Near-Infrared-II Fluorescence Imaging of Tumors with Organic Small-Molecule Fluorophores
Abstract
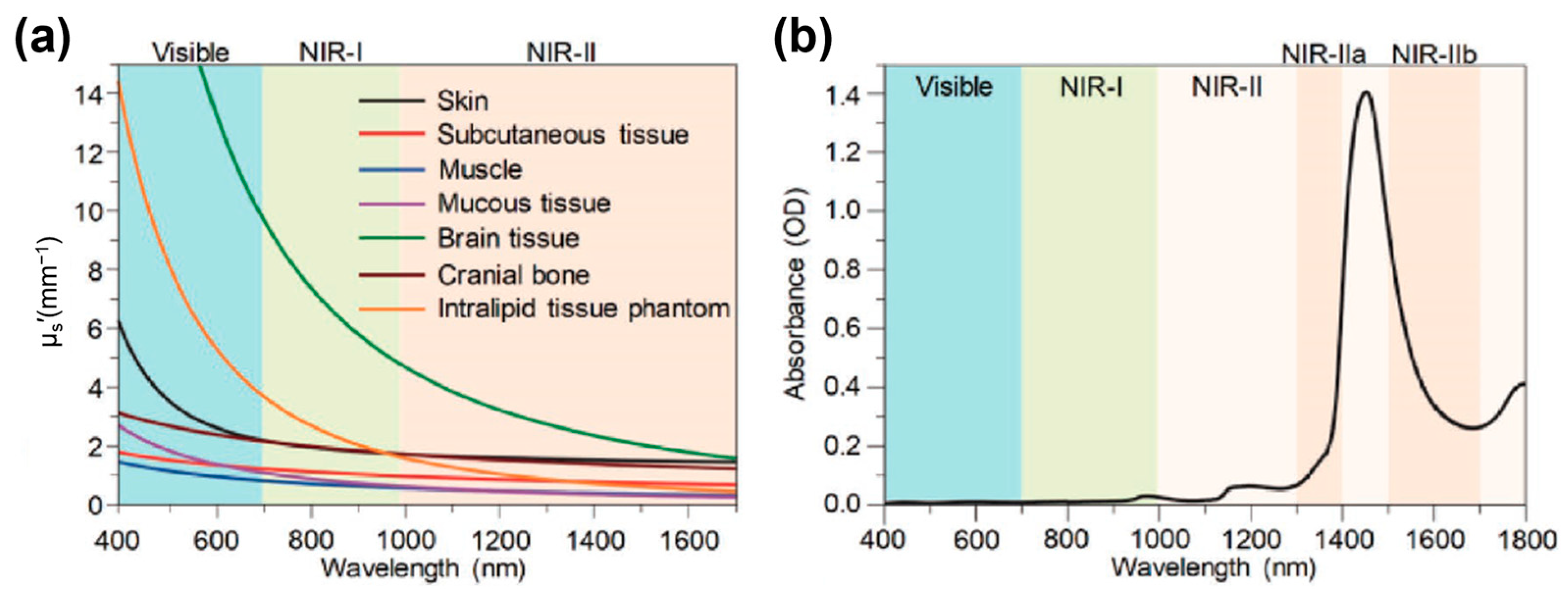
1. Introduction
2. NIR-II Organic Small-Molecule Fluorophores
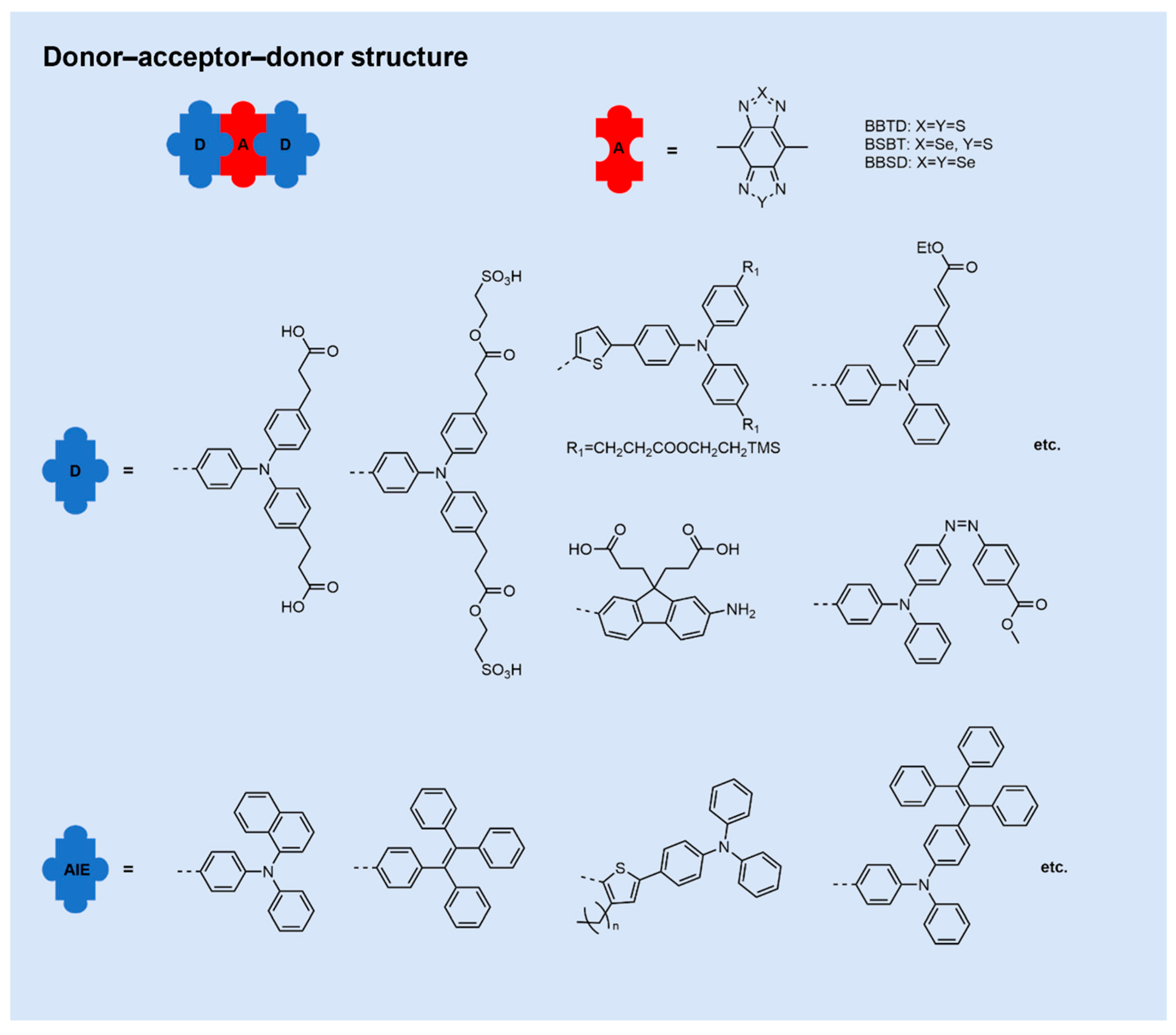
2.1. Donor–Acceptor–Donor NIR-II Small-Molecule Fluorophores
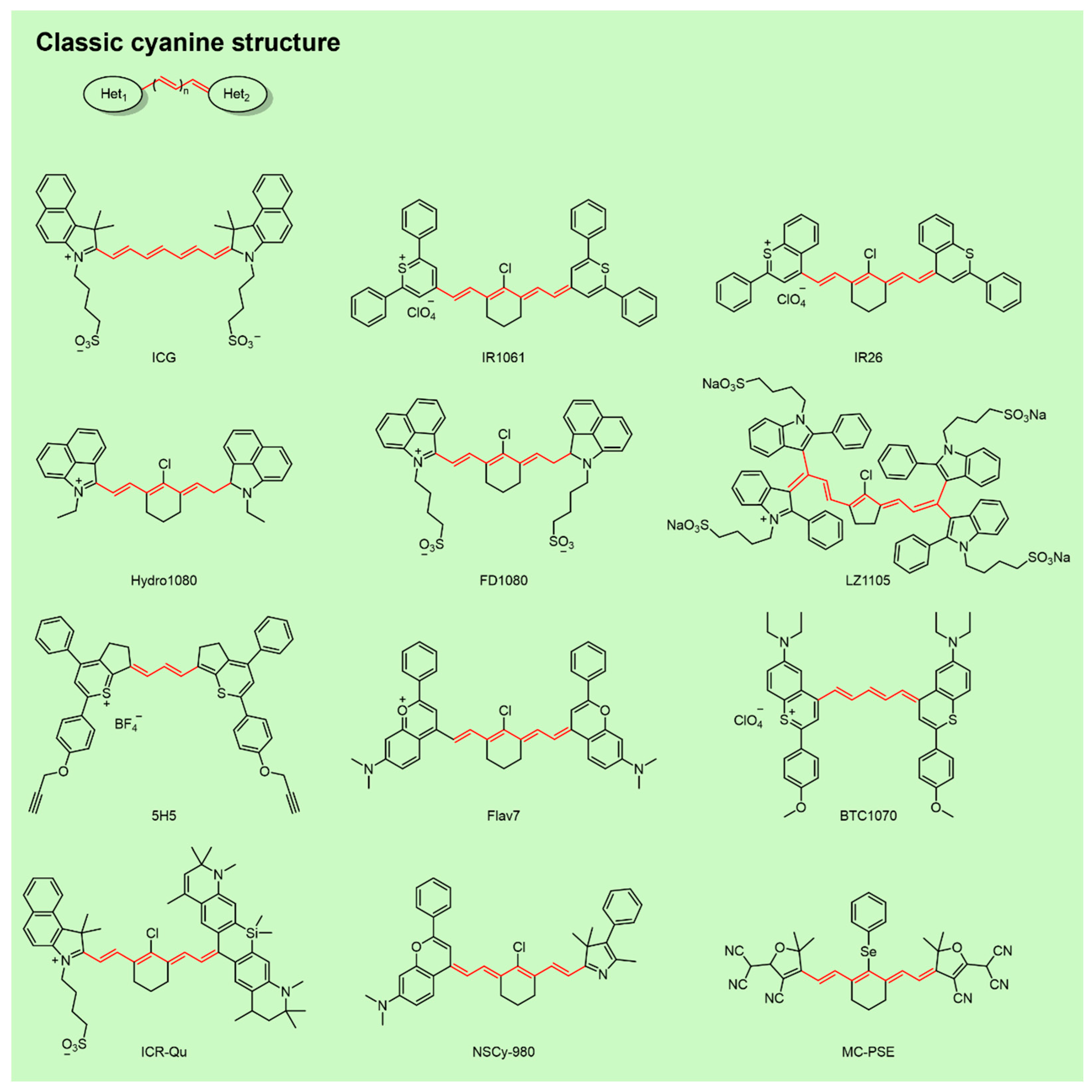
2.2. NIR-II Cyanine Fluorophores
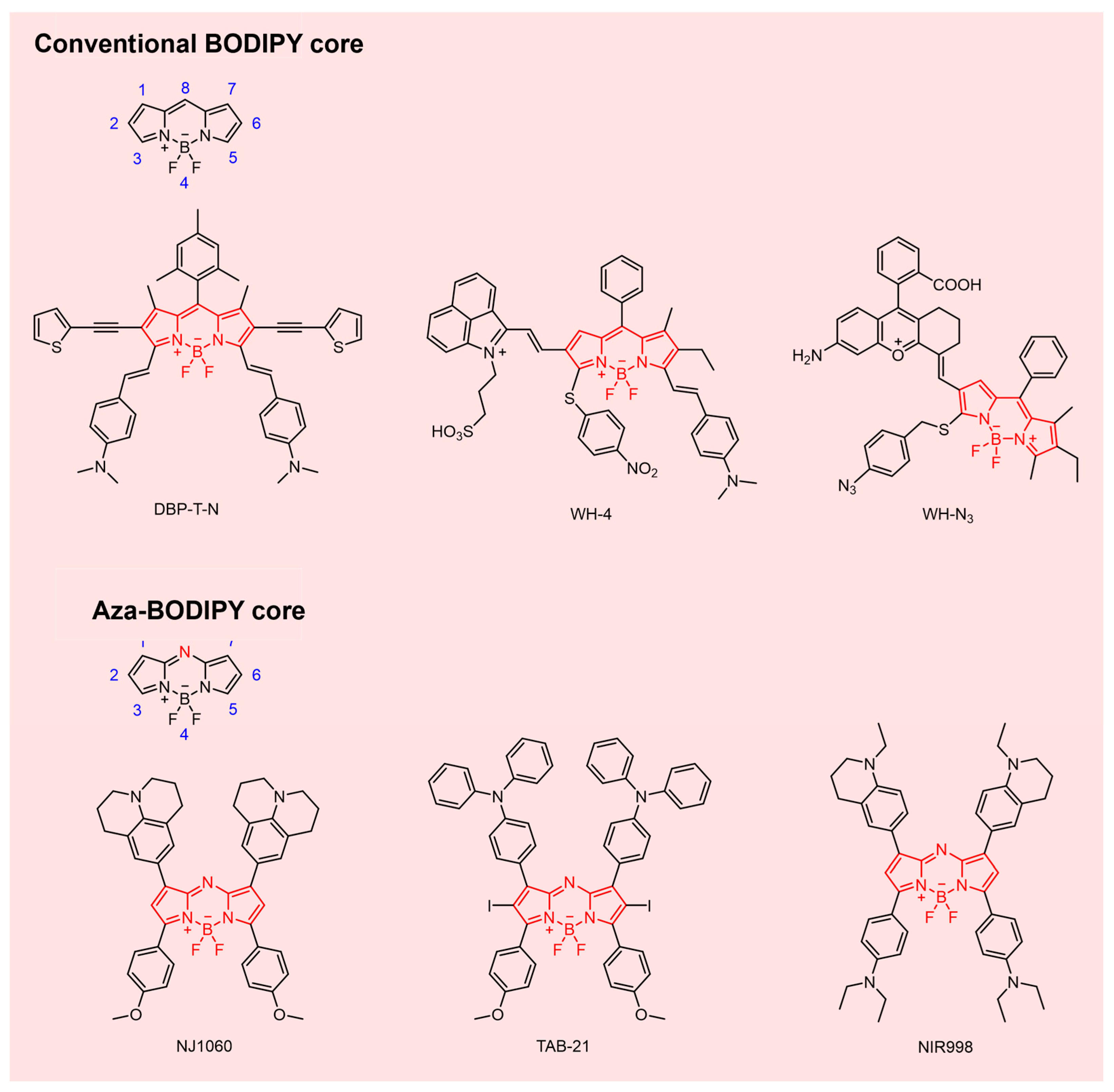
2.3. NIR-II BODIPY Fluorophores
2.4. NIR-II Xanthene Fluorophores
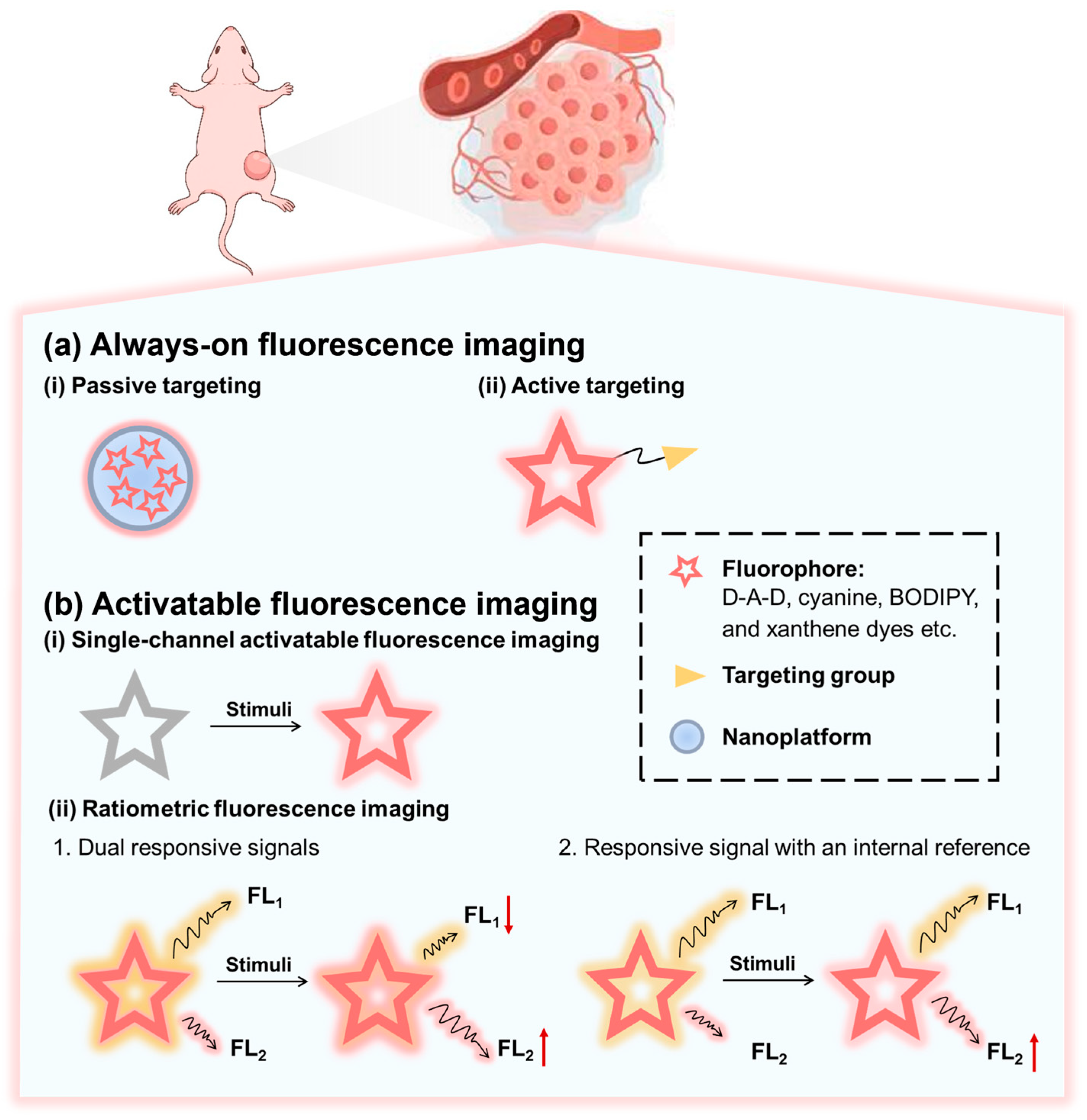
3. Design Strategy of NIR-II Fluorescent Probes Based on Organic Small-Molecule Fluorophores for Tumor Imaging
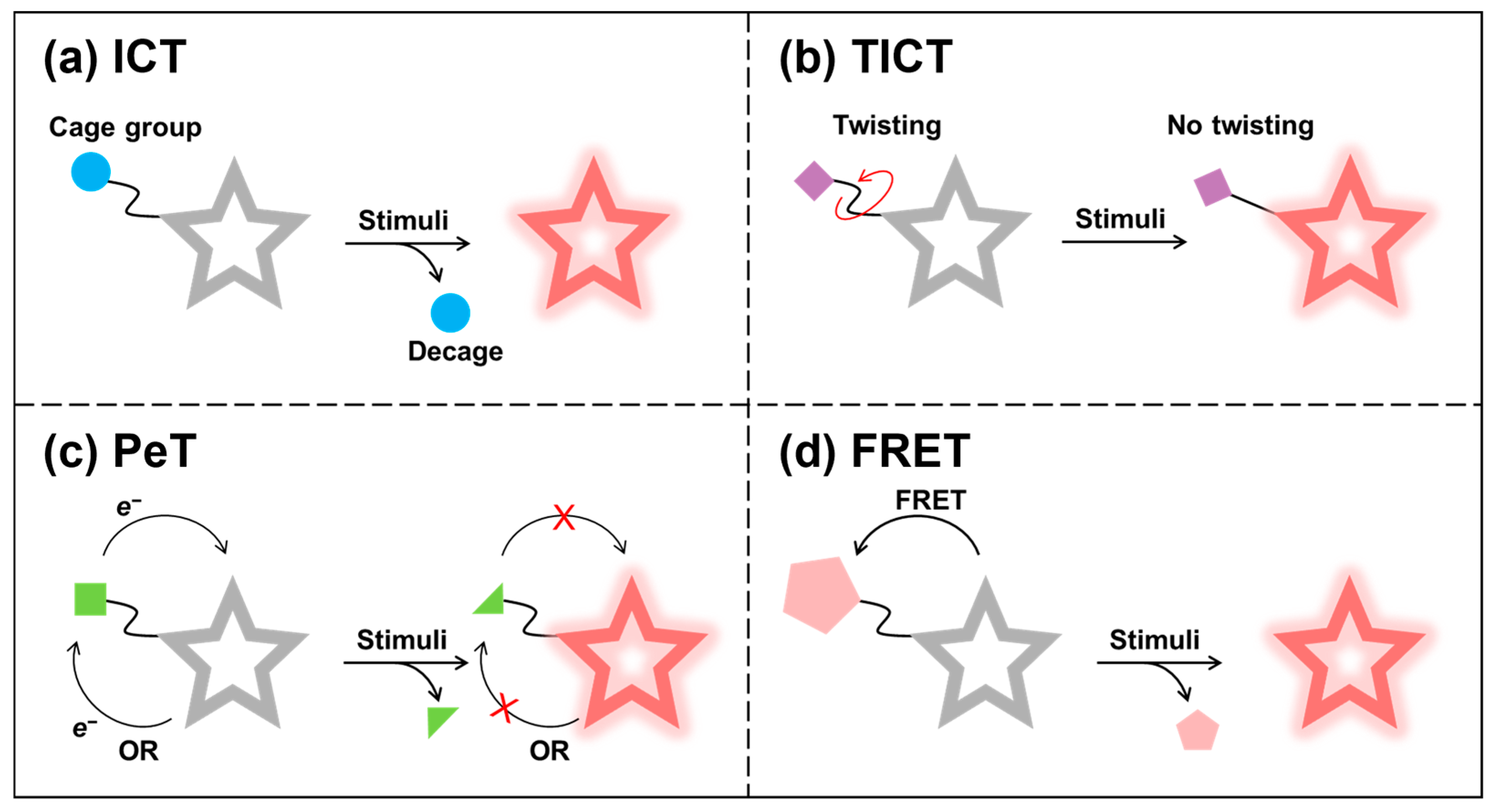
3.1. Always-On Fluorescence Imaging
3.2. Activatable Fluorescence Imaging
4. Advances in NIR-II Fluorescent Probes Based on Organic Small-Molecule Fluorophores for Tumor Imaging
4.1. Always-On NIR-II Fluorescent Probes
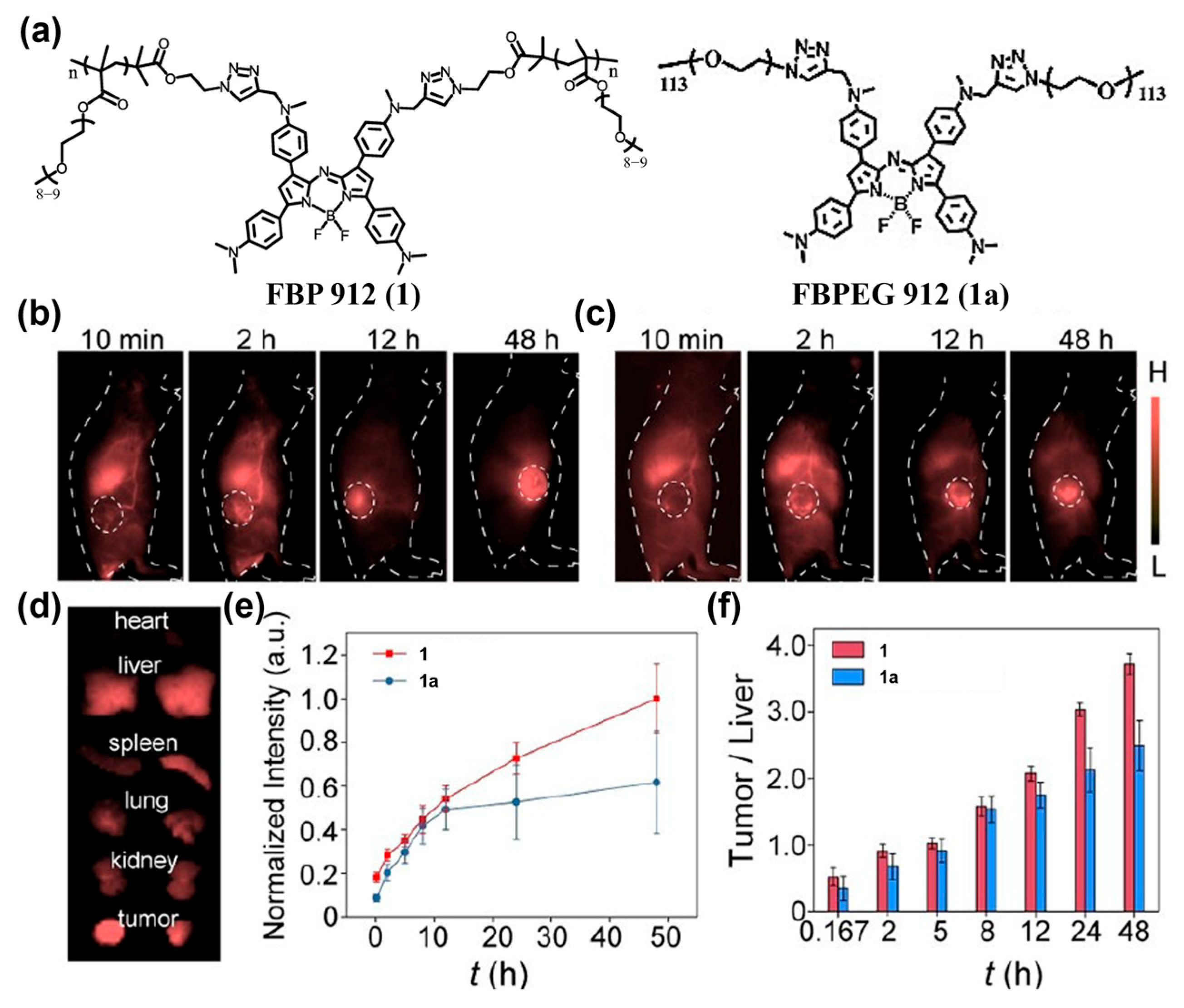
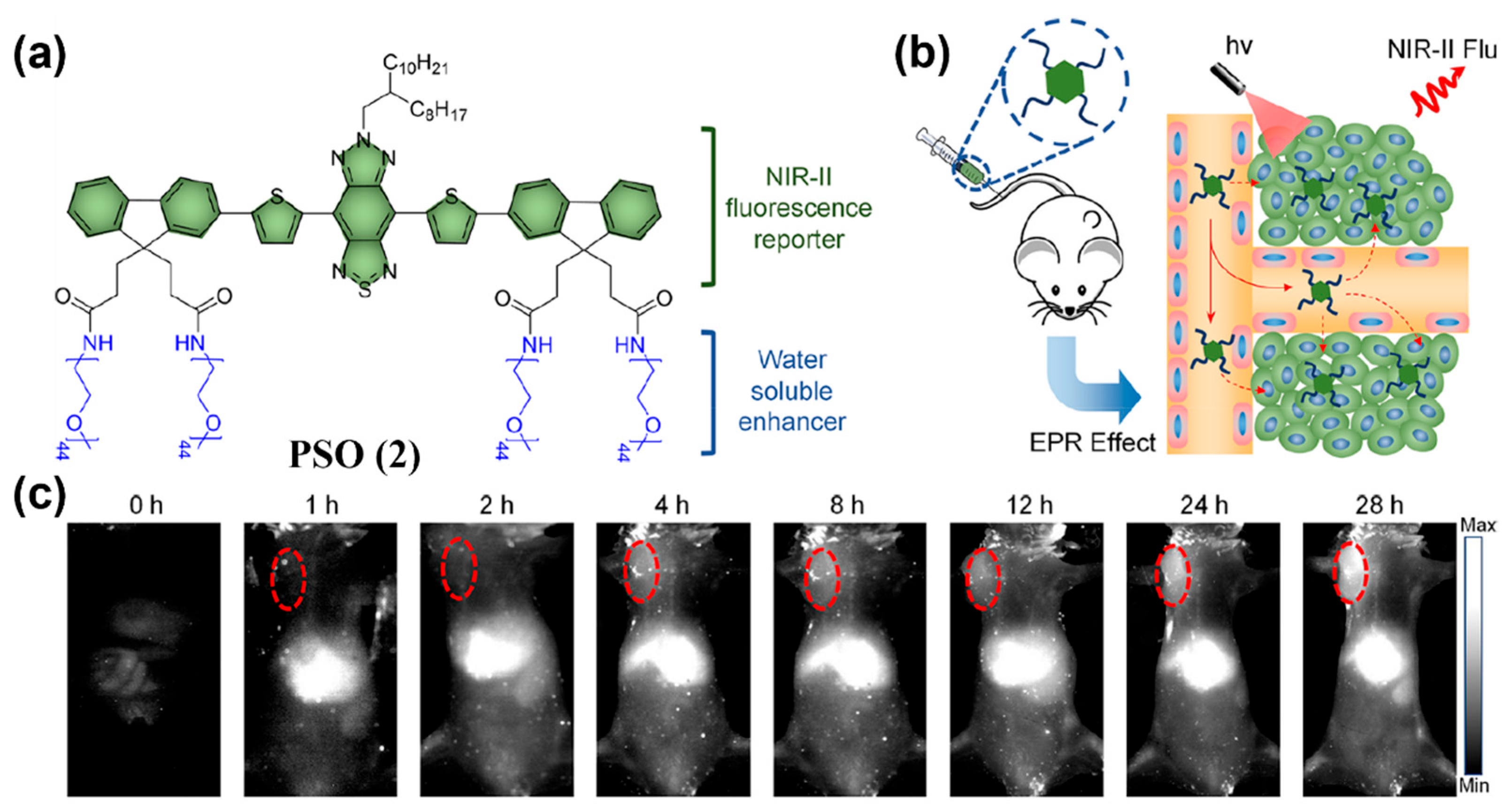
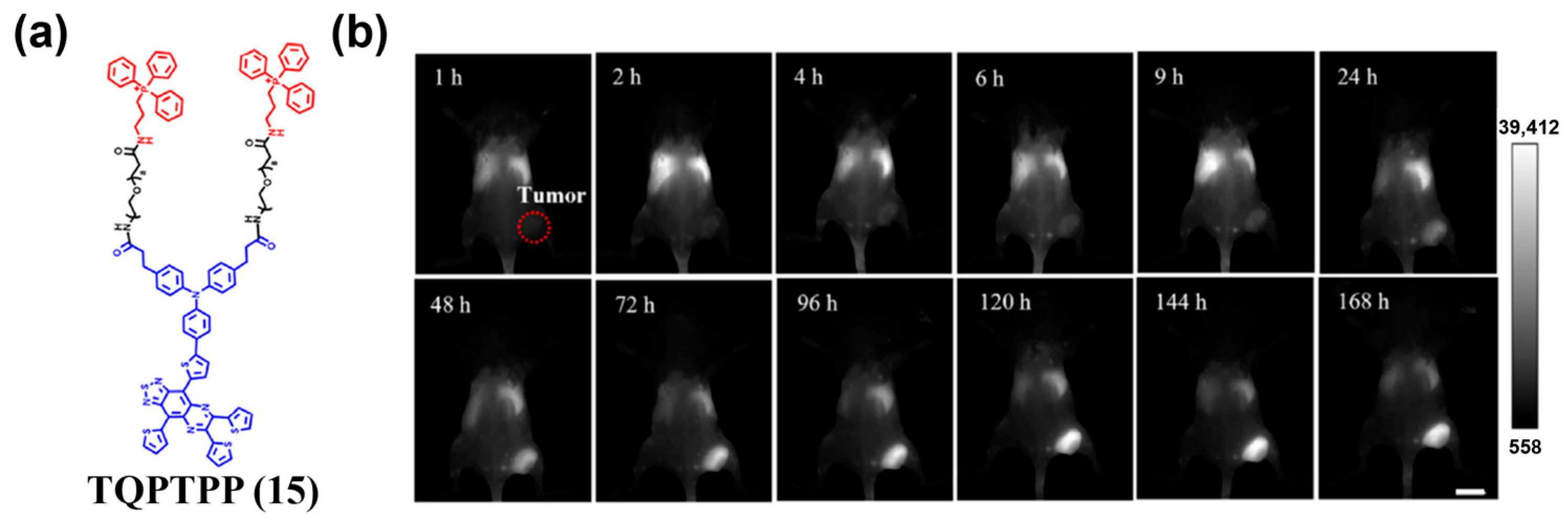
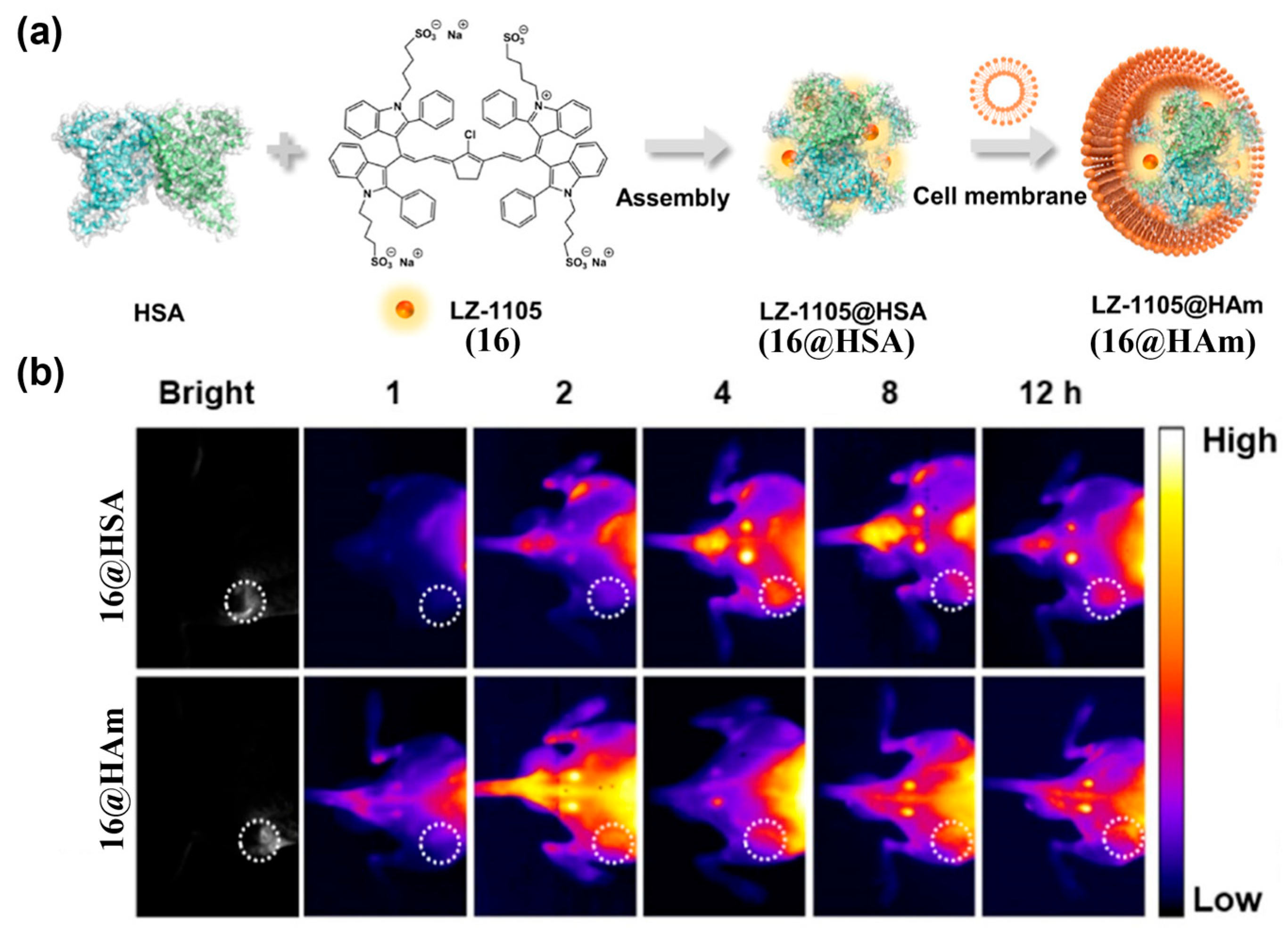
4.1.1. Passive Targeting Probes
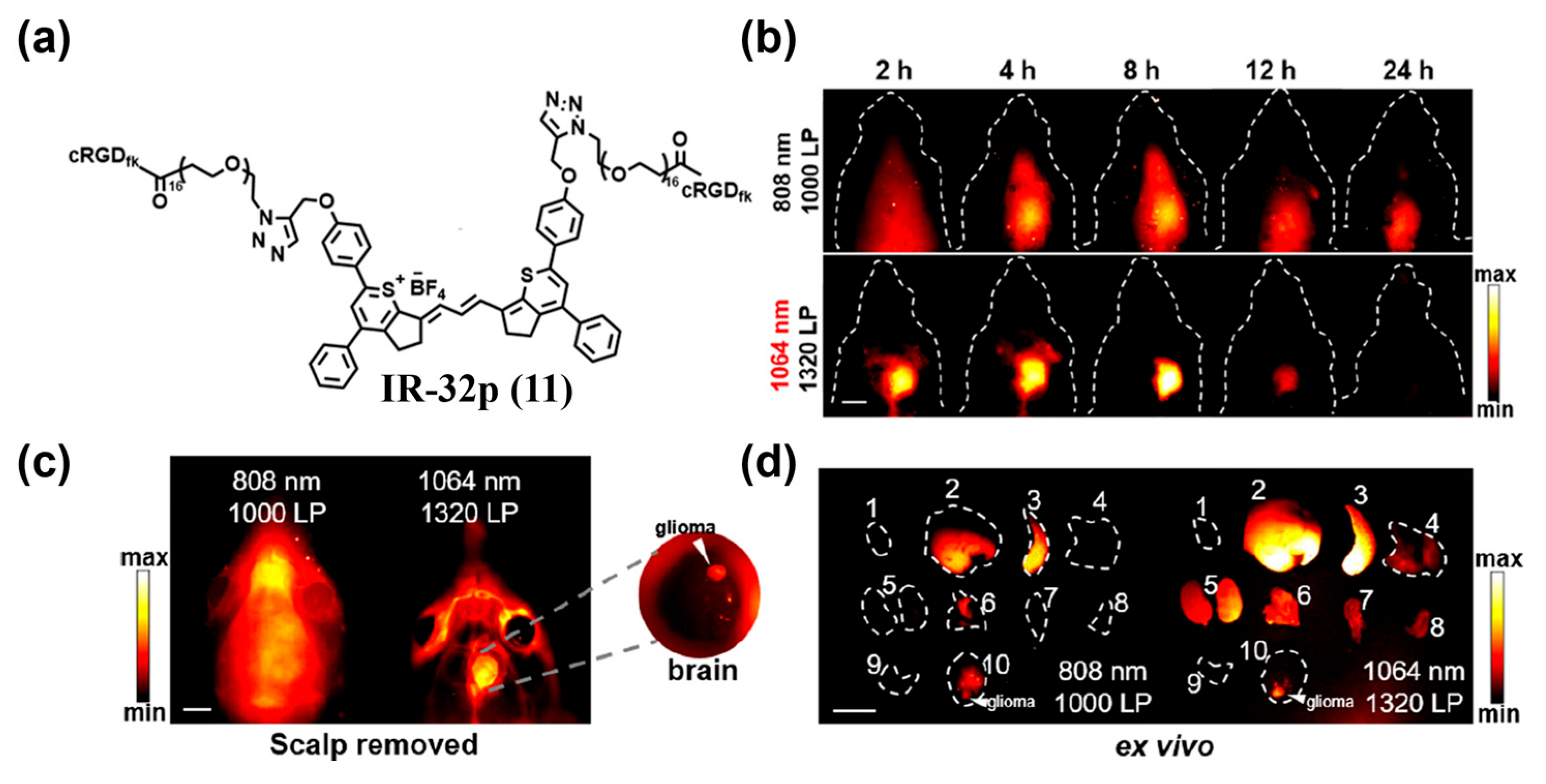
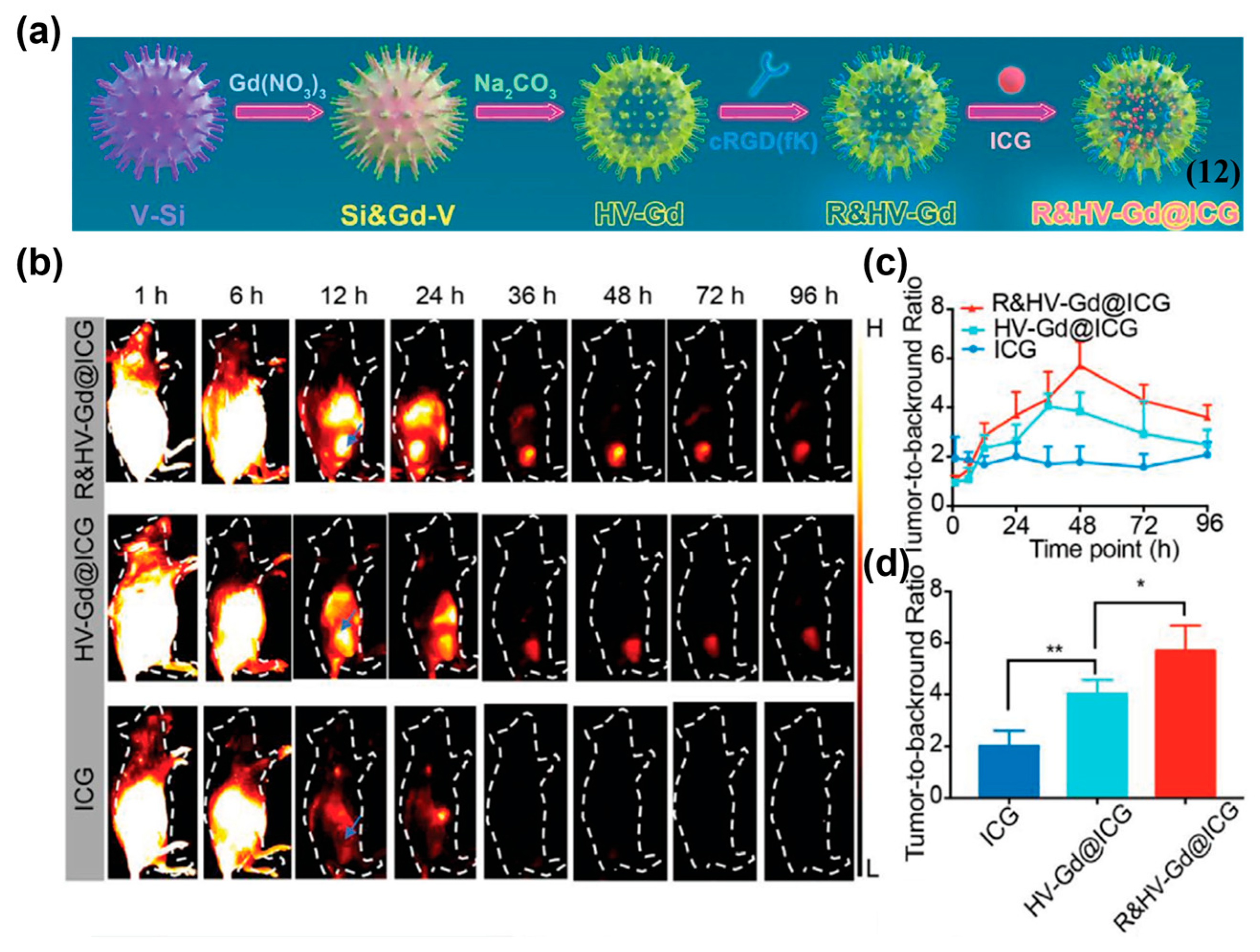
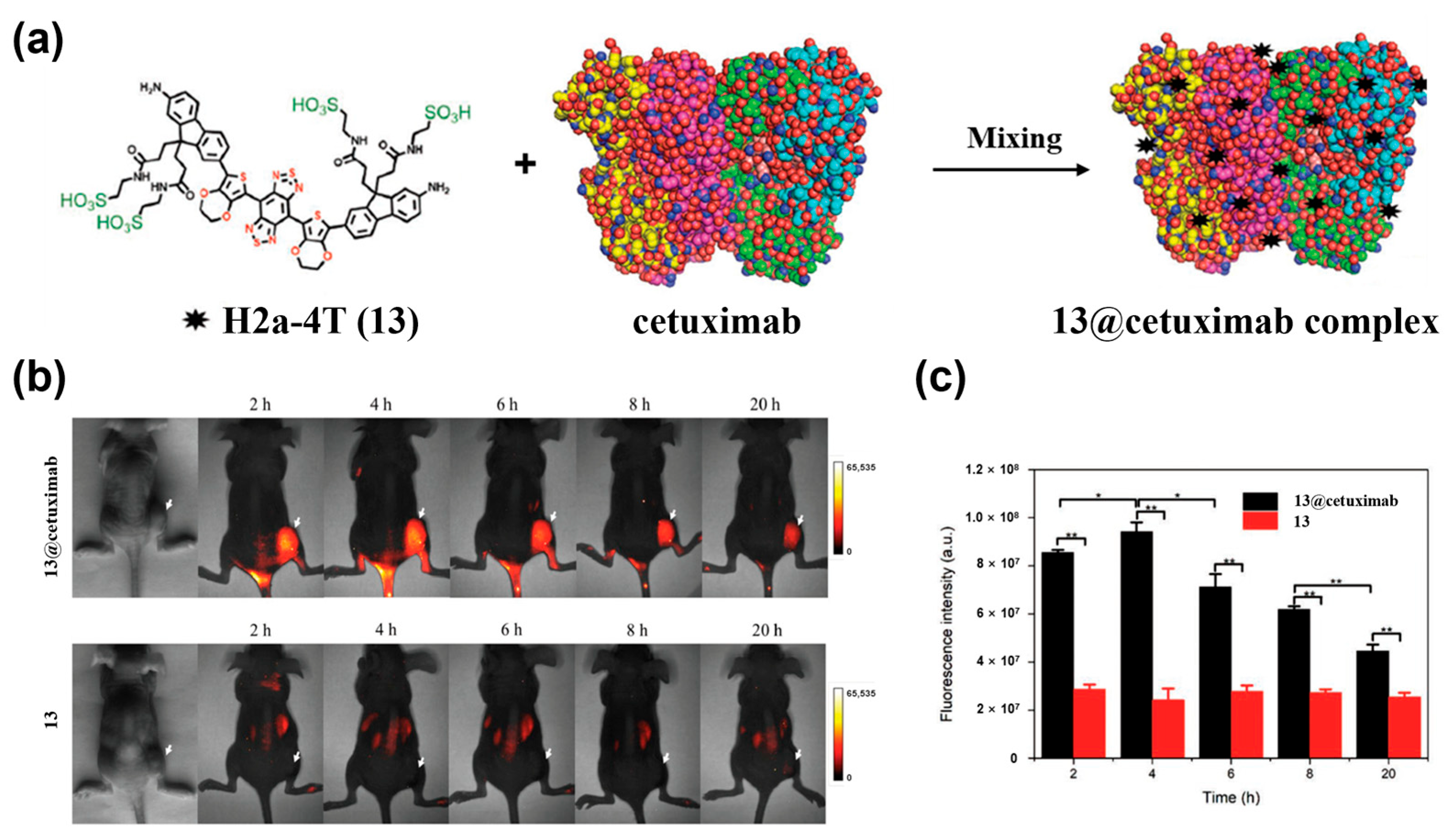
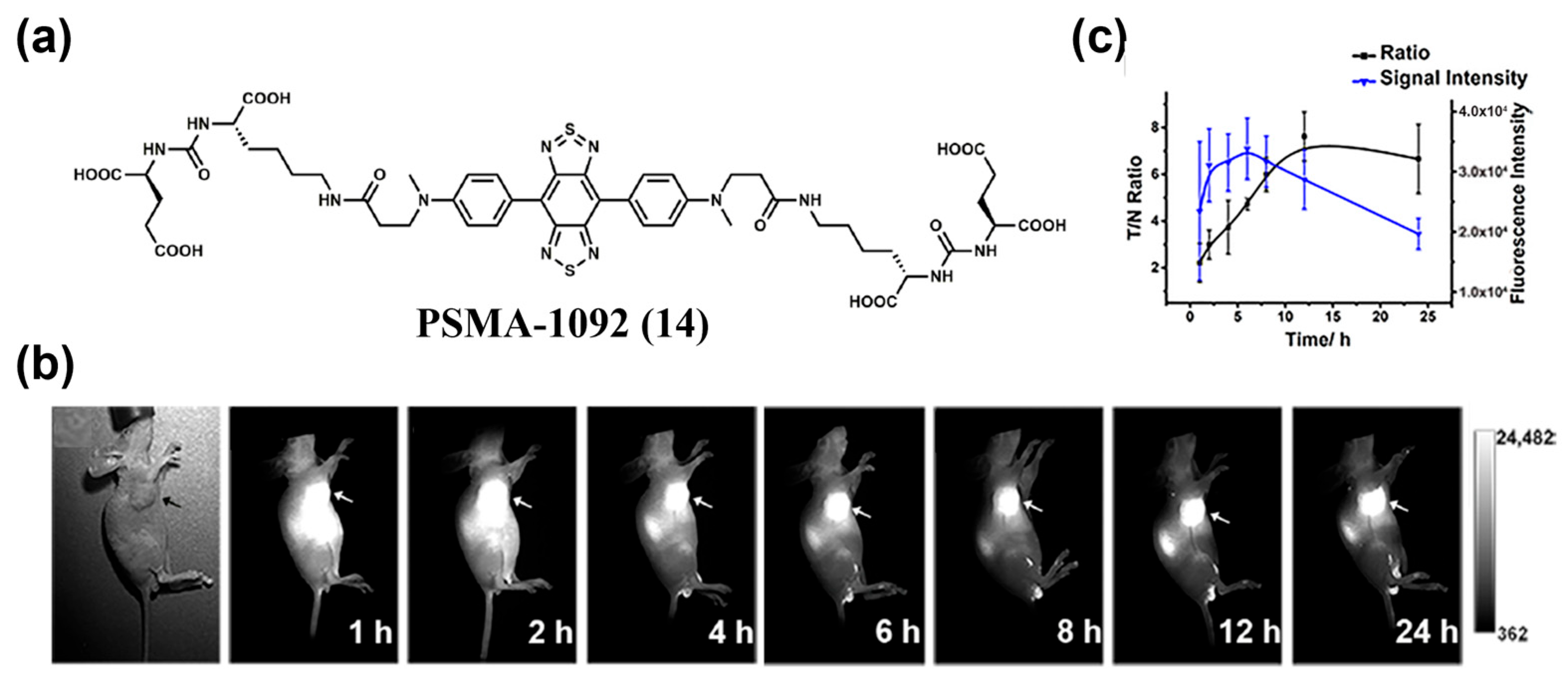
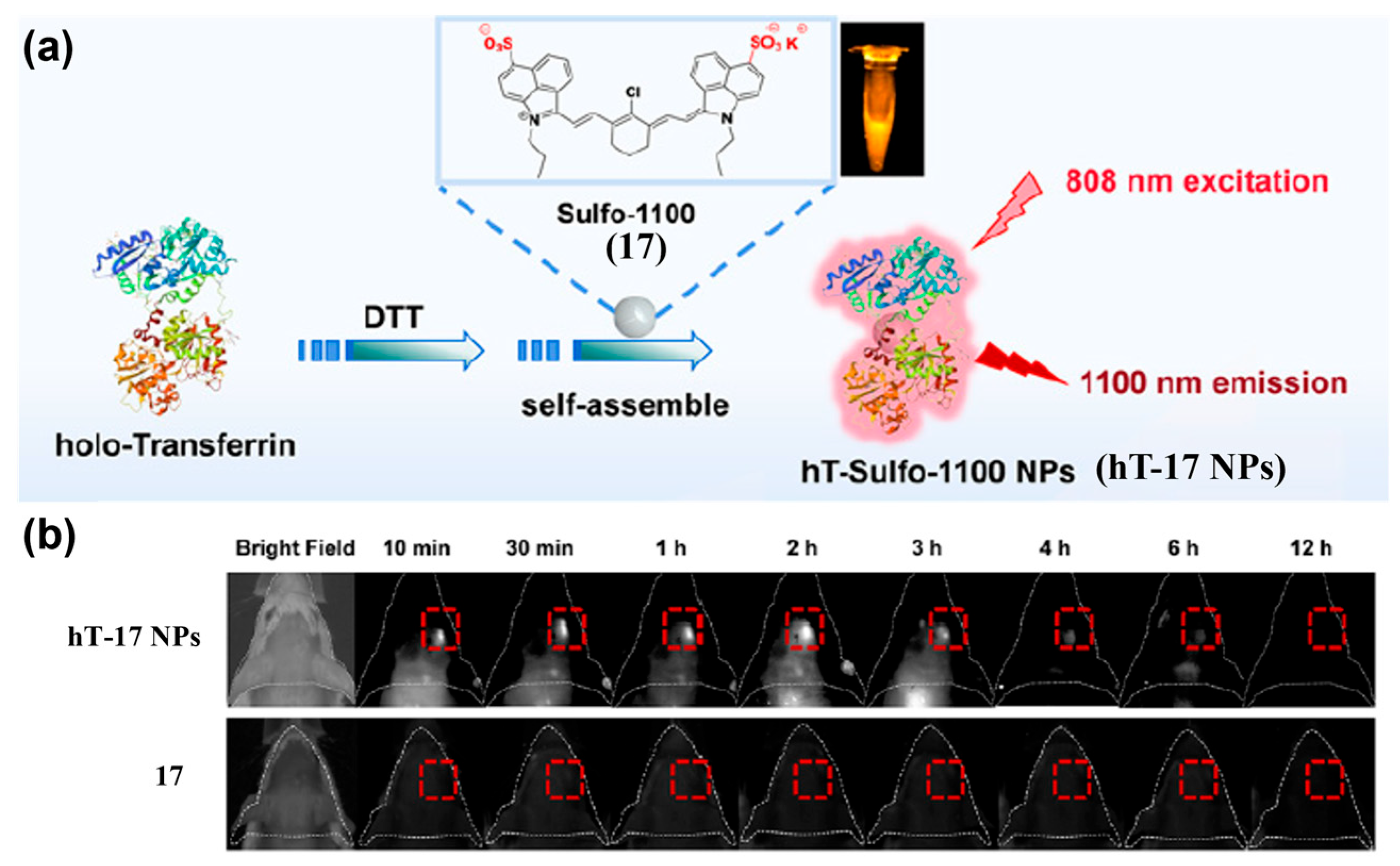
4.1.2. Active Targeting Probes
4.2. Activatable NIR-II Fluorescent Probes
4.2.1. Single-Channel Probes
Redox-Activated Probes
Enzyme-Activated Probes
PH-Activated Probes
Dual-Activated Probes
4.2.2. Ratiometric Probes
Dual-Wavelength Response Probes
Internal Reference Probes
5. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ansell, S.M.; Vonderheide, R.H. Cellular Composition of the Tumor Microenvironment. Am. Soc. Clin. Oncol. Educ. Book 2013, 31, e91–e97. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Yu, Y. Tumor Microenvironment--the Critical Element of Tumor Metastasis. Chin. J. Lung Cancer 2015, 18, 48–54. [Google Scholar]
- Wang, D.; Lu, J. Effects of Tumor Microenvironment on Tumor Angiogenesis. Tumor 2018, 38, 379–385. [Google Scholar]
- Satilmis, B.; Sahin, T.T.; Cicek, E.; Akbulut, S.; Yilmaz, S. Hepatocellular Carcinoma Tumor Microenvironment and Its Implications in Terms of Anti-Tumor Immunity: Future Perspectives for New Therapeutics. J. Gastrointest. Cancer 2021, 52, 1198–1205. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, W.; Li, J.; Zhang, Y. Optical Imaging of Tumor Microenvironment. Am. J. Nucl. Med. Molec. Imaging 2013, 3, 1–15. [Google Scholar]
- Yang, Y.; Jiang, Y.; Hu, X.; Shao, Z. Research Progress of Breast Tumor Microenvironment-Targeted Therapy. China Oncol. 2019, 29, 977–984. [Google Scholar]
- Yang, P.; Sun, Y.; Wang, Z.; Zheng, Y.; Chen, G.; Shi, Y. Research Progress on Effect of Tumor Acidic Microenvironment on Immune Cells. Chin. J. Immunol. 2021, 37, 613–617. [Google Scholar]
- Xiao, Y.; Yu, D. Tumor Microenvironment as A Therapeutic Target in Cancer. Pharmacol. Ther. 2021, 221, 107753. [Google Scholar] [CrossRef] [PubMed]
- Mieog, J.S.D.; Achterberg, F.B.; Zlitni, A.; Hutteman, M.; Burggraaf, J.; Swijnenburg, R.J.; Gioux, S.; Vahrmeijer, A.L. Fundamentals and Developments in Fluorescence-Guided Cancer Surgery. Nat. Rev. Clin. Oncol. 2022, 19, 9–22. [Google Scholar] [CrossRef]
- Tian, M.C.; Wu, R.L.G.; Xiang, C.H.; Niu, G.L.; Guan, W.J. Recent Advances in Fluorescent Probes for Cancer Biomarker Detection. Molecules 2024, 29, 1168. [Google Scholar] [CrossRef]
- Ijaz, M.; Hasan, I.; Aslam, B.; Yan, Y.Q.; Zeng, W.J.; Gu, J.S.; Jin, J.; Zhang, Y.H.; Wang, S.H.; Xing, L.; et al. Diagnostics of Brain Tumor in The Early Stage: Current Status and Future Perspectives. Biomater. Sci. 2025, 13, 2580–2605. [Google Scholar] [CrossRef]
- Komekbay, Z.; Shirazi, R.; Yessultanova, G.; Garifollin, A.; Tulyayeva, A.; Kereyeva, N.; Akhmetova, S.; Kaliev, A. Bibliometric Analysis of Tumor Marker Application in Gastric Cancer Diagnosis From 2019 To 2024. Front. Med. 2025, 12, 1547850. [Google Scholar] [CrossRef]
- Rosch, T.; Lorenz, R.; Braig, C.; Feuerbach, S.; Siewert, J.R.; Schusdziarra, V.; Classen, M. Endoscopic Ultrasound in Pancreatic Tumor-Diagnosis. Gastrointest. Endosc. 1991, 37, 347–352. [Google Scholar] [CrossRef]
- Raghavan, M. Conventional Modalities and Novel, Emerging Imaging Techniques for Musculoskeletal Tumors. Cancer Control 2017, 24, 161–171. [Google Scholar] [CrossRef]
- Savastano, L.E.; Zhou, Q.; Smith, A.; Vega, K.; Murga-Zamalloa, C.; Gordon, D.; McHugh, J.; Zhao, L.L.; Wang, M.M.; Pandey, A.; et al. Multimodal Laser-Based Angioscopy for Structural, Chemical and Biological Imaging of Atherosclerosis. Nat. Biomed. Eng. 2017, 1, 0023. [Google Scholar] [CrossRef]
- Ma, Y.Y.; Yan, C.X.; Guo, Z.Q.; Tan, G.; Niu, D.C.; Li, Y.S.; Zhu, W.H. Spatio-Temporally Reporting Dose-Dependent Chemotherapy via Uniting Dual-Modal MRI/NIR Imaging. Angew. Chem. Int. Ed. 2020, 59, 21143–21150. [Google Scholar] [CrossRef]
- Debs, P.; Ahlawat, S.; Fayad, L.M. Bone Tumors: State-of-the-Art Imaging. Skelet. Radiol. 2024, 53, 1783–1798. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.Y.; Lim, T.K.; Chong, V.; Huang, J. Segmentation and Visualization of Nasopharyngeal Carcinoma Using MRI. Comput. Biol. Med. 2003, 33, 407–424. [Google Scholar] [CrossRef] [PubMed]
- Rockall, A.G.; Reznek, R.H. Imaging of Neuroendocrine Tumours (CT/MR/US). Best Pract. Res. Clin. Endoc. Metab. 2007, 21, 43–68. [Google Scholar] [CrossRef] [PubMed]
- Budäus, L.; Leyh-Bannurah, S.R.; Salomon, G.; Michl, U.; Heinzer, H.; Huland, H.; Graefen, M.; Steuber, T.; Rosenbaum, C. Initial Experience of 68Ga-PSMA PET/CT Imaging in High-Risk Prostate Cancer Patients Prior to Radical Prostatectomy. Eur. Urol. 2016, 69, 393–396. [Google Scholar] [CrossRef]
- Cai, Q.Y.; Kim, S.H.; Choi, K.S.; Kim, S.Y.; Byun, S.J.; Kim, K.W.; Park, S.H.; Juhng, S.K.; Yoon, K.H. Colloidal Gold Nanoparticles as A Blood-Pool Contrast Agent for X-Ray Computed Tomography in Mice. Investig. Radiol. 2007, 42, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lee, J.M.; Lee, M.W.; Kim, G.H.; Han, J.K.; Choi, B.I. Sonography Transmission Gel as Endorectal Contrast Agent for Tumor Visualization in Rectal Cancer. Am. J. Roentgenol. 2008, 191, 186–189. [Google Scholar] [CrossRef]
- Hoogstins, C.E.S.; Handgraaf, H.J.M.; Boogerd, L.S.F.; Burggraaf, J.; Vahrmeijer, A.L. Image Guided Surgery Using Near-Infrared Fluorescence: Road to Clinical Translation of Novel Probes for Real Time Tumor Visualization. In Proceedings of the Conference on Molecular-Guided Surgery—Molecules, Devices, and Applications III, San Francisco, CA, USA, 28–30 January 2017. [Google Scholar]
- Digital Medical Association of Chinese Medical Association; Digital Intelligent Surgery Professional Committee of Chinese Research Hospital; Liver Cancer Professional Committee of Chinese Medical Doctor Association; Clinical Precise Medicine Professional Committee; Medical Imaging and Equipment Professional Committee of China Graphics Society; Molecular Imaging Professional Committee of China Biophysical Society. Guidelines for Application of Computer-Assisted Indocyanine Green Molecular Fluorescence Imaging in Diagnosis and Surgical Navigation of Liver Tumors (2019). J. South. Med. Univ. 2019, 39, 1127–1140. [Google Scholar]
- Meng, X.; Pang, X.; Zhang, K.; Gong, C.; Yang, J.; Dong, H.; Zhang, X. Recent Advances in Near-Infrared-II Fluorescence Imaging for Deep-Tissue Molecular Analysis and Cancer Diagnosis. Small 2022, 18, 2202035. [Google Scholar] [CrossRef]
- Jo, G.; Park, Y.; Park, M.H.; Hyun, H. Rational Design of a Small Molecular Near-Infrared Fluorophore for Improved In Vivo Fluorescence Imaging. Materials 2023, 16, 7227. [Google Scholar] [CrossRef]
- Lim, I.; Yu Lin, E.; Garcia, J.; Jia, S.; Sommerhalter, R.E.; Ghosh, S.K.; Gladysz, J.A.; Sletten, E.M. Shortwave Infrared Fluorofluorophores for Multicolor in Vivo Imaging. Angew. Chem. Int. Ed. 2023, 62, e202215200. [Google Scholar] [CrossRef] [PubMed]
- Meador, W.E.; Lin, E.Y.; Lim, I.; Friedman, H.C.; Ndaleh, D.; Shaik, A.K.; Hammer, N.I.; Yang, B.; Caram, J.R.; Sletten, E.M.; et al. Silicon-Rosindolizine Fluorophores with Shortwave Infrared Absorption and Emission Profiles Enable In Vivo Fluorescence Imaging. Nat. Chem. 2024, 16, 970–978. [Google Scholar] [CrossRef]
- Hu, C.; Liu, H.; Zhang, Z.; Li, L.; Mao, G.-j.; Cheng, W.; Zhou, L. A Self-Calibrating Fluorescent-Photoacoustic Integrated Probe Enables Fast Visualizing Pancreatic Cancer and Imaging-Guided Tumor Surgery. Small 2025, 21, 2408527. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Dang, Y.J.; Li, F.L.; Ding, C.Y.; Yu, H.J.; Zhang, W.; Xu, Z.A. Reactive Glycolysis Metabolite-Activatable Nanotheranostics for NIR-II Fluorescence Imaging-Guided Phototherapy of Cancer. Adv. Funct. Mater. 2022, 32, 2200016. [Google Scholar] [CrossRef]
- Ren, H.; Zeng, X.; Zhao, X.; Hou, D.; Yao, H.; Yaseen, M.; Zhao, L.; Xu, W.; Wang, H.; Li, L. A Bioactivated In Vivo Assembly Nanotechnology Fabricated NIR Probe for Small Pancreatic Tumor Intraoperative Imaging. Nat. Commun. 2022, 13, 418. [Google Scholar] [CrossRef]
- Song, J.W.; Wang, H.; Meng, X.; Li, W.; Qi, J. A Hypoxia-Activated and Microenvironment-Remodeling Nanoplatform for Multifunctional Imaging and Potentiated Immunotherapy of Cancer. Nat. Commun. 2024, 15, 10395. [Google Scholar] [CrossRef]
- Xing, H.; Han, M.; Zhang, T.; Zeng, G.; He, J.; Xu, Z.; Kang, Y.; Xue, P. Fluorescence-Switchable Iron-Doped Nanodot Assembly as a Robust Redox Dyshomeostasis Amplifier for Noninvasive Treatment of Deep-Seated Tumors. Adv. Healthc. Mater. 2025, 14, 2500083. [Google Scholar] [CrossRef]
- Wan, H.; Yue, J.; Zhu, S.; Uno, T.; Zhang, X.; Yang, Q.; Yu, K.; Hong, G.; Wang, J.; Li, L.; et al. A Bright Organic NIR-II Nanofluorophore for Three-Dimensional Imaging into Biological Tissues. Nat. Commun. 2018, 9, 1171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, L.; Ren, Y.; Li, M.; Tang, Y. Organic NIR-II Nanofluorophore with Ultrahigh Quantum Yield for Vessels Imaging and Fluorescence Image-Guided Surgery. Adv. Funct. Mater. 2025, 35, 2413341. [Google Scholar] [CrossRef]
- Kenry; Duan, Y.; Liu, B. Recent Advances of Optical Imaging in the Second Near-Infrared Window. Adv. Mater. 2018, 30, 1802394. [Google Scholar] [CrossRef]
- Tang, Y.; Li, Y.; Hu, X.; Zhao, H.; Ji, Y.; Chen, L.; Hu, W.; Zhang, W.; Li, X.; Lu, X.; et al. “Dual Lock-and-Key”-Controlled Nanoprobes for Ultrahigh Specific Fluorescence Imaging in the Second Near-Infrared Window. Adv. Mater. 2018, 30, 1801140. [Google Scholar] [CrossRef]
- Lu, L.; Li, B.; Ding, S.; Fan, Y.; Wang, S.; Sun, C.; Zhao, M.; Zhao, C.; Zhang, F. NIR-II Bioluminescence for In Vivo High Contrast Imaging and In Situ ATP-Mediated Metastases Tracing. Nat. Commun. 2020, 11, 4192. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lu, Z.; Zhang, J.; Chen, L.; Tang, X.; Jiang, Q.; Hu, Q.; Li, L.; Liu, J.; Huang, W. Near-Infrared-II Fluorophore with Inverted Dependence of Fluorescence Quantum Yield on Polarity as Potent Phototheranostics for Fluorescence-Image-Guided Phototherapy of Tumors. Adv. Mater. 2023, 35, 2209647. [Google Scholar] [CrossRef]
- Sun, Z.; Li, T.; Wu, F.; Yao, T.; Yang, H.; Yang, X.; Yin, H.; Gao, Y.; Zhang, Y.; Li, C.; et al. Precise Synergistic Photothermal Therapy Guided by Accurate Temperature-Dependent NIR-II Fluorescence Imaging. Adv. Funct. Mater. 2024, 34, 2311622. [Google Scholar] [CrossRef]
- Xu, H.; Yuan, L.; Shi, Q.; Tian, Y.; Hu, F. Ultrabright NIR-II Nanoprobe for Image-Guided Accurate Resection of Tiny Metastatic Lesions. Nano Lett. 2024, 24, 1367–1375. [Google Scholar] [CrossRef]
- Yan, D.; Zhang, Z.; Zhang, J.; Li, X.; Wu, Q.; Gui, Y.; Zhu, J.; Kang, M.; Chen, X.; Tang, B.; et al. An All-Rounder for NIR-II Phototheranostics: Well-Tailored 1064 nm-Excitable Molecule for Photothermal Combating of Orthotopic Breast Cancer. Angew. Chem. Int. Ed. 2024, 63, e202401877. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qu, F.; Wan, F.; Zhong, C.; Rao, J.; Liu, Y.; Li, Z.; Zhu, J.; Li, Z. Aggregation Control of Anionic Pentamethine Cyanine Enabling Excitation Wavelength Selective NIR-II Fluorescence Imaging-Guided Photodynamic Therapy. Nat. Commun. 2025, 16, 762. [Google Scholar] [CrossRef]
- Tang, Y.; Li, Y.; He, C.; Wang, Z.; Huang, W.; Fan, Q.; Liu, B. NIR-II-Excited Off-On-Off Fluorescent Nanoprobes for Sensitive Molecular Imaging In Vivo. Nat. Commun. 2025, 16, 278. [Google Scholar] [CrossRef]
- Jiang, Y.; Pu, K. Dual-Targeted Molecular Fluorescence and Photoacoustic Imaging in the Second Near-Infrared Optical Window Using Organic Contrast Agents. Adv. Biosys. 2018, 2, 1700262. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Y.; Li, C.; Huang, D.; Wang, Q.; Wang, Q. Recent Advances in Tracking the Transplanted Stem Cells Using Near-Infrared Fluorescent Nanoprobes: Turning from the First to the Second Near-Infrared Window. Adv. Healthc. Mater. 2018, 7, 1800497. [Google Scholar] [CrossRef]
- Ceppi, L.; Bardhan, N.M.; Na, Y.; Siegel, A.; Rajan, N.; Fruscio, R.; Del Carmen, M.G.; Belcher, A.M.; Birrer, M.J. Real-Time Single-Walled Carbon Nanotube-Based Fluorescence Imaging Improves Survival after Debulking Surgery in an Ovarian Cancer Model. ACS Nano 2019, 13, 5356–5365. [Google Scholar] [CrossRef] [PubMed]
- Zebibula, A.; Alifu, N.; Xia, L.; Sun, C.; Yu, X.; Xue, D.; Liu, L.; Li, G.; Qian, J. Ultrastable and Biocompatible NIR-II Quantum Dots for Functional Bioimaging. Adv. Funct. Mater. 2018, 28, 2200386. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, H.; An, X.; Wang, Z.; Yang, X.; Yu, M.; Zhang, R.; Sun, Z.; Wang, Q. Controlled Synthesis of Ag2Te@Ag2S Core-Shell Quantum Dots with Enhanced and Tunable Fluorescence in the Second Near-Infrared Window. Small 2020, 16, 2001003. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, L.; Wang, Z.; Liu, S.; Pang, D. Near-Infrared-II Quantum Dots for In Vivo Imaging and Cancer Therapy. Small 2022, 18, 2104567. [Google Scholar] [CrossRef]
- Zhong, Y.; Ma, Z.; Zhu, S.; Yue, J.; Zhang, M.; Antaris, A.L.; Yuan, J.; Cui, R.; Wan, H.; Zhou, Y.; et al. Boosting the Down-Shifting Luminescence of Rare-Earth Nanocrystals for Biological Imaging Beyond 1500 nm. Nat. Commun. 2017, 8, 737. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Chen, H.; Xie, X.; Wan, Y.; Li, Q.; Wu, F.; Yang, R.; Wang, W.; Kong, X. Bright Tm3+-Based Downshifting Luminescence Nanoprobe Operating Around 1800 nm for NIR-IIb and C Bioimaging. Nat. Commun. 2023, 14, 1079. [Google Scholar] [CrossRef]
- Tong, L.; Cao, J.; Wang, K.; Song, J.; Mu, J. Lanthanide-Doped Nanomaterials for Tumor Diagnosis and Treatment by Second Near-Infrared Fluorescence Imaging. Adv. Opt. Mater. 2024, 12, 2301767. [Google Scholar] [CrossRef]
- Dai, H.; Shen, Q.; Shao, J.; Wang, W.; Gao, F.; Dong, X. Small Molecular NIR-II Fluorophores for Cancer Phototheranostics. Innovation 2021, 2, 100082. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, D.; Zhu, A.; Ding, M.; Yu, N.; Li, J. Neutrophil-Targeting Semiconducting Polymer Nanotheranostics for NIR-II Fluorescence Imaging-Guided Photothermal-NO-Immunotherapy of Orthotopic Glioblastoma. Adv. Sci. 2024, 11, 2406750. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, W.; Shao, J.; Chen, J.; Dong, X. Small Molecular Cyanine Dyes for Phototheranostics. Coord. Chem. Rev. 2024, 516, 215986. [Google Scholar] [CrossRef]
- Mei, A.; He, X.; Lei, D.; Wang, L.; Wang, W.; Shao, J.; Shen, Q.; Jiang, F.; Dong, X. Squaraine-Based NIR Dyes for Phototheranostics. Coord. Chem. Rev. 2025, 527, 216419. [Google Scholar] [CrossRef]
- Yang, Q.; Hu, Z.; Zhu, S.; Ma, R.; Ma, H.; Ma, Z.; Wan, H.; Zhu, T.; Jiang, Z.; Liu, W.; et al. Donor Engineering for NIR-II Molecular Fluorophores with Enhanced Fluorescent Performance. J. Am. Chem. Soc. 2018, 140, 1715–1724. [Google Scholar] [CrossRef]
- Tian, R.; Ma, H.; Zhu, S.; Lau, J.; Ma, R.; Liu, Y.; Lin, L.; Chandra, S.; Wang, S.; Zhu, X.; et al. Multiplexed NIR-II Probes for Lymph Node-Invaded Cancer Detection and Imaging-Guided Surgery. Adv. Mater. 2020, 32, 1907365. [Google Scholar] [CrossRef]
- Mu, J.; Xiao, M.; Shi, Y.; Geng, X.; Li, H.; Yin, Y.; Chen, X. The Chemistry of Organic Contrast Agents in the NIR-II Window. Angew. Chem. Int. Ed. 2022, 61, e202114722. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Feng, X.; Wei, L.; Dai, D.; Ma, Y.; Pan, H.; Ge, S.; Bai, L.; Ke, C.; Liu, Y.; et al. A Genetic Engineering Strategy for Editing Near-Infrared-II Fluorophores. Nat. Commun. 2022, 13, 2853. [Google Scholar] [CrossRef]
- Gao, D.; Luo, Z.; He, Y.; Yang, L.; Hu, D.; Liang, Y.; Zheng, H.; Liu, X.; Sheng, Z. Low-Dose NIR-II Preclinical Bioimaging Using Liposome-Encapsulated Cyanine Dyes. Small 2023, 19, 2206544. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Fang, C.; Li, B.; Zhang, Z.; Cao, C.; Cai, M.; Su, S.; Sun, X.; Shi, X.; Li, C.; et al. First-in-Human Liver-Tumor Surgery Guided by Multispectral Fluorescence Imaging in The Visible and Near-Infrared-I/II Windows. Nat. Biomed. Eng. 2020, 4, 259–271. [Google Scholar] [CrossRef]
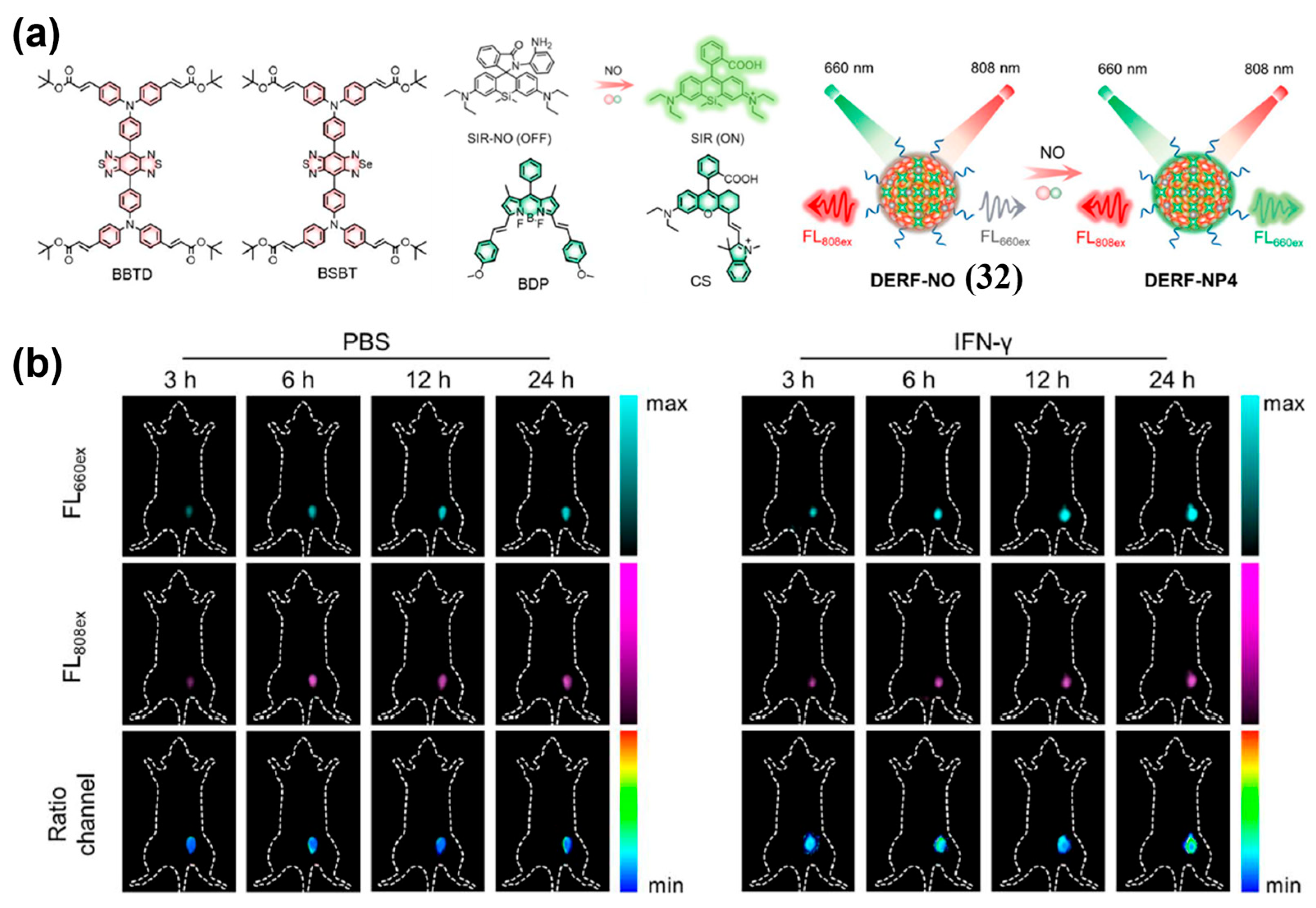
- Li, B.; Tian, J.; Wu, C.; Li, Z.; Qiao, L.; Xie, Z.; Song, B.; Shan, Y.; Chen, S.; Tang, Y.; et al. Nitric Oxide-Activated Bioorthogonal Codelivery Nanoassembly for In Situ Synthesis of Photothermal Agent for Precise and Safe Anticancer Treatment. Adv. Mater. 2024, 36, 2405502. [Google Scholar] [CrossRef]
- Wang, L.; Li, N.; Wang, W.; Mei, A.; Shao, J.; Wang, W.; Dong, X. Benzobisthiadiazole-Based Small Molecular Near-Infrared-II Fluorophores: From Molecular Engineering to Nanophototheranostics. ACS Nano 2024, 18, 4683–4703. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, Q.; Wang, X.; Lin, W. Biomedical Applications of NIR-II Organic Small Molecule Fluorescent Probes in Different Organs. Coord. Chem. Rev. 2024, 519, 216122. [Google Scholar] [CrossRef]
- Zhao, X.; Qin, Z.; Zhang, X.; Yuan, L. Research Progress in Activatable NIR-Ⅱ Small Molecule Fluorescent Probes. Chin. J. Appl. Chem. 2024, 41, 39–59. [Google Scholar]
- Antaris, A.L.; Chen, H.; Cheng, K.; Sun, Y.; Hong, G.; Qu, C.; Diao, S.; Deng, Z.; Hu, X.; Zhang, B.; et al. A Small-Molecule Dye for NIR-II Imaging. Nat. Mater. 2016, 15, 235–242. [Google Scholar] [CrossRef]
- Zeng, X.; Xiao, Y.; Lin, J.; Li, S.; Zhou, H.; Nong, J.; Xu, G.; Wang, H.; Xu, F.; Wu, J.; et al. Near-Infrared II Dye-Protein Complex for Biomedical Imaging and Imaging-Guided Photothermal Therapy. Adv. Healthc. Mater. 2018, 7, 1800589. [Google Scholar] [CrossRef]
- Li, B.; Lu, L.; Zhao, M.; Lei, Z.; Zhang, F. An Efficient 1064 nm NIR-II Excitation Fluorescent Molecular Dye for Deep-Tissue High-Resolution Dynamic Bioimaging. Angew. Chem. Int. Ed. 2018, 57, 7483–7487. [Google Scholar] [CrossRef]
- Cosco, E.D.; Caram, J.R.; Bruns, O.T.; Franke, D.; Day, R.A.; Farr, E.P.; Bawendi, M.G.; Sletten, E.M. Flavylium Polymethine Fluorophores for Near- and Shortwave Infrared Imaging. Angew. Chem. Int. Ed. 2017, 56, 13126–13129. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Sun, P.; Liu, Y.; Zhang, H.; Hu, W.; Zhang, W.; Liu, Z.; Fan, Q.; Li, L.; Huang, W. Novel aza-BODIPY Based Small Molecular NIR-II Fluorophores for In Vivo Imaging. Chem. Commun. 2019, 55, 10920–10923. [Google Scholar] [CrossRef]
- Dou, K.; Feng, W.; Fan, C.; Cao, Y.; Xiang, Y.; Liu, Z. Flexible Designing Strategy to Construct Activatable NIR-II Fluorescent Probes with Emission Maxima beyond 1200 nm. Anal. Chem. 2021, 93, 4006–4014. [Google Scholar] [CrossRef]
- Lei, Z.; Sun, C.; Pei, P.; Wang, S.; Li, D.; Zhang, X.; Zhang, F. Stable, Wavelength-Tunable Fluorescent Dyes in the NIR-II Region for In Vivo High-Contrast Bioimaging and Multiplexed Biosensing. Angew. Chem. Int. Ed. 2019, 58, 8166–8171. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; He, Z.; Zhao, Y.; Yang, Y.; Shi, W.; Li, X.; Ma, H. Xanthene-Based NIR-II Dyes for In Vivo Dynamic Imaging of Blood Circulation. J. Am. Chem. Soc. 2021, 143, 17136–17143. [Google Scholar] [CrossRef]
- Qian, J.; Li, J.; Feng, Z.; Yu, X.; Wu, D.; Wu, T. Aggregation-Induced Emission Fluorophores Towards the Second Near- Infrared Optical Windows with Suppressed Imaging Background. Coord. Chem. Rev. 2022, 472, 214792. [Google Scholar]
- Hu, Z.; Feng, L.; Yang, P. 2, 1, 3-Benzothiadiazole Derivative Small Molecule Fluorophores for NIR-II Bioimaging. Adv. Funct. Mater. 2024, 34, 2310818. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, H.; Bi, R.; Li, X.; Zha, M.; Yang, Y.; Ni, J.; Liew, W.H.; Olivo, M.; Yao, K.; et al. Acceptor Engineering of Small-Molecule Fluorophores for NIR-II Fluorescence and Photoacoustic Imaging. J. Mat. Chem. B 2021, 9, 9951–9960. [Google Scholar] [CrossRef]
- Zhou, H.; Lu, Z.; Zhang, Y.; Li, M.; Xue, D.; Zhang, D.; Liu, J.; Li, L.; Qian, J.; Huang, W. Simultaneous Enhancement of the Long-Wavelength NIR-II Brightness and Photothermal Performance of Semiconducting Polymer Nanoparticles. ACS Appl. Mater. Interfaces 2022, 14, 8705–8717. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Sun, P.; Cai, Y.; Xu, W.; Fan, Q.; Hu, Q.; Han, W. A Novel Multimodal NIR-II Nanoprobe for the Detection of Metastatic Lymph Nodes and Targeting Chemo-Photothermal Therapy in Oral Squamous Cell Carcinoma. Theranostics 2019, 9, 391–404. [Google Scholar] [CrossRef]
- Sun, P.; Chen, Y.; Sun, B.; Zhang, H.; Chen, K.; Miao, H.; Fan, Q.; Huang, W. Thienothiadiazole-Based NIR-II Dyes with D-A-D Structure for NIR-II Fluorescence Imaging Systems. ACS Appl. Bio Mater. 2021, 4, 4542–4548. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, J.; Wang, S.; Du, M.; Hou, X.; Tian, T.; Qiao, X.; Tian, Z.; Stang, P.; Li, S.; et al. Self-assembled NIR-II Fluorophores with Ultralong Blood Circulation for Cancer Imaging and Image-guided Surgery. J. Med. Chem. 2022, 65, 2078–2090. [Google Scholar] [CrossRef]
- Li, Y.; Gao, J.; Wang, S.; Li, S.; Hou, X.; Pan, Y.; Gao, J.; Qiao, X.; Tian, Z.; Chen, D.; et al. Organic NIR-II Dyes with Ultralong Circulation Persistence for Image-Guided Delivery and Therapy. J. Control. Release 2022, 342, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Ji, A.; Lou, H.; Qu, C.; Lu, W.; Hao, Y.; Li, J.; Wu, Y.; Chang, T.; Chen, H.; Cheng, Z. Acceptor Engineering for NIR-II Dyes with High Photochemical and Biomedical Performance. Nat. Commun. 2022, 13, 3815. [Google Scholar] [CrossRef]
- Liu, X.; Yu, B.; Shen, Y.; Cong, H. Design of NIR-II High Performance Organic Small Molecule Fluorescent Probes and Summary of Their Biomedical Applications. Coord. Chem. Rev. 2022, 468, 214609. [Google Scholar] [CrossRef]
- Yi, W.; Zhou, H.; Li, A.; Yuan, Y.; Guo, Y.; Li, P.; Qi, B.; Xiao, Y.; Yu, A.; Hu, X. A NIR-II Fluorescent Probe for Articular Cartilage Degeneration Imaging and Osteoarthritis Detection. Biomater. Sci. 2019, 7, 1043–1051. [Google Scholar] [CrossRef]
- Fang, Y.; Shang, J.; Liu, D.; Shi, W.; Li, X.; Ma, H. Design, Synthesis, and Application of a Small Molecular NIR-II Fluorophore with Maximal Emission beyond 1200 nm. J. Am. Chem. Soc. 2020, 142, 15271–15275. [Google Scholar] [CrossRef]
- Guo, B.; Huang, Z.; Shi, Q.; Middha, E.; Xu, S.; Li, L.; Wu, M.; Jiang, J.; Hu, Q.; Fu, Z.; et al. Organic Small Molecule Based Photothermal Agents with Molecular Rotors for Malignant Breast Cancer Therapy. Adv. Funct. Mater. 2020, 30, 1907093. [Google Scholar] [CrossRef]
- Tian, R.; Ma, H.; Yang, Q.; Wan, H.; Zhu, S.; Chandra, S.; Sun, H.; Kiesewetter, D.O.; Niu, G.; Liang, Y.; et al. Rational Design of a Super-Contrast NIR-II Fluorophore Affords High-Performance NIR-II Molecular Imaging Guided Microsurgery. Chem. Sci. 2019, 10, 326–332. [Google Scholar] [CrossRef]
- Yang, Q.; Ma, H.; Liang, Y.; Dai, H. Rational Design of High Brightness NIR-II Organic Dyes with S-D-A-D-S Structure. Acc. Mater. Res. 2021, 2, 170–183. [Google Scholar] [CrossRef]
- Qu, C.; Xiao, Y.; Zhou, H.; Ding, B.; Li, A.; Lin, J.; Zeng, X.; Chen, H.; Qian, K.; Zhang, X.; et al. Quaternary Ammonium Salt Based NIR-II Probes for In Vivo Imaging. Adv. Opt. Mater. 2019, 7, 1900229. [Google Scholar] [CrossRef]
- Antaris, A.L.; Chen, H.; Diao, S.; Ma, Z.; Zhang, Z.; Zhu, S.; Wang, J.; Lozano, A.X.; Fan, Q.; Chew, L.; et al. A High Quantum Yield Molecule-Protein Complex Fluorophore for Near-Infrared II Imaging. Nat. Commun. 2017, 8, 15269. [Google Scholar] [CrossRef]
- Wu, W.; Mao, D.; Xu, S.; Kenry; Hu, F.; Li, X.; Kong, D.; Liu, B. Polymerization-Enhanced Photosensitization. Chem 2018, 4, 1937–1951. [Google Scholar] [CrossRef]
- Yin, S.; Song, J.; Liu, D.; Wang, K.; Qi, J. NIR-II AIEgens with Photodynamic Effect for Advanced Theranostics. Molecules 2022, 27, 6649. [Google Scholar] [CrossRef]
- Lei, Z.; Zhang, F. Molecular Engineering of NIR-II Fluorophores for Improved Biomedical Detection. Angew. Chem. Int. Ed. 2021, 60, 16294–16308. [Google Scholar] [CrossRef]
- Zhu, S.; Hu, Z.; Tian, R.; Yung, B.; Yang, Q.; Zhao, S.; Kiesewetter, D.O.; Niu, G.; Sun, H.; Antaris, A.L.; et al. Repurposing Cyanine NIR-I Dyes Accelerates Clinical Translation of Near-Infrared-II (NIR-II) Bioimaging. Adv. Mater. 2018, 30, 1802546. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Li, B.; Zhao, M.; Wang, S.; Lei, Z.; Lu, L.; Zhang, H.; Feng, L.; Dou, C.; Yin, D.; et al. J-Aggregates of Cyanine Dye for NIR-II In Vivo Dynamic Vascular Imaging beyond 1500 nm. J. Am. Chem. Soc. 2019, 141, 19221–19225. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, F.; Lei, Z. The Pursuit of Polymethine Fluorophores With NIR-II Emission and High Brightness For In Vivo Applications. Chem. Sci. 2022, 13, 11280–11293. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sun, C.; Wang, S.; Yan, K.; Zhao, M.; Wu, B.; Zhang, F. Counterion-Paired Bright Heptamethine Fluorophores with NIR-II Excitation and Emission Enable Multiplexed Biomedical Imaging. Angew. Chem. Int. Ed. 2022, 61, e202117436. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Hong, G.; Shinji, C.; Chen, C.; Diao, S.; Antaris, A.L.; Zhang, B.; Zou, Y.; Dai, H. Biological Imaging Using Nanoparticles of Small Organic Molecules with Fluorescence Emission at Wavelengths Longer than 1000 nm. Angew. Chem. Int. Ed. 2013, 52, 13002–13006. [Google Scholar] [CrossRef]
- Jose, D.A.; Sakla, R.; Sharma, N.; Gadiyaram, S.; Kaushik, R.; Ghosh, A. Sensing and Bioimaging of the Gaseous Signaling Molecule Hydrogen Sulfide by Near-Infrared Fluorescent Probes. ACS Sens. 2020, 5, 3365–3391. [Google Scholar] [CrossRef]
- Mao, Z.; Kim, J.H.; Lee, J.; Xiong, H.; Zhang, F.; Kim, J.S. Engineering of BODIPY-Based Theranostics for Cancer Therapy. Coord. Chem. Rev. 2023, 476, 214908. [Google Scholar] [CrossRef]
- Lee, S.; Min, S.; Kim, G.; Lee, S. Recent Advances in the Design of Organic Photothermal Agents for Cancer Treatment: A Review. Coord. Chem. Rev. 2024, 506, 215719. [Google Scholar] [CrossRef]
- Boens, N.; Verbelen, B.; Ortiz, M.J.; Jiao, L.J.; Dehaen, W. Synthesis of BODIPY Dyes Through Postfunctionalization of the Boron Dipyrromethene Core. Coord. Chem. Rev. 2019, 399, 213024. [Google Scholar] [CrossRef]
- Godard, A.; Kalot, G.; Pliquett, J.; Busser, B.; Le Guével, X.; Wegner, K.D.; Resch-Genger, U.; Rousselin, Y.; Coll, J.L.; Denat, F.; et al. Water-Soluble Aza-BODIPYs: Biocompatible Organic Dyes for High Contrast In Vivo NIR-II Imaging. Bioconjug. Chem. 2020, 31, 1088–1092. [Google Scholar] [CrossRef]
- Hu, X.; Fang, Z.; Zhu, C.; Yang, Y.; Yang, Z.; Huang, W. Crucial Breakthrough of BODIPY-Based NIR-II Fluorescent Emitters for Advanced Biomedical Theranostics. Adv. Funct. Mater. 2024, 34, 2401325. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, S.; Chen, Z.; Hu, R.; Li, S.; Zhao, Y.; Liu, L.; Qu, J. Highly Stable Organic Photothermal Agent Based on Near-Infrared-II Fluorophores for Tumor Treatment. J. Nanobiotechnol. 2021, 19, 37. [Google Scholar] [CrossRef]
- Mao, Z.; Rha, H.; Kim, J.; You, X.; Zhang, F.; Tao, W.; Kim, J.S. THQ-Xanthene: An Emerging Strategy to Create Next-Generation NIR-I/II Fluorophores. Adv. Sci. 2023, 10, 2301177. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Scott, C.N. Design Strategies to Rhodamine Analogue Fluorophores for Near-Infrared II Biological Imaging Applications. Dye. Pigment. 2021, 196, 109792. [Google Scholar] [CrossRef]
- Mei, Y.; Hu, Q.; Zhou, B.; Zhang, Y.; He, M.; Xu, T.; Li, F.; Kong, J. Fluorescence Quenching Based Alkaline Phosphatase Activity Detection. Talanta 2018, 176, 52–58. [Google Scholar] [CrossRef]
- Surender, E.M.; Bradberry, S.J.; Bright, S.A.; McCoy, C.P.; Williams, D.C.; Gunnlaugsson, T. Luminescent Lanthanide Cyclen-Based Enzymatic Assay Capable of Diagnosing the Onset of Catheter-Associated Urinary Tract Infections Both in Solution and within Polymeric Hydrogels. J. Am. Chem. Soc. 2017, 139, 381–388. [Google Scholar] [CrossRef]
- Xu, H.; Wang, H.; Xu, Z.; Bian, S.; Xu, Z.; Zhang, H. The Multifaceted Roles of Peptides In “Always-On” Near-Infrared Fluorescent Probes for Tumor Imaging. Bioorg. Chem. 2022, 129, 106182. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, F.; Shi, T.; Chen, Y. Advances in Fluorescence Imaging of Malignant Tumors. J. Shanghai Jiao Tong Univ. (Med. Sci.) 2023, 43, 474–479. [Google Scholar]
- Pan, W.; Rafiq, M.; Haider, W.; Guo, Y.; Wang, H.; Xu, M.; Yu, B.; Cong, H.; Shen, Y. Recent Advances in NIR-II Fluorescence/Photoacoustic Dual-Modality Imaging Probes. Coord. Chem. Rev. 2024, 514, 215907. [Google Scholar] [CrossRef]
- Kalyane, D.; Raval, N.; Maheshwari, R.; Tambe, V.; Kalia, K.; Tekade, R.K. Employment of Enhanced Permeability and Retention Effect (EPR): Nanoparticle-Based Precision Tools for Targeting of Therapeutic and Diagnostic Agent in Cancer. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 98, 1252–1276. [Google Scholar] [CrossRef] [PubMed]
- On, K.C.; Rho, J.; Yoon, H.Y.; Chang, H.; Yhee, J.Y.; Yoon, J.S.; Jeong, S.Y.; Kim, H.K.; Kim, K. Tumor-Targeting Glycol Chitosan Nanoparticles for Image-Guided Surgery of Rabbit Orthotopic VX2 Lung Cancer. Pharmaceutics 2020, 12, 621. [Google Scholar] [CrossRef]
- Choyke, P.L.; Kobayashi, H. Medical Uses of Fluorescence Imaging: Bringing Disease to Light. IEEE J. Sel. Top. Quantum Electron. 2012, 18, 1140–1146. [Google Scholar] [CrossRef]
- Zettlitz, K.A.; Tsai, W.-T.K.; Knowles, S.M.; Kobayashi, N.; Donahue, T.R.; Reiter, R.E.; Wu, A.M. Dual-Modality Immuno-PET and Near-Infrared Fluorescence Imaging of Pancreatic Cancer Using an Anti-Prostate Stem Cell Antigen Cys-Diabody. J. Nucl. Med. 2018, 59, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- Boogerd, L.S.F.; Boonstra, M.C.; Prevoo, H.A.J.M.; Handgraaf, H.J.M.; Kuppen, P.J.K.; van de Velde, C.J.H.; Fish, A.; Cordfunke, R.A.; Valentijn, A.R.P.M.; van Scheltinga, A.G.T.; et al. Fluorescence-Guided Tumor Detection with a Novel Anti-Epcam Targeted Antibody Fragment: Preclinical Validation. Surg. Oncol. 2019, 28, 1–8. [Google Scholar] [CrossRef]
- de Valk, K.S.; Deken, M.M.; Handgraaf, H.J.M.; Bhairosingh, S.S.; Bijlstra, O.D.; van Esdonk, M.J.; van Scheltinga, A.G.T.T.; Valentijn, A.R.P.M.; March, T.L.; Vuijk, J.; et al. First-in-Human Assessment of cRGD-ZW800-1, a Zwitterionic, Integrin-Targeted, Near-Infrared Fluorescent Peptide in Colon Carcinoma. Clin. Cancer Res. 2020, 26, 3990–3998. [Google Scholar] [CrossRef]
- Amini, A.; Safdari, Y.; Tash Shamsabadi, F. Near-Infrared Fluorescence Imaging of EGFR-Overexpressing Tumors in the Mouse Xenograft Model Using scFv-IRDye800CW and Cetuximab-IRDye800CW. Mol. Imaging 2022, 2022, 9589820. [Google Scholar] [CrossRef]
- Sofia Silva, A.; Silva, M.C.; Miguel, S.P.; Bonifacio, V.D.B.; Correia, I.J.; Aguiar-Ricardo, A. Nanogold Poxylation: Towards Always-On Fluorescent Lung Cancer Targeting. RSC Adv. 2016, 6, 33631–33635. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, H.; Huang, J.; Qian, W.; Martinson, D.E.; Ji, B.; Li, Y.; Wang, Y.A.; Yang, L.; Mao, H. Probing and Enhancing Ligand-Mediated Active Targeting of Tumors Using Sub-5 nm Ultrafine Iron Oxide Nanoparticles. Theranostics 2020, 10, 2479–2494. [Google Scholar] [CrossRef]
- Li, D.; Pan, J.; Xu, S.; Fu, S.; Chu, C.; Liu, G. Activatable Second Near-Infrared Fluorescent Probes: A New Accurate Diagnosis Strategy for Diseases. Biosensors 2021, 11, 436. [Google Scholar] [CrossRef]
- Zhang, Z.; Mei, A.; Wang, W.; Xu, K.; Wang, M.; Chen, P.; Shao, J.; Dong, X. Stimuli-Responsive Organic Small-Molecule NIR-II Fluorescent Probes. Coord. Chem. Rev. 2025, 545, 217026. [Google Scholar] [CrossRef]
- Chen, Z.K.; Zhou, Y.J.; Li, L.; Ma, W.; Li, Y.Z.; Yang, Z. Activatable Molecular Probes with Clinical Promise for NIR-II Fluorescent Imaging. Small 2025, 21, 2411787. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Feng, J.; Zhang, X.; Sun, J.; Fan, C.; Ge, Z. Responsive Optical Probes for Deep-Tissue Imaging: Photoacoustics and Second Near-Infrared Fluorescence. Adv. Drug Deliv. Rev. 2021, 173, 141–163. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Shan, X.; Zhao, X.; Tang, L.; Zhang, X. Activatable NIR-II Fluorescent Probes Applied in Biomedicine: Progress and Perspectives. ChemMedChem 2021, 16, 2426–2440. [Google Scholar] [CrossRef]
- Wu, X.; Wang, R.; Kwon, N.; Ma, H.; Yoon, J. Activatable Fluorescent Probes for In Situ Imaging of Enzymes. Chem. Soc. Rev. 2022, 51, 450–463. [Google Scholar] [CrossRef]
- Zeng, Z.; Liew, S.; Wei, X.; Pu, K. Hemicyanine-Based Near-Infrared Activatable Probes for Imaging and Diagnosis of Diseases. Angew. Chem. Int. Ed. 2021, 60, 26454–26475. [Google Scholar] [CrossRef] [PubMed]
- Lan, Q.; Yu, P.; Yan, K.; Li, X.; Zhang, F.; Lei, Z. Polymethine Molecular Platform for Ratiometric Fluorescent Probes in the Second near-Infrared Window. J. Am. Chem. Soc. 2022, 144, 21010–21015. [Google Scholar] [CrossRef]
- Miao, X.; Wu, C.; Li, F.; Zhang, M. Fast and Visual Detection of Biogenic Amines and Food Freshness Based on ICT-Induced Ratiometric Fluorescent Probes. Adv. Funct. Mater. 2023, 33, 2212980. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, L.; Ye, Z.; Xu, L.; Li, Y.; Liu, S.; Song, G.; Zhang, X. Engineering of Cyanine-Based Nanoplatform with Tunable Response Toward Reactive Species for Ratiometric NIR-II Fluorescent Imaging in Mice. Sci. Bull. 2023, 68, 2382–2390. [Google Scholar] [CrossRef]
- Shinde, V.R.; Revi, N.; Murugappan, S.; Singh, S.P.; Rengan, A.K. Enhanced Permeability and Retention Effect: A Key Facilitator for Solid Tumor Targeting by Nanoparticles. Photodiagn. Photodyn. Ther. 2022, 39, 102915. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Norret, M.; House, M.J.; Galabura, Y.; Bradshaw, M.; Ho, D.W.; Woodward, R.C.; St Pierre, T.G.; Luzinov, I.; Smith, N.M.; et al. Dose-Dependent Therapeutic Distinction between Active and Passive Targeting Revealed Using Transferrin-Coated PGMA Nanoparticles. Small 2016, 12, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K. Intracellular Targeting Delivery of Liposomal Drugs to Solid Tumors Based on EPR Effects. Adv. Drug Deliv. Rev. 2011, 63, 161–169. [Google Scholar] [CrossRef]
- Zhao, C.; Deng, H.; Xu, J.; Li, S.; Zhong, L.; Shao, L.; Wu, Y.; Liang, X. “Sheddable” PEG-Lipid to Balance the Contradiction of Pegylation Between Long Circulation and Poor Uptake. Nanoscale 2016, 8, 0832–10842. [Google Scholar] [CrossRef]
- Yao, C.; Chen, Y.; Zhao, M.; Wang, S.; Wu, B.; Yang, Y.; Yin, D.; Yu, P.; Zhang, H.; Zhang, F. A Bright, Renal-Clearable NIR-II Brush Macromolecular Probe with Long Blood Circulation Time for Kidney Disease Bioimaging. Angew. Chem. Int. Ed. 2021, 61, e202114273. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Diao, S.; Liang, T.; Zhang, X.; Guo, Z.; Liu, Y.; Zhou, W.; Xie, C.; Fan, Q. A Renal-Clearable PEGylated Semiconducting Oligomer for the NIR-II Fluorescence Imaging of Tumor. ACS Appl. Bio Mater. 2022, 5, 4965–4971. [Google Scholar] [CrossRef]
- Ji, H.; Wang, W.; Li, X.; Han, X.; Zhang, X.; Wang, J.; Liu, C.; Huang, L.; Gao, W. Natural Small Molecules Enabled Efficient Immunotherapy through Supramolecular Self-Assembly in P53-Mutated Colorectal Cancer. ACS Appl. Mater. Interfaces 2022, 14, 2464–2477. [Google Scholar] [CrossRef]
- Sang, M.; Han, L.; Luo, R.; Qu, W.; Zheng, F.; Zhang, K.; Liu, F.; Xue, J.; Liu, W.; Feng, F. CD44 Targeted Redox-Triggered Self-Assembly with Magnetic Enhanced EPR Effects for Effective Amplification of Gambogic Acid to Treat Triple-Negative Breast Cancer. Biomater. Sci. 2020, 8, 212–223. [Google Scholar] [CrossRef] [PubMed]
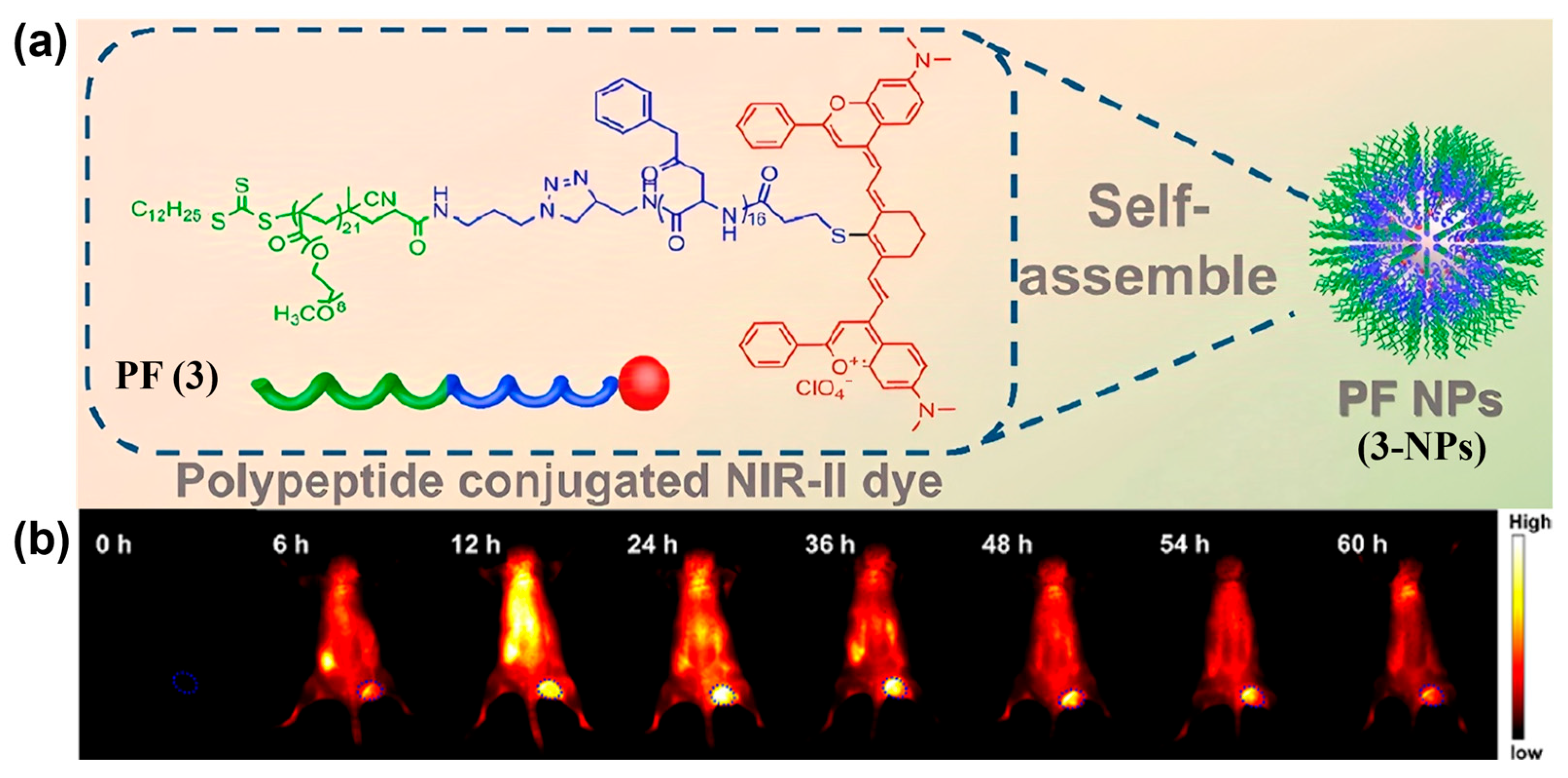
- Li, T.; Li, C.; Ruan, Z.; Xu, P.; Yang, X.; Yuan, P.; Wang, Q.; Yan, L. Polypeptide-Conjugated Second Near-Infrared Organic Fluorophore for Image-Guided Photothermal Therapy. ACS Nano 2019, 13, 3691–3702. [Google Scholar] [CrossRef]
- Arias, J.L. Drug Targeting Strategies in Cancer Treatment: An Overview. Mini-Rev. Med. Chem. 2011, 11, 1–17. [Google Scholar] [CrossRef]
- Biswas, S.; Torchilin, V.P. Nanopreparations for Organelle-Specific Delivery in Cancer. Adv. Drug Deliv. Rev. 2014, 66, 26–41. [Google Scholar] [CrossRef]
- Zhu, L.; Kate, P.; Torchilin, V.P. Matrix Metalloprotease 2-Responsive Multifunctional Liposomal Nanocarrier for Enhanced Tumor Targeting. ACS Nano 2012, 6, 3491–3498. [Google Scholar] [CrossRef]
- Ran, X.; Chen, P.; Liu, Y.; Shi, L.; Chen, X.; Liu, Y.; Zhang, H.; Zhang, L.; Li, K.; Yu, X. Rational Design of Polymethine Dyes with NIR-II Emission and High Photothermal Conversion Efficiency for Multimodal-Imaging-Guided Photo-Immunotherapy. Adv. Mater. 2023, 35, 2210179. [Google Scholar] [CrossRef] [PubMed]
- Bhalodi, K.; Kothari, C.; Butani, S. Next-Generation Cancer Nanotherapeutics: Pluronic® F127 Based Mixed Micelles for Enhanced Drug Delivery. Naunyn-Schmiedebergs Arch. Pharmacol. 2025, 398, 3241–3270. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, Y.; Gong, C.; Li, Y.; Xu, M.; Liu, M.; Liu, W.; Zhou, X.; Wang, L. Design of a High-Performance NIR-II Nanoprobe by Steric Regulation for In Vivo Vasculature and Tumor Imaging. Chem. Commun. 2024, 60, 8059–8062. [Google Scholar] [CrossRef]
- Paul, M.; Itoo, A.M.; Ghosh, B.; Biswas, S. Current Trends in the Use of Human Serum Albumin for Drug Delivery in Cancer. Expert Opin. Drug Deliv. 2022, 19, 1449–1470. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Xu, X.; Xu, J.; Yu, W. Research Progress of Surface Modified Albumin Nanoparticles in Tumor Targeting Therapy. Chin. J. Clin. Pharm. Ther. 2017, 22, 948–954. [Google Scholar]
- Xu, J.; Du, Y.; Han, T.; Zhu, N.; Zhu, S. Protein@Cyanine-Based NIR-II Lymphography Enables the Supersensitive Visualization of Lymphedema and Tumor Lymphatic Metastasis. Adv. Healthc. Mater. 2023, 12, 2301051. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wu, J.; Liu, W.; Zhang, W.; Lee, C.; Wang, P. New Xanthene Dyes with NIR-II Emission Beyond 1200 nm for Efficient Tumor Angiography and Photothermal Therapy. Small 2022, 18, 2202078. [Google Scholar] [CrossRef]
- Cao, R.; Li, R.; Shi, H.; Liu, H.; Cheng, Z. Novel HER2-Targeted Peptide for NIR-II Imaging of Tumor. Mol. Pharm. 2023, 20, 1394–1403. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Zhang, C.; Zhang, J.; Yuan, L.; Jin, S.; Zhou, W.; Guan, X.; Kang, P.; Zhang, C.; et al. Preclinical Assessment of Irdye800cw-Labeled Gastrin-Releasing Peptide Receptor-targeting Peptide for Near Infrared-II Imaging of Brain Malignancies. Bioeng. Transl. Med. 2023, 8, e10532. [Google Scholar] [CrossRef]
- Zhao, M.; Ding, J.; Mao, Q.; Zhang, Y.; Gao, Y.; Ye, S.; Qin, H.; Shi, H. A Novel αvβ3 Integrin-Targeted NIR-II Nanoprobe for Multimodal Imaging-Guided Photothermal Therapy of Tumors In Vivo. Nanoscale 2020, 12, 6953–6958. [Google Scholar] [CrossRef] [PubMed]
- Lyu, S.; Lu, S.; Gui, C.; Guo, C.; Han, J.; Xiao, Y.; Zhang, R.; Hong, X. A NIR-II Photoacoustic/NIR-IIa Fluorescent Probe for Targeted Imaging of Glioma under NIR-II Excitation. J. Med. Chem. 2024, 67, 1861–1871. [Google Scholar] [CrossRef]
- Yang, R.; Wang, P.; Lou, K.; Dang, Y.; Tian, H.; Li, Y.; Gao, Y.; Huang, W.; Zhang, Y.; Liu, X.; et al. Biodegradable Nanoprobe for NIR-II Fluorescence Image-Guided Surgery and Enhanced Breast Cancer Radiotherapy Efficacy. Adv. Sci. 2022, 9, 2104728. [Google Scholar] [CrossRef]
- de Jongh, S.J.; Tjalma, J.J.J.; Koller, M.; Linssen, M.D.; Vonk, J.; Dobosz, M.; Jorritsma-Smit, A.; Kleibeuker, J.H.; Hospers, G.A.P.; Havenga, K.; et al. Back-Table Fluorescence-Guided Imaging for Circumferential Resection Margin Evaluation Using Bevacizumab-800CW in Patients with Locally Advanced Rectal Cancer. J. Nucl. Med. 2020, 61, 655–661. [Google Scholar] [CrossRef]
- Voskuil, F.J.; de Jongh, S.J.; Hooghiemstra, W.T.R.; Linssen, M.D.; Steinkamp, P.J.; de Visscher, S.; Schepman, K.P.; Elias, S.G.; Meersma, G.J.; Jonker, P.K.C.; et al. Fluorescence-Guided Imaging for Resection Margin Evaluation in Head and Neck Cancer Patients Using Cetuximab-800CW: A Quantitative Dose-Escalation Study. Theranostics 2020, 10, 3994–4005. [Google Scholar] [CrossRef]
- Ogawa, M.; Kosaka, N.; Choyke, P.L.; Kobayashi, H. In Vivo Molecular Imaging of Cancer with a Quenching Near-Infrared Fluorescent Probe Using Conjugates of Monoclonal Antibodies and Indocyanine Green. Cancer Res. 2009, 69, 1268–1272. [Google Scholar] [CrossRef] [PubMed]
- Dréau, D.; Moore, L.J.; Das Roy, L.; Wu, S.T.; Puri, R.; Mukherjee, P. Early Detection of Mammary Tumors In Vivo Using a Highly Specific Tumor Antibody. J. Clin. Oncol. 2015, 33, 14. [Google Scholar] [CrossRef]
- Suo, Y.; Wu, F.; Xu, P.; Shi, H.; Wang, T.; Liu, H.; Cheng, Z. NIR-II Fluorescence Endoscopy for Targeted Imaging of Colorectal Cancer. Adv. Healthc. Mater. 2019, 8, 1900974. [Google Scholar] [CrossRef]
- Arsene, D.; Galais, M.P.; Bouhier-Leporrier, K.; Reimund, J.M. Recent Developments in Colorectal Cancer Treatment by Monoclonal Antibodies. Expert Opin. Biol. Ther. 2006, 6, 1175–1192. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Li, Y.; Xiong, L.; Wang, W.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T.; et al. Small Molecules in Targeted Cancer Therapy: Advances, Challenges, and Future Perspectives. Signal Transduct. Target. Ther. 2021, 6, 201. [Google Scholar] [CrossRef]
- Bai, X.; Wang, C.; Bian, L.; Zhang, X. Recent Advances in the Folic Acid-Targeted Anti-Tumor Drugs. Chin. J. Med. Chem. 2021, 31, 632–649. [Google Scholar]
- Shi, X.; Xu, P.; Cao, C.; Cheng, Z.; Tian, J.; Hu, Z. PET/NIR-II Fluorescence Imaging and Image-Guided Surgery of Glioblastoma Using a Folate Receptor α-Targeted Dual-Modal Nanoprobe. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 325–4337. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, X.; Li, Y.; Duan, X.; Zhang, Z.; Fu, H.; Yang, X.; Tian, J.; Hu, Z.; Cui, M. Visualizing Tumors in Real Time: A Highly Sensitive PSMA Probe for NIR-II Imaging and Intraoperative Tumor Resection. J. Med. Chem. 2021, 64, 7735–7745. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wu, Y.; He, C.; Li, X.; Tao, J.; Qu, C.; Chen, W.; Cheng, Z. Mitochondria-Targeting Small-Molecule NIR-II Fluorescent Probes for Imaging and Treatment of Tumor. Adv. Therap. 2023, 6, 2300151. [Google Scholar] [CrossRef]
- Zhu, L.; Zhong, Y.; Wu, S.; Yan, M.; Cao, Y.; Mou, N.L.; Wang, G.X.; Sun, D.; Wu, W. Cell Membrane Camouflaged Biomimetic Nanoparticles: Focusing on Tumor Theranostics. Mater. Today Bio 2022, 14, 100228. [Google Scholar] [CrossRef]
- Jiang, T.; Zhan, Y.; Ding, J.; Song, Z.; Zhang, Y.; Li, J.; Su, T. Biomimetic Cell Membrane-Coated Nanoparticles for Cancer Theranostics. ChemMedChem 2024, 19, e202400410. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Xu, Y.; Wu, J.; Wu, D.H.; Zhou, L.; Xia, X. TME-Related Biomimetic Strategies Against Cancer. Int. J. Nanomed. 2024, 19, 109–135. [Google Scholar] [CrossRef]
- Jiang, S.; Li, W.; Li, B.; Chen, S.; Lei, S.; Liu, Y.; Lin, J.; Huang, P. Albumin-Energized NIR-II Cyanine Dye for Fluorescence/Photoacoustic/Photothermal Multi-Modality Imaging-Guided Tumor Homologous Targeting Photothermal Therapy. J. Med. Chem. 2025, 68, 3324–3334. [Google Scholar] [CrossRef]
- Wei, Q.; Jia, H.; Zhao, H.; Tang, W.; Duan, X. A Novel Water-Soluble NIR-II Cyanine Dye for Fast Targeted Through-Skull Fluorescence Imaging of Orthotopic Glioma. Chem. Commun. 2024, 60, 14451–14454. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Y.; Ye, D. Activatable Multimodal Probes for In Vivo Imaging and Theranostics. Angew. Chem. Int. Ed. 2022, 61, e202209512. [Google Scholar] [CrossRef]
- Zhang, X.; Li, S.; Ma, H.; Wang, H.; Zhang, R.; Zhang, X. Activatable NIR-II Organic Fluorescent Probes for Bioimaging. Theranostics 2022, 12, 3345–3371. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Weng, J.; Men, Y.; Yang, Y.; Ye, D. Dual-Targeted Fluorogenic Probes for Precise In Vivo Imaging: Design Principles, Activation Mechanisms, and Applications. Chin. J. Chem. 2025, 43, 2683–2701. [Google Scholar] [CrossRef]
- Yan, R.; Hu, Y.; Liu, F.; Wei, S.; Fang, D.; Shuhendler, A.J.; Liu, H.; Chen, H.; Ye, D. Activatable NIR Fluorescence/MRI Bimodal Probes for In Vivo Imaging by Enzyme-Mediated Fluorogenic Reaction and Self-Assembly. J. Am. Chem. Soc. 2019, 141, 10331–10341. [Google Scholar] [CrossRef]
- Ding, C.; Ren, T. Near Infrared Fluorescent Probes for Detecting and Imaging Active Small Molecules. Coord. Chem. Rev. 2023, 482, 215080. [Google Scholar] [CrossRef]
- Fernandes, R.S.; Himaja, A.; Ghosh, B.; Dey, N. Fluorescent TICT Probe for Precise Monitoring of Cellular Lipid Droplets. ACS Appl. Bio Mater. 2024, 7, 8248–8260. [Google Scholar] [CrossRef]
- Wang, C.; Chi, W.; Qiao, Q.; Tan, D.; Xu, Z.; Liu, X. Twisted Intramolecular Charge Transfer (TICT) and Twists Beyond TICT: From Mechanisms to Rational Designs of Bright and Sensitive Fluorophores. Chem. Soc. Rev. 2021, 50, 12656–12678. [Google Scholar] [CrossRef] [PubMed]
- Chi, W.; Chen, J.; Liu, W.; Wang, C.; Qi, Q.; Qiao, Q.; Tan, T.; Xiong, K.; Liu, X.; Kang, K.; et al. A General Descriptor ΔE Enables the Quantitative Development of Luminescent Materials Based on Photoinduced Electron Transfer. J. Am. Chem. Soc. 2020, 142, 6777–6785. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Liu, J.; O’Connor, H.M.; Gunnlaugsson, T.; James, T.D.; Zhang, H. Photoinduced Electron Transfer (PeT) Based Fluorescent Probes for Cellular Imaging and Disease Therapy. Chem. Soc. Rev. 2023, 52, 2322–2357. [Google Scholar] [CrossRef]
- Xiang, X.; Dong, C.; Zhou, L.; Liu, J.; Rabinowitz, Z.M.; Zhang, Y.; Guo, H.; He, F.; Chen, X.; Wang, Y.; et al. Novel PET Imaging Probe for Quantitative Detection of Senescence In Vivo. J. Med. Chem. 2024, 67, 5924–5934. [Google Scholar] [CrossRef]
- Fang, B.; Shen, Y.; Peng, B.; Bai, H.; Wang, L.; Zhang, J.; Hu, W.; Fu, L.; Zhang, W.; Li, L.; et al. Small-Molecule Quenchers for Förster Resonance Energy Transfer: Structure, Mechanism, and Applications. Angew. Chem. Int. Ed. 2022, 61, e202207188. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Huang, C.; Emery, B.; Sedgwick, A.C.; Bull, S.D.; He, X.; Tian, H.; Yoon, J.; Sessler, J.L.; James, T.D. Förster Resonance Energy Transfer (FRET)-Based Small-Molecule Sensors and Imaging Agents. Chem. Soc. Rev. 2020, 49, 5110–5139. [Google Scholar] [CrossRef]
- Yang, D.; Wu, X.; Ning, J.; Wei, B.; Miao, J.; Zhao, B.; Lin, Z. Novel Fluorescence Probe for Clo in Living Cells: Based on FRET Mechanism. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2024, 321, 124754. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Yang, C.; Wang, X.; Chen, X.; Wang, Y.; Le, W. Oxygen Metabolism Abnormality and Alzheimer’s Disease: An Update. Redox Biol. 2023, 68, 102955. [Google Scholar] [CrossRef]
- Pezone, A.; Olivieri, F.; Napoli, M.V.; Procopio, A.; Avvedimento, E.V.; Gabrielli, A. Inflammation and DNA Damage: Cause, Effect or Both. Nat. Rev. Rheumatol. 2023, 19, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhang, T.; Jiao, A.; Mou, X.; Dong, X.; Cai, Y. Molecular Endoperoxides for Optical Imaging and Photodynamic Therapy. Coord. Chem. Rev. 2025, 523, 216258. [Google Scholar] [CrossRef]
- Shen, L.; Li, J.; Wen, C.; Wang, H.; Liu, N.; Su, X.; Chen, J.; Li, X. A Firm-Push-to-Open and Light-Push-to-Lock Strategy for a General Chemical Platform to Develop Activatable Dual-modality NIR-II Probes. Sci. Adv. 2024, 10, eado2037. [Google Scholar] [CrossRef]
- Zhang, R.; Ran, X.; Yu, K.; Zhao, Y.; Zhang, L.; Lv, X.; Zhang, H.; Yu, X.; Li, K. Rational Design of NIR-II Fluorescence/Photoacoustic Nanosensor Tailored for Mechanisms of Diabetes-Related Breast Cancer. Adv. Mater. 2025, 37, 2415891. [Google Scholar] [CrossRef]
- Pan, Y.; Lei, S.; Zhang, J.; Qu, J.; Huang, P.; Lin, J. Activatable NIR-II Fluorescence Probe for Highly Sensitive and Selective Visualization of Glutathione In Vivo. Anal. Chem. 2021, 93, 17103–17109. [Google Scholar] [CrossRef]
- Yang, H.; Li, D.; Wu, J.; Pu, K. Shortwave Infrared Hemicyanine-6 for Cancer-Activated and Shaving-Free Preclinical Imaging of Lung Metastasis. J. Am. Chem. Soc. 2025, 147, 30794–30802. [Google Scholar] [CrossRef] [PubMed]
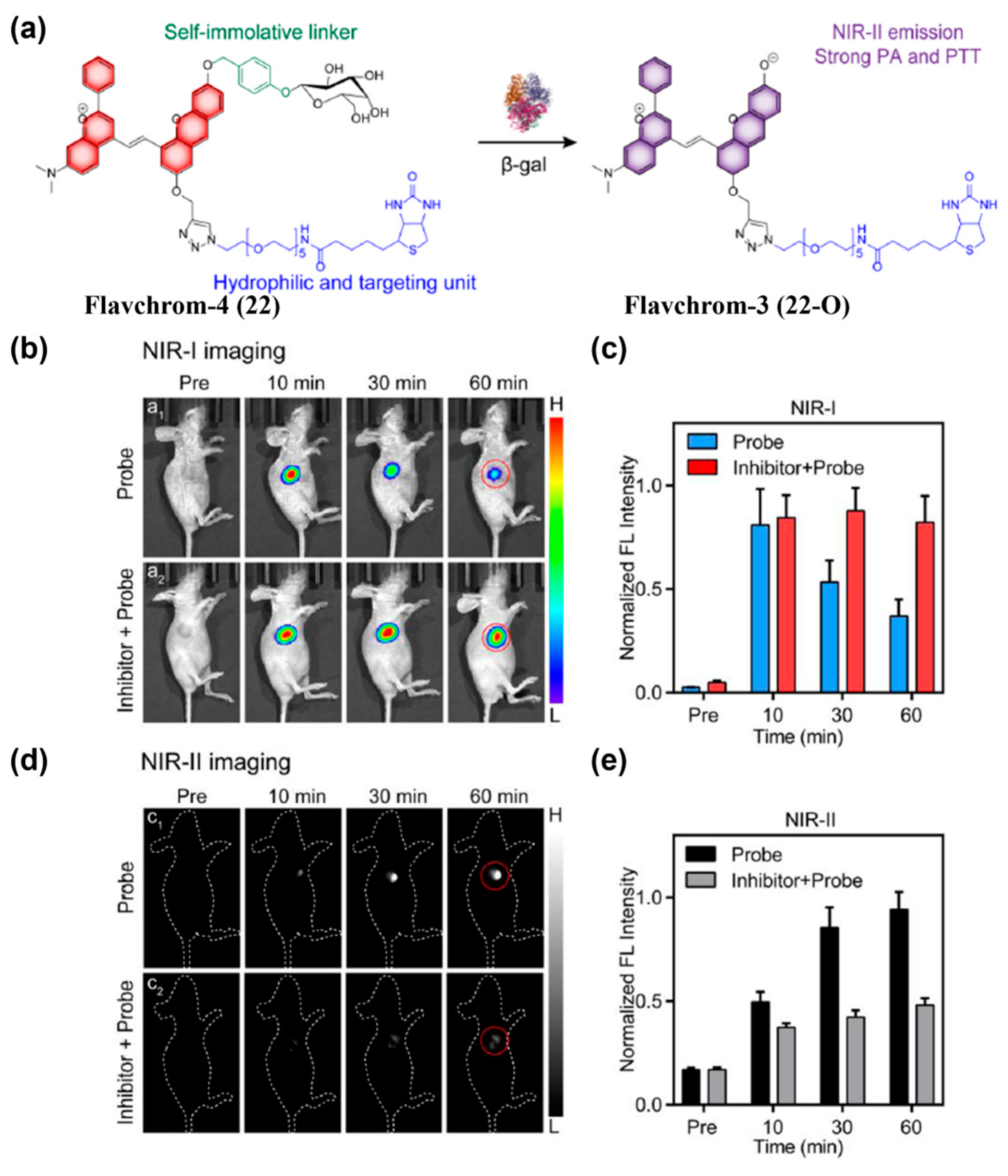
- Zhang, L.; Zhang, Y.; Chi, W.; Yan, C.; Zhao, Z.; Liu, X.; Zhu, W.; Guo, Z. “Crossbreeding” Small-Molecular Weight NIR-II Flavchromenes Endows Activatable Multiplexed In Vivo Imaging. ACS Mater. Lett. 2022, 4, 1493–1502. [Google Scholar] [CrossRef]
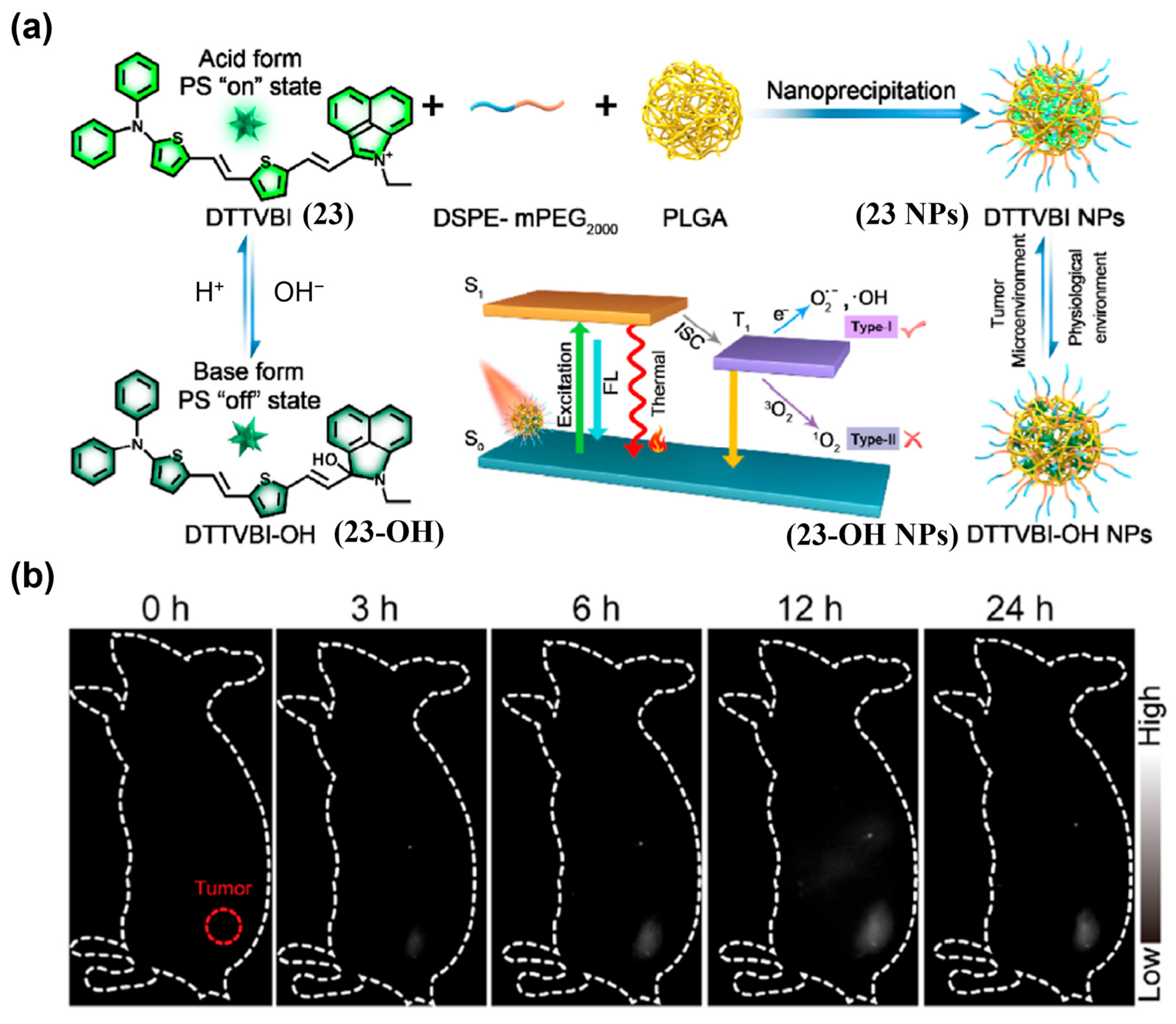
- Xiao, P.; Xie, W.; Zhang, J.; Wu, Q.; Shen, Z.; Guo, C.; Wu, Y.; Wang, F.; Tang, B.; Wang, D. De Novo Design of Reversibly pH-Switchable NIR-II Aggregation-Induced Emission Luminogens for Efficient Phototheranostics of Patient-Derived Tumor Xenografts. J. Am. Chem. Soc. 2022, 145, 334–344. [Google Scholar] [CrossRef]
- Han, Z.; Xiong, J.; Ren, T.; Zhang, X. Recent Advances in Dual-target-activated Fluorescent Probes for Biosensing and Bioimaging. Chem.-Asian J. 2022, 17, e202200387. [Google Scholar] [CrossRef] [PubMed]
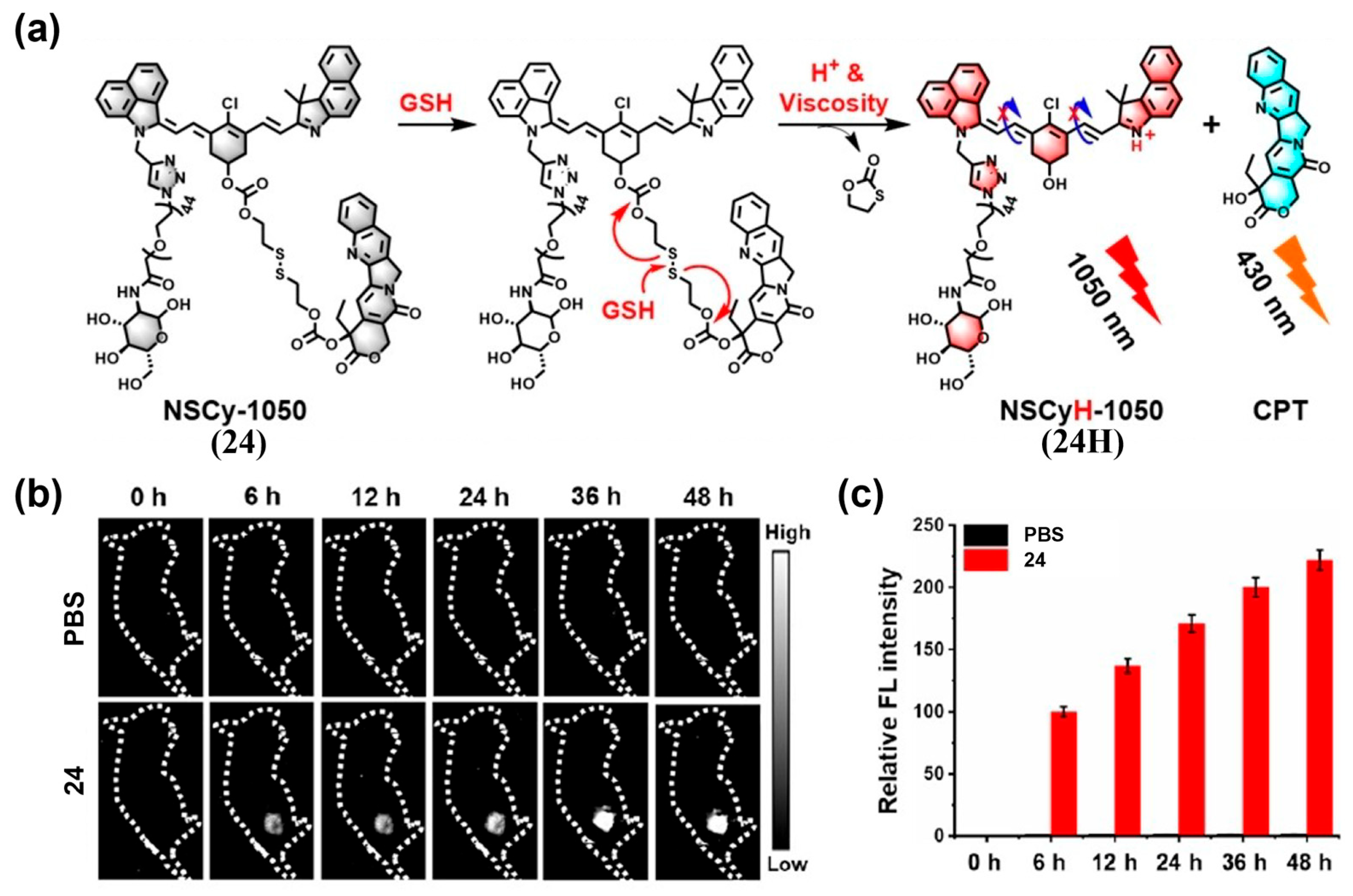
- Tian, Y.; Chen, Z.; Liu, S.; Wu, F.; Cao, W.; Pang, D.; Xiong, H. “Dual-Key-and-Lock” NIR-II NSCyanines Enable High-Contrast Activatable Phototheranostics in Extrahepatic Diseases. Angew. Chem. Int. Ed. 2023, 62, e202309768. [Google Scholar] [CrossRef]
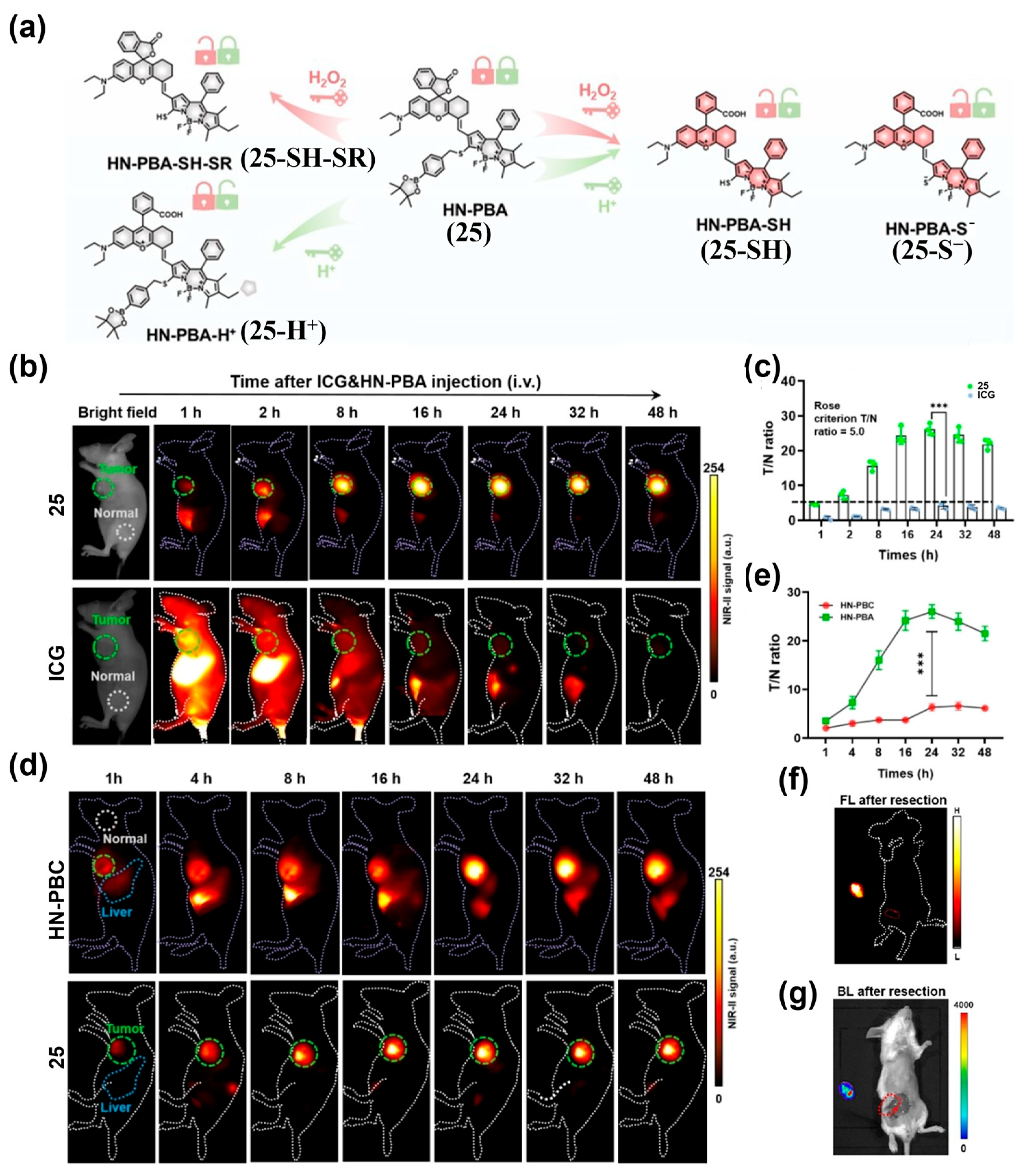
- Dou, K.; Lu, J.; Xing, Y.; Wang, R.; Won, M.; Kim, J.; Yu, F.; Seung Kim, J. Metabolic Acidity/H2O2 Dual-Cascade-Activatable Molecular Imaging Platform Toward Metastatic Breast Tumor Malignancy. Angew. Chem. Int. Ed. 2024, 64, e202419191. [Google Scholar] [CrossRef] [PubMed]
- Bu, L.; Shen, B.; Cheng, Z. Fluorescent Imaging of Cancerous Tissues for Targeted Surgery. Adv. Drug Deliv. Rev. 2014, 76, 21–38. [Google Scholar] [CrossRef]
- Wang, K.; Liu, L.; Mao, D.; Xu, S.; Tan, C.; Cao, Q.; Mao, Z.; Liu, B. A Polarity-Sensitive Ratiometric Fluorescence Probe for Monitoring Changes in Lipid Droplets and Nucleus during Ferroptosis. Angew. Chem. Int. Ed. 2021, 60, 15095–15100. [Google Scholar] [CrossRef]
- Li, T.; Zhang, Y.; Wu, F.; Chen, G.; Li, C.; Wang, Q. Rational Design of NIR-II Ratiometric Fluorescence Probes for Accurate Bioimaging and Biosensing In Vivo. Small Methods 2025, 9, 2400132. [Google Scholar] [CrossRef]
- Chu, F.; Feng, B.; Zhou, Y.; Liu, M.; Zhang, H.; Liu, M.; Chen, Q.; Zhang, S.; Ma, Y.; Dong, J.; et al. Debut of Enzyme-Responsive Anionic Cyanine for Overlap-free NIR-II-to-I Dual-channel Tumour Imaging. Chem. Sci. 2025, 16, 4490–4500. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Su, L.; Chen, Z.; Zhu, K.; Zhang, X.; Wu, Y.; Song, J. A Radio-Pharmaceutical Fluorescent Probe for Synergistic Cancer Radiotherapy and Ratiometric Imaging of Tumor Reactive Oxygen Species. Angew. Chem. Int. Ed. 2023, 62, e202305744. [Google Scholar] [CrossRef] [PubMed]
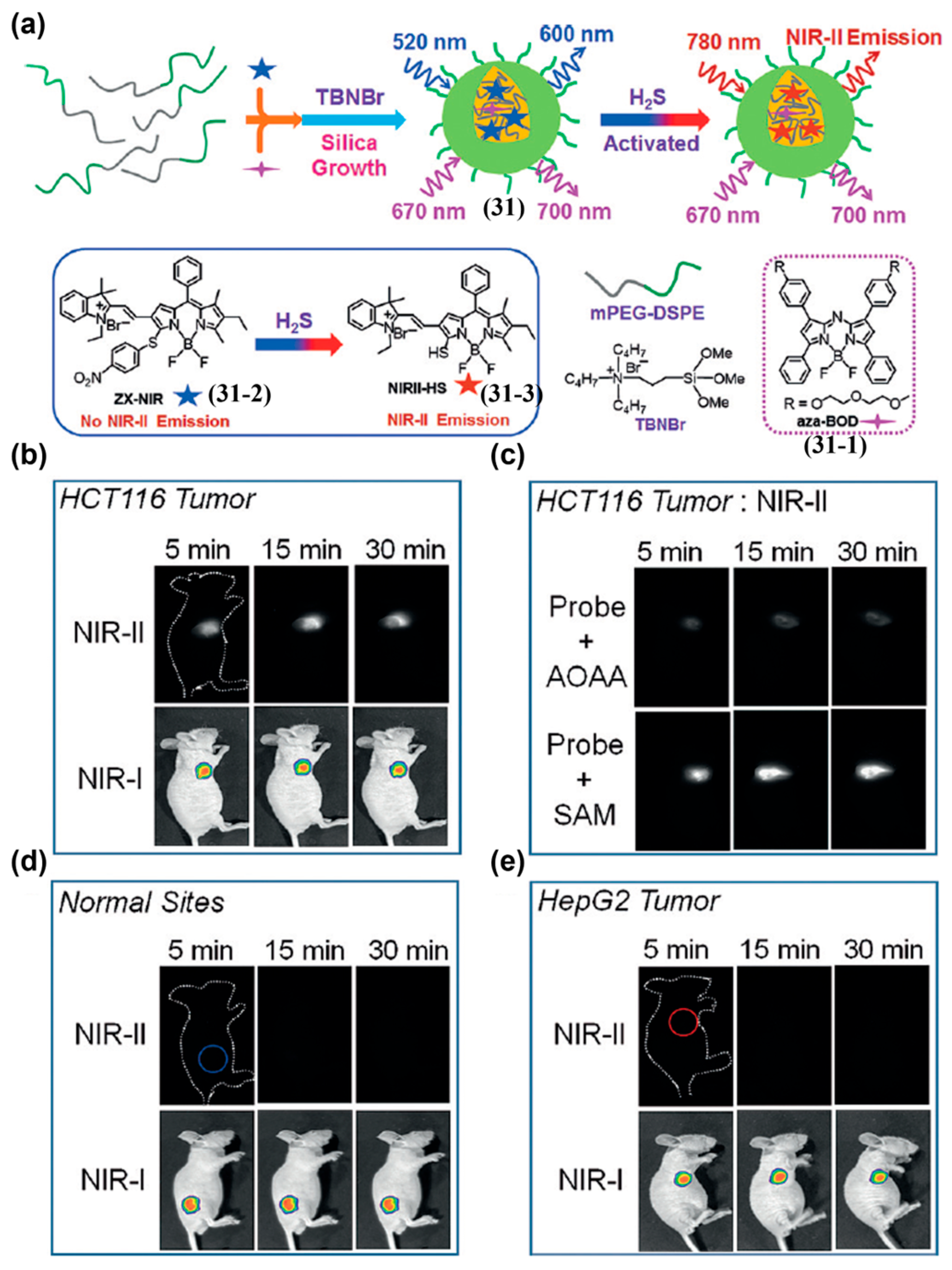
- Lei, S.; Jiang, K.; Zhang, C.; Sun, W.; Pan, Y.; Wang, D.; Huang, P.; Lin, J. A FRET-Based Ratiometric H2S Sensor for Sensitive Optical Molecular Imaging in Second Near-Infrared Window. Research 2023, 6, 0286. [Google Scholar] [CrossRef]
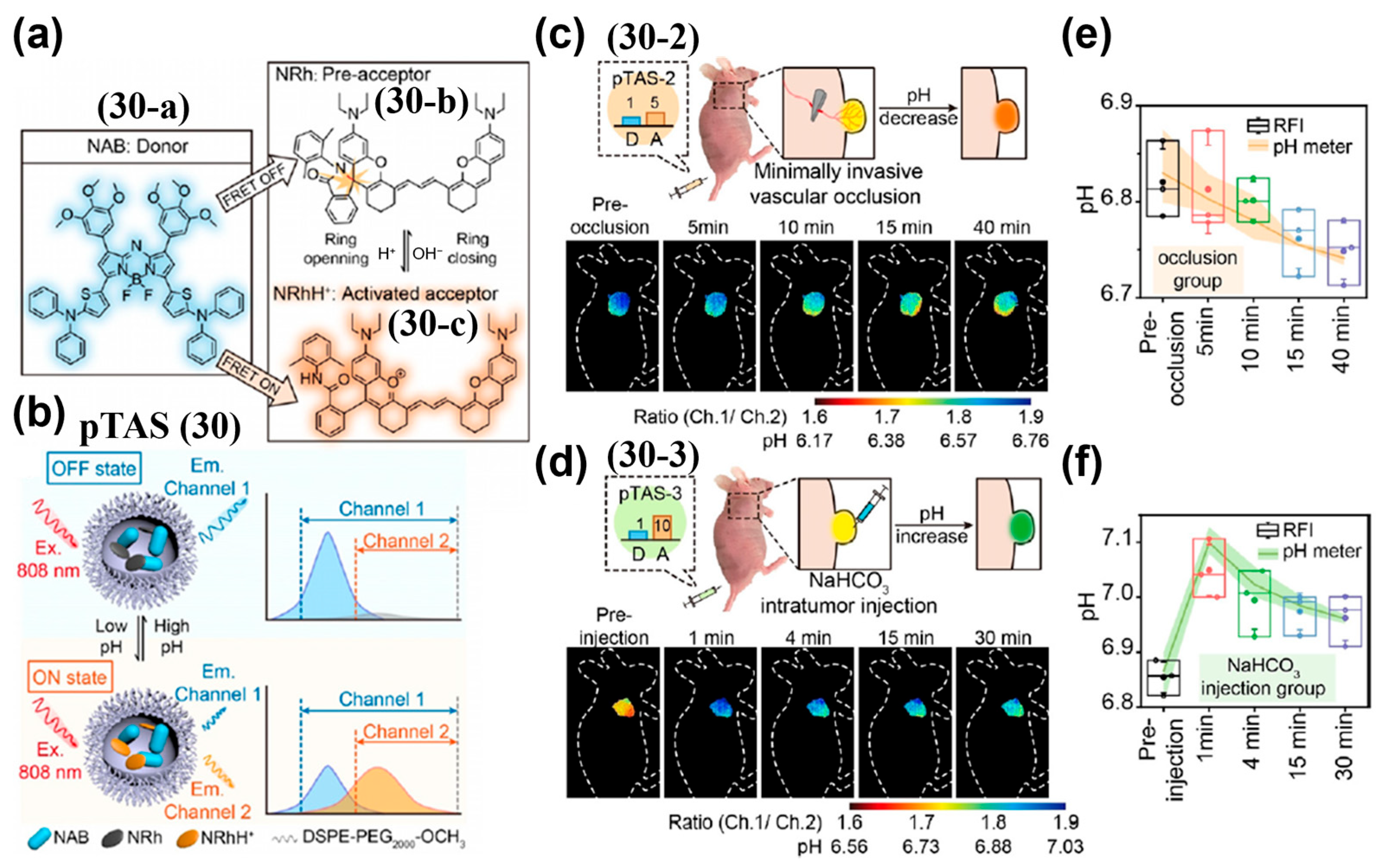
- Zhao, M.; Wang, J.; Lei, Z.; Lu, L.; Wang, S.; Zhang, H.; Li, B.; Zhang, F. NIR-II pH Sensor with a FRET Adjustable Transition Point for In Situ Dynamic Tumor Microenvironment Visualization. Angew. Chem. Int. Ed. 2021, 60, 5091–5095. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Sun, Z.; Yang, H.; Yang, X.; Wu, F.; Sun, Y.; Li, C.; Tian, M.; Zhang, H.; Wang, Q. Precise Examination of Peripheral Vascular Disease for Diabetics with a Novel Multiplexed NIR-II Fluorescence Imaging Technology. Nano Today 2022, 43, 101378. [Google Scholar] [CrossRef]
- Xu, G.; Yan, Q.; Lv, X.; Zhu, Y.; Xin, K.; Shi, B.; Wang, R.; Chen, J.; Gao, W.; Shi, P.; et al. Imaging of Colorectal Cancers Using Activatable Nanoprobes with Second Near-Infrared Window Emission. Angew. Chem. Int. Ed. 2018, 57, 3626–3630. [Google Scholar] [CrossRef]
- Liu, Y.; Teng, L.; Zhang, B.; Zhang, X.-B.; Song, G. A Dual-excitation Ratiometric NIR-II Fluorescent Nanoplatform Enables High Contrast In Vivo Imaging. Chem. Sci. 2025, 16, 13784–13793. [Google Scholar] [CrossRef] [PubMed]
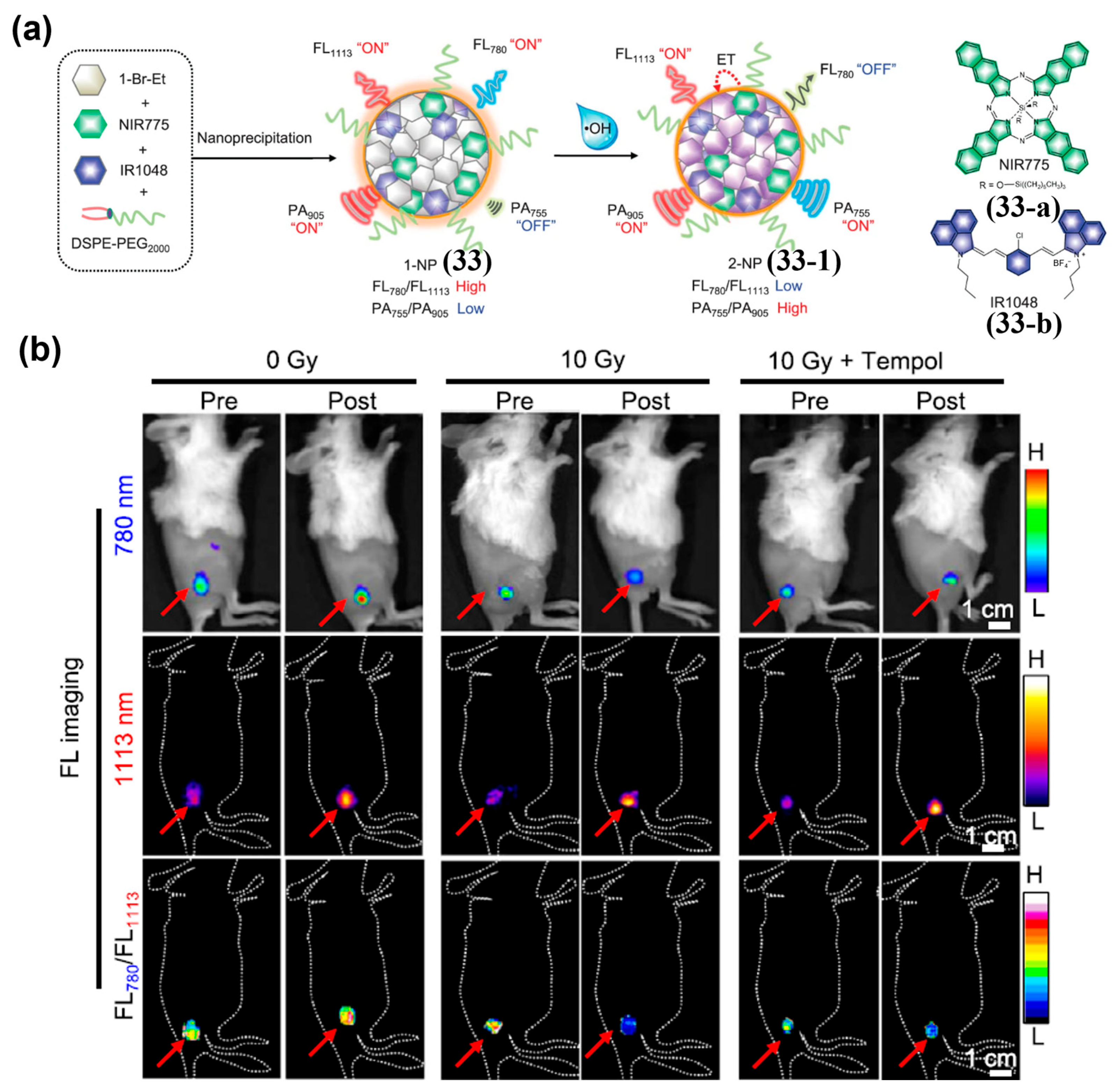
- Wu, L.; Ishigaki, Y.; Zeng, W.; Harimoto, T.; Yin, B.; Chen, Y.; Liao, S.; Liu, Y.; Sun, Y.; Zhang, X.; et al. Generation of Hydroxyl Radical-Activatable Ratiometric Near-Infrared Bimodal Probes for Early Monitoring of Tumor Response to Therapy. Nat. Commun. 2021, 12, 6145. [Google Scholar] [CrossRef] [PubMed]
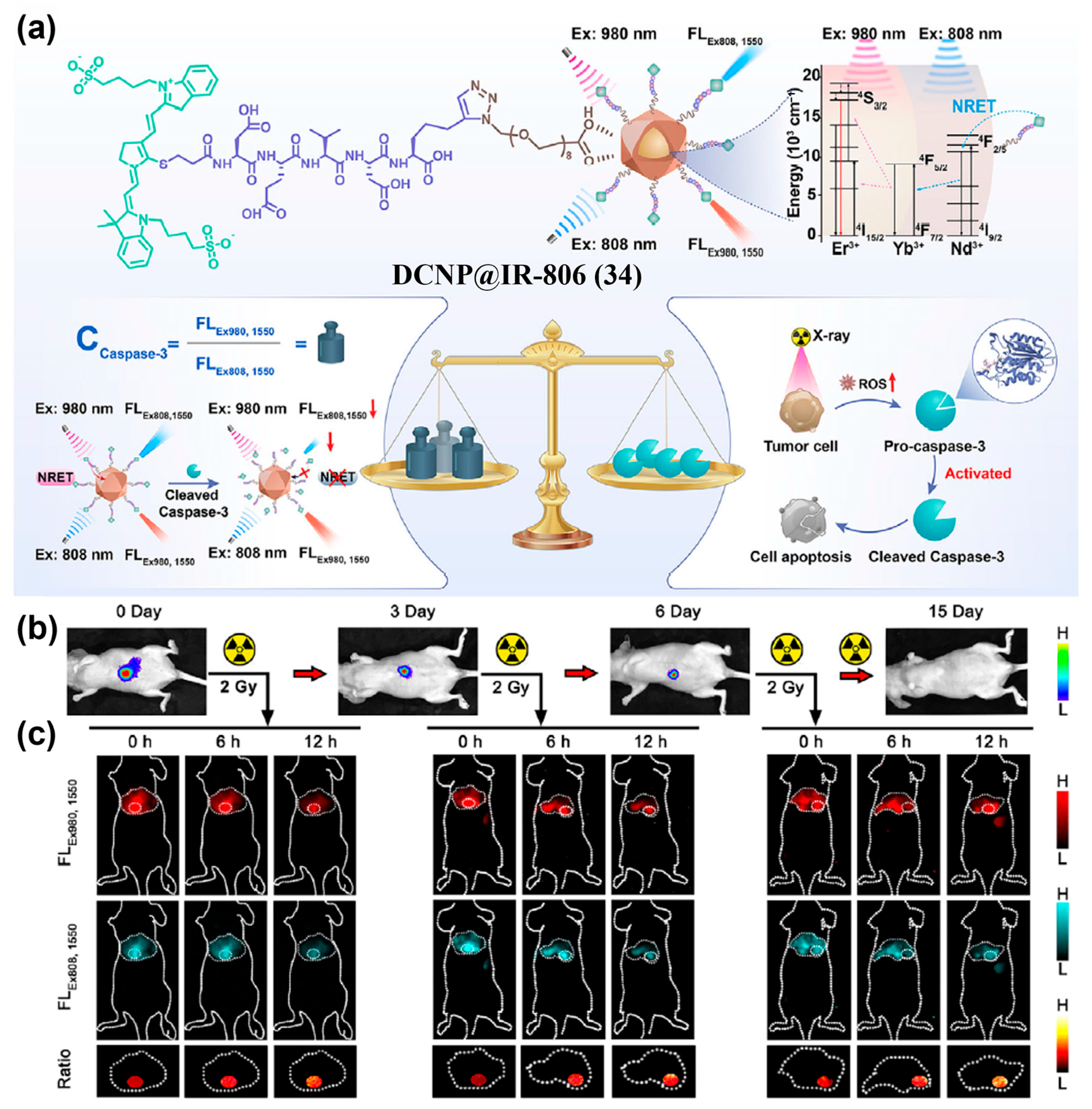
- Wu, Y.; Wang, Q.; Zhu, K.; Zheng, L.; Li, Q.; Huang, W.; Du, Y.; Chen, L.; Song, J.; Yang, H. Quantitative Tracking of Apoptotic Caspase-3 In Vivo for Early Evaluation of Radiation Therapy Efficacy. Angew. Chem. Int. Ed. 2025, 64, e202502811. [Google Scholar] [CrossRef]
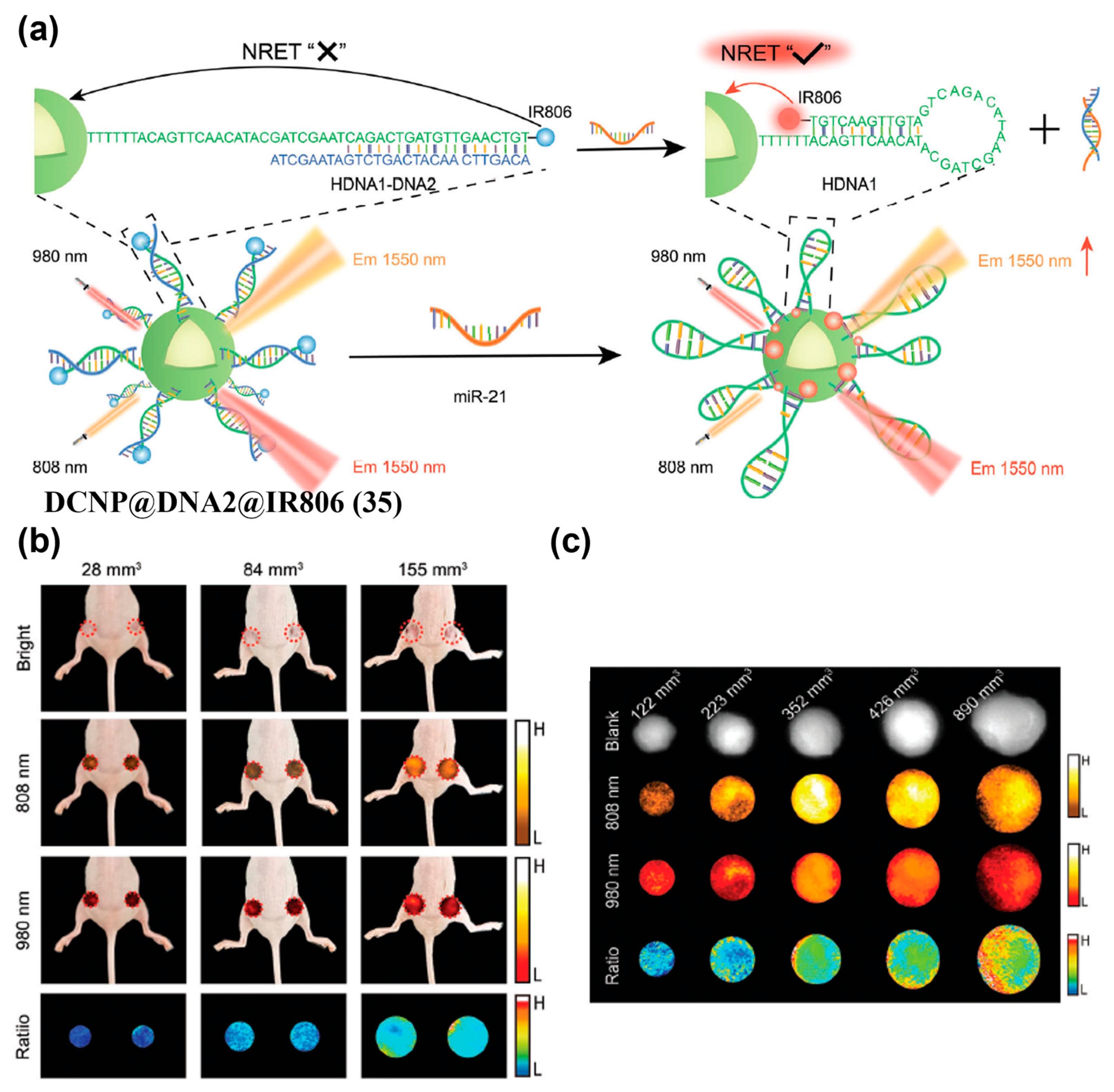
- Zheng, L.; Wu, Y.; Wang, Q.; Du, W.; Chen, L.; Song, J.; Yang, H. Quantitative Imaging of MicroRNA-21 In Vivo for Real-Time Monitoring of the Cancer Initiation and Progression. Adv. Funct. Mater. 2024, 34, 2407348. [Google Scholar] [CrossRef]
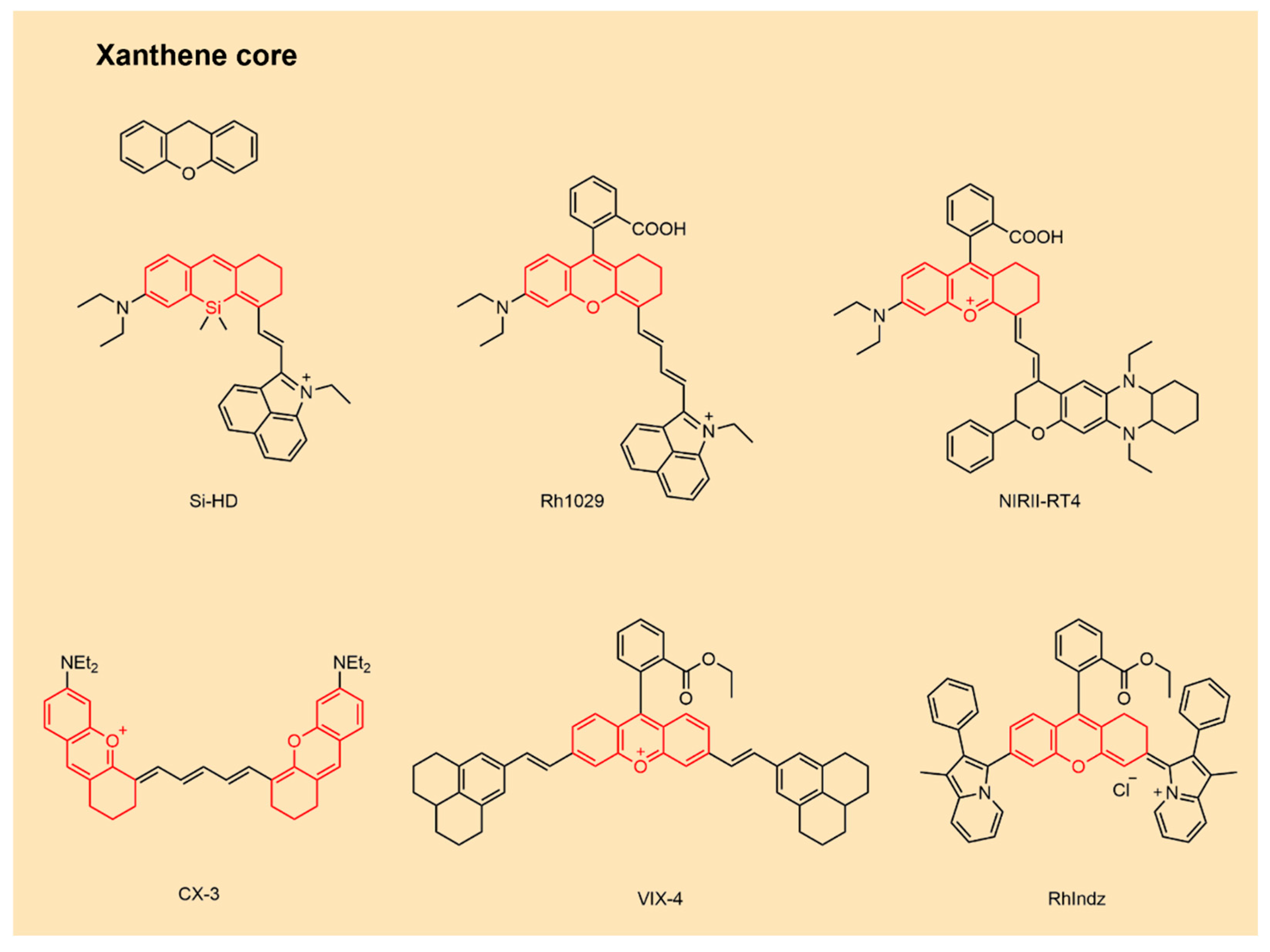
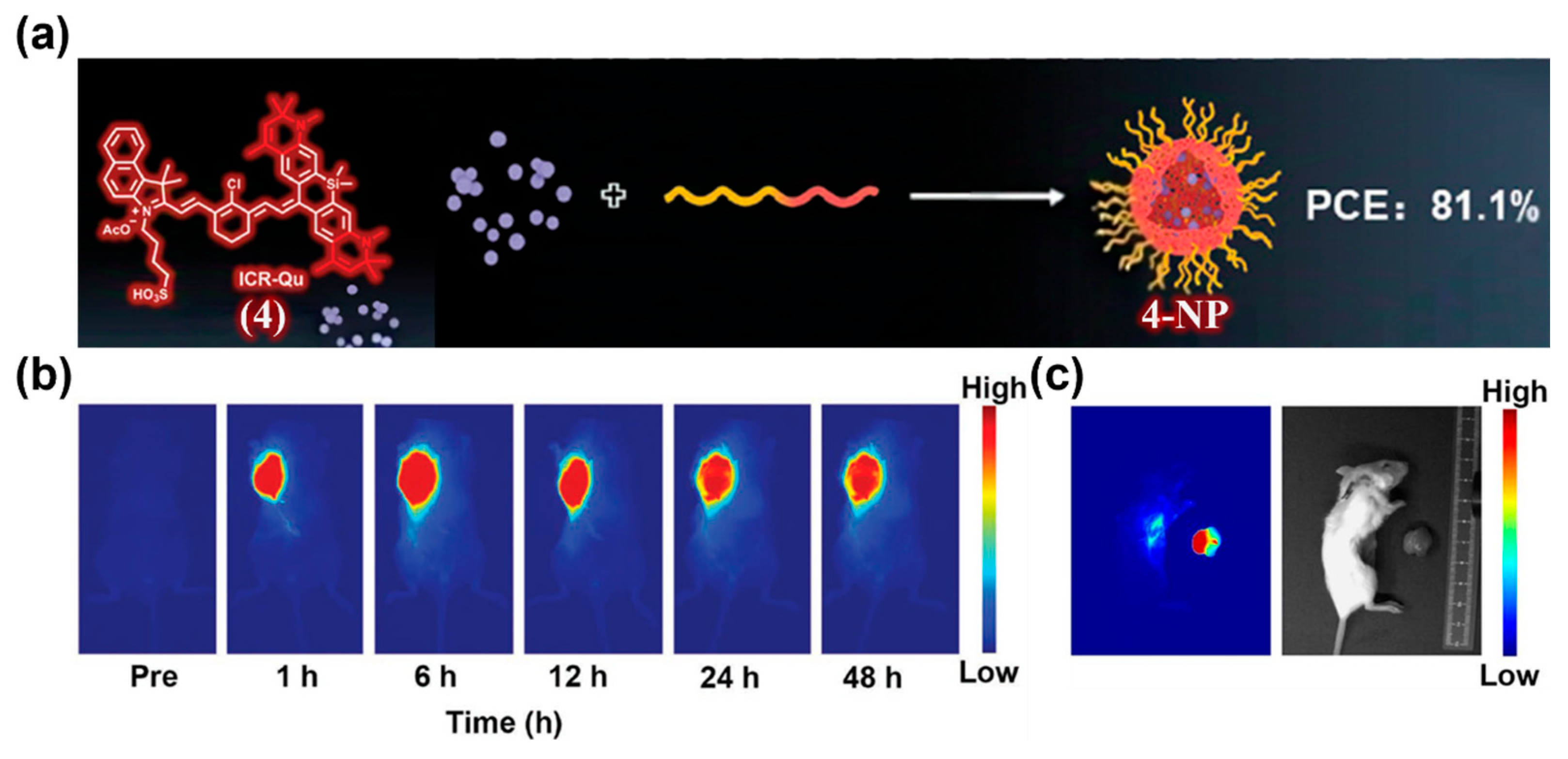
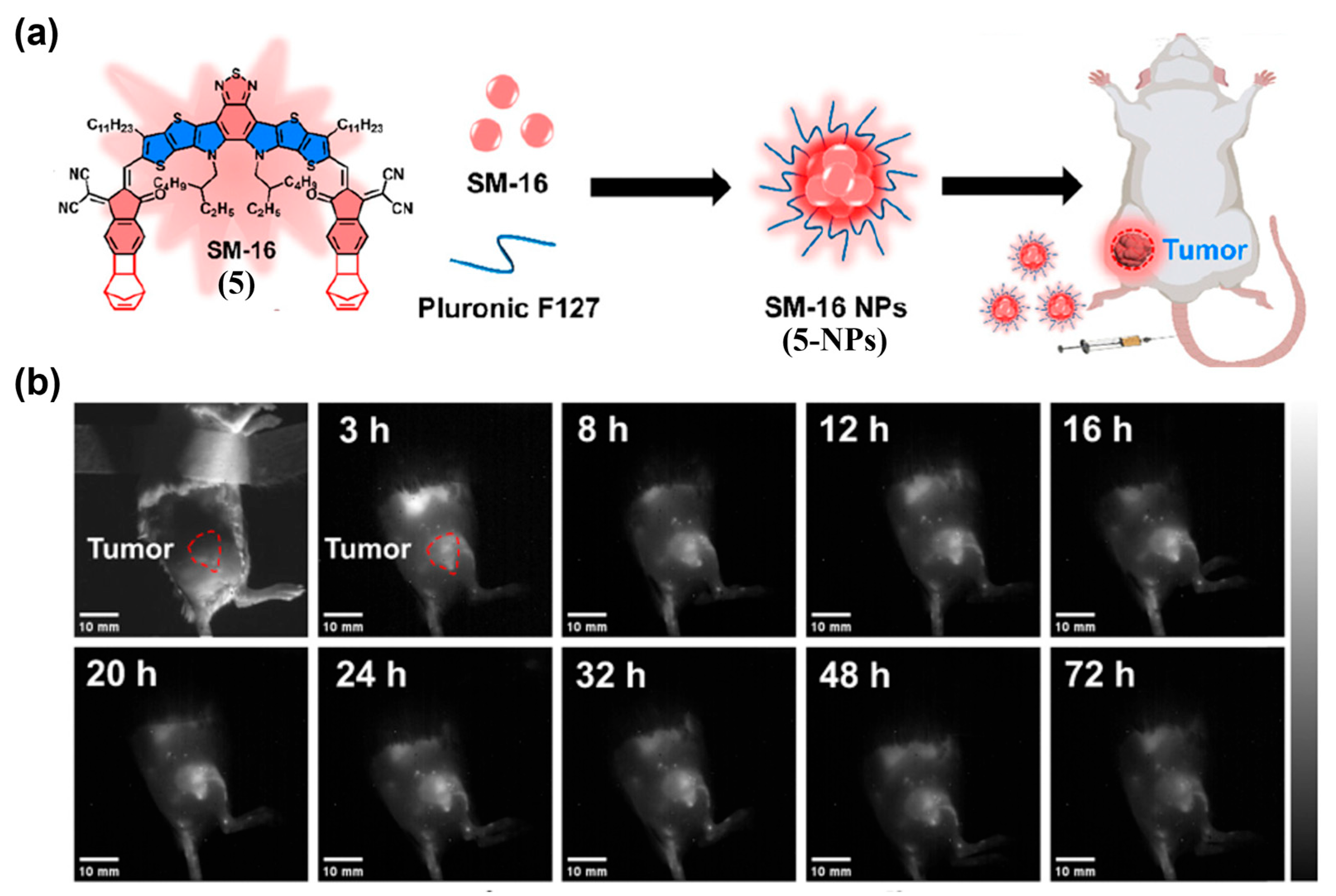
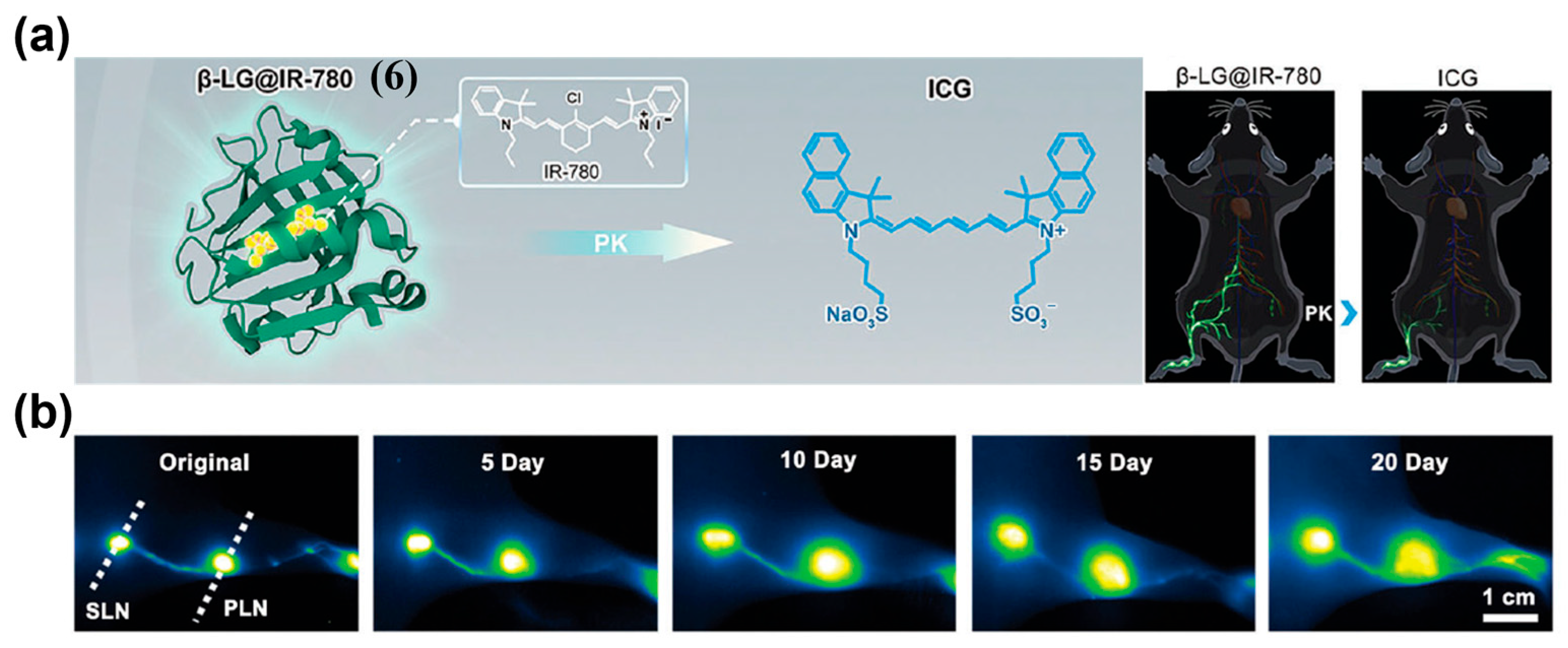
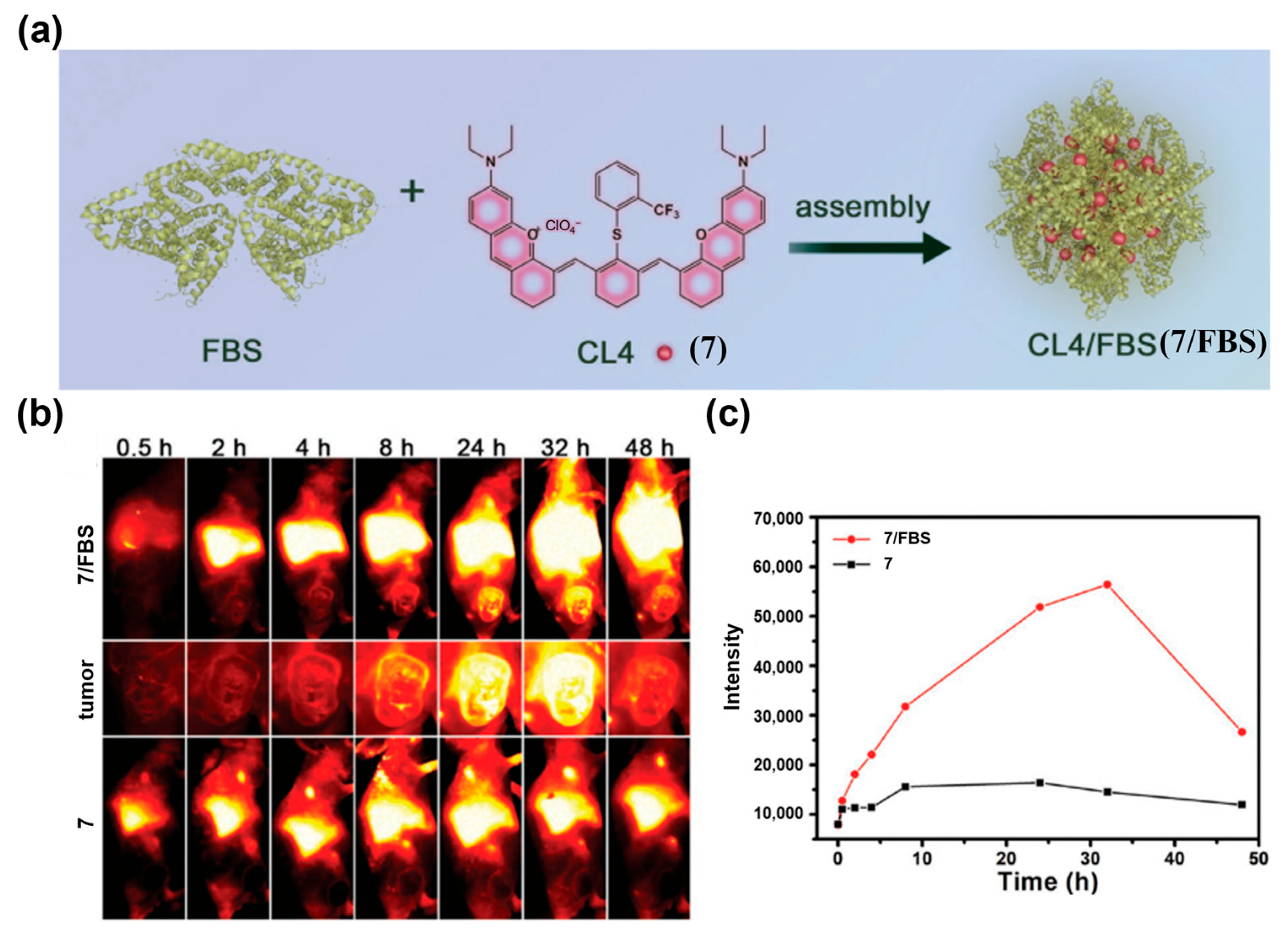
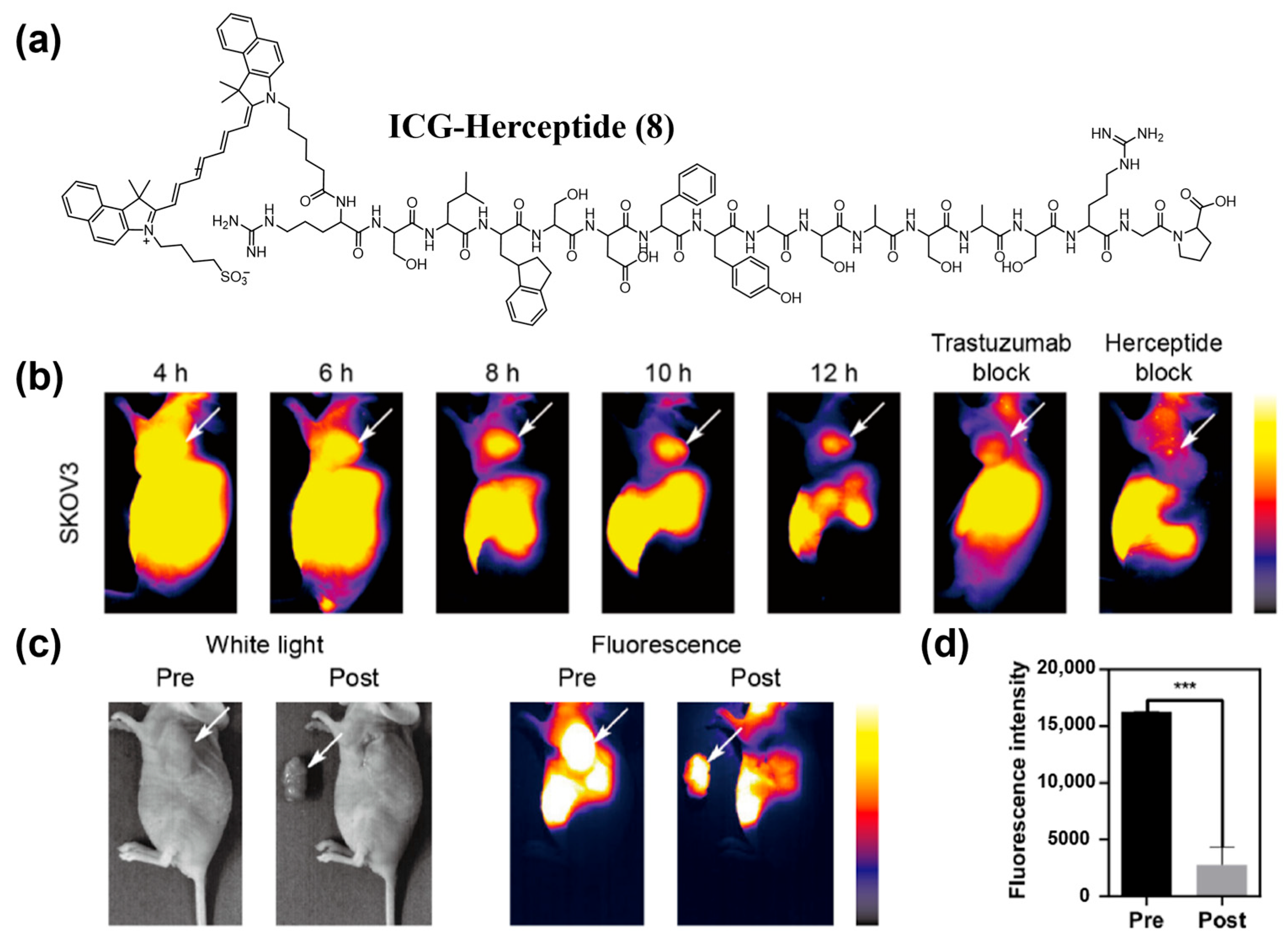
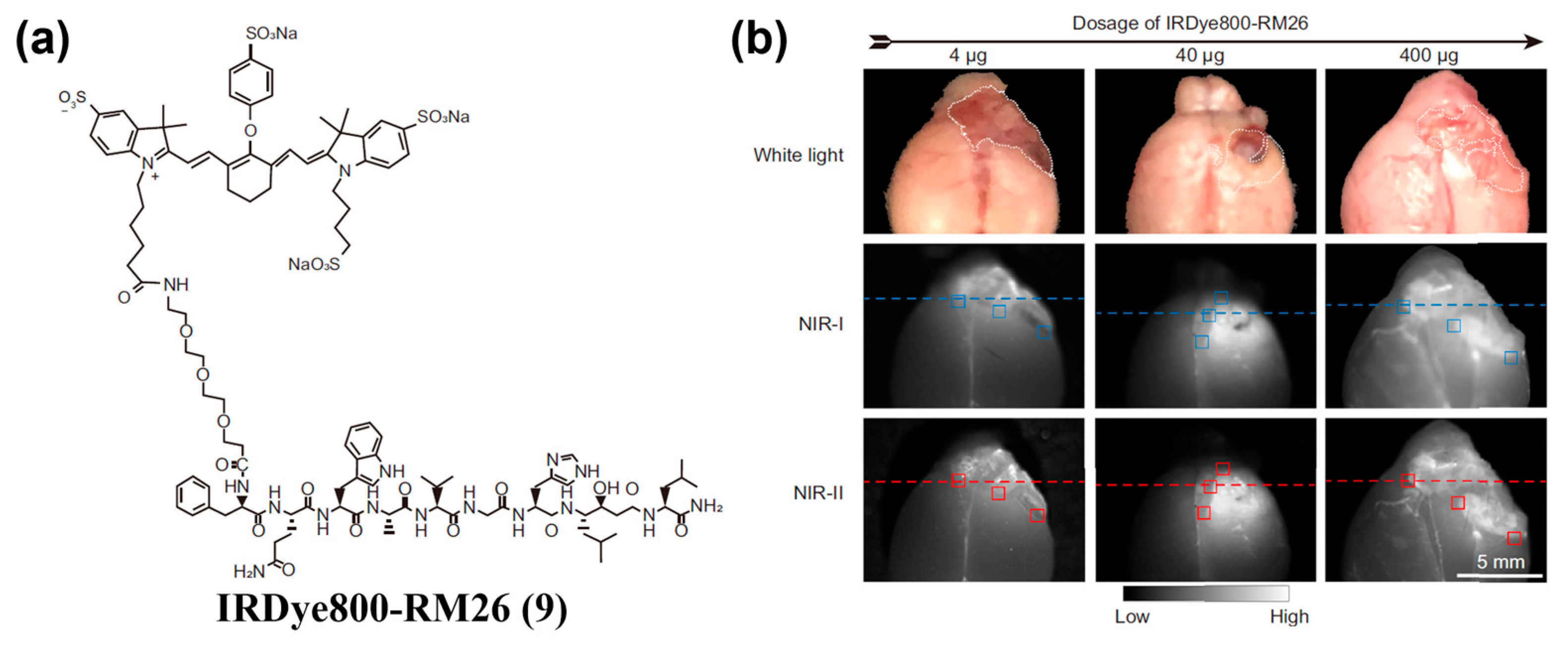
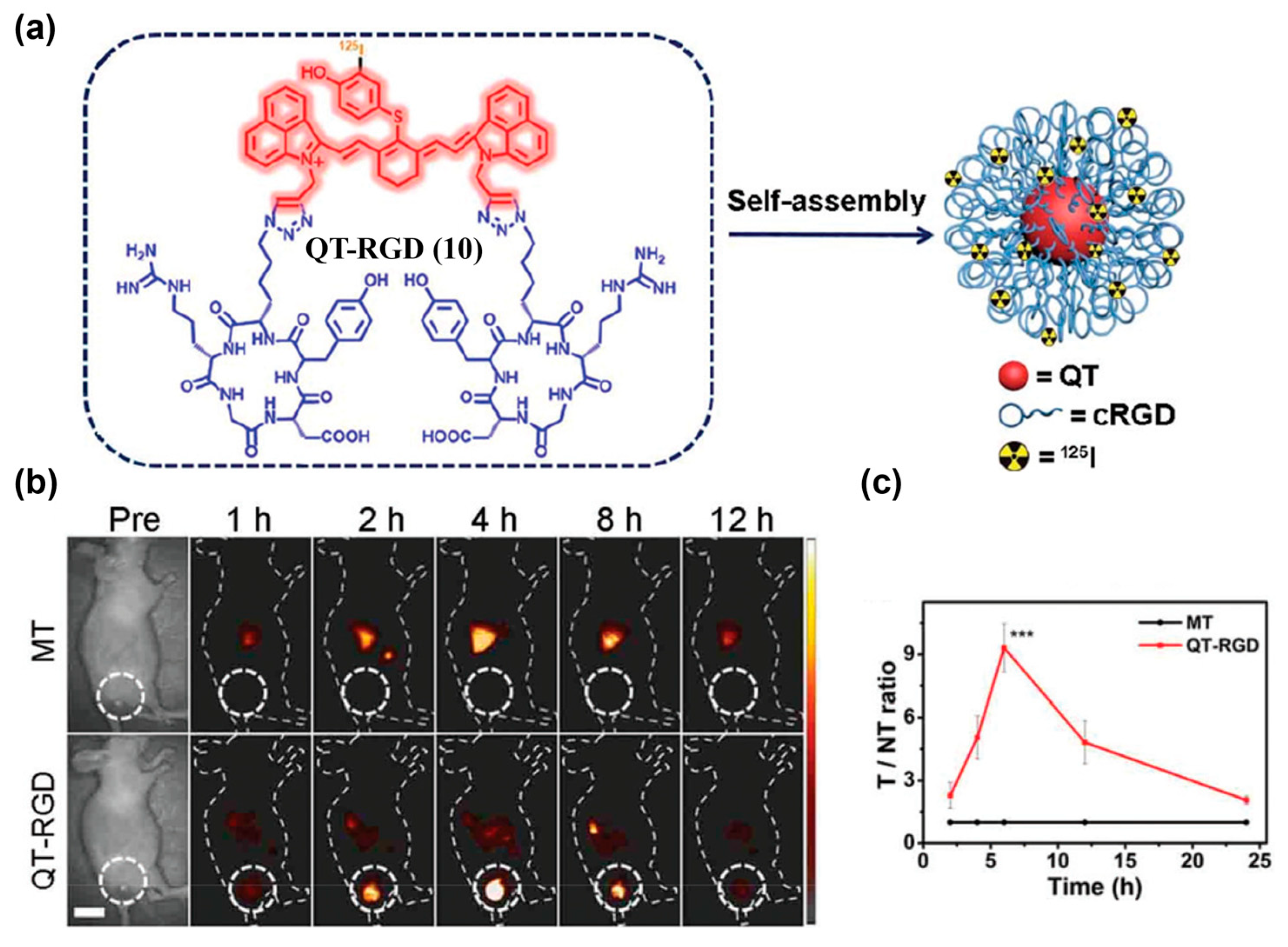
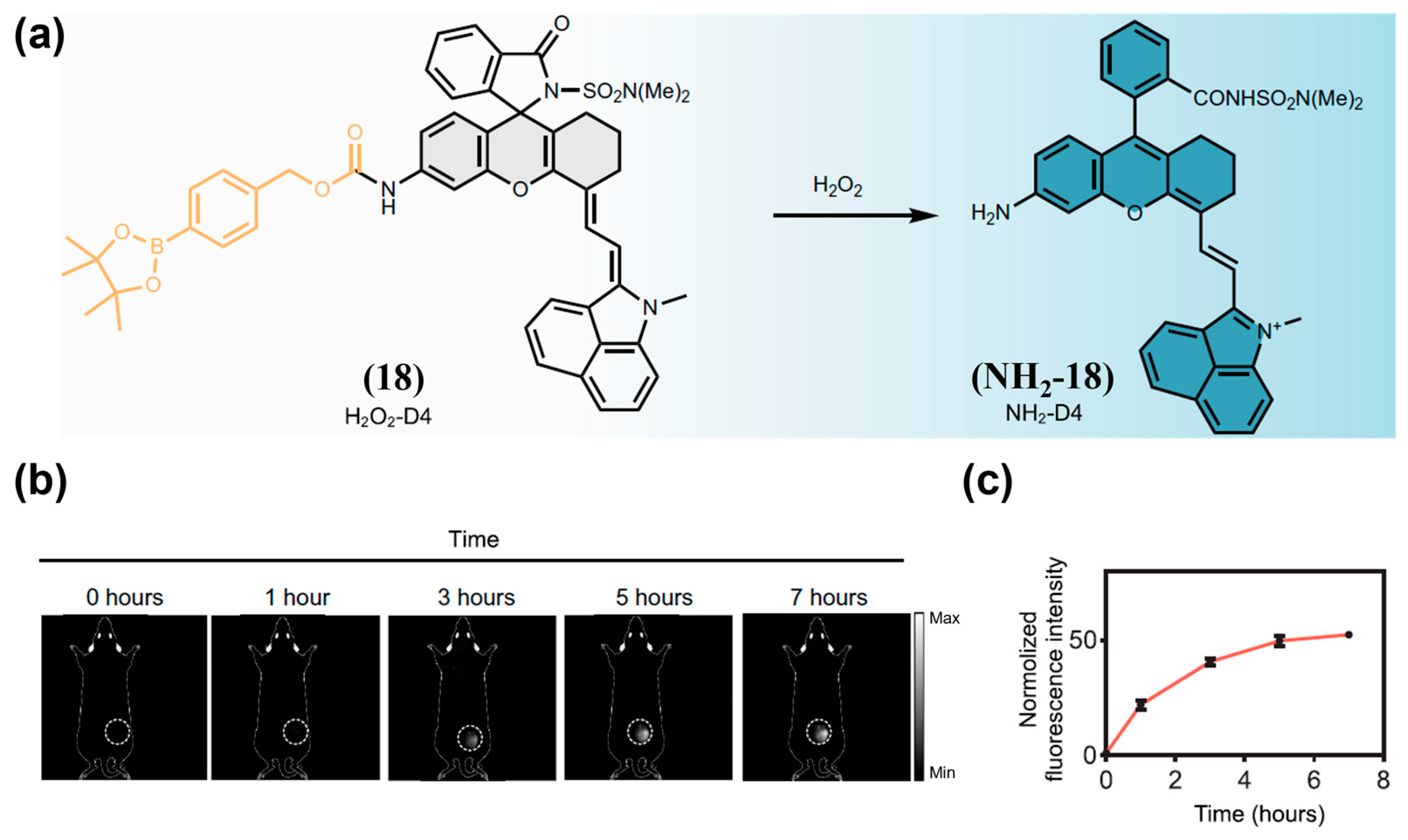
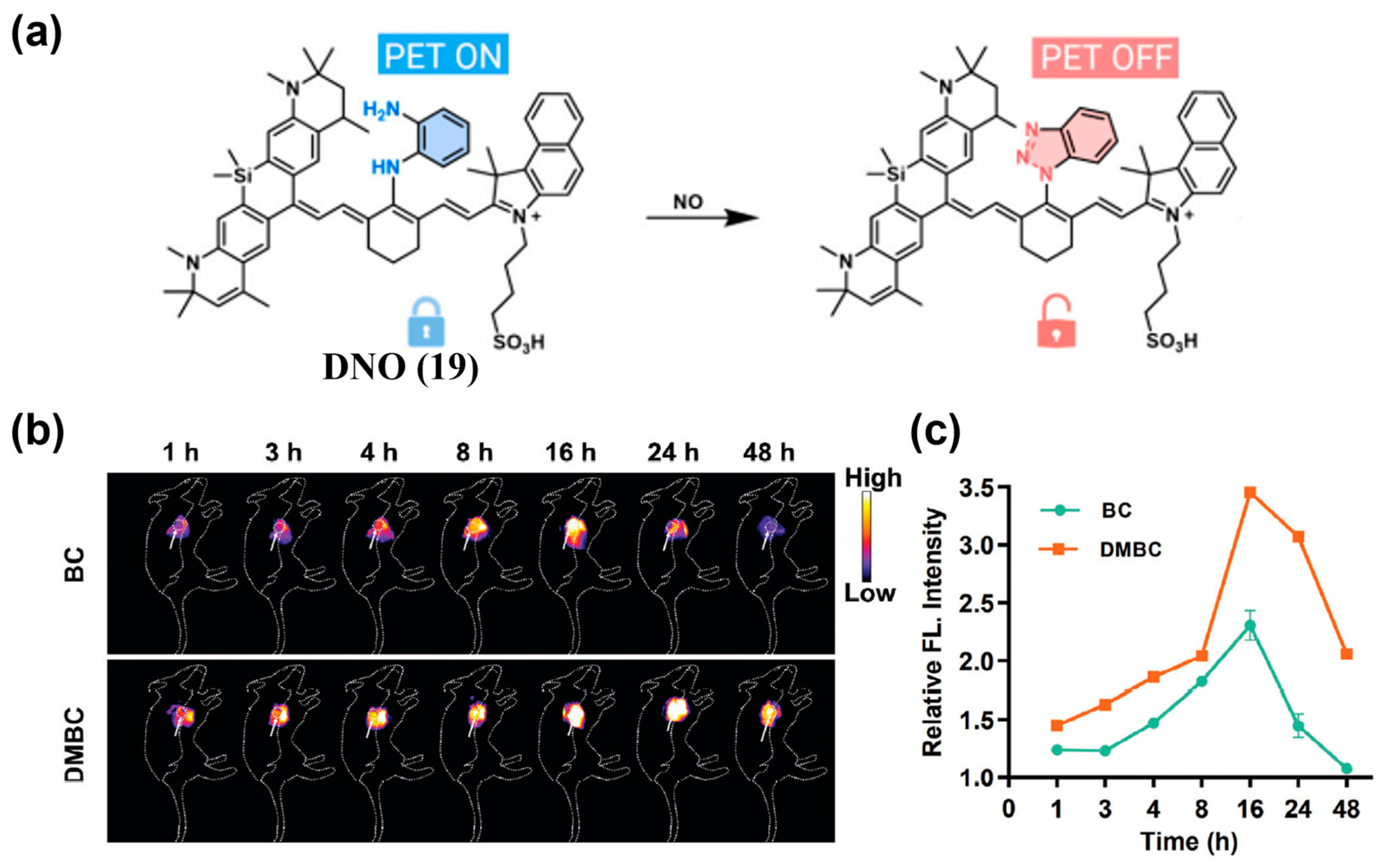
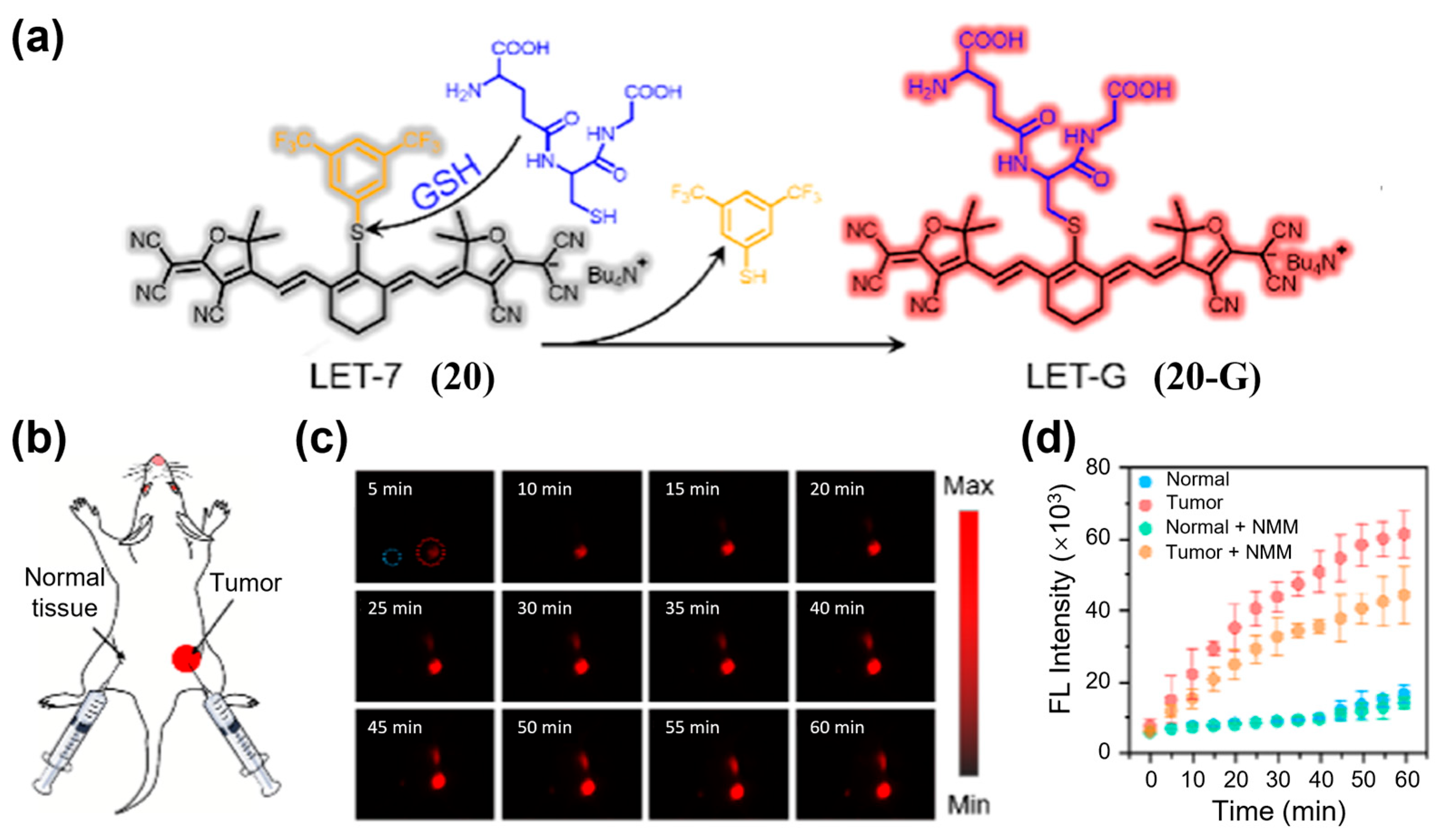
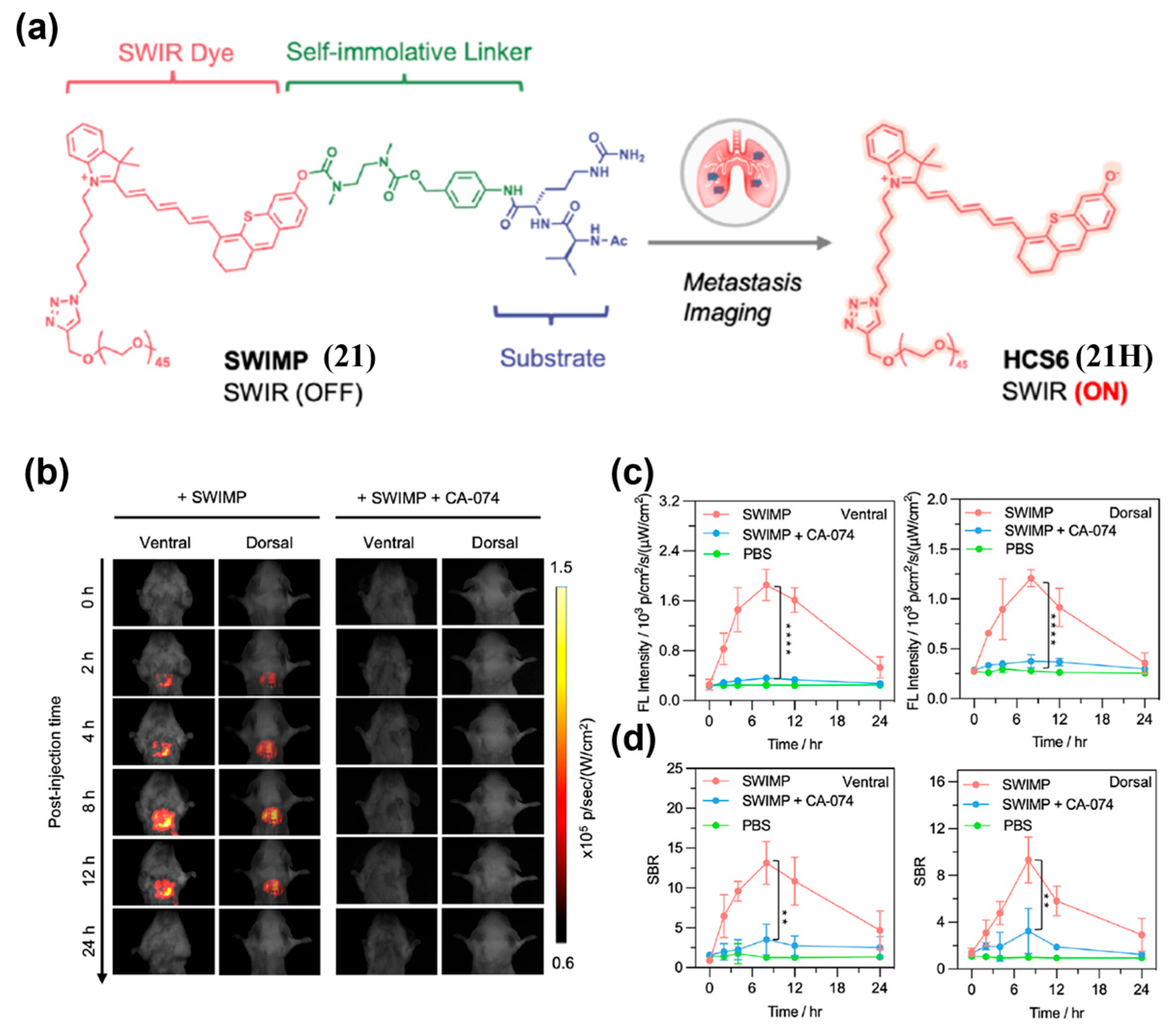
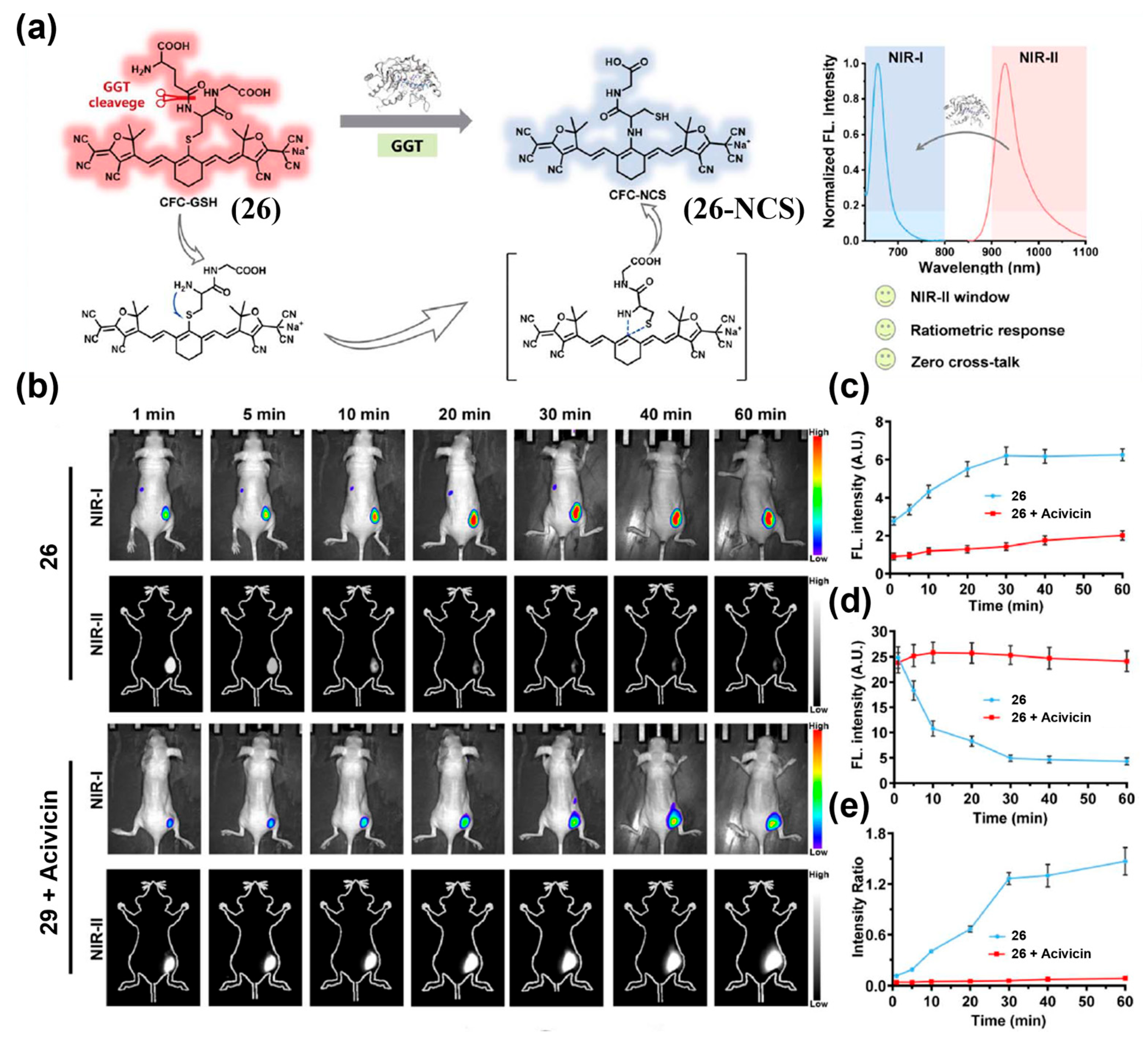
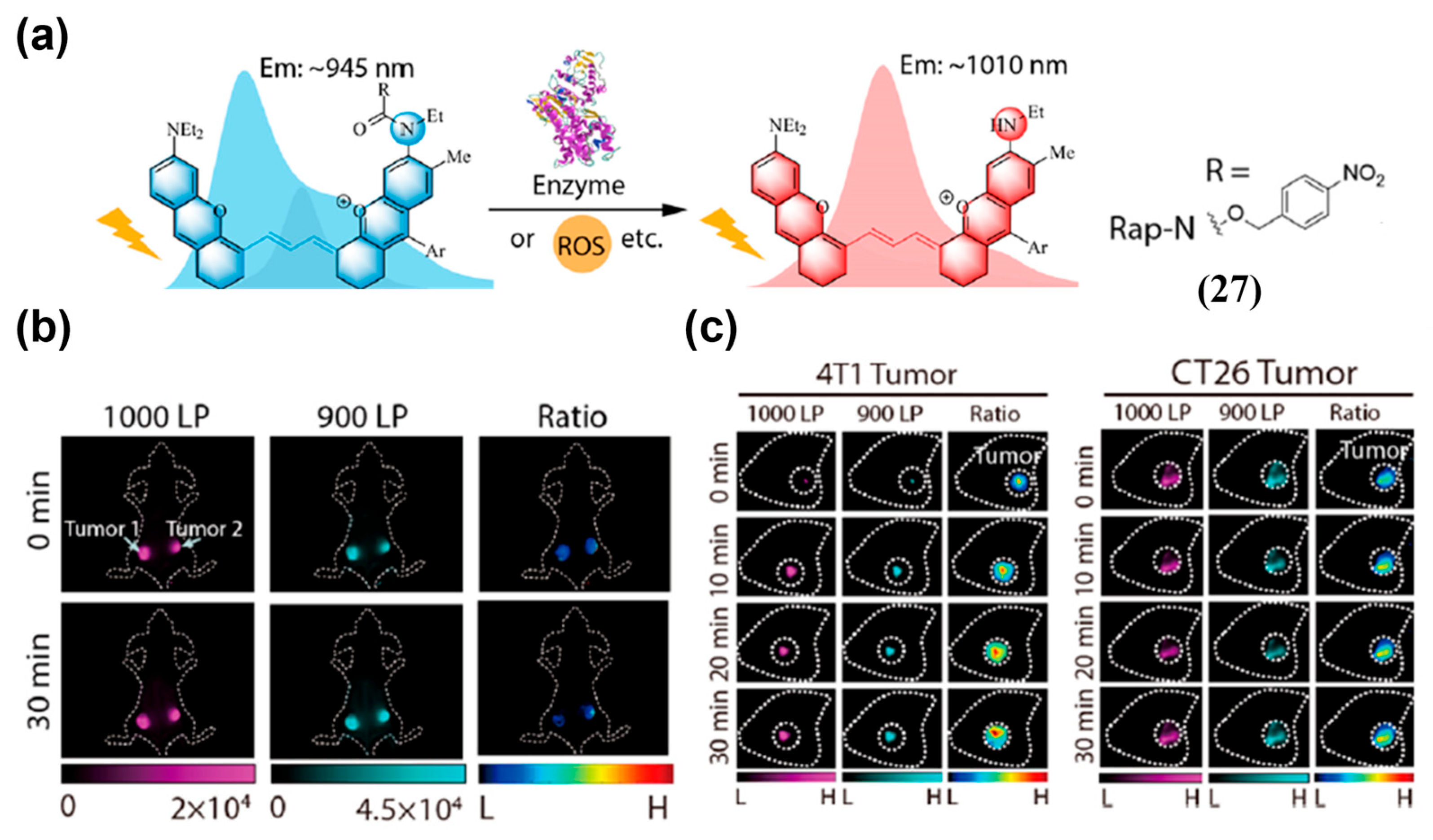
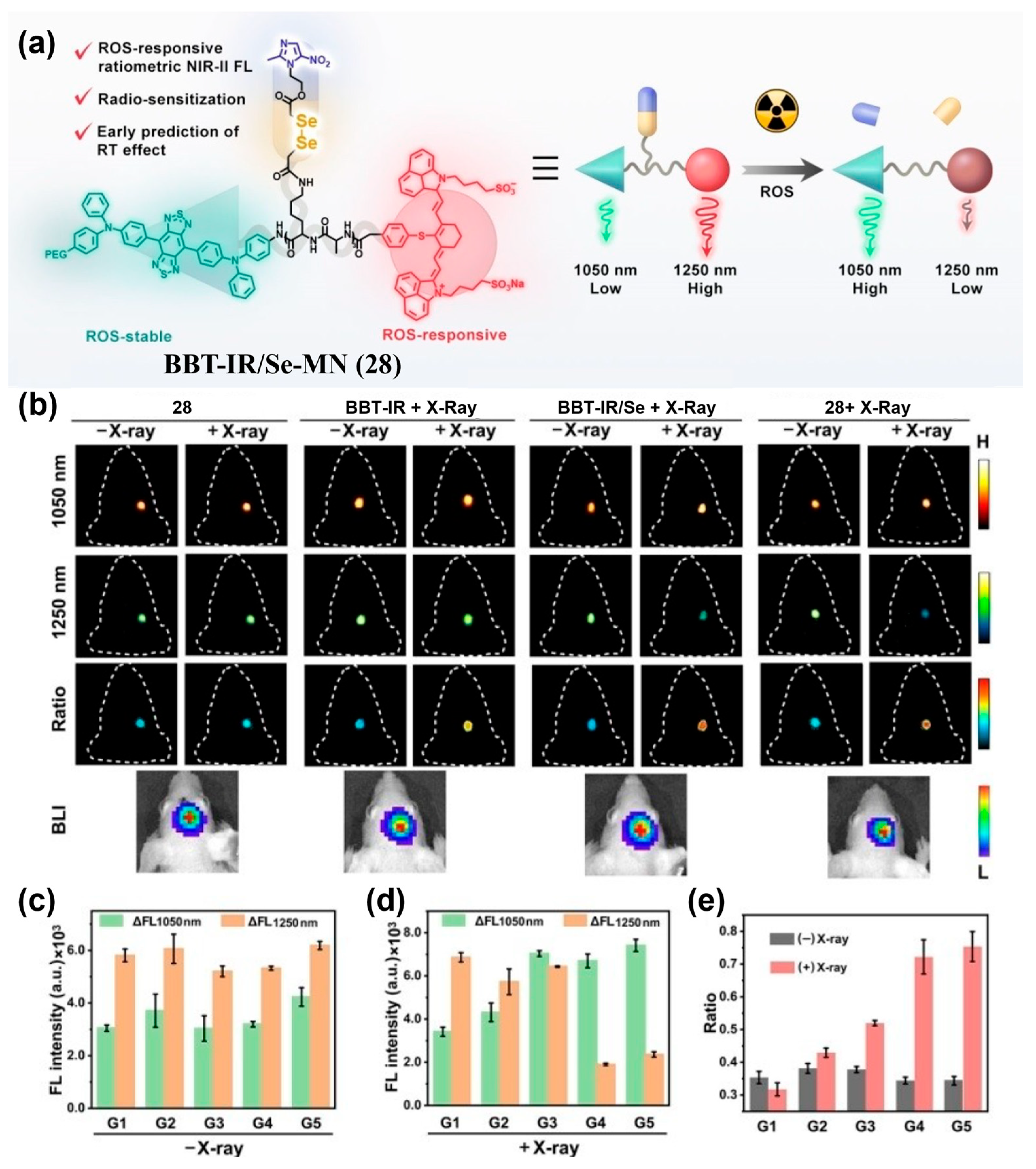
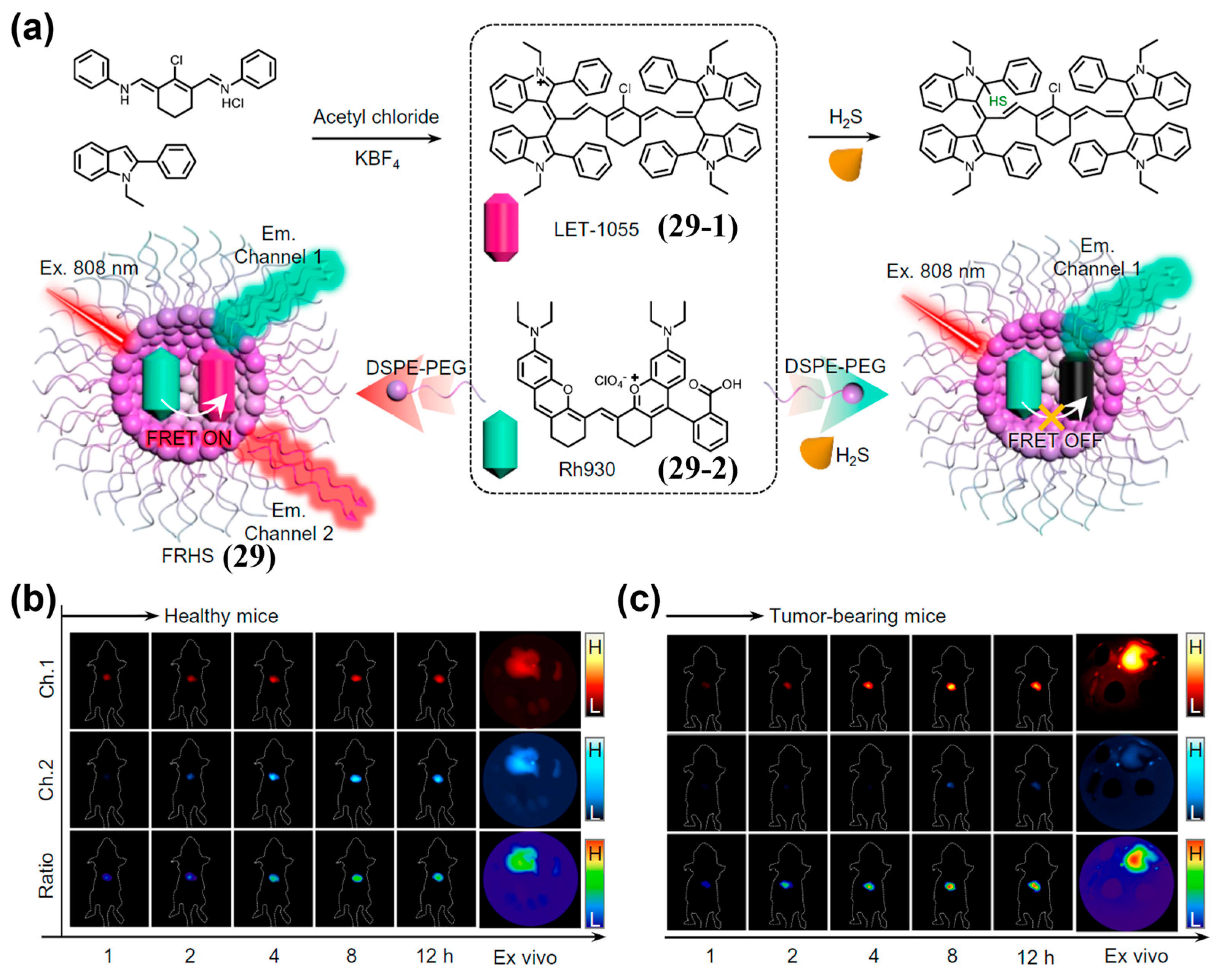
| Fluorophore Class | Representative Example | λabs (nm) | λem (nm) | Solvent | QY (%) | Ref. |
|---|---|---|---|---|---|---|
| D-A-D fluorophores | CH1055 | 750 | 1055 | H2O | 0.03 [a] | [68] |
| H2a-4T | 738 | 1024 | H2O | 0.01 [a] | [69] | |
| Cyanine fluorophores | FD1080 | 1064 | 1080 | H2O | 0.31 [a] | [70] |
| Flav 7 | 1026 | 1045 | H2O | 0.53 [a] | [71] | |
| BODIPY fluorophores | NJ1060 | 910 | 1060 | H2O | 0.50 [a] | [72] |
| WH-4 | 960 | 1205 | H2O | 0.05 [b] | [73] | |
| Xanthene fluorophores | CX-3 | 1089 | 1140 | CHCl3 | 0.09 [a] | [74] |
| VIX-4 | 1014 | 1210 | DCM | 0.03 [a] | [75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, M.; Hu, X.; Du, W. Near-Infrared-II Fluorescence Imaging of Tumors with Organic Small-Molecule Fluorophores. Sensors 2025, 25, 7080. https://doi.org/10.3390/s25227080
Guo M, Hu X, Du W. Near-Infrared-II Fluorescence Imaging of Tumors with Organic Small-Molecule Fluorophores. Sensors. 2025; 25(22):7080. https://doi.org/10.3390/s25227080
Chicago/Turabian StyleGuo, Mao, Xiaomu Hu, and Wei Du. 2025. "Near-Infrared-II Fluorescence Imaging of Tumors with Organic Small-Molecule Fluorophores" Sensors 25, no. 22: 7080. https://doi.org/10.3390/s25227080
APA StyleGuo, M., Hu, X., & Du, W. (2025). Near-Infrared-II Fluorescence Imaging of Tumors with Organic Small-Molecule Fluorophores. Sensors, 25(22), 7080. https://doi.org/10.3390/s25227080






