Case Series Evaluating the Relationship of SGLT2 Inhibition to Pulmonary Artery Pressure and Non-Invasive Cardiopulmonary Parameters in HFpEF/HFmrEF Patients—A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Guideline-Directed Medical Therapy (GDMT)
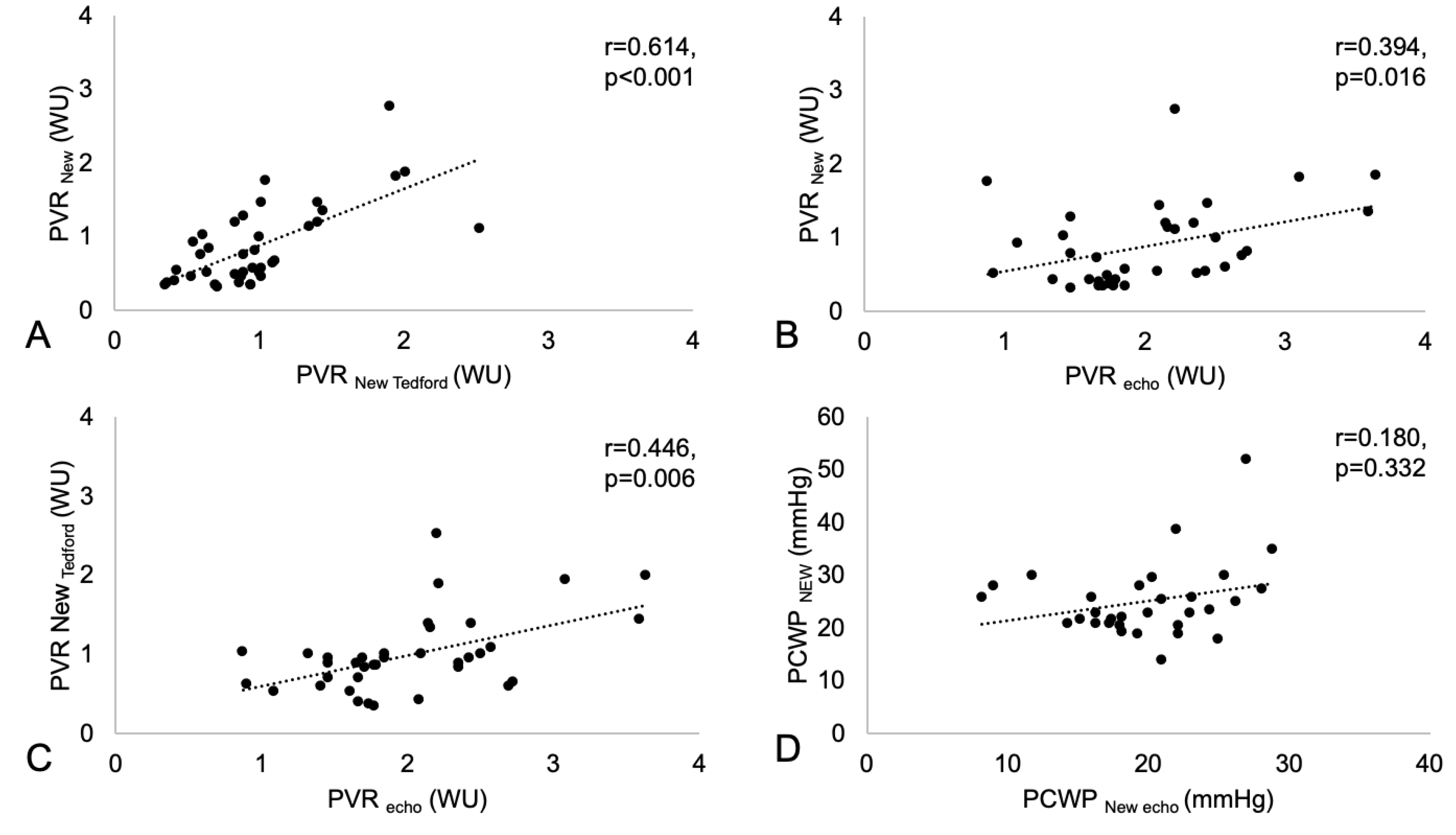
2.2. Pulmonary Vascular Resistance and Pulmonary Capillary Wedge Pressure
- The resistance/capacitance method:
- 2.
- The resistance/capacitance method corrected by Tedford:
- 3.
- Echocardiographic-based assessment:
2.3. Pulmonary Arterial Capacitance
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Temporal Course of Hemodynamic and Laboratory Parameters Prior to and After SGLT2 Inhibitor Therapy
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef] [PubMed]
- Cowie, M.R.; Fisher, M. SGLT2 inhibitors: Mechanisms of cardiovascular benefit beyond glycaemic control. Nat. Rev. Cardiol. 2020, 17, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner-La Rocca, H.-P.; Choi, D.-J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; McMurray, J.J.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.; Filippatos, G.; Siddiqi, T.J.; Brueckmann, M.; Böhm, M.; Chopra, V.K.; Ferreira, J.P.; Januzzi, J.L.; Kaul, S.; Piña, I.L.; et al. Empagliflozin, Health Status, and Quality of Life in Patients with Heart Failure and Preserved Ejection Fraction: The EMPEROR-Preserved Trial. Circulation 2022, 145, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Kosiborod, M.N.; Bhatt, A.S.; Claggett, B.L.; Vaduganathan, M.; Kulac, I.J.; Lam, C.S.; Hernandez, A.F.; Martinez, F.A.; Inzucchi, S.E.; Shah, S.J.; et al. Effect of Dapagliflozin on Health Status in Patients with Preserved or Mildly Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2023, 81, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Frantz, R.P. Group 2 pulmonary hypertension: From diagnosis to treatment. Curr. Opin. Pulm. Med. 2023, 29, 391–398. [Google Scholar] [CrossRef]
- Mahapatra, S.; Nishimura, R.A.; Oh, J.K.; McGoon, M.D. The prognostic value of pulmonary vascular capacitance determined by doppler echocardiography in patients with pulmonary arterial hypertension. J. Am. Soc. Echocardiogr. 2006, 19, 1045–1050. [Google Scholar] [CrossRef]
- Martelli, G.; Congedi, S.; Lorenzoni, G.; Nardelli, M.; Lucchetta, V.; Gregori, D.; Tiberio, I. Echocardiographic assessment of pulmonary capillary wedge pressure by E/e’ ratio: A systematic review and meta-analysis. J. Crit. Care 2023, 76, 154281. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Metkus, T.S.; Mullin, C.J.; Grandin, E.W.; Rame, J.E.; Tampakakis, E.; Hsu, S.; Kolb, T.M.; Damico, R.; Hassoun, P.M.; Kass, D.A.; et al. Heart Rate Dependence of the Pulmonary Resistance x Compliance (RC) Time and Impact on Right Ventricular Load. PLoS ONE 2016, 11, e0166463. [Google Scholar] [CrossRef]
- Tedford, R.J.; Hassoun, P.M.; Mathai, S.C.; Girgis, R.E.; Russell, S.D.; Thiemann, D.R.; Cingolani, O.H.; Mudd, J.O.; Borlaug, B.A.; Redfield, M.M.; et al. Pulmonary capillary wedge pressure augments right ventricular pulsatile loading. Circulation 2012, 125, 289–297. [Google Scholar] [CrossRef]
- Abbas, A.E.; Fortuin, F.; Schiller, N.B.; Appleton, C.P.; A Moreno, C.; Lester, S.J. A simple method for noninvasive estimation of pulmonary vascular resistance. J. Am. Coll. Cardiol. 2003, 41, 1021–1027. [Google Scholar] [CrossRef]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731, Erratum in: Eur Heart J. 2023, 44, 1312. [Google Scholar] [CrossRef] [PubMed]
- Obokata, M.; Kane, G.C.; Reddy, Y.N.V.; Olson, T.P.; Melenovsky, V.; Borlaug, B.A. Role of Diastolic Stress Testing in the Evaluation for Heart Failure with Preserved Ejection Fraction: A Simultaneous Invasive-Echocardiographic Study. Circulation 2017, 135, 825–838. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, A.; Yasumura, Y.; Yoshida, C.; Okumura, T.; Tateishi, J.; Yoshida, J.; Abe, H.; Tamaki, S.; Yano, M.; Hayashi, T.; et al. Prognostic Importance of Right Ventricular-Vascular Uncoupling in Acute Decompensated Heart Failure with Preserved Ejection Fraction. Circ. Cardiovasc. Imaging 2020, 13, e011430. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, A.; Yasumura, Y.; Yoshida, C.; Okumura, T.; Tateishi, J.; Yoshida, J.; Tamaki, S.; Yano, M.; Hayashi, T.; Nakagawa, Y.; et al. Distinctive prognostic factor of heart failure with preserved ejection fraction stratified with admission blood pressure. ESC Heart Fail. 2021, 8, 3145–3155. [Google Scholar] [CrossRef] [PubMed]
- Ghio, S.; Schirinzi, S.; Pica, S. Pulmonary arterial compliance: How and why should we measure it? Glob. Cardiol. Sci. Pract. 2015, 2015, 58. [Google Scholar] [CrossRef]
- Nakagawa, A.; Yasumura, Y.; Yoshida, C.; Okumura, T.; Tateishi, J.; Yoshida, J.; Abe, H.; Tamaki, S.; Yano, M.; Hayashi, T.; et al. Prognostic Importance of Pulmonary Arterial Capacitance in Acute Decompensated Heart Failure with Preserved Ejection Fraction. J. Am. Heart Assoc. 2021, 10, e023043. [Google Scholar] [CrossRef]
- Guazzi, M.; Dixon, D.; Labate, V.; Beussink-Nelson, L.; Bandera, F.; Cuttica, M.J.; Shah, S.J. RV Contractile Function and its Coupling to Pulmonary Circulation in Heart Failure with Preserved Ejection Fraction. JACC Cardiovasc. Imaging 2017, 10 Pt B, 1211–1221. [Google Scholar] [CrossRef]
- Al-Naamani, N.; Preston, I.R.; Paulus, J.K.; Hill, N.S.; Roberts, K.E. Pulmonary Arterial Capacitance Is an Important Predictor of Mortality in Heart Failure with a Preserved Ejection Fraction. JACC Heart Fail. 2015, 3, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Dupont, M.; Mullens, W.; Skouri, H.N.; Abrahams, Z.; Wu, Y.; Taylor, D.O.; Starling, R.C.; Tang, W.H.W. Prognostic role of pulmonary arterial capacitance in advanced heart failure. Circ. Heart Fail. 2012, 5, 778–785. [Google Scholar] [CrossRef]
- Kussmaul, W.G.; A Altschuler, J.; Herrmann, H.C.; Laskey, W.K. Effects of pacing tachycardia and balloon valvuloplasty on pulmonary artery impedance and hydraulic power in mitral stenosis. Circulation 1992, 86, 1770–1779. [Google Scholar] [CrossRef] [PubMed]
- Janicki, J.S.; Weber, K.T.; Likoff, M.J.; Fishman, A.P. The pressure-flow response of the pulmonary circulation in patients with heart failure and pulmonary vascular disease. Circulation 1985, 72, 1270–1278. [Google Scholar] [CrossRef]
- Tedford, R.J. Determinants of right ventricular afterload (2013 Grover Conference series). Pulm. Circ. 2014, 4, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Assmus, B.; Angermann, C.E.; Alkhlout, B.; Asselbergs, F.W.; Schnupp, S.; Brugts, J.J.; Nordbeck, P.; Zhou, Q.; Brett, M.; Ginn, G.; et al. Effects of remote haemodynamic-guided heart failure management in patients with different subtypes of pulmonary hypertension: Insights from the MEMS-HF study. Eur. J. Heart. Fail. 2022, 24, 2320–2330. [Google Scholar] [CrossRef] [PubMed]
- Brugts, J.J.; Radhoe, S.P.; Clephas, P.R.D.; Aydin, D.; van Gent, M.W.F.; Szymanski, M.K.; Rienstra, M.; Heuvel, M.H.v.D.; da Fonseca, C.A.; Linssen, G.C.M.; et al. Remote haemodynamic monitoring of pulmonary artery pressures in patients with chronic heart failure (MONITOR-HF): A randomised clinical trial. Lancet 2023, 401, 2113–2123. [Google Scholar] [CrossRef] [PubMed]
- Clephas, P.R.D.; Zwartkruis, V.W.; Malgie, J.; van Gent, M.W.F.; Rocca, H.-P.B.-L.; Szymanski, M.K.; van Halm, V.P.; Handoko, M.L.; Kok, W.E.M.; Asselbergs, F.W.; et al. Pulmonary artery pressure monitoring in chronic heart failure: Effects across clinically relevant subgroups in the MONITOR-HF trial. Eur. Heart J. 2024, 45, 2954–2964. [Google Scholar] [CrossRef] [PubMed]



| Variables | Study Cohort (n = 13) |
|---|---|
| Age, years | 77 ± 4 |
| Sex | |
| 5 (38.5%) |
| 8 (61.5%) |
| BMI, kg/m2 | 29 ± 5 |
| NYHA functional class | |
| 13 (100%) |
| Medical history | |
| 1 (7.7%) |
| 1 (7.7%) |
| 1 (7.7%) |
| 3 (23.1%) |
| 1 (7.7%) |
| 9 (69.2%) |
| 12 (92.3%) |
| Years since heart failure diagnosis | 2 (1.5–5) |
| Cause | |
| 1 (7.7%) |
| Heart rate, beats per min | 72 ± 7 |
| Systolic blood pressure, mmHg | 129 ± 18 |
| 6-min walking test, m | 307 ± 54 |
| Type of heart failure | |
| 11 (84.6%) |
| 2 (15.4%) |
| 0 (0%) |
| Serum creatinine, mg/dL | 1.3 ± 0.5 |
| eGFR, mL/min | 51 ± 16 |
| Chronic kidney disease | |
| 0 (0%) |
| 1 (7.7%) |
| 5 (38.5%) |
| 1 (7.7%) |
| 0 (0%) |
| NT-proBNP, pg/mL | 1109 (349–2133) |
| Hemotocrit, % | 0.39 ± 0.04 |
| Hemoglobin, g/dL | 13.0 ± 12.0 |
| Implanted cardioverter defibrillator | 2 (15.4%) |
| Cardiac resynchronization therapy | 2 (15.4%) |
| Medical therapy | |
| 9 (69.2%) |
| 5 (38.5%) |
| - Angiotensin converting enzyme inhibitor | 4 (30.8%) |
| - Angiotensin receptor blocker | 1 (7.7%) |
| 4 (30.8%) |
| 8 (61.5%) |
| 0 (0%) |
| 12 (92.3%) |
| 20 ± 16 |
| 3 (23.1%) |
| 3 (23.1%) |
| Variables | At Sensor Implantation | 3 Months Prior to SGLT2-I Therapy (Baseline) | Start of SGLT2-I Therapy | 3 Months After SGLT2-I Therapy | 6 Months After SGLT2-I Therapy | p Values |
|---|---|---|---|---|---|---|
| Echocardiography | ||||||
| Quantitative left ventricular ejection fraction, % | 60 (53–60) | 60 (51–60) | 60 (49–60) | 60 (50–65) | 60 (50–64) | p > 0.05 (1,2,3,4,5,6) |
| TAPSE, mm | 22 ± 5 | 21 ± 5 | 20 ± 2 | 21 ± 3 | 21 ± 5 | p > 0.05 (1,2,3,4,5,6) |
| Right ventricular fractional area change, % | 41 ± 6 | 42 ± 5 | 44 ± 5 | 38 ± 10 | 45 ± 6 | p > 0.05 (1,2,3,4,5,6) |
| TAPSE/PASP ratio (echo), mm/mm Hg | 0.56 ± 0.21 | 0.60 ± 0.21 | 0.62 ± 0.24 | 0.60 ± 0.10 | 0.58 ± 0.21 | p > 0.05 (1,2,3,4,5,6) |
| TAPSE/PASP ratio (CardioMEMS™), mm/mmHg | 0.52 ± 0.20 | 0.48 ± 0.15 | 0.47 ± 0.16 | 0.48 ± 0.12 | 0.48 ± 0.13 | p > 0.05 (1,2,3,4,5,6) |
| E/E’mean | 12 (10–15) | 11.4 (8.5–15.8) | 10 (8.3–15.4) | 11.0 (10.3–16.0) | 9.1 (8.1–15.3) | p > 0.05 (1,2,3,4,5,6) |
| LV-CO, L/min | 5.4 (5.1–7.4) | 5.2 (4.2–5.7) | 4.5 (3.6–5.2) | 4.4 (3.8–5.0) | 4.6 (4.1–5.3) | p > 0.05 (1,2,3,4,5,6) |
| RV-CO, L/min | 6.1 (5.8–7.0) | 5.5 (4.5–6.4) | 5.1 (4.8–6.8) | 4.2 (3.5–5.5) | 4.6 (3.45–6.7) | p > 0.05 (1,2,3,4,5,6) |
| LV-SV, mL | 87 (78–94) | 85 (73–95) | 67 (53–77) | 75 (58–84) | 73 (55–85) | p > 0.05 (1,2,3,4,5,6) |
| RV-SV, mL | 91 (80–103) | 87 (78–103) | 92 (74–117) | 82 (62–98) | 78 (53–97) | p > 0.05 (1,2,3,4,5,6) |
| Pulmonary arterial capacitance (PAC, Echo-SVRV/CardioMEMS™-Pulse pressure), mL/mmHg | - | 3.5 (3.1–5.2) | 3.8 (3.4–6.2) | 3.5 (2.4–3.7) | 3.3 (2.2–4.6) | p > 0.05 (1,2,3,4,5,6) |
| CardioMEMS™-measured values | ||||||
| Systolic PAP, mmHg | 46 ± 12 | 45 ± 14 | 45 ± 13 | 45 ± 9 | p > 0.05 (1,2,3,4,5,6) | |
| Diastolic PAP, mmHg | 22 ± 7 | 22 ± 8 | 21 ± 7 | 21 ± 5 | p > 0.05 (1,2,3,4,5,6) | |
| Mean PAP, mmHg | 32 ± 9 | 32 ± 10 | 31 ± 10 | 31 ± 7 | p > 0.05 (1,2,3,4,5,6) | |
| Pulse pressure, mmHg | 24 ± 7 | 24 ± 7 | 25 ± 7 | 24 ± 6 | p > 0.05 (1,2,3,4,5,6) | |
| Proportional pulse pressure | 0.52 ± 0.07 | 0.52 ± 0.07 | 0.54 ± 0.07 | 0.53 ± 0.07 | p > 0.05 (1,2,3,4,5,6) | |
| Comparison of different methods | ||||||
| PVREcho, WU | 1.8 (1.3–2.4) | 1.7 (1.5–2.1) | 2.1 (1.6–2.5) | 1.9 (1.8–2.2) | 2.20 (1.18–2.63) | p > 0.05 (1,2,3,4,5,6) |
| PVRNew, WU | 0.7 (0.4–1.1) | 0.8 (0.5–0.9) | 0.6 (0.4–1.3) | 0.76 (0.55–1.53) | p > 0.05 (1,2,3,4,5,6) | |
| PVRNew Tedford, WU | 1.0 (0.7–1.1) | 0.9 (0.6–1.0) | 1.0 (0.9–1.4) | 0.95 (0.75–1.55) | p > 0.05 (1,2,3,4,5,6) | |
| PCWPNew | 23 (19–25) | 23 (19–27) | 20 (18–29) | 23 (21–27) | p > 0.05 (1,2,3,4,5,6) | |
| PCWPNew echo | 14 (10–20) | 20 (16–22) | 21 (18–23) | 17 (16–25) | p > 0.05 (1,2,3,4,5,6) |
| Variables | At Sensor Implantation | 3 Months Prior toInitiation of SGLT2-I Therapy | Initiation of SGLT2-I Therapy | 3 Months After Initiation of SGLT2-I Therapy | 6 Months After Initiation of SGLT2-I Therapy | p Values |
|---|---|---|---|---|---|---|
| NYHA functional class | ||||||
| 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | p > 0.05 (1,2,3,4,5,6) | |
| 0 (0%) | 1 (7.7%) | 1 (7.7%) | 0 (0%) | ||
| 1 (7.7%) | 2 (15.4%) | 3 (23.1%) | 4 (30.8%) | ||
| 2 (15.4%) | 3 (23.1%) | 2 (15.4%) | 2 (15.4%) | ||
| 13 (100%) | 10 (76.9%) | 6 (46.2%) | 7 (53.8%) | 7 (53.8%) | |
| 0 (0%) | 1 (7.7%) | 0 (0%) | 0 (0%) | ||
| NT-proBNP, pg/mL | 1109 (349–2133) | 1412 (562–2011) | 1536 (790–3152) | 1170 (754–2373) | 1017 (568–3513) | p > 0.05 (1,2,3,4,5,6) |
| Hematocrit, % | 0.39 ± 0.04 | 0.38 ± 0.02 | 0.39 ± 0.03 | 0.38 ± 0.03 | 0.40 ± 0.04 | p > 0.05 (1,2,3,4,5,6) |
| Hemoglobin, g/dL | 13.0 ± 12.0 | 12.8 ± 0.7 | 12.8 ± 1.1 | 13.2 ± 1.3 | 13.1 ± 1.3 | p > 0.05 (1,2,3,4,5,6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrmann, E.J.; Guckert, M.; Gruen, D.; Keller, T.; Tello, K.; Seeger, W.; Sossalla, S.; Assmus, B. Case Series Evaluating the Relationship of SGLT2 Inhibition to Pulmonary Artery Pressure and Non-Invasive Cardiopulmonary Parameters in HFpEF/HFmrEF Patients—A Pilot Study. Sensors 2025, 25, 605. https://doi.org/10.3390/s25030605
Herrmann EJ, Guckert M, Gruen D, Keller T, Tello K, Seeger W, Sossalla S, Assmus B. Case Series Evaluating the Relationship of SGLT2 Inhibition to Pulmonary Artery Pressure and Non-Invasive Cardiopulmonary Parameters in HFpEF/HFmrEF Patients—A Pilot Study. Sensors. 2025; 25(3):605. https://doi.org/10.3390/s25030605
Chicago/Turabian StyleHerrmann, Ester Judith, Michael Guckert, Dimitri Gruen, Till Keller, Khodr Tello, Werner Seeger, Samuel Sossalla, and Birgit Assmus. 2025. "Case Series Evaluating the Relationship of SGLT2 Inhibition to Pulmonary Artery Pressure and Non-Invasive Cardiopulmonary Parameters in HFpEF/HFmrEF Patients—A Pilot Study" Sensors 25, no. 3: 605. https://doi.org/10.3390/s25030605
APA StyleHerrmann, E. J., Guckert, M., Gruen, D., Keller, T., Tello, K., Seeger, W., Sossalla, S., & Assmus, B. (2025). Case Series Evaluating the Relationship of SGLT2 Inhibition to Pulmonary Artery Pressure and Non-Invasive Cardiopulmonary Parameters in HFpEF/HFmrEF Patients—A Pilot Study. Sensors, 25(3), 605. https://doi.org/10.3390/s25030605






