Humidity-Sensing Performance of TiO2/RGO and α-Fe2O3/RGO Composites
Abstract
1. Introduction
2. Experiment
2.1. Raw Materials and Instrument
2.2. Synthesis of Materials
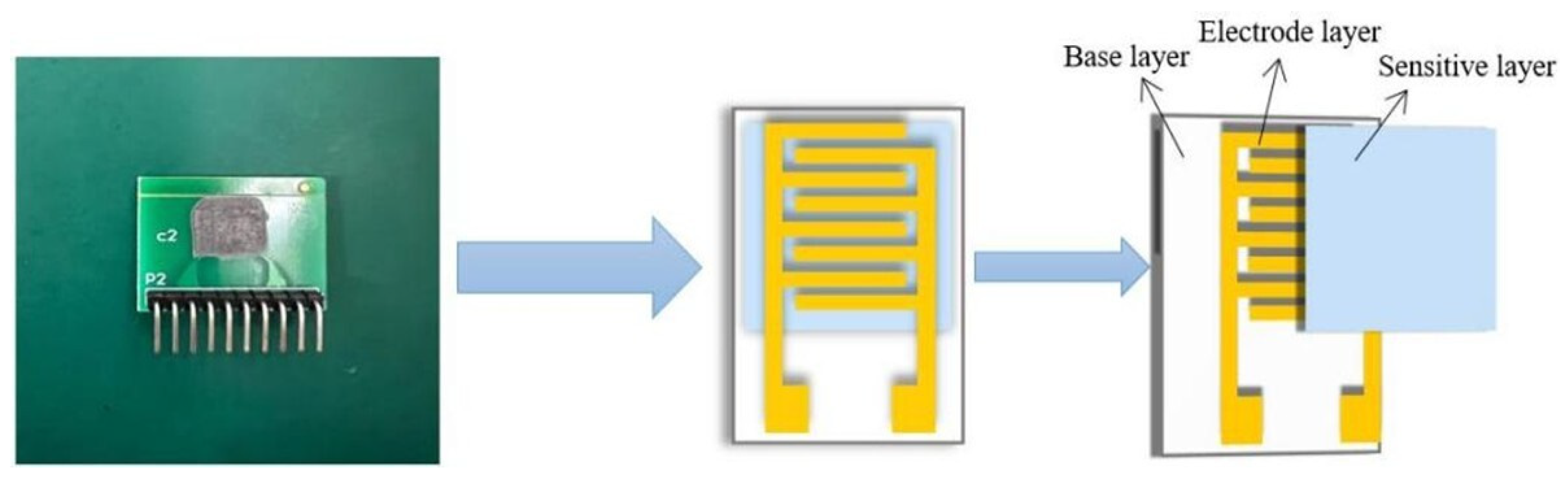
2.3. Fabrication of Sensors
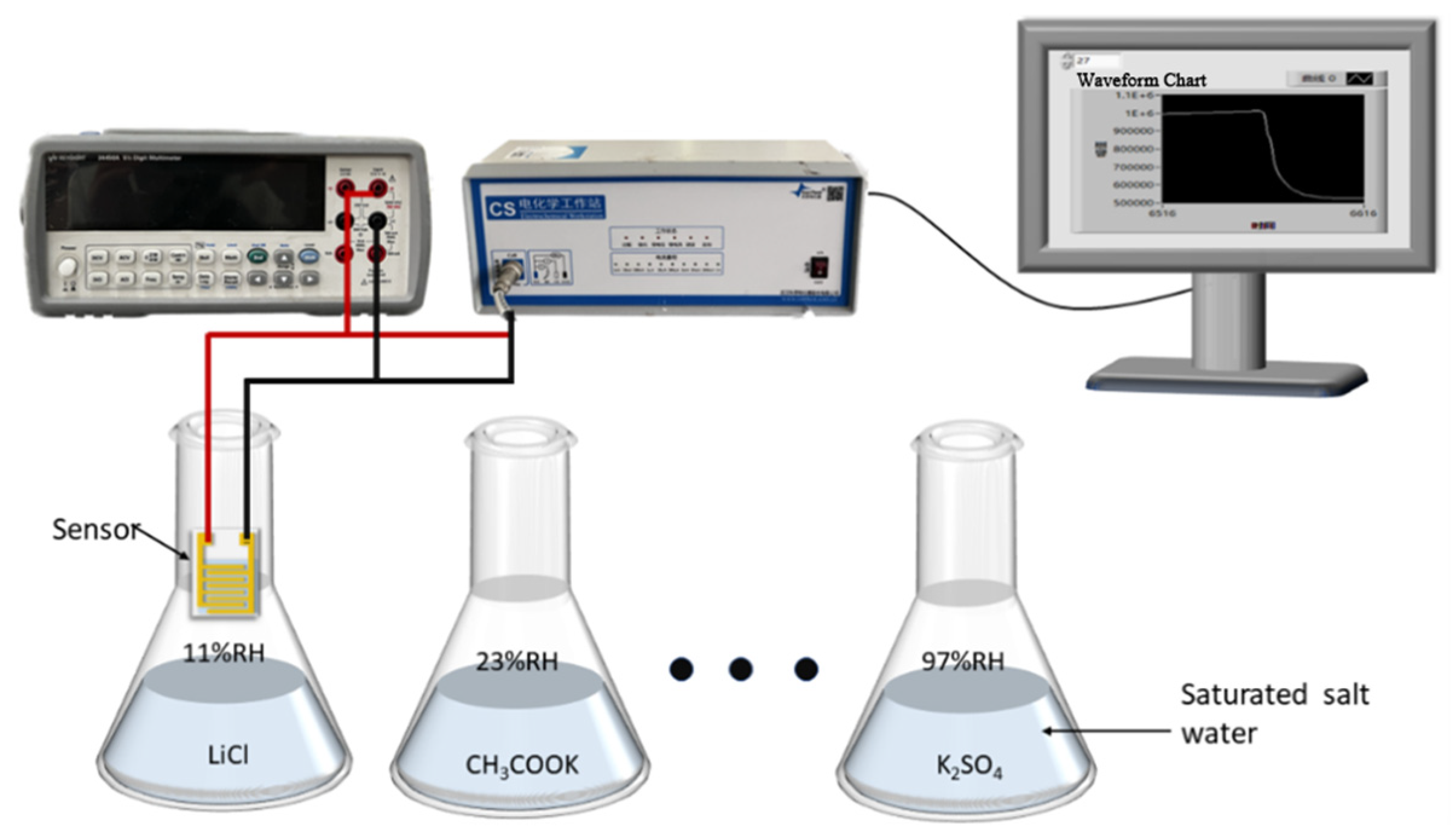
2.4. Experiment System
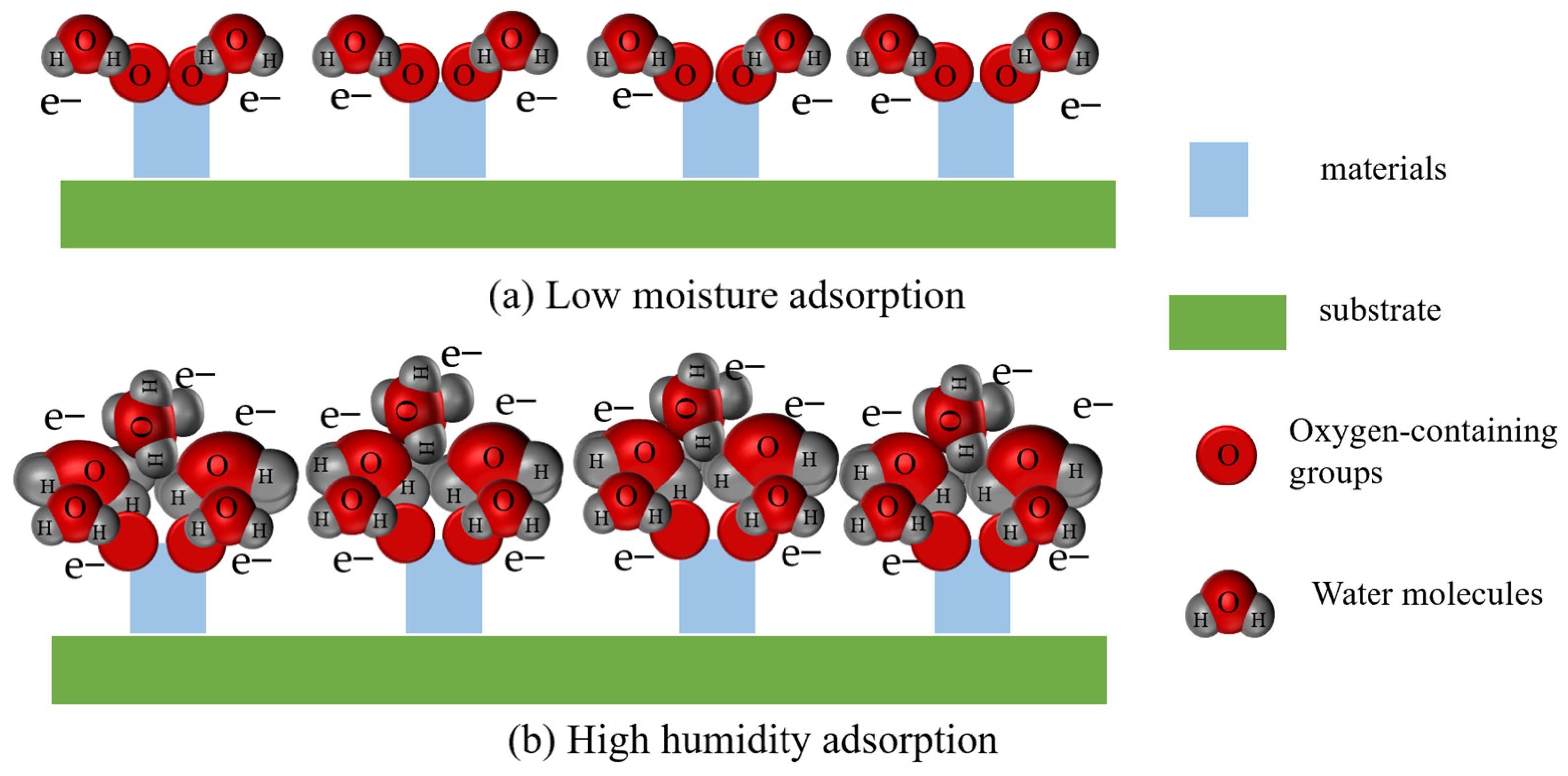
3. Humidity-Sensing Mechanism
4. Results and Discussions
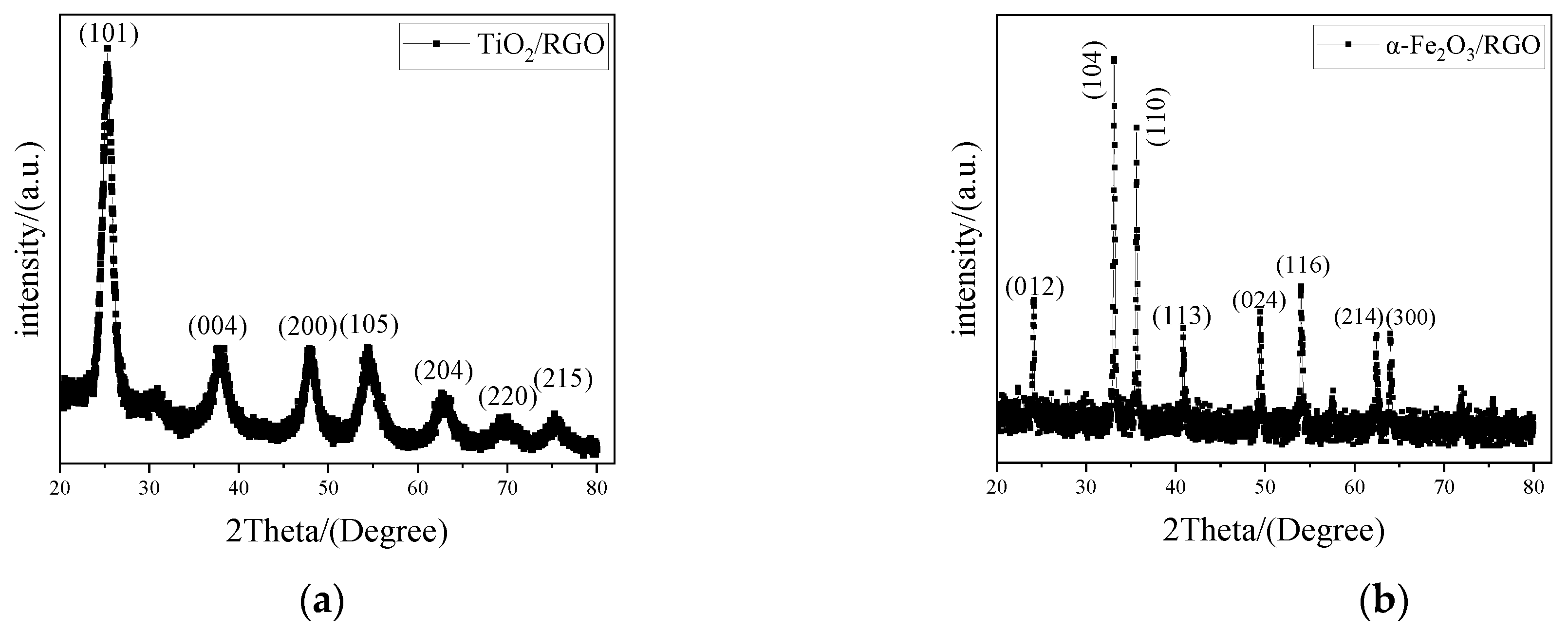
4.1. Material Characterization
4.2. Sensitivity and Hysteresis
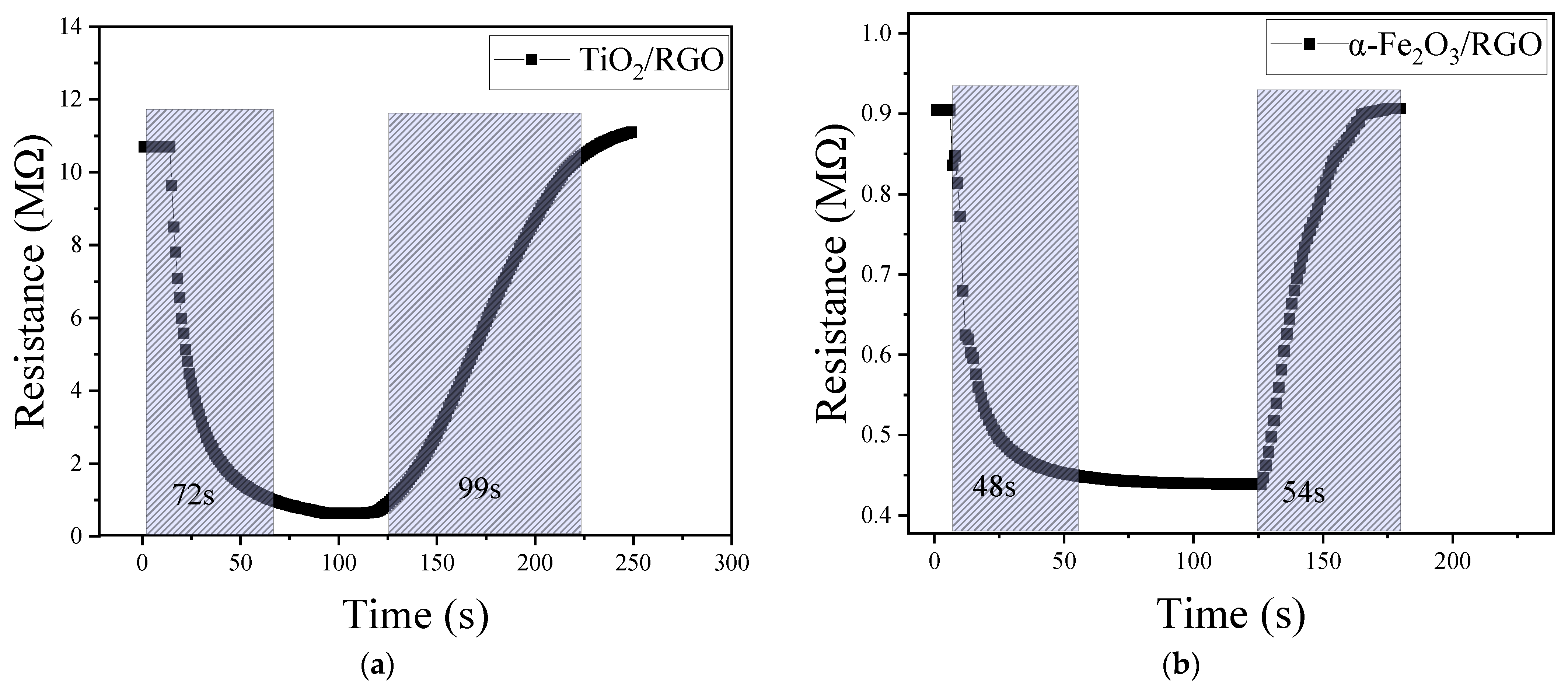
4.3. Response/Recovery Time
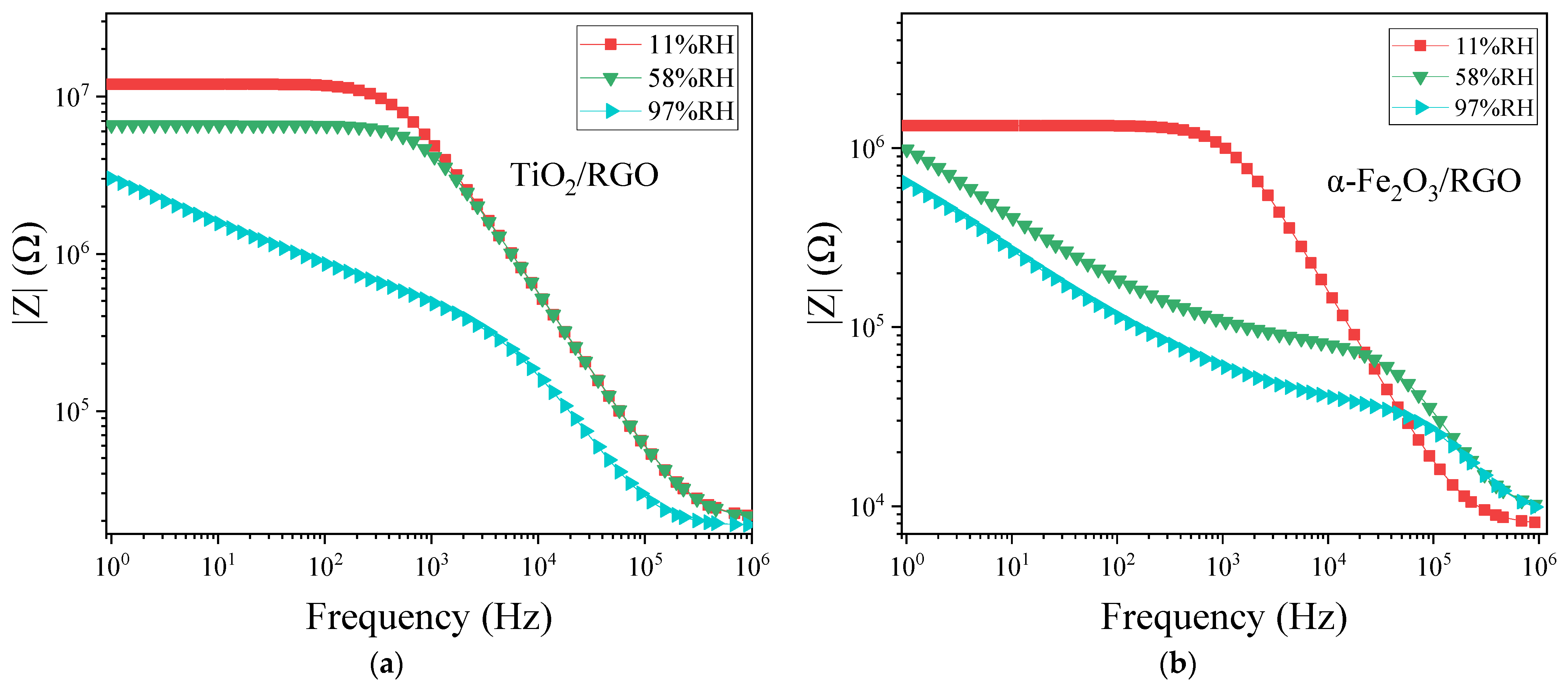
4.4. Complex Impedance and Frequency
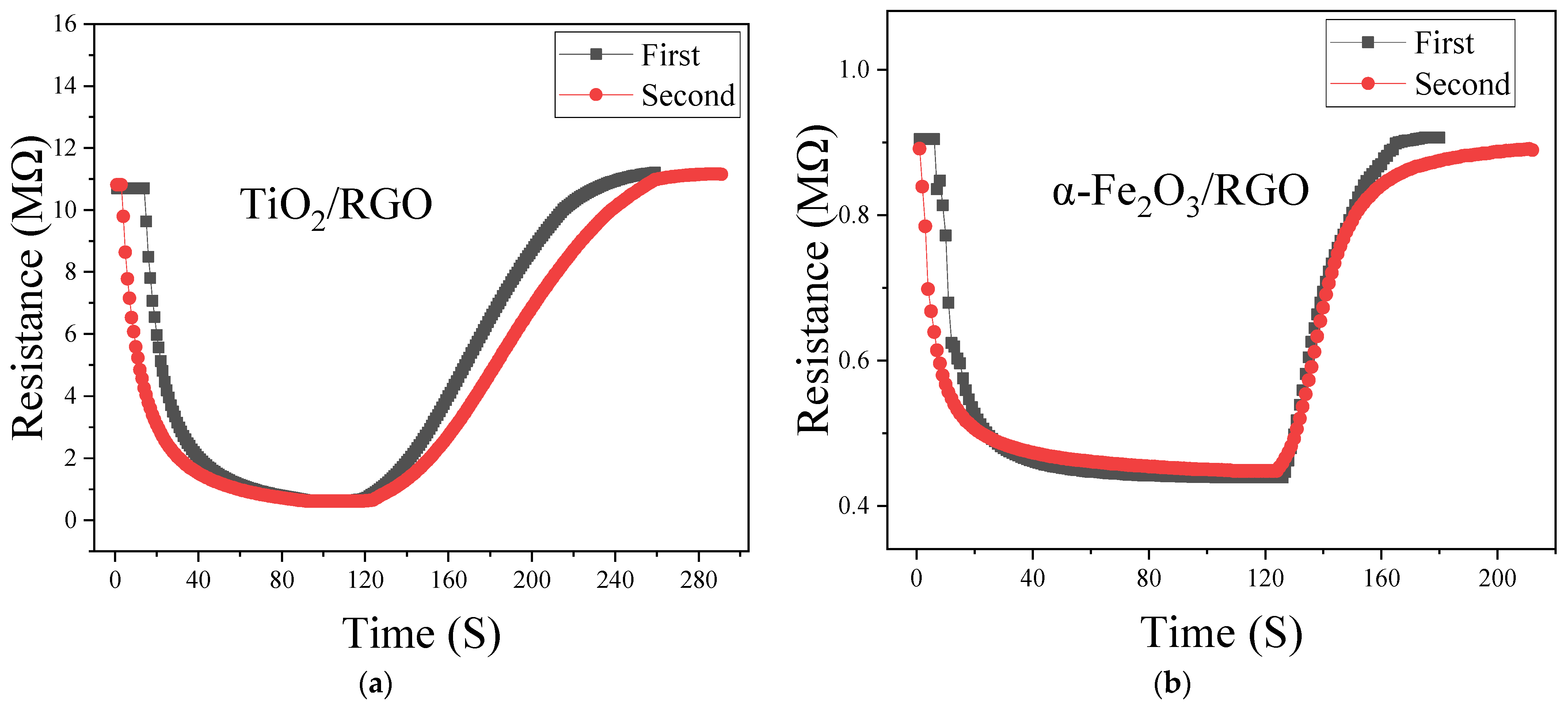
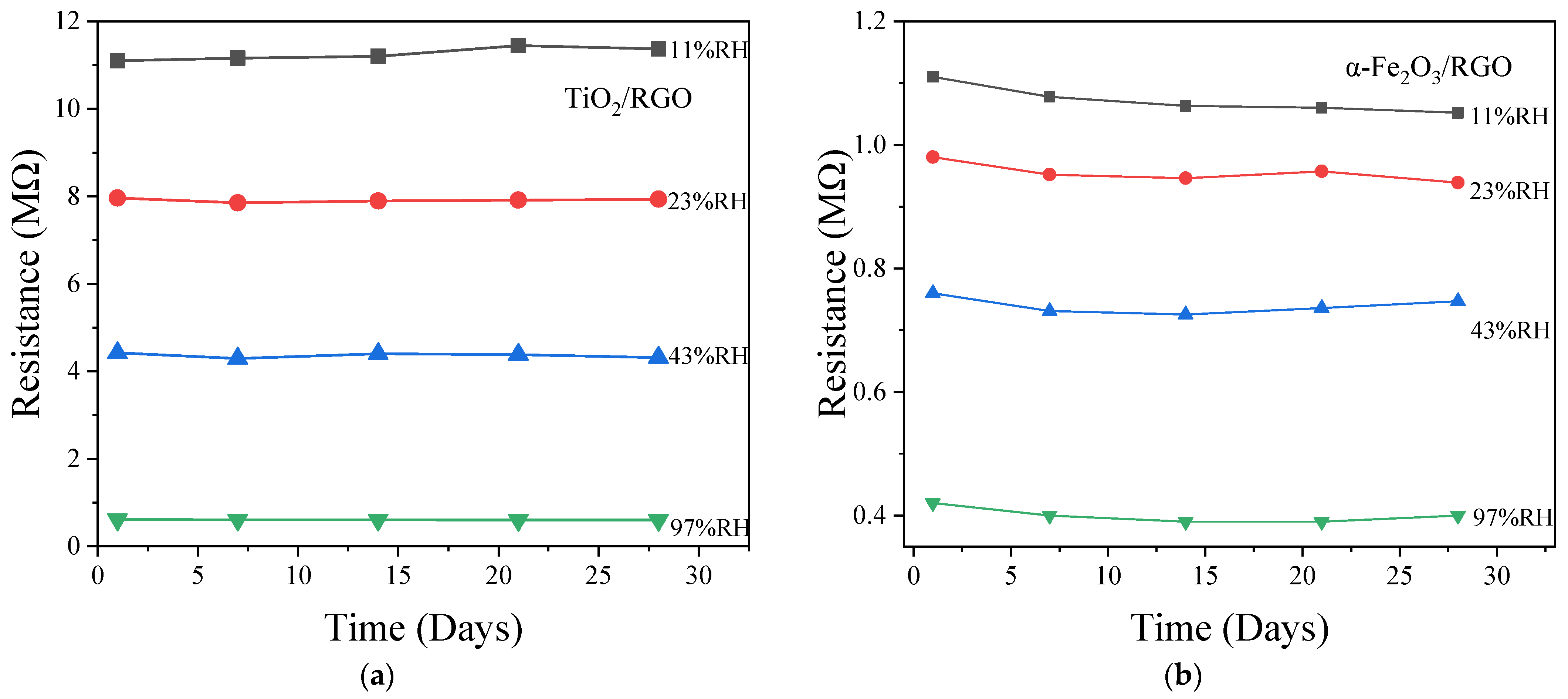
4.5. Repeatability and Stability
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SMO | Semiconductor metal oxide |
| RGO | Reduced Graphene Oxide |
| FESEM | Field emission scanning electron microscopy |
| XRD | X-ray diffractometer |
| QCM | Quartz Crystal Microbalance |
| CMUT | Capacitive Micro-machined Ultrasonic Transducer |
| FBAR | Film Bulk Acoustic Resonator |
| AC | Alternating current |
| EIS | Electrochemical impedance spectroscopy |
| RH | Relative humidity |
| PCB | Printed circuit board |
References
- Peng, Y.; Zhao, Y.; Chen, M.Q.; Xia, F. Research advances in microfiber humidity sensors. Small 2018, 14, 1800524. [Google Scholar] [CrossRef]
- Ding, X.; Yan, J.; Chen, N.; Jin, T.; Zhang, R. Highly sensitive balloon-like fiber interferometer based on GO nanomaterial coated for humidity measurement. Opt. Laser Technol. 2023, 158, 108798. [Google Scholar] [CrossRef]
- Shaukat, R.A.; Khan, M.U.; Saqib, Q.M.; Chougale, M.Y.; Kim, J.; Bae, J. All range highly linear and sensitive humidity sensor based on 2D material TiSi2 for real-time monitoring. Sens. Actuators B Chem. 2021, 345, 130371. [Google Scholar] [CrossRef]
- Qiao, C.; Yao, Y.; Ao, J.; Yu, X.; Wu, D.; Shou, M.; Li, R.; Yang, P. Advances in quartz crystal microbalance relative humidity sensors: A review. Measurement 2025, 243, 116415. [Google Scholar]
- Park, Y.D.; Kang, B.; Lim, H.S.; Cho, K.; Kang, M.S.; Cho, J.H. Polyelectrolyte Interlayer for ultra-sensitive organic transistor humidity sensors. Appl. Mater. Interfaces 2013, 5, 8591–8596. [Google Scholar] [CrossRef]
- Zhang, W.; Webb, D.J.; Peng, G. Investigation into time response of polymer fiber bragg grating based humidity sensors. J. Light. Technol. 2012, 30, 1090–1096. [Google Scholar] [CrossRef]
- Khan, M.U.; Hassan, G.; Awais, M.; Bae, J. All printed full range humidity sensor based on Fe2O3. Sens. Actuators A Phys. 2020, 311, 112072. [Google Scholar] [CrossRef]
- Oliveira, R.d.M.; Nono, M.d.C.d.A.; Oliveira, G.d.S. A capacitive-type humidity sensor using porous ceramics for environmental monitoring. Adv. Mater. Res. 2014, 3258, 194–198. [Google Scholar] [CrossRef]
- Yun, P.S.; Lee, J.E.; Kim, Y.H.; Kim, J.J.; Shim, Y.-S.; Kim, S.Y.; Lee, M.H.; Jang, H.W. Room temperature humidity sensors based on RGO/MoS2 hybrid composites synthesized by hydrothermal method-ScienceDirect. Sens. Actuators B Chem. 2018, 258, 775–782. [Google Scholar]
- Patial, P.; Deshwal, M. Selectivity and sensitivity property of metal oxide semiconductor based gas sensor with dopants variation: A review. Trans. Electr. Electron. Mater. 2021, 23, 6–18. [Google Scholar] [CrossRef]
- Compton, O.C.; Nguyen, S.B.T. Graphene oxide, highly reduced graphene oxide, and graphene: Versatile building blocks for carbon-based materials. Small 2010, 6, 711–723. [Google Scholar] [CrossRef]
- Yan, H.; Chen, Z.; Zeng, L.; Wang, Z.; Zheng, G.; Zhou, R. The effect of RGO-doping on the performance of SnO2/RGO flexible humidity sensor. Nanomaterials 2021, 11, 3368. [Google Scholar] [CrossRef]
- Morsy, M.; Mokhtar, M.M.; Ismail, H.S.; Mohamed, G.G.; Ibrahim, M. Humidity sensing behaviour of lyophilized RGO/Fe2O3 nanocomposite. J. Inorg. Organomet. Polym. Mater. 2020, 30, 4180–4190. [Google Scholar] [CrossRef]
- Yang, F.; Li, P. Preparation and humidity sensing performance study of SnO2 in situ loaded RGO. Mater. Sci. Eng. B 2023, 290, 116329. [Google Scholar] [CrossRef]
- Shilpa, M.; Chethan, B.; Shetty, J.S.; Murari, M.S.; Waikar, M.R.; Sonkawade, R.G.; Gurumurthy, S.C. Highly responsive reduced graphene oxide embedded PVDF flexible film-based room temperature operable humidity sensor. Sens. Actuators A Phys. 2024, 366, 115011. [Google Scholar] [CrossRef]
- Kumar, A.; Gupta, G.; Bapna, K.; Shivagan, D.D. Semiconductor-metal-oxide-based nano-composites for humidity sensing applications. Mater. Res. Bull. 2023, 158, 112023. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, Q.; Chen, C.; Shi, Y.; Li, L. High Performance and Cost-Effective Flexible Humidity Sensor Based on Polydopamine Coated Cellulose Nanocrystals/Graphene Oxide Nanocomposite. IEEE Sens. J. 2024, 24, 20386–20393. [Google Scholar] [CrossRef]
- Yao, Y.; Huang, X.; Chen, Q.; Zhang, Z.; Ling, W. High sensitivity and high stability QCM humidity sensors based on polydopamine coated cellulose nanocrystals/graphene oxide nanocomposite. Nanomaterials 2020, 10, 2210. [Google Scholar] [CrossRef]
- Jyothilal, H.; Shukla, G.; Walia, S.; Kundu, S.; Angappane, S. Humidity sensing and breath analyzing applications of TiO2 slanted nanorod arrays. Sens. Actuators A Phys. 2020, 301, 111758. [Google Scholar] [CrossRef]
- Sharma, N.; Sharma, C.; Sharma, S.; Sharma, S.; Paul, S. The synergetic effect of PdCr based bimetallic catalysts supported on RGO-TiO2 for organic transformations. Results Chem. 2022, 4, 100524. [Google Scholar] [CrossRef]
- Li, M.; Li, X.; Ge, S.; Li, W.; Li, R.; Wei, G. High Sensing performance of n-butanol gas sensor based on mesoporous Cu-Doped α- Fe2O3 nanoparticle materials. IEEE Sens. J. 2024, 224, 35406–35412. [Google Scholar] [CrossRef]
- Ruidíaz-Martínez, M.; Álvarez, M.A.; López-Ramón, M.V.; Cruz-Quesada, G.; Rivera-Utrilla, J.; Sánchez-Polo, M. Hydrothermal synthesis of rGO-TiO2 composites as high-performance UV Photocatalysts for Ethylparaben degradation. Catalysts 2020, 10, 520. [Google Scholar] [CrossRef]
- Kumar, P.; Khan, M.Q.; Khan, R.A.; Ahmad, K.; Kim, H. Hydrothermal synthesis of MnO2/Reduced graphene oxide composite for 4-Nitrophenol sensing applications. Inorganics 2022, 10, 219. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, H.; Zhang, J.; Hu, Z. High-sensitivity RGO-TiO2 humidity sensor driven by triboelectric nanogenerators for non-contact monitoring of human respiration. J. Alloys Compd. 2023, 935, 168006. [Google Scholar] [CrossRef]
- Wu, J.; Chen, Y.; Shen, W.; Wu, Y.; Corriou, J.P. Highly sensitive, flexible and transparent TiO2/Nanocellulose humidity sensor for respiration and skin monitoring. Ceram. Int. 2023, 49, 2204–2214. [Google Scholar] [CrossRef]
- Gu, L.; Zheng, K.; Zhou, Y.; Li, J.; Mo, X.; Patzke, G.R.; Chen, G. Humidity sensors based on ZnO/TiO2 core/shell nanorod arrays with enhanced sensitivity. Sens. Actuators B Chem. 2011, 159, 1–7. [Google Scholar] [CrossRef]
- Dutt, M.; Suhasini, K.; Ratan, A.; Shah, J.; Kotnala, R.K.; Singh, V. Mesoporous silica mediated synthesis of α-Fe2O3 porous structures and their application as humidity sensors. J. Mater. Sci. Mater. Electron. 2018, 29, 20506–20516. [Google Scholar] [CrossRef]
- Biju, K.P.; Jain, M.K. Sol–gel derived TiO2:ZrO2 multilayer thin films for humidity sensing application. Sens. Actuators B Chem. 2008, 128, 407–413. [Google Scholar] [CrossRef]
- Singh, P.; Kushwaha, C.S.; Shukla, S.K.; Dubey, G.C. Synthesis and humidity sensing property of α-Fe2O3 and polyaniline composite. Mater. Today Proc. 2018, 5, 9118–9125. [Google Scholar] [CrossRef]
- Huang, J. Diffusion impedance of electroactive materials, electrolytic solutions and porous electrodes: Warburg impedance and beyond. Electrochim. Acta 2018, 281, 170–188. [Google Scholar] [CrossRef]
- Scribner Associates. (n.d.). ZView Software: User Manual. Southern Pines, NC: Scribner Associates Inc. Available online: https://www.scribner.com (accessed on 12 May 2024).



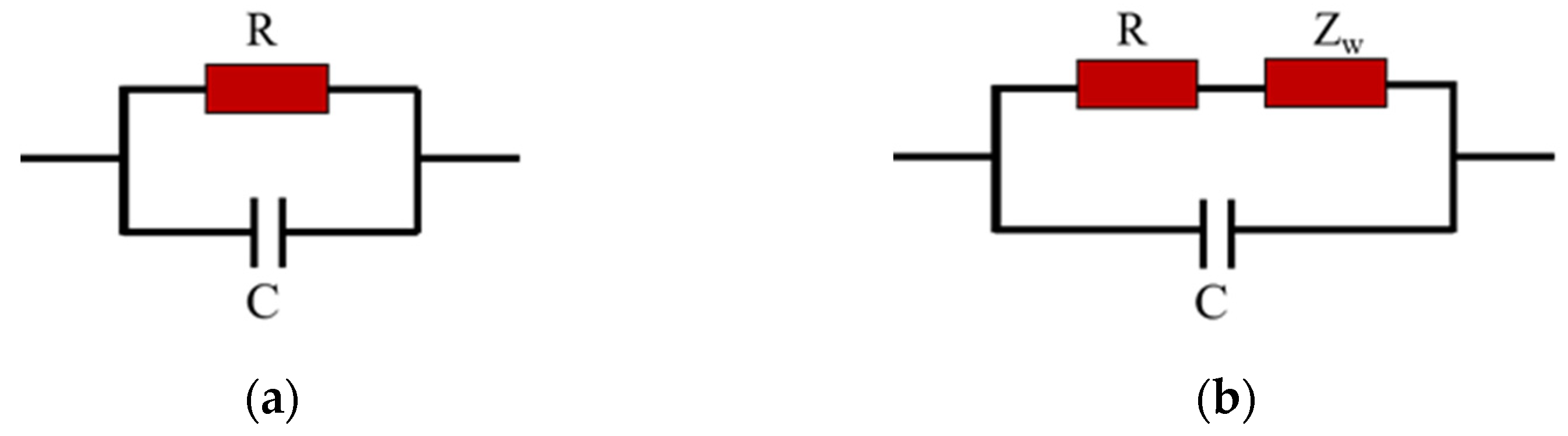
| Saturated Salt Solution | Relative Humidity (100%) |
|---|---|
| LiCl | 11 |
| CH3COOK | 23 |
| MgCl2 | 33 |
| K2CO3 | 43 |
| NaBr | 58 |
| NaCl | 75 |
| KCl | 85 |
| K2SO4 | 97 |
| Materials | Response (s) | Recovery (s) | Sensitivity | Sensing Type | Ref. |
|---|---|---|---|---|---|
| ZnO/TiO2 | 774.9 | 19.7 | 2.04 × 103 pF/%RH | capacitance | [26] |
| RGO/SnO2 | 80 | 4 | 2.36 × 103 pF/%RH | capacitance | [12] |
| α-Fe2O3 | 60 | 140 | 0.16 MΩ/%RH | impedance | [27] |
| ZrO2/TiO2 | 54 | 124 | 0.84 MΩ/%RH | impedance | [28] |
| RGO/Fe2O3 | 63 | 48 | 20.35 MΩ/%RH | impedance | [13] |
| SnO2/RGO | 45 | 8 | 13.10 MΩ/%RH | impedance | [14] |
| PANI/α-Fe2O3 | 70 | 90 | 0.22 KΩ/%RH | resistance | [29] |
| α-Fe2O3/RGO | 48 | 54 | 0.83 MΩ/%RH | resistance | This work |
| RGO/TiO2 | 72 | 99 | 12.20 MΩ/%RH | resistance | This work |
| Humidity | R(Ω) | C(pF) | WOR(Ω) | WoT | WOP |
|---|---|---|---|---|---|
| 11%RH (TiO2/RGO) | 11.97 M | 28.26 | - | - | - |
| 58%RH (TiO2/RGO) | 6.573 M | 28.04 | - | - | - |
| 97%RH (TiO2/RGO) | 498.370 K | 91.33 | 2.6257 M | 0.17308 | 0.17417 |
| 11%RH (α-Fe2O3/RGO) | 1.3432 M | 98.67 | - | - | - |
| 58%RH (α-Fe2O3/RGO) | 72.411 K | 52.29 | 2.0592 M | 0.83674 | 0.43813 |
| 97%RH (α-Fe2O3/RGO) | 28.875 K | 57.92 | 1.3143 M | 0.80889 | 0.42651 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, W.; Wu, C.; Zhao, W. Humidity-Sensing Performance of TiO2/RGO and α-Fe2O3/RGO Composites. Sensors 2025, 25, 691. https://doi.org/10.3390/s25030691
Zou W, Wu C, Zhao W. Humidity-Sensing Performance of TiO2/RGO and α-Fe2O3/RGO Composites. Sensors. 2025; 25(3):691. https://doi.org/10.3390/s25030691
Chicago/Turabian StyleZou, Wanghui, Chenhui Wu, and Wei Zhao. 2025. "Humidity-Sensing Performance of TiO2/RGO and α-Fe2O3/RGO Composites" Sensors 25, no. 3: 691. https://doi.org/10.3390/s25030691
APA StyleZou, W., Wu, C., & Zhao, W. (2025). Humidity-Sensing Performance of TiO2/RGO and α-Fe2O3/RGO Composites. Sensors, 25(3), 691. https://doi.org/10.3390/s25030691





