Exploring the Link Between Motor Functions and the Relative Use of the More Affected Arm in Adults with Cerebral Palsy
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
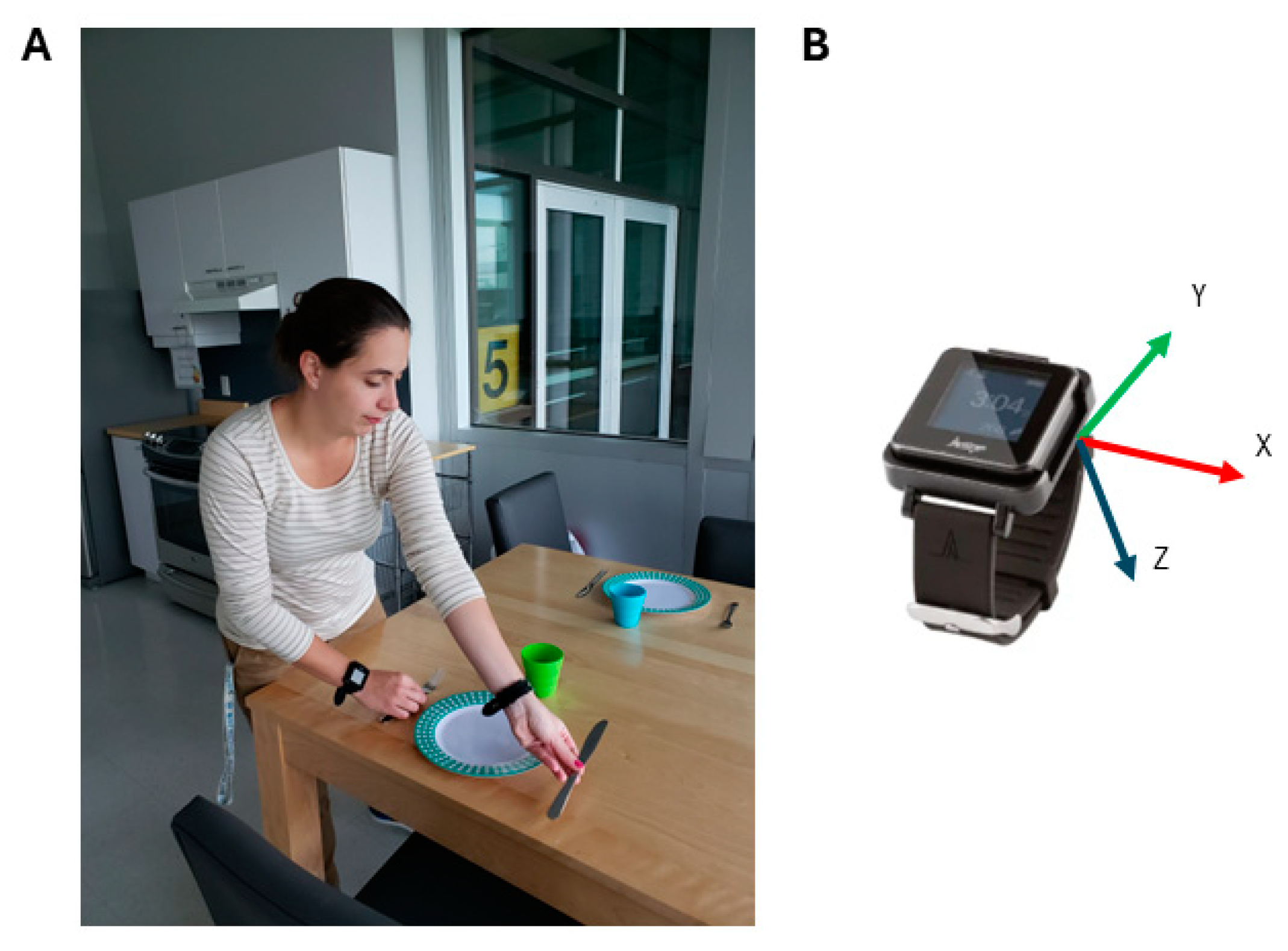
2.2. Experimental Setup
2.3. Accelerometry Processing
2.4. Metrics Calculations
2.4.1. Magnitude Ratio (Metric Focusing on Intensity + Global Relative Use)
2.4.2. BAUI (Metric Focusing on Intensity + Bilateral Use)
2.4.3. Use Ratio (Metric Focusing on Duration + Global Relative Use)
2.4.4. Percentage of Bilateral Use (Metric Focusing on Duration + Bilateral Use)
2.4.5. Bilateral Magnitude
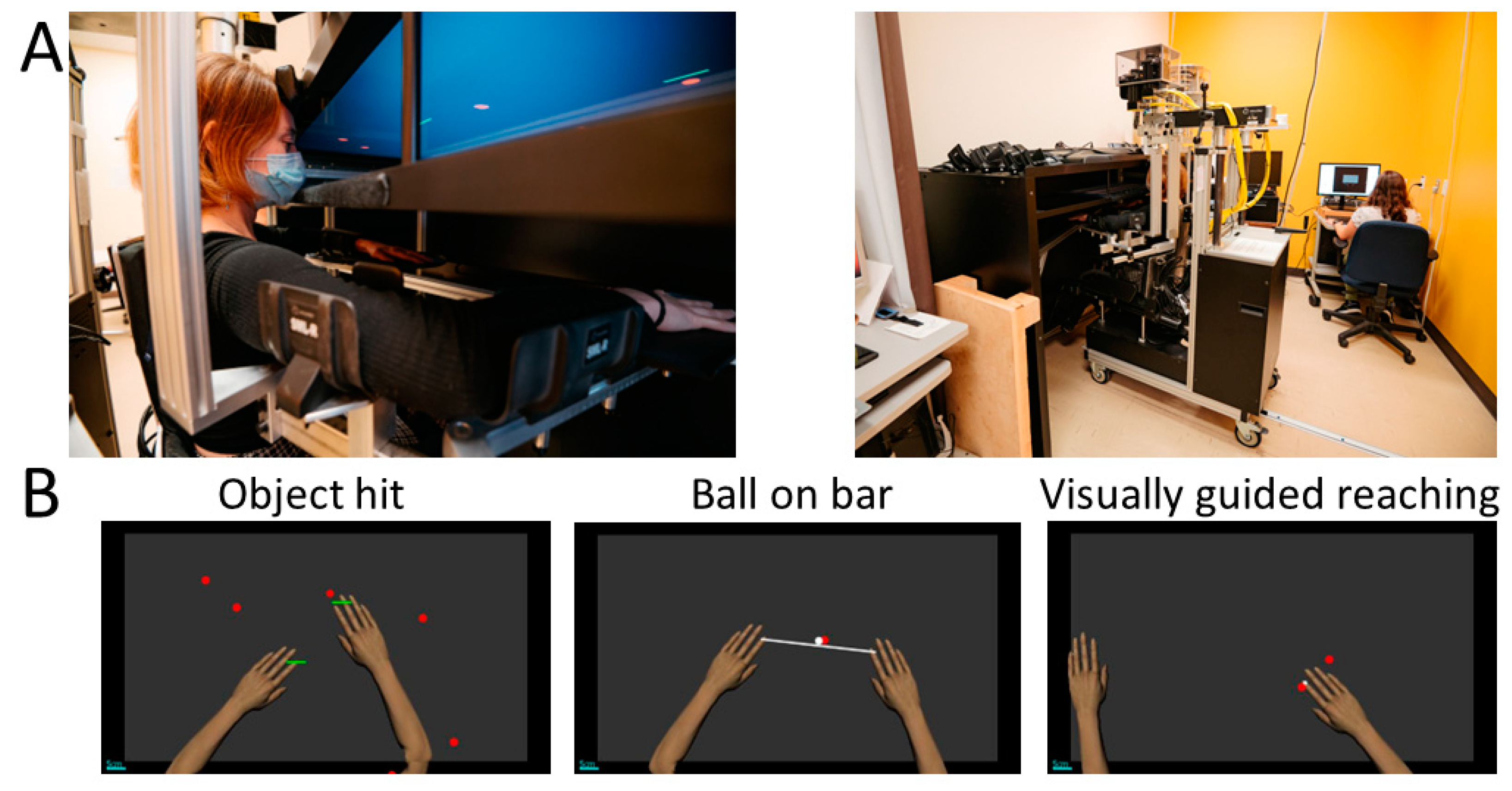
2.5. Robotic Assessments
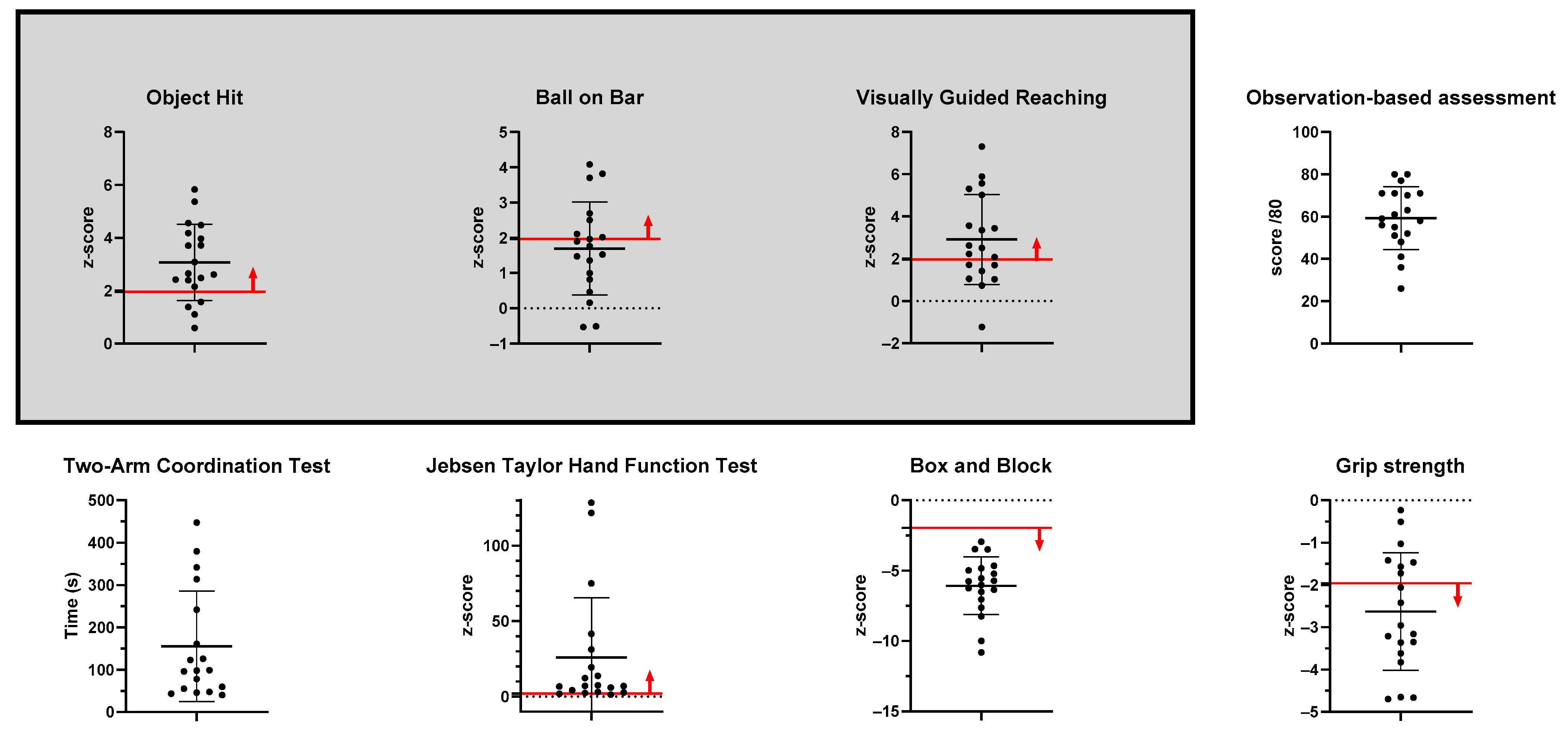
2.5.1. Object Hit
2.5.2. Ball on Bar
2.5.3. Visually Guided Reaching
2.6. Clinical Assessments
2.6.1. Observation-Based Assessment of the Involvement of the MA Arm
2.6.2. Two-Arm Coordination Test
2.6.3. Jebsen–Taylor Hand Function Test
2.6.4. Box and Block Test
2.6.5. Grip Strength
2.7. Statistical Analysis
3. Results
3.1. Sample Description
3.2. Description of Upper Extremity Use Using Accelerometry During Kitchen Tasks
3.3. Relationship Between Robotic and Clinical Assessments and Upper Limb Use in Real-Life Activities
4. Discussion
4.1. Clinical Relavance
4.2. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Basu, A.P.; Pearse, J.E.; Baggaley, J.; Watson, R.M.; Rapley, T. Participatory design in the development of an early therapy intervention for perinatal stroke. BMC Pediatr. 2017, 17, 33. [Google Scholar] [CrossRef]
- Rich, T.L.; Menk, J.S.; Rudser, K.D.; Feyma, T.; Gillick, B.T. Less-Affected Hand Function in Children with Hemiparetic Unilateral Cerebral Palsy: A Comparison Study with Typically Developing Peers. Neurorehabilit. Neural Repair 2017, 31, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Arnould, C.; Penta, M.; Renders, A.; Thonnard, J.L. ABILHAND-Kids: A measure of manual ability in children with cerebral palsy. Neurology 2004, 63, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Krumlinde-Sundholm, L.; Holmefur, M.; Kottorp, A.; Eliasson, A.-C. The Assisting Hand Assessment: Current evidence of validity, reliability, and responsiveness to change. Dev. Med. Child. Neurol. 2007, 49, 259–264. [Google Scholar] [CrossRef]
- Choi, B.C.; Pak, A.W. A catalog of biases in questionnaires. Prev. Chronic Dis. 2005, 2, A13. [Google Scholar] [PubMed]
- Mahtani, K.; Spencer, E.A.; Brassey, J.; Heneghan, C. Catalogue of bias: Observer bias. BMJ Evid.-Based Med. 2018, 23, 23. [Google Scholar] [CrossRef] [PubMed]
- Gagné-Pelletier, L.; Poitras, I.; Flamand, V.H.; Mercier, C. Reliability of an observation-based scoring grid to assess bimanual performance during unstandardized tasks in adults living with cerebral palsy. Disabil. Rehabil. 2023, 46, 3733–3738. [Google Scholar] [CrossRef]
- Langan, J.; Doyle, S.T.; Hurvitz, E.A.; Brown, S.H. Influence of Task on Interlimb Coordination in Adults with Cerebral Palsy. Arch. Phys. Med. Rehabil. 2010, 91, 1571–1576. [Google Scholar] [CrossRef] [PubMed]
- Lott, C.; Johnson, M.J. Upper limb kinematics of adults with cerebral palsy on bilateral functional tasks. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Orlando, FL, USA, 16–20 August 2016; IEEE Engineering in Medicine and Biology Society. Annual International Conference. Volume 2016, pp. 5676–5679. [Google Scholar]
- Riquelme, I.; Arnould, C.; Hatem, S.M.; Bleyenheuft, Y. The Two-Arm Coordination Test: Maturation of Bimanual Coordination in Typically Developing Children and Deficits in Children with Unilateral Cerebral Palsy. Dev. Neurorehabil 2019, 22, 312–320. [Google Scholar] [CrossRef]
- Coderre, A.M.; Amr Abou, Z.; Dukelow, S.P.; Demmer, M.J.; Moore, K.D.; Demers, M.J.; Bretzke, H.; Herter, T.M.; Glasgow, J.I.; Norman, K.E.; et al. Assessment of Upper-Limb Sensorimotor Function of Subacute Stroke Patients Using Visually Guided Reaching. Neurorehabilit. Neural Repair 2010, 24, 528–541. [Google Scholar] [CrossRef] [PubMed]
- Simmatis, L.E.; Jin, A.Y.; Taylor, S.W.; Bisson, E.J.; Scott, S.H.; Baharnoori, M. The feasibility of assessing cognitive and motor function in multiple sclerosis patients using robotics. Mult. Scler. J.-Exp. Transl. Clin. 2020, 6, 2055217320964940. [Google Scholar] [CrossRef] [PubMed]
- Kuczynski, A.M.; Semrau, J.A.; Kirton, A.; Dukelow, S.P. Kinesthetic deficits after perinatal stroke: Robotic measurement in hemiparetic children. J. Neuroeng. Rehabil. 2017, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Poitras, I.; Dukelow, S.P.; Campeau-Lecours, A.; Mercier, C. Robotic assessment of bilateral and unilateral upper limb functions in adults living with cerebral palsy. J. Neuroeng. Rehabil. 2024, 21, 144. [Google Scholar] [CrossRef]
- Scott, S.H.; Lowrey, C.R.; Brown, I.E.; Dukelow, S.P. Assessment of Neurological Impairment and Recovery Using Statistical Models of Neurologically Healthy Behavior. Neurorehabil Neural Repair 2022, 37, 394–408. [Google Scholar] [CrossRef] [PubMed]
- Kantak, S.; Jax, S.; Wittenberg, G. Bimanual coordination: A missing piece of arm rehabilitation after stroke. Restor. Neurol. Neurosci. 2017, 35, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Decraene, L.; Orban de Xivry, J.-J.; Kleeren, L.; Crotti, M.; Verheyden, G.; Ortibus, E.; Feys, H.; Mailleux, L.; Klingels, K. In-depth quantification of bimanual coordination using the Kinarm exoskeleton robot in children with unilateral cerebral palsy. J. Neuroeng. Rehabil. 2023, 20, 154. [Google Scholar] [CrossRef] [PubMed]
- Poitras, I.; Campeau-Lecours, A.; Mercier, C. Relationship between somatosensory and visuo-perceptual impairments and motor functions in adults with hemiparetic cerebral palsy. Neurorehabilit. Neural Repair 2024, 15, 1425124. [Google Scholar] [CrossRef] [PubMed]
- Rudisch, J.; Butler, J.; Izadi, H.; Zielinski, I.M.; Aarts, P.; Birtles, D.; Green, D. Kinematic parameters of hand movement during a disparate bimanual movement task in children with unilateral Cerebral Palsy. Hum. Mov. Sci. 2016, 46, 239–250. [Google Scholar] [CrossRef]
- Volman, M.J. Spatial coupling in children with hemiplegic cerebral palsy during bimanual circle and line drawing. Mot. Control 2005, 9, 395–416. [Google Scholar] [CrossRef]
- Hoyt, C.R.; Van, A.N.; Ortega, M.; Koller, J.M.; Everett, E.A.; Nguyen, A.L.; Lang, C.E.; Schlaggar, B.L.; Dosenbach, N.U.F. Detection of Pediatric Upper Extremity Motor Activity and Deficits with Accelerometry. JAMA Netw. Open 2019, 2, e192970. [Google Scholar] [CrossRef]
- Lang, C.E.; Wagner, J.M.; Edwards, D.F.; Dromerick, A.W. Upper extremity use in people with hemiparesis in the first few weeks after stroke. J. Neurol. Phys. Ther. 2007, 31, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R. Assessment of Real-World Upper Limb Activity in Adults with Chronic Stroke. Ph.D. Thesis, Washington University, Washington, DC, USA, 2015; 148p. [Google Scholar]
- Poitras, I.; Clouâtre, J.; Campeau-Lecours, A.; Mercier, C. Accelerometry-Based Metrics to Evaluate the Relative Use of the More Affected Arm during Daily Activities in Adults Living with Cerebral Palsy. Sensors 2022, 22, 1022. [Google Scholar] [CrossRef]
- Poitras, I.; Gagné-Pelletier, L.; Clouâtre, J.; Flamand, V.H.; Campeau-Lecours, A.; Mercier, C. Optimizing Epoch Length and Activity Count Threshold Parameters in Accelerometry: Enhancing Upper Extremity Use Quantification in Cerebral Palsy. Sensors 2024, 24, 1100. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.R.; Klaesner, J.W.; Lang, C.E. Quantifying Real-World Upper-Limb Activity in Nondisabled Adults and Adults with Chronic Stroke. Neurorehabil Neural Repair 2015, 29, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Uswatte, G.; Giuliani, C.; Winstein, C.; Zeringue, A.; Hobbs, L.; Wolf, S.L. Validity of Accelerometry for Monitoring Real-World Arm Activity in Patients with Subacute Stroke: Evidence from the Extremity Constraint-Induced Therapy Evaluation Trial. Arch. Phys. Med. Rehabil. 2006, 87, 1340–1345. [Google Scholar] [CrossRef] [PubMed]
- Heye, A.-L.; Kersting, C.; Kneer, M.; Barzel, A. Suitability of accelerometry as an objective measure for upper extremity use in stroke patients. BMC Neurol. 2022, 22, 220. [Google Scholar] [CrossRef]
- Zielinski, I.M.; Jongsma, M.L.; Baas, C.; Aarts, P.B.; Steenbergen, B. Unravelling developmental disregard in children with unilateral cerebral palsy by measuring event-related potentials during a simple and complex task. BMC Neurol. 2014, 14, 6. [Google Scholar] [CrossRef]
- Zielinski, I.M.; Steenbergen, B.; Aarts, P.B.M.; Green, D.; Jongsma, M.L.A. Developmental disregard in children with unilateral cerebral palsy is accompanied by a lack of movement related mu-suppression. Dev. Med. Child. Neurol. 2016, 58 (Suppl. S6), 34. [Google Scholar]
- Houwink, A.; Geerdink, Y.A.; Steenbergen, B.; Geurts, A.C.; Aarts, P.B. Assessment of upper-limb capacity, performance, and developmental disregard in children with cerebral palsy: Validity and reliability of the revised Video-Observation Aarts and Aarts module: Determine Developmental Disregard (VOAA-DDD-R). Dev. Med. Child. Neurol. 2013, 55, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, I.M.; Steenbergen, B.; Baas, C.; Aarts, P.B.; Jongsma, M.L. Neglect-like characteristics of developmental disregard in children with cerebral palsy revealed by event related potentials. BMC Neurol. 2014, 14, 221. [Google Scholar] [CrossRef]
- Poitras, I.; Clouâtre, J.; Bouyer, L.J.; Routhier, F.; Mercier, C.; Campeau-Lecours, A. Development and Validation of Open-Source Activity Intensity Count and Activity Intensity Classification Algorithms from Raw Acceleration Signals of Wearable Sensors. Sensors 2020, 20, 6767. [Google Scholar] [CrossRef] [PubMed]
- Tyryshkin, K.; Coderre, A.M.; Glasgow, J.I.; Herter, T.M.; Bagg, S.D.; Dukelow, S.P.; Scott, S.H. A robotic object hitting task to quantify sensorimotor impairments in participants with stroke. J. Neuroeng. Rehabil. 2014, 11, 47. [Google Scholar] [CrossRef]
- Mang, C.S.; Whitten, T.A.; Cosh, M.S.; Scott, S.H.; Wiley, J.P.; Debert, C.T.; Dukelow, S.P.; Benson, B.W. Test-retest reliability of the KINARM end-point robot for assessment of sensory, motor and neurocognitive function in young adult athletes. PLoS ONE 2018, 13, e0196205. [Google Scholar] [CrossRef] [PubMed]
- Lowrey, C.; Jackson, C.; Bagg, S.; Dukelow, S.; Scott, S. A Novel Robotic Task for Assessing Impairments in Bimanual Coordination Post-Stroke. Int. J. Phys. Med. Rehabil. 2014, S3, 002. [Google Scholar] [CrossRef]
- Lafayette Instrument. Two-Hand Coordination Test—User Instructions, 2nd ed.; Lafayette Instrument: Lafayette, IN, USA, 2016; Available online: https://lafayetteevaluation.com/products/two-arm-coordination/ (accessed on 20 January 2025).
- Jebsen, R.H.; Taylor, N.; Trieschmann, R.B.; Trotter, M.J.; Howard, L.A. An objective and standardized test of hand function. Arch. Phys. Med. Rehabil. 1969, 50, 311–319. [Google Scholar] [PubMed]
- Araneda, R.; Ebner-Karestinos, D.; Paradis, J.; Saussez, G.; Friel, K.M.; Gordon, A.M.; Bleyenheuft, Y. Reliability and responsiveness of the Jebsen-Taylor Test of Hand Function and the Box and Block Test for children with cerebral palsy. Dev. Med. Child. Neurol. 2019, 61, 1182–1188. [Google Scholar] [CrossRef] [PubMed]
- Mathiowetz, V.; Volland, G.; Kashman, N.; Weber, K. Adult norms for the Box and Block Test of manual dexterity. Am. J. Occup. Ther. 1985, 39, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.-J.; Chen, H.-L.; Shieh, J.-Y.; Wang, T.-N. Measurement properties of the box and block test in children with unilateral cerebral palsy. Sci. Rep. 2021, 11, 20955. [Google Scholar] [CrossRef]
- AbilityOne, H. Hydraulic Hand Dynamometer Owner’s Manual; Sammons Preston Rolyan: Bolingbrook, IL, USA, 2003. [Google Scholar]
- Sugiyama, T.; Whitney, D.G.; Schmidt, M.; Haapala, H.; Bowman, A.; Peterson, M.D.; Hurvitz, E.A. Measuring grip strength in adolescents and adults with cerebral palsy in a clinic setting: Feasibility, reliability, and clinical associations. Dev. Med. Child. Neurol. 2024, 66, 87–94. [Google Scholar] [CrossRef]
- Bailey, R.R.; Klaesner, J.W.; Lang, C.E. An Accelerometry-Based Methodology for Assessment of Real-World Bilateral Upper Extremity Activity. PLoS ONE 2014, 9, e103135. [Google Scholar] [CrossRef]
- Bailey, R.R.; Birkenmeier, R.L.; Lang, C.E. Real-world affected upper limb activity in chronic stroke: An examination of potential modifying factors. Top. Stroke Rehabil. 2015, 22, 26–33. [Google Scholar] [CrossRef]
- Bailey, R.R.; Lang, C.E. Upper-limb activity in adults: Referent values using accelerometry. J. Rehabil. Res. Dev. 2013, 50, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Essers, B.; Biering Lundquist, C.; Verheyden, G.; Brunner, I.C. Determinants of Different Aspects of Upper-Limb Activity after Stroke. Sensors 2022, 22, 2273. [Google Scholar] [CrossRef]
- Goodwin, B.M.; Sabelhaus, E.K.; Pan, Y.C.; Bjornson, K.F.; Pham, K.L.D.; Walker, W.O.; Steele, K.M. Accelerometer Measurements Indicate That Arm Movements of Children with Cerebral Palsy Do Not Increase After Constraint-Induced Movement Therapy (CIMT). Am. J. Occup. Ther. 2020, 74, 7405205100p1–7405205100p9. [Google Scholar] [CrossRef] [PubMed]
- Porta, M.; Leban, B.; Orrù, P.F.; Pau, M. Use of bilateral wrist-worn accelerometers to characterize upper limb activity time, intensity and asymmetry of use in physically demanding and sedentary working task. Int. J. Ind. Ergon. 2022, 92, 103359. [Google Scholar] [CrossRef]
- Dawe, J.; Yang, J.F.; Fehlings, D.; Likitlersuang, J.; Rumney, P.; Zariffa, J.; Musselman, K.E. Validating Accelerometry as a Measure of Arm Movement for Children with Hemiplegic Cerebral Palsy. Phys. Ther. 2019, 99, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Howcroft, J.; Fehlings, D.; Zabjek, K.; Fay, L.; Liang, J.; Biddiss, E. Wearable wrist activity monitor as an indicator of functional hand use in children with cerebral palsy. Dev. Med. Child. Neurol. 2011, 53, 1024–1029. [Google Scholar] [CrossRef]
- Hurvitz, E.A.; Whitney, D.G.; Waldron-Perrine, B.; Ryan, D.; Haapala, H.J.; Schmidt, M.; Gray, C.; Peterson, M.D. Navigating the Pathway to Care in Adults with Cerebral Palsy. Front. Neurol. 2021, 12, 734139. [Google Scholar] [CrossRef] [PubMed]
- Swinnen, S.P.; Heuninckx, S.; Van Impe, A.; Goble, D.J.; Coxon, J.P.; Wenderoth, N. Aging and Movement ControlThe Neural Basis of Age-related Compensatory Recruitment. In Motor Control: Theories, Experiments, and Applications; Danion, P.F., Latash, P.M., Eds.; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Yan, J.H.; Thomas, J.R.; Stelmach, G.E. Aging and rapid aiming arm movement control. Exp. Aging Res. 1998, 24, 155–168. [Google Scholar]
- Liutsko, L.; Muiños, R.; Tous Ral, J.M.; Contreras, M.J. Fine Motor Precision Tasks: Sex Differences in Performance with and without Visual Guidance across Different Age Groups. Behav. Sci. 2020, 10, 36. [Google Scholar] [CrossRef]
- Charles, J. Upper extremity muscle activation in children with unilateral cerebral palsy during an auditory cued repetitive task: Effects on bimanual coordination. J. Pediatr. Rehabil. Med. 2017, 10, 19–26. [Google Scholar] [CrossRef]
- Basu, A.P.; Pearse, J.; Kelly, S.; Wisher, V.; Kisler, J. Early Intervention to Improve Hand Function in Hemiplegic Cerebral Palsy. Front. Neurol. 2015, 5, 281. [Google Scholar] [CrossRef] [PubMed]

| Session 1 (<3 h) | Session 2 (~3 h) |
|---|---|
|
|
| Total Number of Participants | 19 |
|---|---|
| Age (years) | 34.3 ± 11.5 |
| Sex (female (F), male (M)) | F = 58% M = 42% |
| More affected side (right (R), left (L)) * | R = 63% L = 37% |
| Handedness (right (R), left (L)) * | R= 42% L = 58% |
| Percentage of participants for each level of the Manual Ability Classification System | I = 37% II = 31.5% III = 31.5% |
| Mean [95%—Confidence Interval] | ||
|---|---|---|
| Robotic Assessments | Object Hit | 3.1 [2.37–3.76] |
| Ball on Bar | 1.7 [1.66–3.9] | |
| Visually Guided Reaching | 2.9 [1.9–3.9] | |
| Clinical Assessments | Observation-based assessment of the involvement of the MA arm | 59.3 [52.1–66.4] |
| Two-Arm Coordination | 155.5 [90.6–220.4] | |
| Jebsen–Taylor Hand Function Test | 25.9 [7.0–44.9] | |
| Box and Block test | −6.1 [−10.8–−2.9] | |
| Grip strength | −2.6 [−3.3–−1.96] |
| MACS I | MACS II | MACS III | Total | p-Value | ||
|---|---|---|---|---|---|---|
| Intensity | Magnitude Ratio | −0.69 ± 0.46 | −1.02 ± 0.28 | −1.63 ± 0.75 | −1.09 ± 0.64 | 0.047 |
| Bilateral Arm Use Index | 0.71 ± 0.18 | 0.52 ± 0.10 | 0.47 ± 0.14 | 0.58 ± 0.18 | 0.03 | |
| Duration | Use Ratio | 0.47 ± 0.09 | 0.37 ± 0.10 | 0.30 ± 0.16 | 0.38 ± 0.13 | 0.15 |
| Percentage of Bilateral Use | 26.1 ± 13.5 | 24.2 ± 6.9 | 16.2 ± 14.0 | 22.4 ± 12.1 | 0.42 |
| Robotic Assessment Model | Clinical Assessment Model | ||
|---|---|---|---|
| Intensity | Magnitude Ratio | Ball on Bar (r2 = 0.43, p = 0.001) |
|
| Bilateral Arm Use Index | Ball on Bar (r2 = 0.23, p = 0.02) | Observation-based assessment of the involvement of the MA arm (r2 = 0.60, p < 0.001) | |
| Duration | Use Ratio | Ball on Bar (r2 = 0.27, p = 0.02) | Observation-based assessment of the involvement of the MA arm (r2 = 0.64, p < 0.001) |
| Percentage of Bilateral Use | Ball on Bar (r2 = 0.25, p = 0.02) | Observation-based assessment of the involvement of the MA arm (r2 = 0.43, p = 0.002) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poitras, I.; Clouâtre, J.; Campeau-Lecours, A.; Mercier, C. Exploring the Link Between Motor Functions and the Relative Use of the More Affected Arm in Adults with Cerebral Palsy. Sensors 2025, 25, 660. https://doi.org/10.3390/s25030660
Poitras I, Clouâtre J, Campeau-Lecours A, Mercier C. Exploring the Link Between Motor Functions and the Relative Use of the More Affected Arm in Adults with Cerebral Palsy. Sensors. 2025; 25(3):660. https://doi.org/10.3390/s25030660
Chicago/Turabian StylePoitras, Isabelle, Jade Clouâtre, Alexandre Campeau-Lecours, and Catherine Mercier. 2025. "Exploring the Link Between Motor Functions and the Relative Use of the More Affected Arm in Adults with Cerebral Palsy" Sensors 25, no. 3: 660. https://doi.org/10.3390/s25030660
APA StylePoitras, I., Clouâtre, J., Campeau-Lecours, A., & Mercier, C. (2025). Exploring the Link Between Motor Functions and the Relative Use of the More Affected Arm in Adults with Cerebral Palsy. Sensors, 25(3), 660. https://doi.org/10.3390/s25030660









