AI-Driven Analysis of Wrist-Worn Sensor Data for Monitoring Individual Treatment Response and Optimizing Levodopa Dosing in Parkinson’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Vote and Patient Consent
2.2. Study Cohort
2.3. Algorithm
2.4. Statistics
- Levodopa cycles
- Motor state at time of medication intake
- OFF if PD9TM ≤ −1
- ON if −1 < PD9TM < +1
- DYS if PD9TM ≥ 1
3. Results
3.1. Study Population and Data Collected
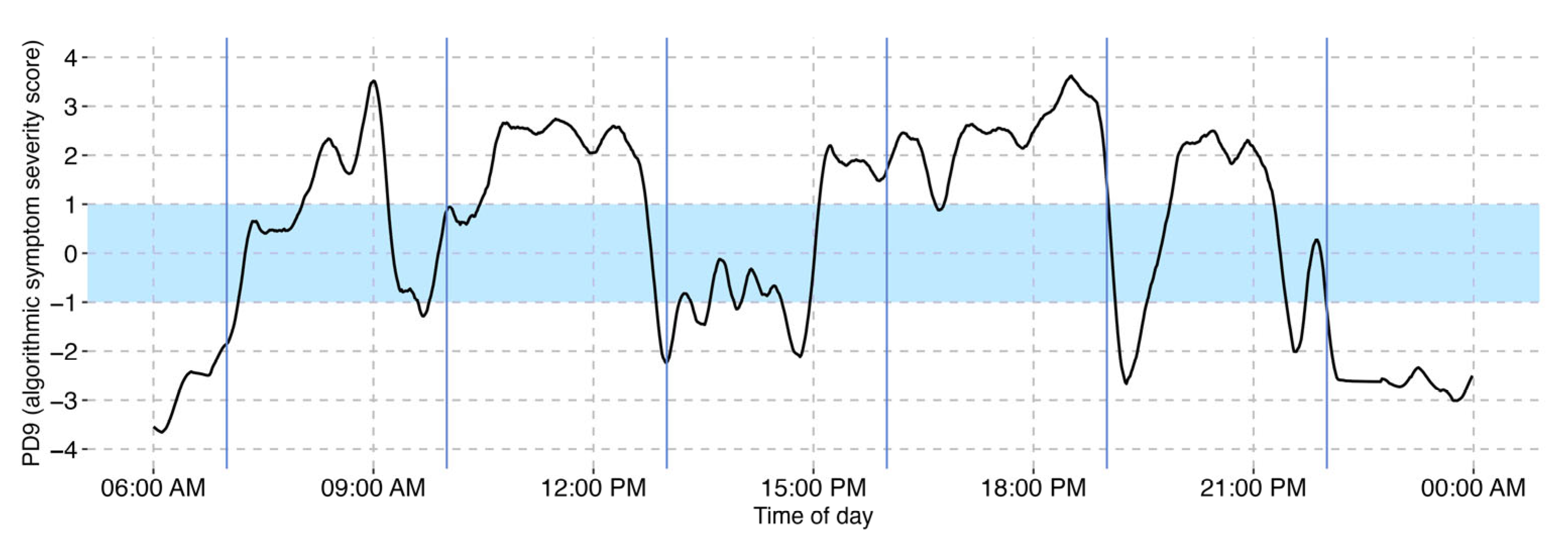
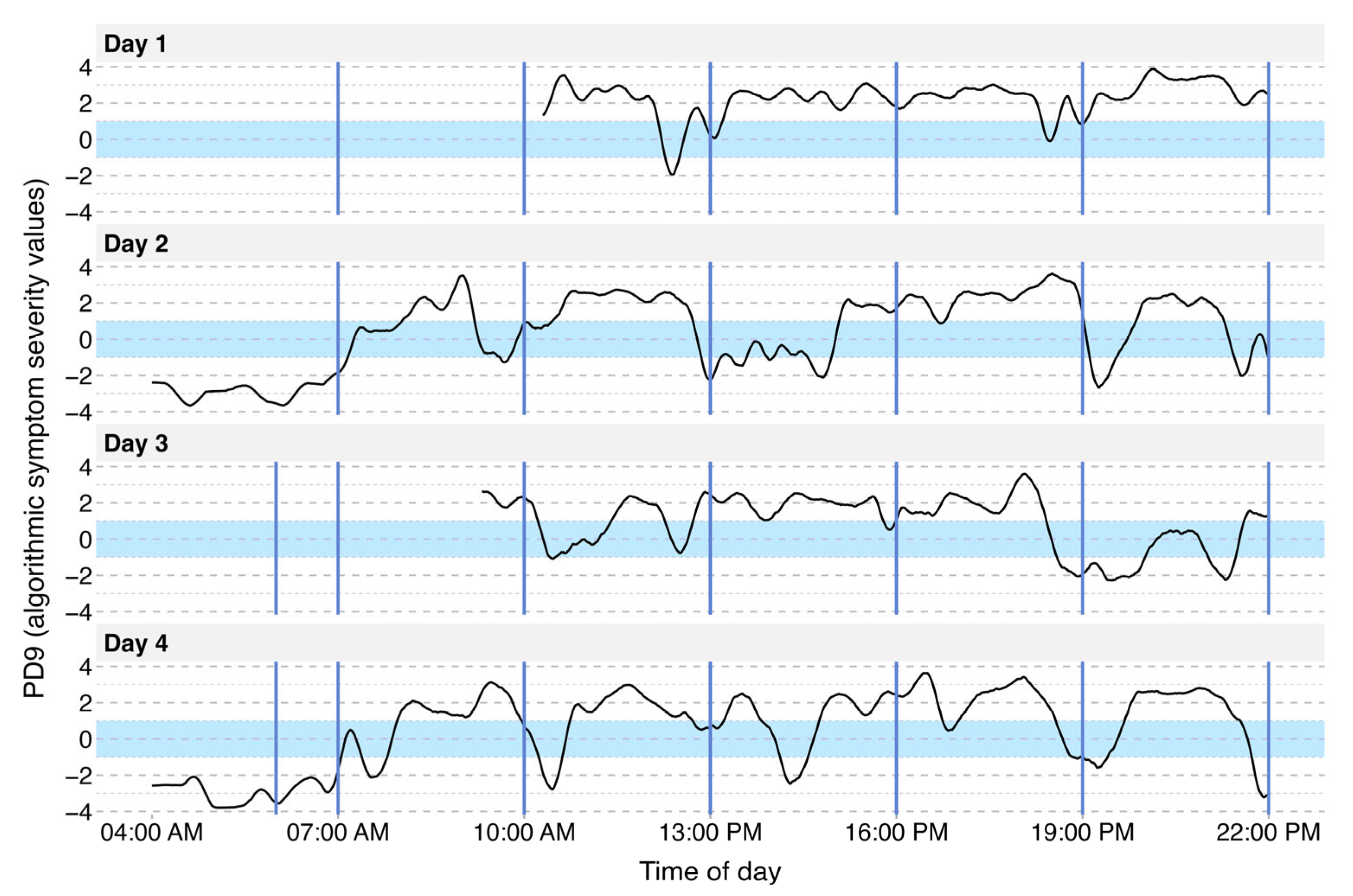
3.2. Visualization of Motor Symptom Severity
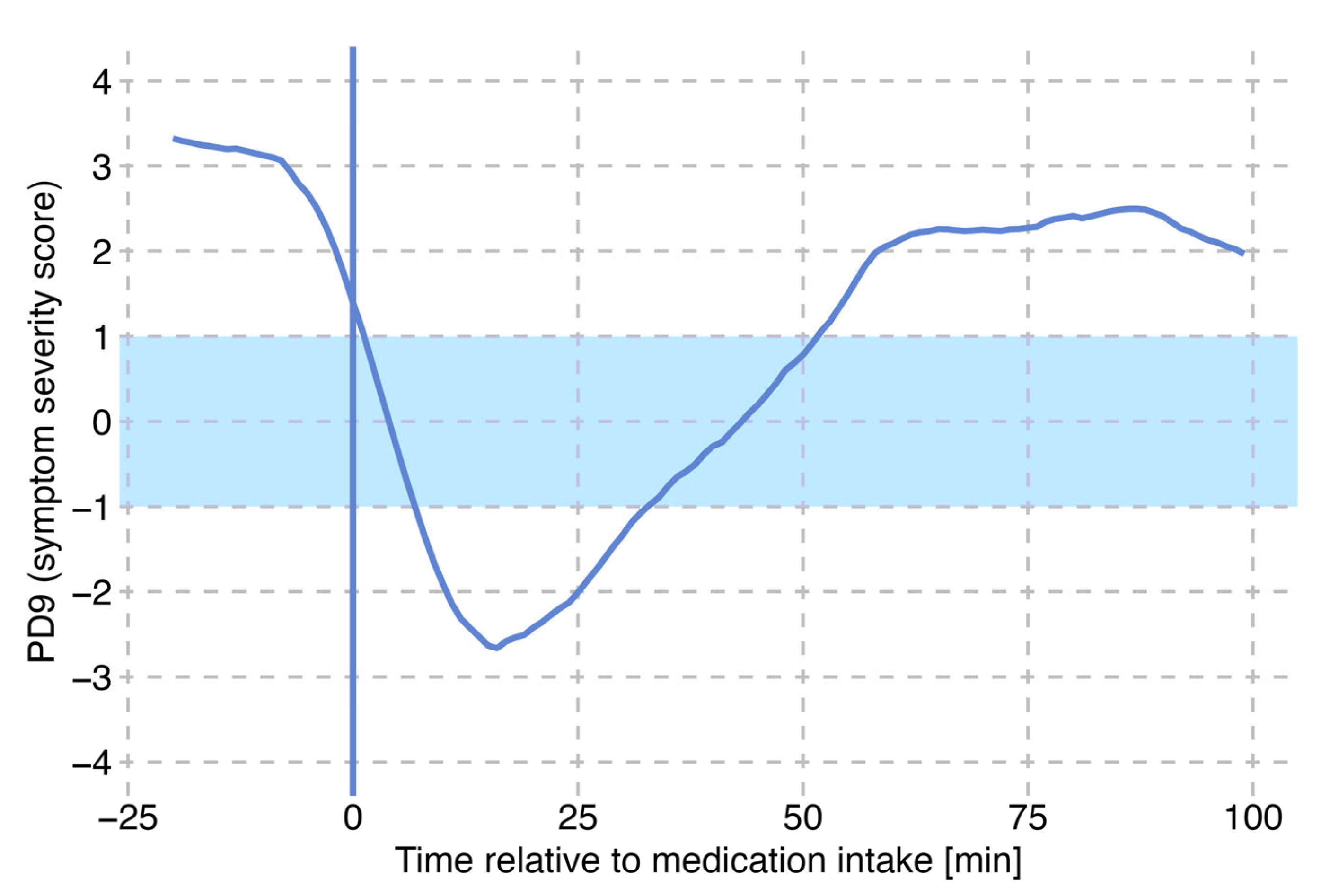
3.3. Identification of Single L-DOPA Cycles
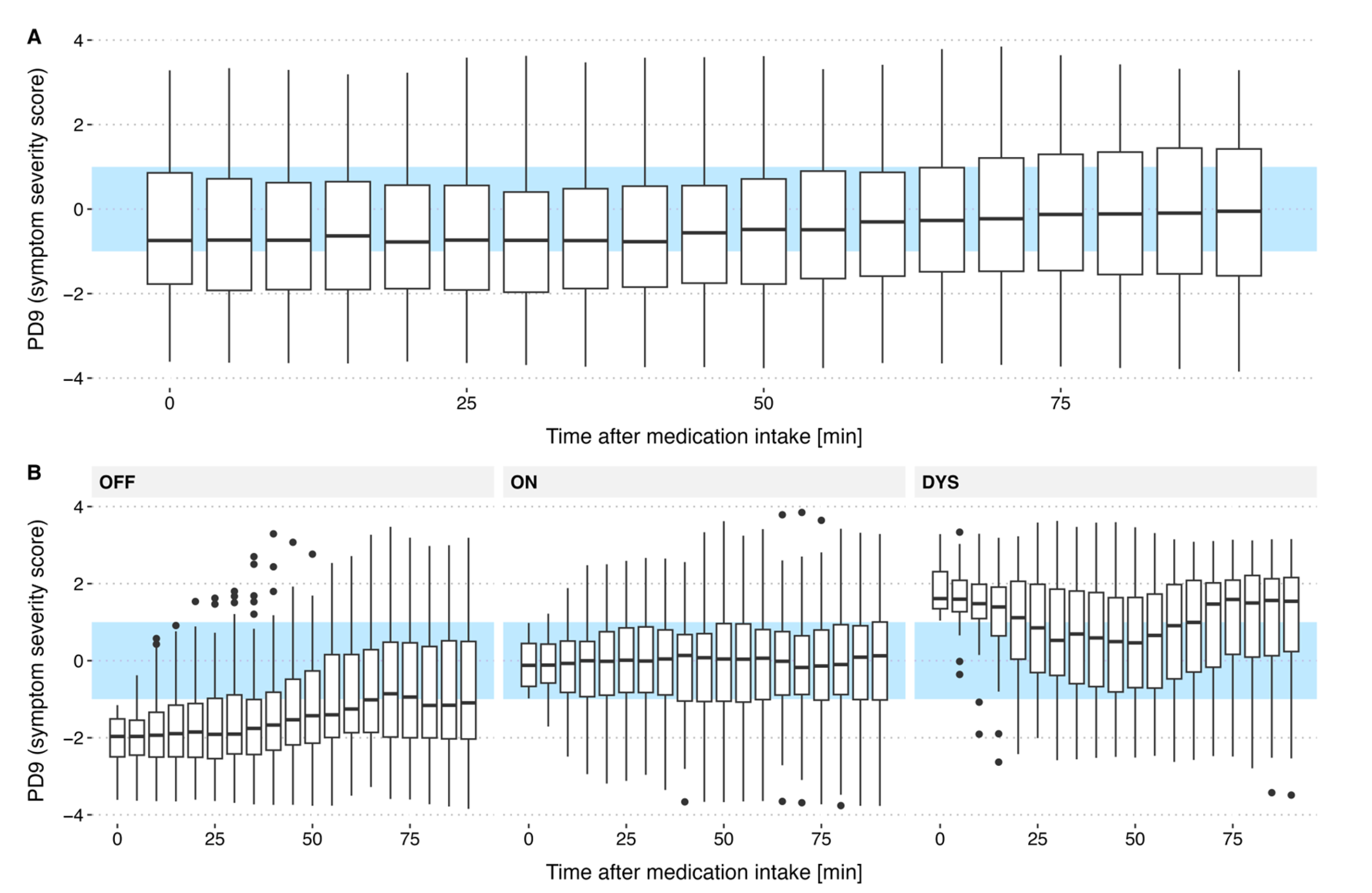
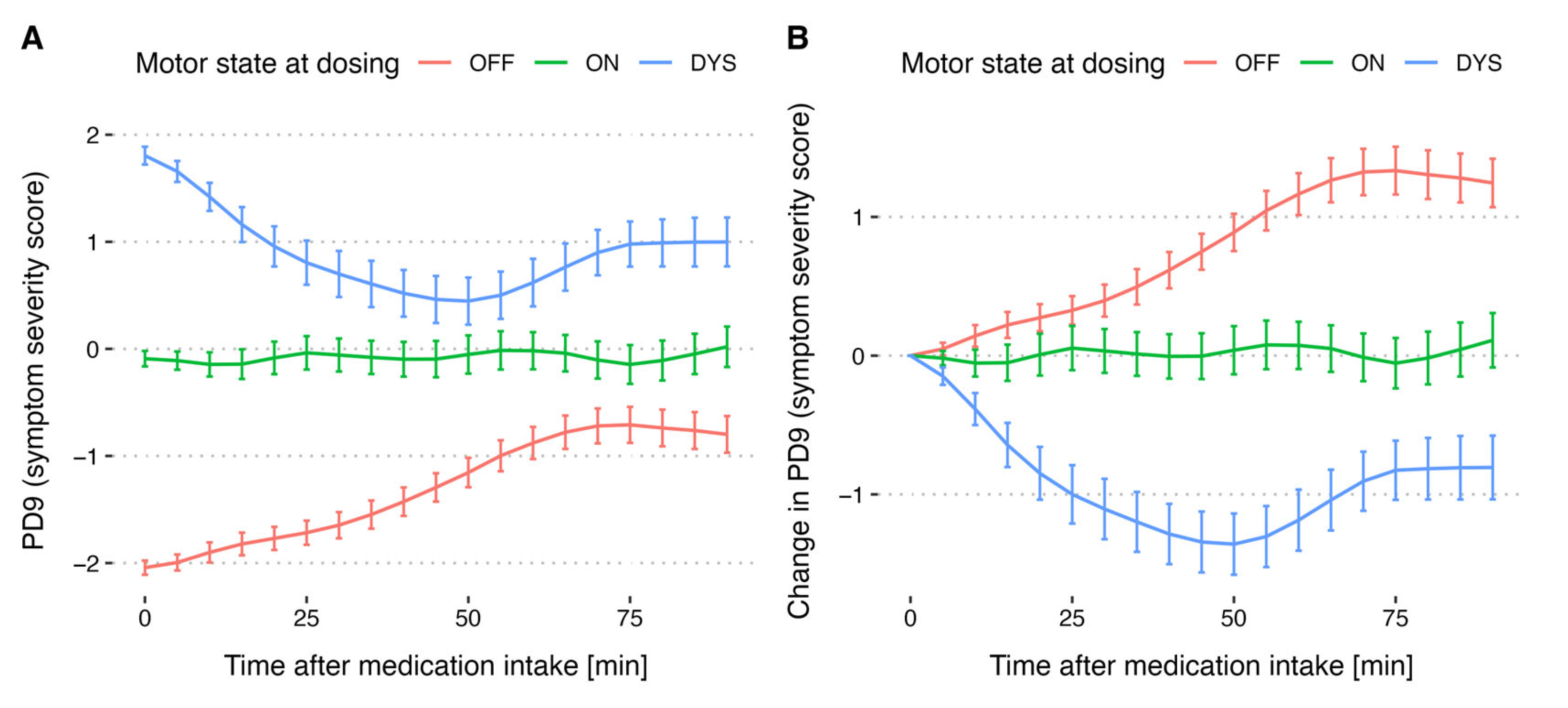
3.4. Motor States as an Effect of Levodopa Cycle
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dorsey, E.R.; Constantinescu, R.; Thompson, J.P.; Biglan, K.M.; Holloway, R.G.; Kieburtz, K.; Marshall, F.J.; Ravina, B.M.; Schifitto, G.; Siderowf, A.; et al. Projected Number of People with Parkinson Disease in the Most Populous Nations, 2005 through 2030. Neurology 2007, 68, 384–386. [Google Scholar] [CrossRef]
- Maserejian, N.; Vinikoor-Imler, L.; Dilley, A. Estimation of the 2020 Global Population of Parkinson’s Disease (PD). In Proceedings of the MDS Virtual Congress 2020, Virtual, 12–16 September 2020; Volume 35. [Google Scholar]
- Dorsey, E.R.; Sherer, T.; Okun, M.S.; Bloem, B.R. The Emerging Evidence of the Parkinson Pandemic. J. Park. Dis. 2018, 8, S3–S8. [Google Scholar] [CrossRef]
- Su, D.; Cui, Y.; He, C.; Yin, P.; Bai, R.; Zhu, J.; Lam, J.S.T.; Zhang, J.; Yan, R.; Zheng, X.; et al. Projections for Prevalence of Parkinson’s Disease and Its Driving Factors in 195 Countries and Territories to 2050: Modelling Study of Global Burden of Disease Study 2021. BMJ 2025, 388, e080952. [Google Scholar] [CrossRef] [PubMed]
- Stoker, T.B.; Greenland, J.C. (Eds.) Parkinson’s Disease: Pathogenesis and Clinical Aspects; Exon Publications: Brisbane, Australia, 2018; ISBN 978-0-9944381-6-4. [Google Scholar]
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020, 323, 548. [Google Scholar] [CrossRef] [PubMed]
- Balestrino, R.; Schapira, A.H.V. Parkinson Disease. Eur. J. Neurol. 2020, 27, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shlomo, Y.; Darweesh, S.; Llibre-Guerra, J.; Marras, C.; San Luciano, M.; Tanner, C. The Epidemiology of Parkinson’s Disease. Lancet 2024, 403, 283–292. [Google Scholar] [CrossRef]
- Tanner, C.M.; Ostrem, J.L. Parkinson’s Disease. N. Engl. J. Med. 2024, 391, 442–452. [Google Scholar] [CrossRef]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s Disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef]
- Church, F.C. Treatment Options for Motor and Non-Motor Symptoms of Parkinson’s Disease. Biomolecules 2021, 11, 612. [Google Scholar] [CrossRef]
- Othman, A.A.; Rosebraugh, M.; Chatamra, K.; Locke, C.; Dutta, S. Levodopa-Carbidopa Intestinal Gel Pharmacokinetics: Lower Variability than Oral Levodopa-Carbidopa. J. Park. Dis. 2017, 7, 275–278. [Google Scholar] [CrossRef]
- Nyholm, D.; Odin, P.; Johansson, A.; Chatamra, K.; Locke, C.; Dutta, S.; Othman, A.A. Pharmacokinetics of Levodopa, Carbidopa, and 3-O-Methyldopa Following 16-Hour Jejunal Infusion of Levodopa-Carbidopa Intestinal Gel in Advanced Parkinson’s Disease Patients. AAPS J. 2013, 15, 316–323. [Google Scholar] [CrossRef] [PubMed]
- LeWitt, P.A.; Fahn, S. Levodopa Therapy for Parkinson Disease: A Look Backward and Forward. Neurology 2016, 86, S3–S12. [Google Scholar] [CrossRef]
- Marsden, C.D.; Parkes, J.D. “On-off” effects in patients with Parkinson’s disease on chronic levodopa therapy. Lancet 1976, 307, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Obeso, J.A.; Grandas, F.; Vaamonde, J.; Luquin, M.R.; Artieda, J.; Lera, G.; Rodriguez, M.E.; Martinez-Lage, J.M. Motor Complications Associated with Chronic Levodopa Therapy in Parkinson’s Disease. Neurology 1989, 39, 11–19. [Google Scholar]
- Watts, J.; Khojandi, A.; Vasudevan, R.; Nahab, F.B.; Ramdhani, R.A. Improving Medication Regimen Recommendation for Parkinson’s Disease Using Sensor Technology. Sensors 2021, 21, 3553. [Google Scholar] [CrossRef]
- Pahwa, R.; Pagan, F.L.; Kremens, D.E.; Saint-Hilaire, M. Clinical Use of On-Demand Therapies for Patients with Parkinson’s Disease and OFF Periods. Neurol. Ther. 2023, 12, 1033–1049. [Google Scholar] [CrossRef]
- Connolly, B.S.; Lang, A.E. Pharmacological Treatment of Parkinson Disease: A Review. JAMA 2014, 311, 1670. [Google Scholar] [CrossRef]
- Di Biase, L.; Pecoraro, P.M.; Carbone, S.P.; Caminiti, M.L.; Di Lazzaro, V. Levodopa-Induced Dyskinesias in Parkinson’s Disease: An Overview on Pathophysiology, Clinical Manifestations, Therapy Management Strategies and Future Directions. J. Clin. Med. 2023, 12, 4427. [Google Scholar] [CrossRef]
- Agnieszka, W.; Paweł, P.; Małgorzata, K. How to Optimize the Effectiveness and Safety of Parkinson’s Disease Therapy?—A Systematic Review of Drugs Interactions with Food and Dietary Supplements. Curr. Neuropharmacol. 2022, 20, 1427–1447. [Google Scholar] [CrossRef]
- Jankovic, J.; Tan, E.K. Parkinson’s Disease: Etiopathogenesis and Treatment. J. Neurol. Neurosurg. Psychiatry 2020, 91, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Emamzadeh, F.N.; Surguchov, A. Parkinson’s Disease: Biomarkers, Treatment, and Risk Factors. Front. Neurosci. 2018, 12, 612. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, S.H.; Pagan, F.L.; Lew, M.F.; Pahwa, R. Should “on-Demand” Treatments for Parkinson’s Disease OFF Episodes Be Used Earlier? Clin. Park. Relat. Disord. 2022, 7, 100161. [Google Scholar] [CrossRef]
- Höglinger, G.; German Parkinson’s Guidelines Committee; Bähr, M.; Becktepe, J.; Berg, D.; Brockmann, K.; Buhmann, C.; Ceballos-Baumann, A.; Claßen, J.; Deuschl, C.; et al. Diagnosis and Treatment of Parkinson´s Disease (Guideline of the German Society for Neurology). Neurol. Res. Pract. 2024, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Tönges, L.; Buhmann, C.; Eggers, C.; Lorenzl, S.; Warnecke, T.; for the German Parkinson Guideline Group; Bähr, M.; Becktepe, J.; Berg, D.; Brockmann, K.; et al. Guideline “Parkinson’s Disease” of the German Society of Neurology (Deutsche Gesellschaft Für Neurologie): Concepts of Care. J. Neurol. 2024, 271, 7377–7386. [Google Scholar] [CrossRef]
- Ancona, S.; Faraci, F.D.; Khatab, E.; Fiorillo, L.; Gnarra, O.; Nef, T.; Bassetti, C.L.A.; Bargiotas, P. Wearables in the Home-Based Assessment of Abnormal Movements in Parkinson’s Disease: A Systematic Review of the Literature. J. Neurol. 2022, 269, 100–110. [Google Scholar] [CrossRef]
- Del Din, S.; Kirk, C.; Yarnall, A.J.; Rochester, L.; Hausdorff, J.M. Body-Worn Sensors for Remote Monitoring of Parkinson’s Disease Motor Symptoms: Vision, State of the Art, and Challenges Ahead. J. Park. Dis. 2021, 11, S35–S47. [Google Scholar] [CrossRef]
- Espay, A.J.; Bonato, P.; Nahab, F.B.; Maetzler, W.; Dean, J.M.; Klucken, J.; Eskofier, B.M.; Merola, A.; Horak, F.; Lang, A.E.; et al. Technology in Parkinson’s Disease: Challenges and Opportunities: Technology in PD. Mov. Disord. 2016, 31, 1272–1282. [Google Scholar] [CrossRef]
- Giannakopoulou, K.-M.; Roussaki, I.; Demestichas, K. Internet of Things Technologies and Machine Learning Methods for Parkinson’s Disease Diagnosis, Monitoring and Management: A Systematic Review. Sensors 2022, 22, 1799. [Google Scholar] [CrossRef]
- Monje, M.H.G.; Foffani, G.; Obeso, J.; Sánchez-Ferro, Á. New Sensor and Wearable Technologies to Aid in the Diagnosis and Treatment Monitoring of Parkinson’s Disease. Annu. Rev. Biomed. Eng. 2019, 21, 111–143. [Google Scholar] [CrossRef]
- Ossig, C.; Antonini, A.; Buhmann, C.; Classen, J.; Csoti, I.; Falkenburger, B.; Schwarz, M.; Winkler, J.; Storch, A. Wearable Sensor-Based Objective Assessment of Motor Symptoms in Parkinson’s Disease. J. Neural Transm. Vienna Austria 1996 2016, 123, 57–64. [Google Scholar] [CrossRef] [PubMed]
- National Institue for Health and Care Excellence (NICE). NICE Guideline: Devices for Remote Monitoring of Parkinson’s Disease; NICE: London, UK, 2023. [Google Scholar]
- Dominey, T.; Kehagia, A.A.; Gorst, T.; Pearson, E.; Murphy, F.; King, E.; Carroll, C. Introducing the Parkinson’s KinetiGraph into Routine Parkinson’s Disease Care: A 3-Year Single Centre Experience. J. Park. Dis. 2020, 10, 1827–1832. [Google Scholar] [CrossRef]
- Pfister, F.M.J.; Um, T.T.; Pichler, D.C.; Goschenhofer, J.; Abedinpour, K.; Lang, M.; Endo, S.; Ceballos-Baumann, A.O.; Hirche, S.; Bischl, B.; et al. High-Resolution Motor State Detection in Parkinson’s Disease Using Convolutional Neural Networks. Sci. Rep. 2020, 10, 5860. [Google Scholar] [CrossRef] [PubMed]
- Goschenhofer, J.; Pfister, F.M.J.; Yuksel, K.A.; Bischl, B.; Fietzek, U.; Thomas, J. Wearable-Based Parkinson’s Disease Severity Monitoring Using Deep Learning. In Machine Learning and Knowledge Discovery in Databases; Brefeld, U., Fromont, E., Hotho, A., Knobbe, A., Maathuis, M., Robardet, C., Eds.; Lecture Notes in Computer Science; Springer International Publishing: Cham, Switzerland, 2020; Volume 11908, pp. 400–415. ISBN 978-3-030-46132-4. [Google Scholar]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-sponsored Revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale Presentation and Clinimetric Testing Results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef]
- Lane, R.D.; Glazer, W.M.; Hansen, T.E.; Berman, W.H.; Kramer, S.I. Assessment of Tardive Dyskinesia Using the Abnormal Involuntary Movement Scale. J. Nerv. Ment. Dis. 1985, 173, 353–357. [Google Scholar] [CrossRef]
- Fietzek, U.M. Closing the Loop mit NeptuneTM—Erste Erfahrungen mit der KI-Sensorlösung zur Erfassung des motorischen Zustandes bei Menschen bit Parkinson. Neuro Aktuell 2024, 4, 1–6. [Google Scholar]
- Nyholm, D.; Stepien, V. Levodopa Fractionation in Parkinson’s Disease. J. Park. Dis. 2014, 4, 89–96. [Google Scholar] [CrossRef]
- Cabreira, V.; Soares-da-Silva, P.; Massano, J. Contemporary Options for the Management of Motor Complications in Parkinson’s Disease: Updated Clinical Review. Drugs 2019, 79, 593–608. [Google Scholar] [CrossRef]
- Moreau, C.; Rouaud, T.; Grabli, D.; Benatru, I.; Remy, P.; Marques, A.-R.; Drapier, S.; Mariani, L.-L.; Roze, E.; Devos, D.; et al. Overview on Wearable Sensors for the Management of Parkinson’s Disease. Npj Park. Dis. 2023, 9, 153. [Google Scholar] [CrossRef] [PubMed]
- FitzGerald, J.J.; Lu, Z.; Jareonsettasin, P.; Antoniades, C.A. Quantifying Motor Impairment in Movement Disorders. Front. Neurosci. 2018, 12, 202. [Google Scholar] [CrossRef]
- Sundgren, M.; Andréasson, M.; Svenningsson, P.; Noori, R.-M.; Johansson, A. Does Information from the Parkinson KinetiGraphTM (PKG) Influence the Neurologist’s Treatment Decisions?-An Observational Study in Routine Clinical Care of People with Parkinson’s Disease. J. Pers. Med. 2021, 11, 519. [Google Scholar] [CrossRef]
- Rodríguez-Molinero, A.; Samà, A.; Pérez-López, C.; Rodríguez-Martín, D.; Alcaine, S.; Mestre, B.; Quispe, P.; Giuliani, B.; Vainstein, G.; Browne, P.; et al. Analysis of Correlation between an Accelerometer-Based Algorithm for Detecting Parkinsonian Gait and UPDRS Subscales. Front. Neurol. 2017, 8, 431. [Google Scholar] [CrossRef] [PubMed]
- Thomas, I.; Alam, M.; Bergquist, F.; Johansson, D.; Memedi, M.; Nyholm, D.; Westin, J. Sensor-Based Algorithmic Dosing Suggestions for Oral Administration of Levodopa/Carbidopa Microtablets for Parkinson’s Disease: A First Experience. J. Neurol. 2019, 266, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Pulliam, C.L.; Heldman, D.A.; Orcutt, T.H.; Mera, T.O.; Giuffrida, J.P.; Vitek, J.L. Motion Sensor Strategies for Automated Optimization of Deep Brain Stimulation in Parkinson’s Disease. Park. Relat. Disord. 2015, 21, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Suescun, J.; Schiess, M.C.; Jiang, X. Computational Medication Regimen for Parkinson’s Disease Using Reinforcement Learning. Sci. Rep. 2021, 11, 9313. [Google Scholar] [CrossRef]
- Shuqair, M.; Jimenez-Shahed, J.; Ghoraani, B. Reinforcement Learning-Based Adaptive Classification for Medication State Monitoring in Parkinson’s Disease. IEEE J. Biomed. Health Inform. 2024, 28, 6168–6179. [Google Scholar] [CrossRef]
| Overall (n = 67) | |
|---|---|
| Gender | |
| Male | 37 (55.2%) |
| Female | 30 (44.8%) |
| Age | |
| Mean (SD) | 67.0 (10.4) |
| Median [Min, Max] | 68.2 [35.9, 86.0] |
| BMI | |
| Mean (SD) | 25.3 (4.32) |
| Median [Min, Max] | 24.8 [17.0, 36.1] |
| Disease duration | |
| Mean (SD) | 9.09 (6.13) |
| Median [Min, Max] | 9.00 [0, 26.0] |
| Hoehn & Yahr Stage | |
| 1 | 3 (4.5%) |
| 2 | 20 (29.9%) |
| 3 | 29 (43.3%) |
| 4 | 14 (20.9%) |
| 5 | 1 (1.5%) |
| LED | |
| Mean (SD) | 1070 (554) |
| Median [Min, Max] | 1050 [100, 3750] |
| Motor State | Count (%) (N = 218) |
|---|---|
| OFF | 99 (45.4) |
| ON | 68 (31.2) |
| Dyskinetic | 51 (23.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sander, M.; Messner, M.R.; Knapp, S.K.; Pfister, F.M.J.; Fietzek, U.M. AI-Driven Analysis of Wrist-Worn Sensor Data for Monitoring Individual Treatment Response and Optimizing Levodopa Dosing in Parkinson’s Disease. Sensors 2025, 25, 7273. https://doi.org/10.3390/s25237273
Sander M, Messner MR, Knapp SK, Pfister FMJ, Fietzek UM. AI-Driven Analysis of Wrist-Worn Sensor Data for Monitoring Individual Treatment Response and Optimizing Levodopa Dosing in Parkinson’s Disease. Sensors. 2025; 25(23):7273. https://doi.org/10.3390/s25237273
Chicago/Turabian StyleSander, Mathias, Moritz R. Messner, Sina K. Knapp, Franz M. J. Pfister, and Urban M. Fietzek. 2025. "AI-Driven Analysis of Wrist-Worn Sensor Data for Monitoring Individual Treatment Response and Optimizing Levodopa Dosing in Parkinson’s Disease" Sensors 25, no. 23: 7273. https://doi.org/10.3390/s25237273
APA StyleSander, M., Messner, M. R., Knapp, S. K., Pfister, F. M. J., & Fietzek, U. M. (2025). AI-Driven Analysis of Wrist-Worn Sensor Data for Monitoring Individual Treatment Response and Optimizing Levodopa Dosing in Parkinson’s Disease. Sensors, 25(23), 7273. https://doi.org/10.3390/s25237273






