Sensor-Based Assessment of Groove Music and Sports Dance on Cognitive–Emotional and Neuromuscular Functions in Older Adults
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Research Design
2.3. Groove Music Selection
2.4. Sports Dance Intervention Protocol
2.5. Assessment Indices
2.5.1. Emotional Regulation
2.5.2. Executive Function
Behavior Rating Inventory of Executive Function
Stroop Test
2.5.3. Body Movement Control Measurement
2.5.4. Functional Brain Connectivity Measurement
2.6. Statistical Analysis
3. Results
4. Discussion
4.1. Improvements in Cognitive–Emotional Functions
4.2. Neuromuscular Coordination and Brain Functional Connectivity
4.3. Stability-Oriented Perspective on Neurophysiological Signal Processing
4.4. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, S.; Ye, N.; Liu, X.; Li, Y.; Ai, Y.; Wang, X.; Zhou, P.; Hu, H. Association of motoric cognitive risk syndrome with depression in older adults: A meta-analysis and systematic review of cross-sectional and cohort studies. BMC Geriatr. 2024, 24, 973. [Google Scholar] [CrossRef]
- Yen, H.Y.; Chiu, H.L. Virtual Reality Exergames for Improving Older Adults’ Cognition and Depression: A Systematic Review and Meta-Analysis of Randomized Control Trials. J. Am. Med. Dir. Assoc. 2021, 22, 995–1002. [Google Scholar] [CrossRef]
- Zwingmann, I.; Hoffmann, W.; Michalowsky, B.; Dreier-Wolfgramm, A.; Hertel, J.; Wucherer, D.; Eichler, T.; Kilimann, I.; Thiel, F.; Teipel, S.; et al. Supporting family dementia caregivers: Testing the efficacy of dementia care management on multifaceted caregivers’ burden. Aging Ment. Health 2018, 22, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Zwar, L.; König, H.H.; Delfin, E.; Hajek, A. Development and validation of the Perceived Care Stigma Scale (PerCSS): Measuring cognitive, emotional and behavioral reactions to (unpaid) caregivers of older family members and friends. Aging Ment. Health 2025, 29, 2154–2162, Advance online publication. [Google Scholar] [CrossRef] [PubMed]
- Zuidersma, M.; Müller, F.; Snippe, E.; Zuidema, S.U.; Oude Voshaar, R.C. Feasibility, usability and clinical value of intensive longitudinal diary assessments in older persons with cognitive impairment and depressive symptoms. Aging Ment. Health 2023, 27, 1466–1475. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Meng, F.; He, M.; Li, Z. The efficacy of a transdiagnostic group cognitive behavioral intervention for Chinese elderly with emotional disorders: A one-year follow-up randomized clinical trial. Front. Psychiatry 2022, 13, 1027994. [Google Scholar] [CrossRef]
- Zwicker, A.; Fabbri, C.; Rietschel, M.; Hauser, J.; Mors, O.; Maier, W.; Zobel, A.; Farmer, A.; Aitchison, K.J.; McGuffin, P.; et al. Genetic disposition to inflammation and response to antidepressants in major depressive disorder. J. Psychiatr. Res. 2018, 105, 17–22. [Google Scholar] [CrossRef]
- Zygmont, M.; Prigerson, H.G.; Houck, P.R.; Miller, M.D.; Shear, M.K.; Jacobs, S.; Reynolds, C.F., 3rd. A post hoc comparison of paroxetine and nortriptyline for symptoms of traumatic grief. J. Clin. Psychiatry 1998, 59, 241–245. [Google Scholar] [CrossRef]
- Zu, S.; Xiang, Y.T.; Liu, J.; Zhang, L.; Wang, G.; Ma, X.; Kilbourne, A.M.; Ungvari, G.S.; Chiu, H.F.; Lai, K.Y.; et al. A comparison of cognitive-behavioral therapy, antidepressants, their combination and standard treatment for Chinese patients with moderate-severe major depressive disorders. J. Affect. Disord. 2014, 152–154, 262–267. [Google Scholar] [CrossRef]
- Zuo, X.; Dong, Z.; Zhang, P.; Zhang, P.; Zhu, X.; Qiao, C.; Yang, Y.; Lou, P. Cognitive-behavioral therapy on psychological stress and quality of life in subjects with pulmonary tuberculosis: A community-based cluster randomized controlled trial. BMC Public Health 2022, 22, 2160. [Google Scholar] [CrossRef]
- Talar, K.; Vetrovsky, T.; van Haren, M.; Négyesi, J.; Granacher, U.; Váczi, M.; Martín-Arévalo, E.; Del Olmo, M.F.; Kałamacka, E.; Hortobágyi, T. The effects of aerobic exercise and transcranial direct current stimulation on cognitive function in older adults with and without cognitive impairment: A systematic review and meta-analysis. Ageing Res. Rev. 2022, 81, 101738. [Google Scholar] [CrossRef]
- Ye, M.; Song, T.; Xia, H.; Hou, Y.; Chen, A. Effects of aerobic exercise on executive function of healthy middle-aged and older adults: A systematic review and meta-analysis. Int. J. Nurs. Stud. 2024, 160, 104912. [Google Scholar] [CrossRef]
- Jiang, J.; Guo, W.; Wang, B. Effects of exergaming on executive function of older adults: A systematic review and meta-analysis. PeerJ 2022, 10, e13194. [Google Scholar] [CrossRef]
- Zygmont, A.; Doliński, W.; Zawadzka, D.; Pezdek, K. Uplifted by Dancing Community: From Physical Activity to Well-Being. Int. J. Environ. Res. Public Health 2023, 20, 3535. [Google Scholar] [CrossRef]
- Zilidou, V.I.; Frantzidis, C.A.; Romanopoulou, E.D.; Paraskevopoulos, E.; Douka, S.; Bamidis, P.D. Functional Re-organization of Cortical Networks of Senior Citizens After a 24-Week Traditional Dance Program. Front. Aging Neurosci. 2018, 10, 422. [Google Scholar] [CrossRef] [PubMed]
- Wöllner, C. Music in the Air and in the Body: An Interoceptive System’s Perspective on Musical Emotions, Awareness, and Time. Curr. Top. Behav. Neurosci. 2024, 70, 269–284. [Google Scholar] [CrossRef]
- Vuust, P.; Gebauer, L.K.; Witek, M.A. Neural underpinnings of music: The polyrhythmic brain. Adv. Exp. Med. Biol. 2014, 829, 339–356. [Google Scholar] [CrossRef]
- Burrai, F.; Apuzzo, L.; Zanotti, R. Effectiveness of Rhythmic Auditory Stimulation on Gait in Parkinson Disease: A Systematic Review and Meta-analysis. Holist. Nurs. Pract. 2024, 38, 109–119. [Google Scholar] [CrossRef]
- Ngo, J.K.; Lu, J.; Cloak, R.; Wong, D.P.; Devonport, T.; Wyon, M.A. Strength and conditioning in dance: A systematic review and meta-analysis. Eur. J. Sport Sci. 2024, 24, 637–652. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Yang, X.; Lv, J. Efficacy of tinnitus retraining therapy in the treatment of tinnitus: A meta-analysis and systematic review. Am. J. Otolaryngol. 2021, 42, 103151. [Google Scholar] [CrossRef]
- Huang, H.; Jin, L.; Zeng, Z. A Momentum Recurrent Neural Network for Sparse Motion Planning of Redundant Manipulators With Majorization-Minimization. IEEE Trans. Ind. Electronics. 2025, 3, 1–10. [Google Scholar] [CrossRef]
- Yin, Q.; Wen, J.; Chen, S.; Hou, T.; Liu, Y.; Yang, D.; Liu, G.; Shi, P.; Dong, W. Uncovering the neural basis of risk preferences in cooperative Dyads: A fNIRS study. NeuroImage 2025, 310, 121167. [Google Scholar] [CrossRef]
- Dias, J.W.; McClaskey, C.M.; Alvey, A.P.; Lawson, A.; Matthews, L.J.; Dubno, J.R.; Harris, K.C. Effects of age and noise exposure history on auditory nerve response amplitudes: A systematic review, study, and meta-analysis. Hear. Res. 2024, 447, 109010. [Google Scholar] [CrossRef]
- Sihvonen, A.J.; Leo, V.; Ripollés, P.; Lehtovaara, T.; Ylönen, A.; Rajanaro, P.; Laitinen, S.; Forsblom, A.; Saunavaara, J.; Autti, T.; et al. Vocal music enhances memory and language recovery after stroke: Pooled results from two RCTs. Ann. Clin. Transl. Neurol. 2020, 7, 2272–2287. [Google Scholar] [CrossRef]
- Nasser, A.; Hull, J.T.; Chaturvedi, S.A.; Liranso, T.; Odebo, O.; Kosheleff, A.R.; Fry, N.; Cutler, A.J.; Rubin, J.; Schwabe, S.; et al. A Phase III, Randomized, Double-Blind, Placebo-Controlled Trial Assessing the Efficacy and Safety of Viloxazine Extended-Release Capsules in Adults with Attention-Deficit/Hyperactivity Disorder. CNS Drugs 2022, 36, 897–915. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H.; Shie, J.J.; Yu, N.Y. Enhancing Executive Functions and Handwriting with a Concentrative Coordination Exercise in Children with ADHD: A Randomized Clinical Trial. Percept. Mot. Ski. 2022, 129, 1014–1035. [Google Scholar] [CrossRef]
- Chung, C.L.; Mak, M.K.; Hallett, M. Transcranial Magnetic Stimulation Promotes Gait Training in Parkinson Disease. Ann. Neurol. 2020, 88, 933–945. [Google Scholar] [CrossRef] [PubMed]
- Struckmann, W.; Bodén, R.; Gingnell, M.; Fällmar, D.; Persson, J. Modulation of dorsolateral prefrontal cortex functional connectivity after intermittent theta-burst stimulation in depression: Combining findings from fNIRS and fMRI. NeuroImage Clin. 2022, 34, 103028. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xie, Y.; Wang, Y.; Wu, L.; Yu, X.; Bai, L.; Shao, S.; Zhou, M.; Zhang, M.; Yu, X.; et al. Effect of acupuncture on patients with chronic obstructive pulmonary disease: A multicenter randomized controlled trial. Complement. Ther. Med. 2025, 89, 103146. [Google Scholar] [CrossRef]
- Ready, E.A.; Holmes, J.D.; Grahn, J.A. Gait in younger and older adults during rhythmic auditory stimulation is influenced by groove, familiarity, beat perception, and synchronization demands. Hum. Mov. Sci. 2022, 84, 102972. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, G.; Han, J.; Su, P.; Zhang, H.; Tang, D. The Effect of Perceived Groove in Music on Effective Brain Connectivity during Cycling: An fNIRS Study. Med. Sci. Sports Exerc. 2025, 57, 857–866. [Google Scholar] [CrossRef]
- Znazen, H.; Hammami, A.; Bragazzi, N.L.; Hadadi, A.; Slimani, M. Effects of Different Acute Plyometric Training Intensities on Attention and Psychological States. Int. J. Environ. Res. Public Health 2022, 19, 14959. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, H.; Qi, M.; Wang, S.; Zhang, Q.; Zhou, L.; Wang, S.; Wang, W.; Wu, T.; Xiao, M.; et al. Effects of a specially designed aerobic dance routine on mild cognitive impairment. Clin. Interv. Aging 2018, 13, 1691–1700. [Google Scholar] [CrossRef]
- Siponkoski, S.T.; Martínez-Molina, N.; Kuusela, L.; Laitinen, S.; Holma, M.; Ahlfors, M.; Jordan-Kilkki, P.; Ala-Kauhaluoma, K.; Melkas, S.; Pekkola, J.; et al. Music Therapy Enhances Executive Functions and Prefrontal Structural Neuroplasticity after Traumatic Brain Injury: Evidence from a Randomized Controlled Trial. J. Neurotrauma 2020, 37, 618–634. [Google Scholar] [CrossRef]
- Zuk, J.; Benjamin, C.; Kenyon, A.; Gaab, N. Behavioral and neural correlates of executive functioning in musicians and non-musicians. PLoS ONE 2014, 9, e99868. [Google Scholar] [CrossRef] [PubMed]
- Stegemöller, E.L.; Izbicki, P.; Hibbing, P. The influence of moving with music on motor cortical activity. Neurosci. Lett. 2018, 683, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, C.; Poisoner, H.; Toiviainen, P.; Huotilainen, M.; Mathiak, K.; Ristaniemi, T.; Cong, F. Exploring Frequency-Dependent Brain Networks from Ongoing EEG Using Spatial ICA During Music Listening. Brain Topogr. 2020, 33, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Lin, H.; Chen, F. An fNIRS Study on the Effect of Music Style on Cognitive Activities. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Montreal, QC, Canada, 20–24 July 2020; pp. 3200–3203. [Google Scholar] [CrossRef]
- Wu, C.C.; Yang, J.; Wang, X.Q. Analgesic effect of dance movement therapy: An fNIRS study. NeuroImage 2024, 301, 120880. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhou, R.; Wei, W.; Luan, R.; Li, K. Effects of music-based movement therapy on motor function, balance, gait, mental health, and quality of life for patients with Parkinson’s disease: A systematic review and meta-analysis. Clin. Rehabil. 2021, 35, 937–951. [Google Scholar] [CrossRef]
- Zaferiou, A.; Hirsch, Z.; Bacani, T.; Dahl, L. A review of concurrent sonified biofeedback in balance and gait training. J. Neuroeng. Rehabil. 2025, 22, 38. [Google Scholar] [CrossRef]
| Characteristic | GODA (n = 25) | CODA (n = 26) | Control (n = 24) |
|---|---|---|---|
| Age, years, mean (SD) | 67.4 (4.1) | 67.8 (3.9) | 67.2 (4.3) |
| Gender, (%) | |||
| Female | 60.0 | 57.7 | 50.0 |
| Male | 40.0 | 42.3 | 50.0 |
| Race/ethnicity, (%) | |||
| Han Chinese | 100.0 | 100.0 | 100.0 |
| Education, years, mean (SD) | 12.3 (2.7) | 12.1 (2.9) | 12.4 (2.6) |
| MMSE baseline, mean (SD) | 28.1 (1.4) | 28.0 (1.5) | 28.2 (1.3) |
| Group | Participants Enrolled (n) | Dropouts (n) | Participants Completing the Study (n) | Attendance Rate (%) (Mean ± SD) |
|---|---|---|---|---|
| GODA | 26 | 2 | 24 | 92.5 ± 5.2 |
| CODA | 26 | 0 | 26 | 95.8 ± 3.8 |
| CON | 26 | 1 | 25 | 94.3 ± 4.5 |
| Total | 78 | 3 | 75 | 94.2 ± 4.5 |
| GODA | CODA | CON | PBetween-group | ||
|---|---|---|---|---|---|
| Acceptance use: low intensity (%) | Pre | 62.8 (21.3) | 61.3 (20.2) | 62.9 (24.1) | 0.70 |
| Post | 69.7 (25.1) | 65.2 (23.7) | 62.0 (21.4) | <0.001 | |
| PWithin-group | <0.001 | 0.03 | 0.80 | ||
| Acceptance use: high intensity (%) | Pre | 52.1 (24.4) | 51.6 (34.1) | 53.3 (26.5) | 0.90 |
| Post | 57.6 (27.3) | 53.8 (24.6) | 53.0 (27.8) | 0.30 | |
| PWithin-group | 0.10 | 0.20 | 0.90 |
| GODA | CODA | CON | PBetween-group | ||
|---|---|---|---|---|---|
| Inhibition | Pre | 14.9 (5.9) | 13.9 (7.1) | 15.1 (5.9) | 0.050 |
| Post | 12.9 (6.1) | 13.1 (6.9) | 14.9 (5.1) | 0.020 | |
| PWithin-group | 0.06 | 0.15 | 0.80 | ||
| Shifting | Pre | 10.2 (3.9) | 10.0 (3.1) | 10.1 (2.2) | 0.80 |
| Post | 8.9 (3.2) | 10.1 (3.0) | 9.9 (4.1) | 0.30 | |
| PWithin-group | 0.02 | 0.50 | 0.90 | ||
| Emotional control | Pre | 15.8 (6.1) | 15.1 (6.3) | 15.9 (4.9) | 0.40 |
| Post | 12.9 (5.8) | 14.2 (7.1) | 15.8 (5.1) | 0.01 | |
| PWithin-group | <0.001 | 0.08 | 0.50 | ||
| Initiation | Pre | 12.9 (4.8) | 12.1 (4.1) | 12.8 (2.6) | 0.20 |
| Post | 9.8 (5.1) | 10.9 (5.2) | 12.9 (4.1) | 0.002 | |
| PWithin-group | <0.001 | 0.04 | 0.70 | ||
| Working memory | Pre | 18.8 (6.7) | 20.1 (5.3) | 18.9 (6.1) | 0.70 |
| Post | 11.2 (7.3) | 16.9 (6.8) | 17.8 (7.1) | <0.001 | |
| PWithin-group | <0.001 | <0.001 | 0.10 | ||
| Planning | Pre | 20.9 (7.1) | 19.8 (6.3) | 19.8 (6.1) | 0.30 |
| Post | 13.1 (5.1) | 16.9 (6.8) | 18.9 (6.1) | <0.001 | |
| PWithin-group | <0.001 | <0.001 | 0.20 | ||
| Organization | Pre | 9.1 (3.9) | 7.9 (5.1) | 8.9 (3.1) | 0.10 |
| Post | 8.9 (5.1) | 9.1 (5.2) | 9.0 (5.1) | 0.90 | |
| PWithin-group | 0.50 | 0.03 | 0.80 | ||
| Monitoring | Pre | 12.1 (5.2) | 12.9 (4.7) | 11.9 (4.1) | 0.20 |
| Post | 13.2 (5.0) | 11.1 (5.3) | 13.1 (5.1) | 0.01 | |
| PWithin-group | 0.20 | 0.003 | 0.30 |
| GODA | CODA | CON | PBetween-group | ||
|---|---|---|---|---|---|
| Color interference (s) | Pre | 6.1 (4.9) | 5.1 (4.1) | 5.0 (3.3) | 0.30 |
| Post | 4.9 (5.1) | 5.0 (5.3) | 5.1 (3.1) | 0.70 | |
| PWithin-group | 0.20 | 1.00 | 1.00 | ||
| Word interference (s) | Pre | 19.8 (9.9) | 19.9 (10.8) | 20.1 (10.3) | 1.00 |
| Post | 17.2 (7.8) | 19.1 (9.2) | 20.8 (11.6) | 0.01 | |
| PWithin-group | <0.001 | 0.10 | 0.50 |
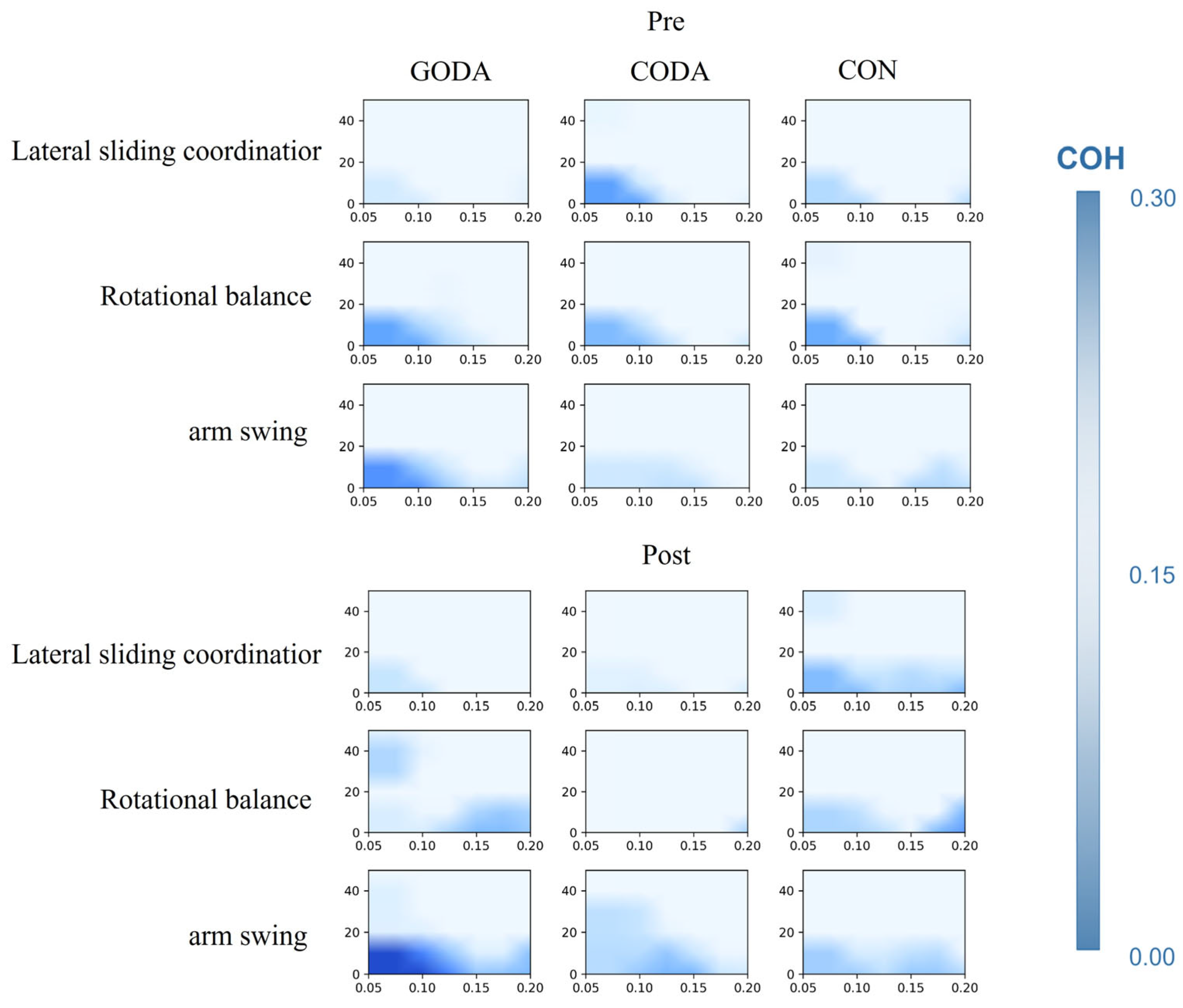
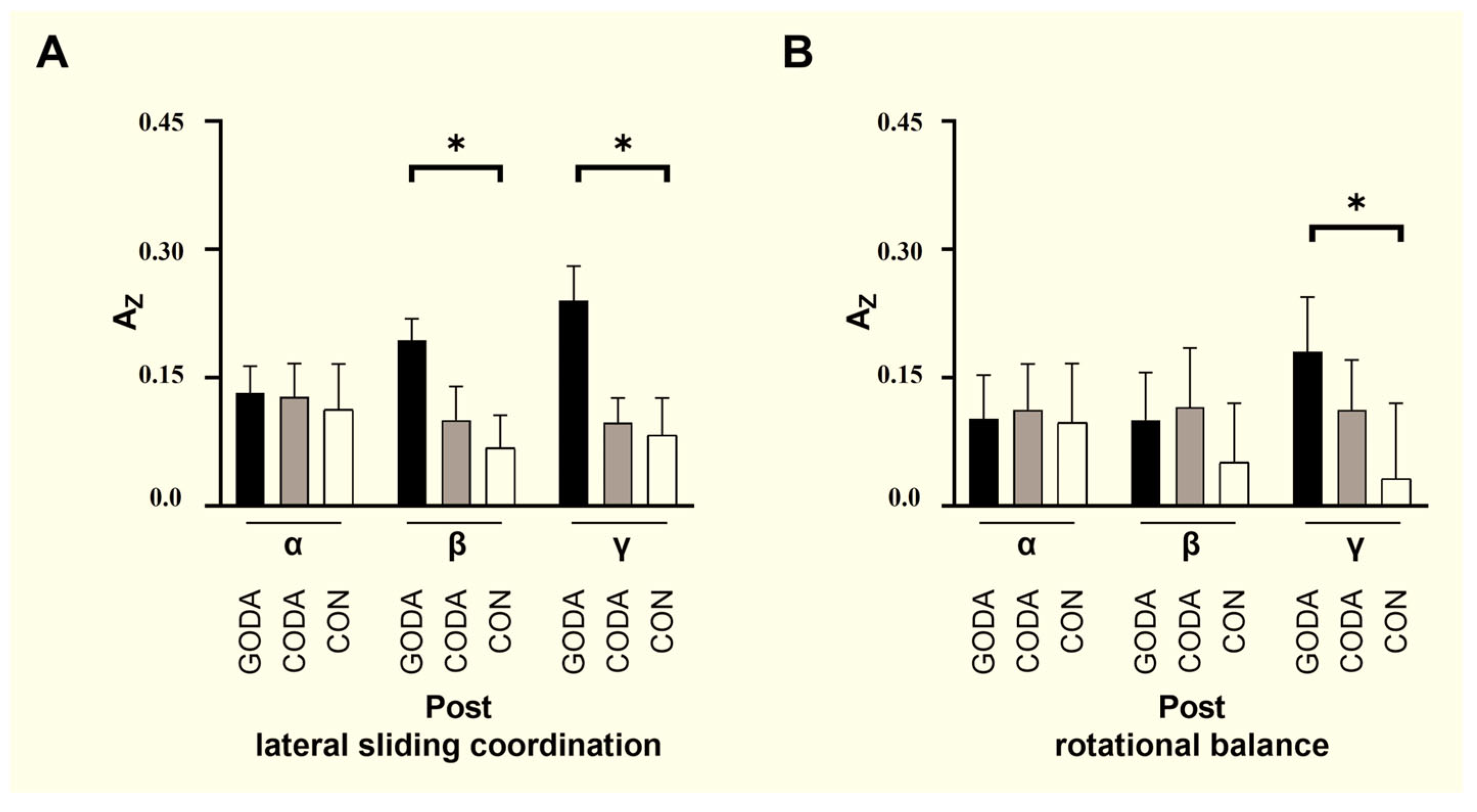
| Functional Connectivity of mPFC-lPFC | |
|---|---|
| Lateral sliding coordination: | |
| β-band COH | 0.013 |
| γ-band COH | 0.236 |
| Rotational balance: | |
| γ-band COH | 0.066 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, J.; Zhao, J.; Li, H.; He, J. Sensor-Based Assessment of Groove Music and Sports Dance on Cognitive–Emotional and Neuromuscular Functions in Older Adults. Sensors 2025, 25, 7162. https://doi.org/10.3390/s25237162
Ye J, Zhao J, Li H, He J. Sensor-Based Assessment of Groove Music and Sports Dance on Cognitive–Emotional and Neuromuscular Functions in Older Adults. Sensors. 2025; 25(23):7162. https://doi.org/10.3390/s25237162
Chicago/Turabian StyleYe, Jun, Junya Zhao, Haojie Li, and Jiao He. 2025. "Sensor-Based Assessment of Groove Music and Sports Dance on Cognitive–Emotional and Neuromuscular Functions in Older Adults" Sensors 25, no. 23: 7162. https://doi.org/10.3390/s25237162
APA StyleYe, J., Zhao, J., Li, H., & He, J. (2025). Sensor-Based Assessment of Groove Music and Sports Dance on Cognitive–Emotional and Neuromuscular Functions in Older Adults. Sensors, 25(23), 7162. https://doi.org/10.3390/s25237162





