Scalp electrode impedance, infection risk, and EEG data quality. Clin. Neurophysiol. 2001, 112, 536–544. [Google Scholar] [CrossRef]
- Foreman, S.W.; Thorngate, L.; Burr, R.L.; Thomas, K.A. Electrode challenges in amplitude-integrated electroencephalography (aEEG): Research application of a novel noninvasive measure of brain function in preterm infants. Biol. Res. Nurs. 2011, 13, 251–259. [Google Scholar] [CrossRef]
- Walls-Esquivel, E.; Vecchierini, M.F.; Héberlé, C.; Wallois, F. Electroencephalography (EEG) recording techniques and artefact detection in early premature babies. Neurophysiol. Clin. Clin. Neurophysiol. 2007, 37, 299–309. [Google Scholar] [CrossRef]
- O’Sullivan, M.; Temko, A.; Bocchino, A.; O’Mahony, C.; Boylan, G.; Popovici, E. Analysis of a low-cost EEG monitoring system and dry electrodes toward clinical use in the neonatal ICU. Sensors 2019, 19, 2637. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Z.; Lu, C.; Shu, F.; Chen, C.; Wang, L.; Chen, W. Neonatal seizure detection using a wearable multi-sensor system. Bioengineering 2023, 10, 658. [Google Scholar] [CrossRef]
- Fabregat-Sanjuan, A.; Rodríguez-Ballabriga, Á.; Rigo-Vidal, A.; Pàmies-Vilà, R.; Larrosa-Capaces, S.; Rius-Costa, V.; Pascual-Rubio, V. Analysis of electrode performance on amplitude integrated electroencephalography in neonates: Evaluation of a new electrode aCUP-E vs. liquid gel electrodes. Front. Pediatr. 2024, 12, 1452862. [Google Scholar] [CrossRef]
- O’Sullivan, M.; Pena, J.P.; Bocchino, A.; O’Mahony, C.; Costello, D.; Popovici, E.; Temko, A. Comparison of electrode technologies for dry and portable EEG acquisition. In Proceedings of the 7th IEEE International Workshop on Advances in Sensors and Interfaces (IWASI), Vieste, Italy, 15–16 June 2017; IEEE: New York, NY, USA, 2017; pp. 15–20. [Google Scholar]
- Lopez-Gordo, M.A.; Sanchez-Morillo, D.; Valle, F.P. Dry EEG electrodes. Sensors 2014, 14, 12847–12870. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, P.; Cunha, L.T.; Pedrosa, P.; Brodkorb, S.; Fonseca, C.; Vaz, F.; Haueisen, J. Novel TiNx-based biosignal electrodes for electroencephalography. Meas. Sci. Technol. 2011, 22, 124007. [Google Scholar] [CrossRef]
- Ng, C.R.; Fiedler, P.; Kuhlmann, L.; Liley, D.; Vasconcelos, B.; Fonseca, C.; Tamburro, G.; Comani, S.; Warsito, I.F.; Supriyanto, E. Multi-center evaluation of gel-based and dry multipin EEG caps. Sensors 2022, 22, 8079. [Google Scholar] [CrossRef] [PubMed]
- Di Flumeri, G.; Aricò, P.; Borghini, G.; Sciaraffa, N.; Di Florio, A.; Babiloni, F. The dry revolution: Evaluation of three different EEG dry electrode types in terms of signal spectral features, mental states classification and usability. Sensors 2019, 19, 1365. [Google Scholar] [CrossRef]
- Leach, S.; Chung, K.; Tüshaus, L.; Huber, R.; Karlen, W. A protocol for comparing dry and wet EEG electrodes during sleep. Front. Neurosci. 2020, 14, 586. [Google Scholar] [CrossRef]
- Krachunov, S.; Casson, A.J. 3D printed dry EEG electrodes. Sensors 2016, 16, 1635. [Google Scholar] [CrossRef]
- Fiedler, P.; Mühle, R.; Griebel, S.; Pedrosa, P.; Fonseca, C.; Vaz, F.; Zanow, F.; Haueisen, J. Contact pressure and flexibility of multipin dry EEG electrodes. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 750–757. [Google Scholar] [CrossRef]
- Heijs, J.J.A.; Havelaar, R.J.; Fiedler, P.; van Wezel, R.J.A.; Heida, T. Validation of soft multipin dry EEG electrodes. Sensors 2021, 21, 6827. [Google Scholar] [CrossRef]
- Erickson, B.; Rich, R.; Shanker, S.; Kim, B.; Driscoll, N.; Mentzelopoulos, G.; Fernandez-Nuñez, G.; Vitale, F.; D Medaglia, J. Evaluating and benchmarking the EEG signal quality of high-density, dry MXene-based electrode arrays against gelled Ag/AgCl electrodes. J. Neural Eng. 2024, 21, 016005. [Google Scholar] [CrossRef]
- Fridman, I.; Cordeiro, M.; Rais-Bahrami, K.; McDonald, N.J.; Reese, J.J.; Massaro, A.N.; Conry, J.A.; Chang, T.; Soussou, W.; Tsuchida, T.N. Evaluation of dry sensors for neonatal EEG recordings. J. Clin. Neurophysiol. 2016, 33, 149–155. [Google Scholar] [CrossRef]
- Taji, B.; Chan, A.D.C.; Shirmohammadi, S. Effect of pressure on skin-electrode impedance in wearable biomedical measurement devices. IEEE Trans. Instrum. Meas. 2018, 67, 1900–1912. [Google Scholar]
- Mietzsch, U.; Cooper, K.L.; Harris, M.L. Successful Reduction in Electrode-Related Pressure Ulcers During EEG Monitoring in Critically Ill Neonates. Adv. Neonatal Care 2019, 19, 262–274. Available online: https://journals.lww.com/advancesinneonatalcare/fulltext/2019/08000/successful_reduction_in_electrode_related_pressure.5.aspx (accessed on 2 February 2025). [CrossRef]
- Warsito, I.F.; Komosar, M.; Bernhard, M.A.; Fiedler, P.; Haueisen, J. Flower electrodes for comfortable dry electroencephalography. Sci. Rep. 2023, 13, 16589. [Google Scholar] [CrossRef]
- Damalerio, R.B.; Lim, R.; Gao, Y.; Zhang, T.-T.; Cheng, M.-Y. Development of Low-Contact-Impedance Dry Electrodes for Electroencephalogram Signal Acquisition. Sensors 2023, 23, 4453. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Guess, M.; Kim, K.R.; Yeo, W.-H. Skin-interfacing wearable biosensors for smart health monitoring of infants and neonates. Commun. Mater. 2024, 5, 72. [Google Scholar] [PubMed]
- O’Sullivan, M.; Popovici, E.; Bocchino, A.; O’Mahony, C.; Boylan, G.; Temko, A. System level framework for assessing the accuracy of neonatal EEG acquisition. In Proceedings of the 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; IEEE: New York, NY, USA, 2018; pp. 4339–4342. [Google Scholar]
- Cordeiro, M.; Peinado, H.; Montes, M.T.; Valverde, E. Evaluation of the suitability and clinical applicability of different electrodes for aEEG/cEEG monitoring in the extremely premature infant. An. De Pediatría 2021, 95, 423–430. [Google Scholar] [CrossRef]
- Tseghai, G.B.; Malengier, B.; Fante, K.A.; Van Langenhove, L. A long-lasting textile-based anatomically realistic head phantom for validation of EEG electrodes. Sensors 2021, 21, 4658. [Google Scholar] [CrossRef]
- Asayesh, A.; Vanhatalo, S.; Tokariev, A. The impact of EEG electrode density on the mapping of cortical activity networks in infants. Neuroimage 2024, 303, 120932. [Google Scholar]
- Tokariev, A.; Breakspear, M.; Videman, M.; Stjerna, S.; Scholtens, L.H.; van den Heuvel, M.P.; Cocchi, L.; Vanhatalo, S. Impact of in utero exposure to antiepileptic drugs on neonatal brain function. Cereb. Cortex 2022, 32, 2385–2397. [Google Scholar]
- Ahtola, E.; Leikos, S.; Tuiskula, A.; Haataja, L.; Smeds, E.; Piitulainen, H.; Jousmäki, V.; Tokariev, A.; Vanhatalo, S. Cortical networks show characteristic recruitment patterns after somatosensory stimulation by pneumatically evoked repetitive hand movements in newborn infants. Cereb. Cortex 2023, 33, 4699–4713. [Google Scholar] [CrossRef]
- Syvälahti, T.; Tuiskula, A.; Nevalainen, P.; Metsäranta, M.; Haataja, L.; Vanhatalo, S.; Tokariev, A. Networks of cortical activity show graded responses to perinatal asphyxia. Pediatr. Res. 2024, 96, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Khazaei, M.; Raeisi, K.; Vanhatalo, S.; Zappasodi, F.; Comani, S.; Tokariev, A. Neonatal cortical activity organizes into transient network states that are affected by vigilance states and brain injury. Neuroimage 2023, 279, 120342. [Google Scholar] [CrossRef] [PubMed]
- Yrjölä, P.; Stjerna, S.; Palva, J.M.; Vanhatalo, S.; Tokariev, A. Phase-based cortical synchrony is affected by prematurity. Cereb. Cortex 2022, 32, 2265–2276. [Google Scholar] [CrossRef] [PubMed]
- Fleming, S.; Thompson, M.; Stevens, P.; Heneghan, C.; Plüddemann, A.; Maconochie, I.; Tarassenko, L.; Mant, D. Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: A systematic review of observational studies. Lancet 2011, 377, 1011–1018. [Google Scholar]
- Wallois, F.; Routier, L.; Heberlé, C.; Mahmoudzadeh, M.; Bourel-Ponchel, E.; Moghimi, S. Back to basics: The neuronal substrates and mechanisms that underlie the electroencephalogram in premature neonates. Neurophysiol. Clin. 2021, 51, 5–33. [Google Scholar]
- Nilsson, S.; Tokariev, A.; Vehviläinen, T.; Fellman, V.; Vanhatalo, S.; Norman, E. Depression of cortical neuronal activity after a low-dose fentanyl in preterm infants. Acta Paediatr. 2024, 114, 109–115. [Google Scholar] [CrossRef]
- Fiedler, P.; Graichen, U.; Zimmer, E.; Haueisen, J. Simultaneous dry and gel-based high-density electroencephalography recordings. Sensors 2023, 23, 9745. [Google Scholar] [CrossRef]
- Boylan, G.B.; Kharoshankaya, L.; Mathieson, S.R. Diagnosis of seizures and encephalopathy using conventional EEG and amplitude integrated EEG. Handb. Clin. Neurol. 2019, 162, 363–400. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asayesh, A.; Warsito, I.F.; Haueisen, J.; Fiedler, P.; Vanhatalo, S. Neonatal Electroencephalogram Recording with a Dry Electrode Cap: A Feasibility Study. Sensors 2025, 25, 966. https://doi.org/10.3390/s25030966
Asayesh A, Warsito IF, Haueisen J, Fiedler P, Vanhatalo S. Neonatal Electroencephalogram Recording with a Dry Electrode Cap: A Feasibility Study. Sensors. 2025; 25(3):966. https://doi.org/10.3390/s25030966
Chicago/Turabian StyleAsayesh, Amirreza, Indhika Fauzhan Warsito, Jens Haueisen, Patrique Fiedler, and Sampsa Vanhatalo. 2025. "Neonatal Electroencephalogram Recording with a Dry Electrode Cap: A Feasibility Study" Sensors 25, no. 3: 966. https://doi.org/10.3390/s25030966
APA StyleAsayesh, A., Warsito, I. F., Haueisen, J., Fiedler, P., & Vanhatalo, S. (2025). Neonatal Electroencephalogram Recording with a Dry Electrode Cap: A Feasibility Study. Sensors, 25(3), 966. https://doi.org/10.3390/s25030966










