Yttrium Doping of Perovskite Oxide La2Ti2O7 Nanosheets for Enhanced Proton Conduction and Gas Sensing Under HighHumidity Levels
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
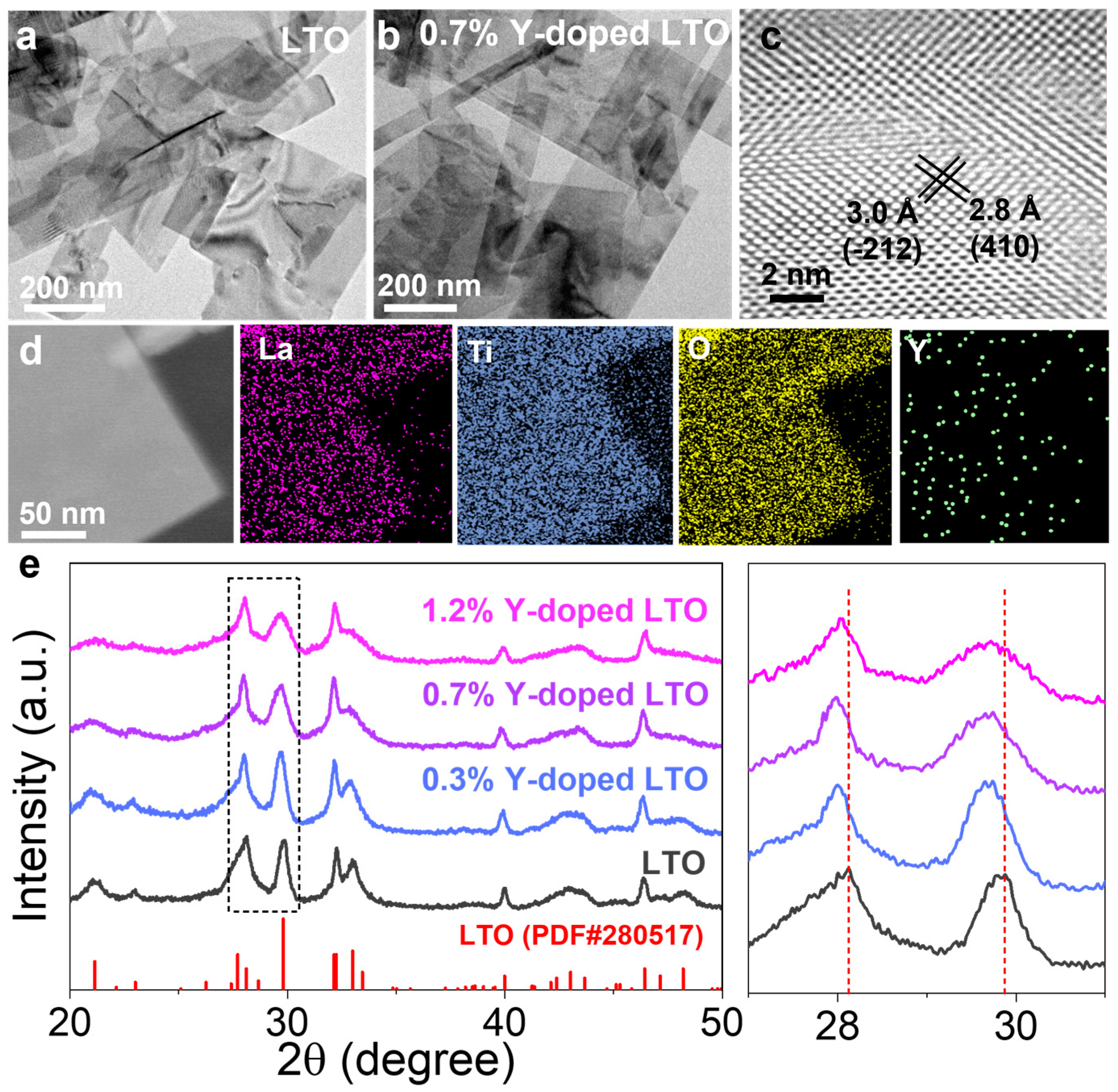
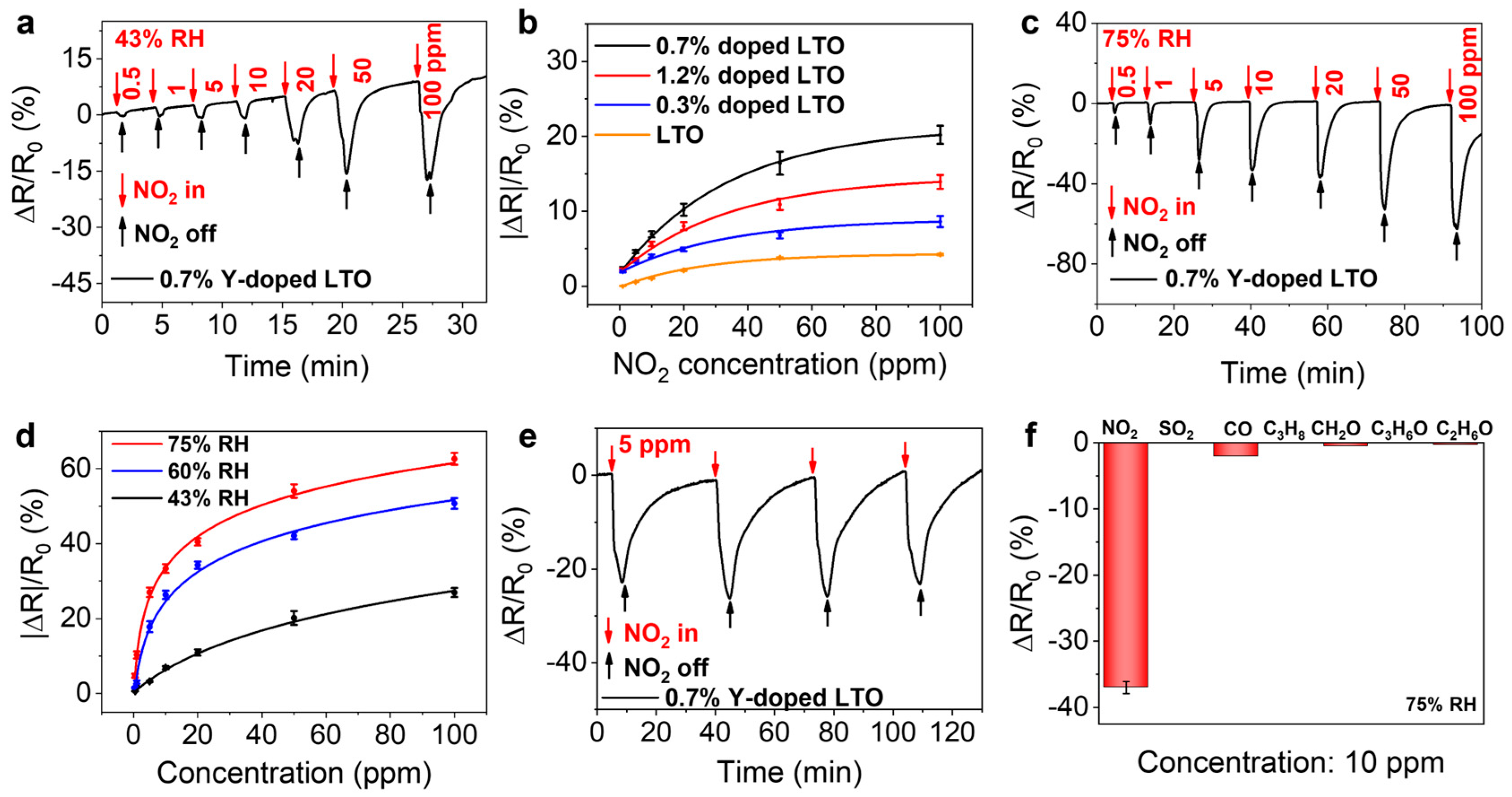
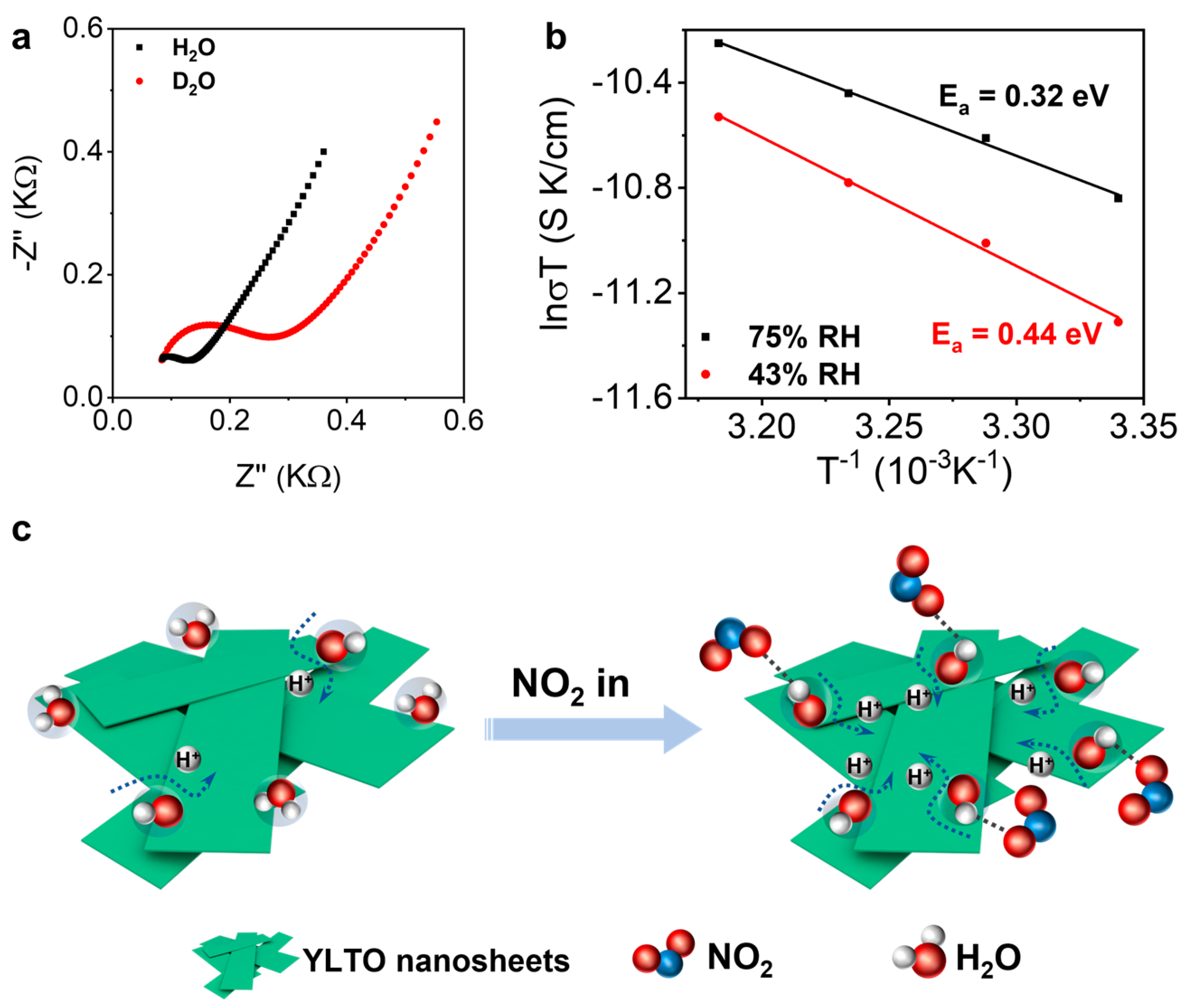
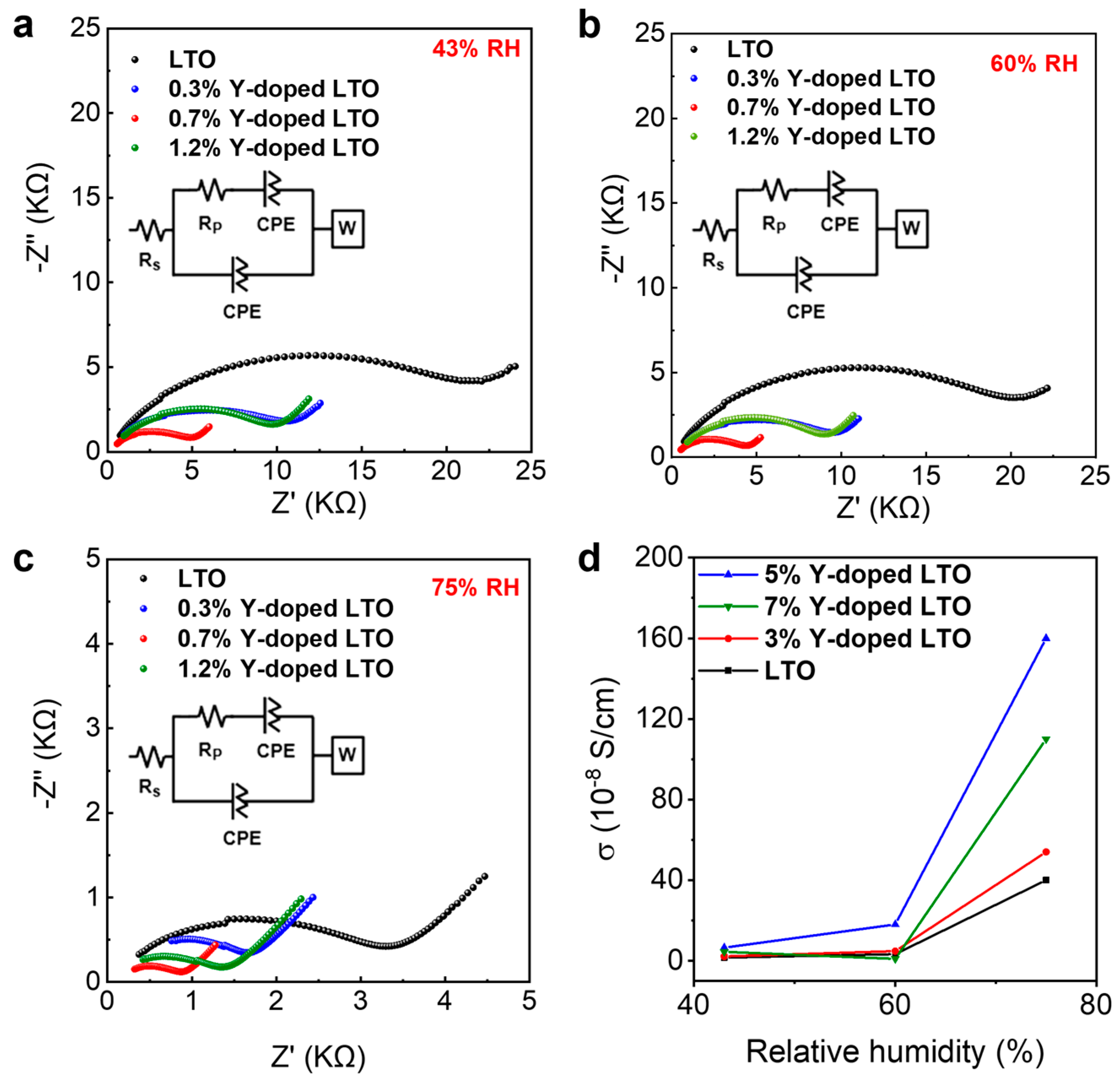
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bai, H.; Guo, H.; Wang, J.; Dong, Y.; Liu, B.; Xie, Z.; Guo, F.; Chen, D.; Zhang, R.; Zheng, Y. A room-temperature NO2 gas sensor based on CuO nanoflakes modified with rGO nanosheets. Sens. Actuators B 2021, 337, 129783–129795. [Google Scholar] [CrossRef]
- Dai, J.; Ogbeide, O.; Macadam, N.; Sun, Q.; Yu, W.; Li, Y.; Su, B.-L.; Hasan, T.; Huang, X.; Huang, W. Printed gas sensors. Chem. Soc. Rev. 2020, 49, 1756–1789. [Google Scholar] [CrossRef]
- Liang, T.; Dai, Z.; Liu, Y.; Zhang, X.; Zeng, H. Suppression of Sn2+ and Lewis acidity in SnS2/black phosphorus heterostructure for ppb-level room temperature NO2 gas sensor. Sci. Bull. 2021, 66, 2471–2478. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Zhang, H.; Jiang, Y.; Yi, J. Superior NO2 sensing of MOF-derived indium-doped ZnO porous hollow cages. ACS Appl. Mater. Interfaces 2020, 12, 37489–37498. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fatima-Ezzahra, E.; Dai, J.; Liu, Y.; Pei, C.; Li, H.; Wang, Z.; Huang, X. Ligand-assisted deposition of ultra-small Au nanodots on Fe2O3/reduced graphene oxide for flexible gas sensors. Nanoscale Adv. 2022, 4, 1345–1350. [Google Scholar] [CrossRef]
- Wei, S.; Li, Z.; Murugappan, K.; Li, Z.; Zhang, F.; Saraswathyvilasam, A.G.; Lysevych, M.; Tan, H.H.; Jagadish, C.; Tricoli, A.; et al. A Self-Powered Portable Nanowire Array Gas Sensor for Dynamic NO2 Monitoring at Room Temperature. Adv. Mater. 2023, 35, e2207199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Z.; Liu, J.; Peng, Z.; Zhou, J.; Zhang, H.; Li, Y. Optoelectronic gas sensor based on few-layered InSe nanosheets for NO2 detection with ultrahigh antihumidity ability. Anal. Chem. 2020, 92, 11277–11287. [Google Scholar] [CrossRef]
- Broza, Y.Y.; Vishinkin, R.; Barash, O.; Nakhleh, M.K.; Haick, H. Synergy between nanomaterials and volatile organic compounds for non-invasive medical evaluation. Chem. Soc. Rev. 2018, 47, 4781–4859. [Google Scholar] [CrossRef]
- Huang, W.; Zhuang, X.; Melkonyan, F.S.; Wang, B.; Zeng, L.; Wang, G.; Han, S.; Bedzyk, M.J.; Yu, J.; Marks, T.J.; et al. UV–Ozone Interfacial Modification in Organic Transistors for High-Sensitivity NO2 Detection. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Yu, J.; Wang, D.; Tipparaju, V.V.; Tsow, F.; Xian, X. Mitigation of Humidity Interference in Colorimetric Sensing of Gases. ACS Sens. 2020, 6, 303–320. [Google Scholar] [CrossRef]
- Nasriddinov, A.; Rumyantseva, M.; Konstantinova, E.; Marikutsa, A.; Tokarev, S.; Yaltseva, P.; Fedorova, O.; Gaskov, A. Effect of Humidity on Light-Activated NO and NO2 Gas Sensing by Hybrid Materials. Nanomaterials 2020, 10, 915. [Google Scholar] [CrossRef]
- Lin, H.; Wang, J.; Xu, S.; Zhang, Q.; Cheng, Y.; Han, D.; Wang, H.; Zhuo, K. Au-WO3 Nanowire-Based Electrodes for NO2 Sensing. ACS Appl. Nano Mater. 2022, 5, 14311–14319. [Google Scholar] [CrossRef]
- Mohanta, D. Ahmaruzzaman Novel Ag-SnO2-βC3N4 ternary nanocomposite based gas sensor for enhanced low-concentration NO2 sensing at room temperature. Sens. Actuators B Chem. 2020, 326, 128910. [Google Scholar] [CrossRef]
- Xu, Y.; Xie, J.; Zhang, Y.; Tian, F.; Yang, C.; Zheng, W.; Liu, X.; Zhang, J.; Pinna, N. Edge-enriched WS2 nanosheets on carbon nanofibers boosts NO2 detection at room temperature. J. Hazard. Mater. 2021, 411, 125120. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Gupta, G.; Bapna, K.; Shivagan, D. Semiconductor-metal-oxide-based nano-composites for humidity sensing applications. Mater. Res. Bull. 2022, 158. [Google Scholar] [CrossRef]
- Qin, Y.; Jiang, Y.; Zhao, L. Enhanced humidity resistance of porous SiNWs via OTS functionalization for rarefied NO2 detection. Sens. Actuators B Chem. 2018, 283, 61–68. [Google Scholar] [CrossRef]
- Mandić, V.; Bafti, A.; Pavić, L.; Panžić, I.; Kurajica, S.; Pavelić, J.-S.; Shi, Z.; Mužina, K.; Ivković, I.K. Humidity Sensing Ceria Thin-Films. Nanomaterials 2022, 12, 521. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Y.; Yu, J.; Zhou, C.; Yang, C.; Wang, L.; Ma, L.; Wang, L.J. Highly-sensitive organic field effect transistor sensors for dual detection of humidity and NO2. Sens. Actuators B Chem. 2022, 374. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, H.; Lin, C.; Bian, M. The enhancement of humidity sensing performance based on Eu-doped ZnO. Curr. Appl. Phys. 2018, 19, 82–88. [Google Scholar] [CrossRef]
- Gao, Z.; Song, G.; Zhang, X.; Li, Q.; Yang, S.; Wang, T.; Li, Y.; Zhang, L.; Guo, L.; Fu, Y. A facile PDMS coating approach to room-temperature gas sensors with high humidity resistance and long-term stability. Sens. Actuators B Chem. 2020, 325, 128810. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Yu, Q.; Liu, Y.; Zhang, X.; Zhu, G.; Jia, Y.; Lu, H.; Gao, J.; Wang, H.; et al. Flexible, Breathable and Hydrophobic SnO2–SnS2–SiO2/SiO2 All-Inorganic Self-Supporting Nanofiber Membrane for Ultralow-Concentration NO2 Sensing Under High Humidity. Adv. Funct. Mater. 2024, 35. [Google Scholar] [CrossRef]
- Ma, Z.; Li, Y.; Lv, Y.; Sa, R.; Li, Q.; Wu, K. Synergistic Effect of Doping and Compositing on Photocatalytic Efficiency: A Case Study of La2Ti2O7. ACS Appl. Mater. Interfaces 2018, 10, 39327–39335. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Asakura, Y.; Hasegawa, T.; Yin, S. High-concentration N-doped La2Ti2O7 nanocrystals: Effects of nano-structuration and doping sites on enhancing the photocatalytic activity. Chem. Eng. J. 2021, 423. [Google Scholar] [CrossRef]
- Cai, X.; Mao, L.; Fujitsuka, M.; Majima, T.; Kasani, S.; Wu, N.; Zhang, J. Effects of Bi-dopant and co-catalysts upon hole surface trapping on La2Ti2O7 nanosheet photocatalysts in overall solar water splitting. Nano Res. 2021, 15, 438–445. [Google Scholar] [CrossRef]
- Gao, Z.; Wu, L.; Lu, C.; Gu, W.; Zhang, T.; Liu, G.; Xie, Q.; Li, M. The anisotropic conductivity of ferroelectric La2Ti2O7 ceramics. J. Eur. Ceram. Soc. 2017, 37, 137–143. [Google Scholar] [CrossRef]
- Gilardi, E.; Fabbri, E.; Bi, L.; Rupp, J.L.M.; Lippert, T.; Pergolesi, D.; Traversa, E. Effect of Dopant–Host Ionic Radii Mismatch on Acceptor-Doped Barium Zirconate Microstructure and Proton Conductivity. J. Phys. Chem. C 2017, 121, 9739–9747. [Google Scholar] [CrossRef]
- Han, D.; Shinoda, K.; Sato, S.; Majima, M.; Uda, T. Correlation between electroconductive and structural properties of proton conductive acceptor-doped barium zirconate. J. Mater. Chem. A 2014, 3, 1243–1250. [Google Scholar] [CrossRef]
- Wang, H.; Li, Q.; Wang, C.; He, H.; Yu, J.; Xu, J. Growth and Dielectric Properties of Ta-Doped La2Ti2O7 Single Crystals. Crystals 2018, 8, 113. [Google Scholar] [CrossRef]
- Lv, L.; Lei, L.; Chen, Q.-W.; Yin, C.-L.; Fan, H.; Zhou, J.-P. Oxygen vacancies-modified S-scheme heterojunction of Bi-doped La2Ti2O7 and La-doped Bi4Ti3O12 to improve the NO gas removal avoiding NO2 product. Appl. Catal. B Environ. 2023, 343. [Google Scholar] [CrossRef]
- Zhu, K.; Shi, N.; Zhang, L.; Huan, D.; Li, X.; Zhang, X.; Song, R.; Xia, C.; Peng, R.; Lu, Y. Engineering oxygen vacancy to accelerate proton conduction in Y-doped BaZrO3. Ceram. Int. 2022, 49, 13321–13329. [Google Scholar] [CrossRef]
- Li, K.; Wang, Y.; Wang, H.; Zhu, M.; Yan, H. Hydrothermal synthesis and photocatalytic properties of layered La2Ti2O7 nanosheets. Nanotechnology 2006, 17, 4863–4867. [Google Scholar] [CrossRef]
- Cai, X.; Zhu, M.; Elbanna, O.A.; Fujitsuka, M.; Kim, S.; Mao, L.; Zhang, J.; Majima, T. Au Nanorod Photosensitized La2Ti2O7 Nanosteps: Successive Surface Heterojunctions Boosting Visible to Near-Infrared Photocatalytic H2 Evolution. ACS Catal. 2017, 8, 122–131. [Google Scholar] [CrossRef]
- Cao, Y.; Tang, P.; Qiu, W.; Zhao, T. Preparation of Y-Doped La2Ti2O7 Flexible Self-Supporting Films and Their Application in High-Performance Flexible All-Solid-State Supercapacitor Devices. ACS Omega 2020, 5, 29722–29732. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Kochanski, G.P.; Jin, S.; Seibles, L. Defect-enhanced electron field emission from chemical vapor deposited diamond. J. Appl. Phys. 1995, 78, 2707–2711. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Leung, Y.H.; Tam, K.H.; Hsu, Y.F.; Ding, L.; Ge, W.K.; Zhong, Y.C.; Wong, K.S.; Chan, W.K.; Tam, H.L.; et al. Defect emissions in ZnO nanostructures. Nanotechnology 2007, 18. [Google Scholar] [CrossRef]
- Z Zhang, X.; Jiang, L.; Guo, S.; Han, D. (La1-xMx)2(Nb0.45Yb0.55)2O7-δ (M=Ca, Sr, Ba) Ionic Conductors Promoted by Foreign/Domestic Dual Acceptor-Doping Strategies. ChemSusChem 2022, 15, e202201879. [Google Scholar] [CrossRef] [PubMed]
- Agmon, N. The Grotthuss mechanism. Chem. Phys. Lett. 1995, 244, 456–462. [Google Scholar] [CrossRef]
- Mertes, S.; Wahner, A. Uptake of Nitrogen Dioxide and Nitrous Acid on Aqueous Surfaces. J. Phys. Chem. 1995, 99, 14000–14006. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, S.; Zhou, P.; Cui, B.; Liu, W.; Wei, D.; Shen, Y. Room-temperature NO2 sensing properties and mechanism of CuO nanorods with Au functionalization. Sens. Actuators B 2021, 328, 129070. [Google Scholar] [CrossRef]
- Ganesan, M.; Jayaraman, V.; Selvaraj, P.; Mani, K.M.; Kim, D.-H. Pyrochlore cerium stannate (Ce2Sn2O7) for highly sensitive NO2 gas sensing at room temperature. Appl. Surf. Sci. 2023, 624, 157135. [Google Scholar] [CrossRef]
- Dwivedi, C.; Srivastava, S.; Singh, P. Highly selective dual gas (NO & NO2) sensing depended on the operating temperature of WO3 thin films sputtered at room temperature. Curr. Appl. Phy. 2025, 69, 70–80. [Google Scholar] [CrossRef]
- Ghosh, S.; Ilango, M.S.; Prajapati, C.S.; Bhat, N. Reduction of Humidity Effect in WO3 Thin Film-Based NO2 Sensor Using Physiochemical Optimization. Cryst. Res. Technol. 2020, 56, 2000155. [Google Scholar] [CrossRef]
- D’Olimpio, G.; Boukhvalov, D.W.; Galstyan, V.; Occhiuzzi, J.; Vorochta, M.; Amati, M.; Milosz, Z.; Gregoratti, L.; Istrate, M.C.; Kuo, C.-N.; et al. Unlocking superior NO2 sensitivity and selectivity: The role of sulfur abstraction in indium sulfide (InS) nanosheet-based sensors. J. Mater. Chem. A 2024, 12, 10329–10340. [Google Scholar] [CrossRef]
- Bang, J.H.; Kwon, Y.J.; Lee, J.H.; Mirzaei, A.; Lee, H.Y.; Choi, H.; Kim, S.S.; Jeong, Y.K.; Kim, H.W. Proton-beam engineered surface-point defects for highly sensitive and reliable NO2 sensing under humid environments. J. Hazard. Mater. 2021, 416, 125841. [Google Scholar] [CrossRef]
- Ambi, R.R.; Mulla, M.G.; Pittala, R.K. NO2 sensing properties of chemically deposited vertically aligned flowerlike hexagonal ZnO nanorods. Sens. Actuators A 2024, 376, 115621. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Sun, C.; Bao, J.; Yang, Z.; Zhang, J.; Huang, X. Yttrium Doping of Perovskite Oxide La2Ti2O7 Nanosheets for Enhanced Proton Conduction and Gas Sensing Under HighHumidity Levels. Sensors 2025, 25, 901. https://doi.org/10.3390/s25030901
Wang J, Sun C, Bao J, Yang Z, Zhang J, Huang X. Yttrium Doping of Perovskite Oxide La2Ti2O7 Nanosheets for Enhanced Proton Conduction and Gas Sensing Under HighHumidity Levels. Sensors. 2025; 25(3):901. https://doi.org/10.3390/s25030901
Chicago/Turabian StyleWang, Jian, Caicai Sun, Jusheng Bao, Zhiwei Yang, Jian Zhang, and Xiao Huang. 2025. "Yttrium Doping of Perovskite Oxide La2Ti2O7 Nanosheets for Enhanced Proton Conduction and Gas Sensing Under HighHumidity Levels" Sensors 25, no. 3: 901. https://doi.org/10.3390/s25030901
APA StyleWang, J., Sun, C., Bao, J., Yang, Z., Zhang, J., & Huang, X. (2025). Yttrium Doping of Perovskite Oxide La2Ti2O7 Nanosheets for Enhanced Proton Conduction and Gas Sensing Under HighHumidity Levels. Sensors, 25(3), 901. https://doi.org/10.3390/s25030901




