Critical Narrative Review of the Applications of Near-Infrared Spectroscopy Technology in Sports Science
Abstract
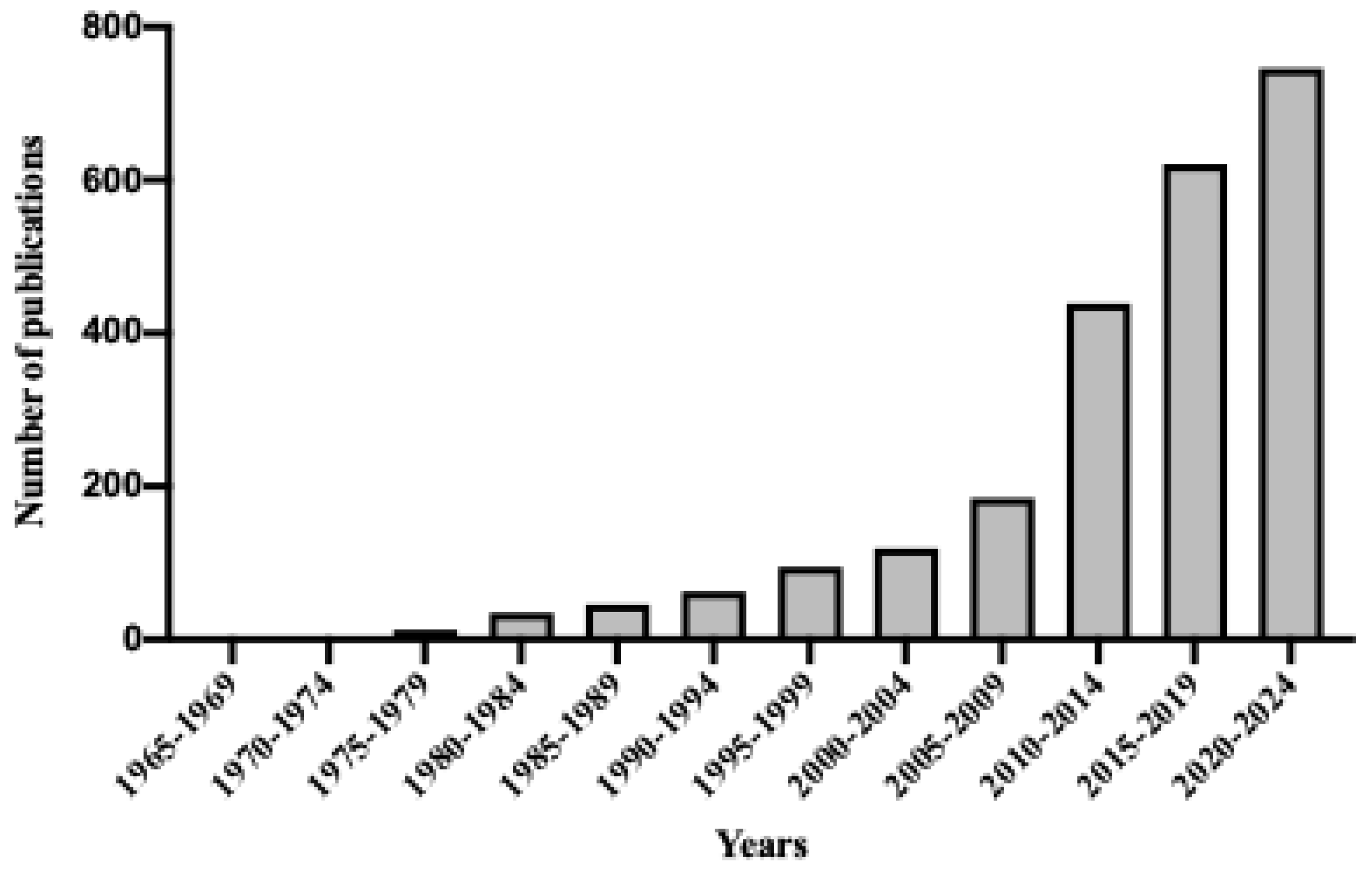
1. Introduction
2. Applications of NIRS Technology in Sports
2.1. Performance Assessment
- -
- Thresholds determination during graded exercise testing in several sports (e.g., cycling, running, and rowing) and muscles (e.g., vastus lateralis or gastrocnemius) [15,16,17]. A recent meta-analysis suggests that determining the second threshold is relatively reliable, whereas the first threshold is somewhat less consistent [15]. Furthermore, there is no consistent evidence suggesting that local thresholds occur at different times than systemic thresholds [18]. Therefore, although NIRS can be considered an interesting non-invasive tool to determine the second metabolic threshold, there is a lack of evidence about its usefulness for determining specific thresholds for different muscles.
- -
- Physiological load assessment through muscle oxygenation responses during strength training (i.e., squat exercise) [19]. Although this application is promising, it remains unclear how factors like fatigue or muscle damage influence SmO2 responses. Evidence indicates that exercise-induced muscle damage can alter muscle oxygenation kinetics at rest and during exercise [20]. Likewise, acute high-intensity efforts can modify SmO2 dynamics in a task-specific manner and are associated with performance loss [21]. Future studies should consider whether specific protocols or exercises should be standardized to improve the reproducibility of internal load assessments.
- -
- Asymmetry limb assessment of muscle oxygenation responses in team [22] and endurance sports [23,24]. In cyclic sports like cycling, muscle oxygen saturation tends to be symmetrical across limbs [24]. However, individual differences have been observed, underscoring the potential utility of individualized assessments [24]. Future research should determine whether such asymmetries have clinical implications or reflect technical inefficiencies.
- -
2.2. Physiological Adaptations
- -
- Monitoring improvements in muscle oxygenation during rehabilitation or training programs. For example, NIRS has been applied to evaluate muscle oxygenation in patients with stroke or chronic obstructive pulmonary disease during rehabilitation, showing potential for clinical application in monitoring functional recovery [27].
- -
- Evaluation of muscle oxygenation responses during intervention (e.g., the use of a tracksuit jacket with heating elements or supplementary inorganic nitrate) [28,29]. As with other physiological markers, NIRS provides a non-invasive means to assess differences in muscle metabolism under various experimental conditions.
- -
- -
- Determination of mitochondrial capacity through the assessment of muscle oxidative function (the maximum rate at which the muscle can utilize O2 to meet the energy demand of exercise) [33]. A widely used and reproducible method for this purpose is the 10 min occlusion test [33,34]. However, the literature reveals high variability in key methodological aspects such as occlusion pressure and duration [2]. Standardized protocols or expert consensus statements are needed to guide future applications.
3. NIRS Technology and Team Sports
4. NIRS Technology and Endurance Sports
5. Limitations of NIRS
6. Future Studies
- ○
- Future studies should incorporate longitudinal designs to better assess training-induced changes.
- ○
- More research is needed in female athlete populations, as sex-based differences in muscle oxygen saturation have been reported [46].
- ○
- Further field-based studies should aim to link NIRS-derived metrics to actual performance outcomes during training, gameplay, and competition scenarios.
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McNulty, J.; Born, M.; Pozos, R.S. Near-Infrared Spectroscopy (NIRS). In Springer Handbook of Medical Technology; Kramme, R., Hoffmann, K.-P., Pozos, R.S., Eds.; Springer Handbooks; Springer: Berlin/Heidelberg, Germany, 2011; pp. 423–438. ISBN 978-3-540-74658-4. [Google Scholar]
- Barstow, T.J. Understanding near Infrared Spectroscopy and Its Application to Skeletal Muscle Research. J. Appl. Physiol. 2019, 126, 1360–1376. [Google Scholar] [CrossRef] [PubMed]
- Boone, J.; Barstow, T.J.; Celie, B.; Prieur, F.; Bourgois, J. The Interrelationship between Muscle Oxygenation, Muscle Activation, and Pulmonary Oxygen Uptake to Incremental Ramp Exercise: Influence of Aerobic Fitness. Appl. Physiol. Nutr. Metab. 2016, 41, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Perrey, S.; Ferrari, M. Muscle Oximetry in Sports Science: A Systematic Review. Sports Med. 2018, 48, 597–616. [Google Scholar] [CrossRef] [PubMed]
- Perrey, S.; Quaresima, V.; Ferrari, M. Muscle Oximetry in Sports Science: An Updated Systematic Review. Sports Med. 2024, 54, 975–996. [Google Scholar] [CrossRef]
- Scheeren, T.W.L.; Schober, P.; Schwarte, L.A. Monitoring Tissue Oxygenation by near Infrared Spectroscopy (NIRS): Background and Current Applications. J. Clin. Monit. Comput. 2012, 26, 279–287. [Google Scholar] [CrossRef]
- Feldmann, A.; Schmitz, R.; Erlacher, D. Near-Infrared Spectroscopy-Derived Muscle Oxygen Saturation on a 0% to 100% Scale: Reliability and Validity of the Moxy Monitor. J. Biomed. Opt. 2019, 24, 115001. [Google Scholar] [CrossRef]
- Crum, E.M.; O’Connor, W.J.; Van Loo, L.; Valckx, M.; Stannard, S.R. Validity and Reliability of the Moxy Oxygen Monitor during Incremental Cycling Exercise. Eur. J. Sport Sci. 2017, 17, 1037–1043. [Google Scholar] [CrossRef]
- Gandia-Soriano, A.; Salas-Montoro, J.-A.; Javaloyes, A.; Lorente-Casaus, C.; Zabala, M.; Priego-Quesada, J.I.; March, M.M. Validity and Reliability of Two Near-infrared Spectroscopy Devices to Measure Resting Hemoglobin in Elite Cyclists. Int. J. Sports Med. 2022, 43, 875–880. [Google Scholar] [CrossRef]
- Yogev, A.; Arnold, J.; Nelson, H.; Clarke, D.C.; Guenette, J.A.; Sporer, B.C.; Koehle, M.S. Comparing the Reliability of Muscle Oxygen Saturation with Common Performance and Physiological Markers across Cycling Exercise Intensity. Front. Sports Act. Living 2023, 5, 1143393. [Google Scholar] [CrossRef]
- Hamaoka, T.; McCully, K.K. Review of Early Development of Near-Infrared Spectroscopy and Recent Advancement of Studies on Muscle Oxygenation and Oxidative Metabolism. J. Physiol. Sci. 2019, 69, 799–811. [Google Scholar] [CrossRef]
- Severinghaus, J.W. Takuo Aoyagi: Discovery of Pulse Oximetry. Anesth. Analg. 2007, 105, S1. [Google Scholar] [CrossRef]
- Jöbsis, F.F. Noninvasive, Infrared Monitoring of Cerebral and Myocardial Oxygen Sufficiency and Circulatory Parameters. Science 1977, 198, 1264–1267. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.; Hesford, C.M.; Cooper, C.E. The Use of Portable NIRS to Measure Muscle Oxygenation and Haemodynamics During a Repeated Sprint Running Test. In Oxygen Transport to Tissue XXXV; Van Huffel, S., Naulaers, G., Caicedo, A., Bruley, D.F., Harrison, D.K., Eds.; Springer: New York, NY, USA, 2013; pp. 185–191. [Google Scholar]
- Sendra-Pérez, C.; Sanchez-Jimenez, J.L.; Marzano-Felisatti, J.M.; Encarnación-Martínez, A.; Salvador-Palmer, R.; Priego-Quesada, J.I. Reliability of Threshold Determination Using Portable Muscle Oxygenation Monitors during Exercise Testing: A Systematic Review and Meta-Analysis. Sci. Rep. 2023, 13, 12649. [Google Scholar] [CrossRef] [PubMed]
- Batterson, P.M.; Kirby, B.S.; Hasselmann, G.; Feldmann, A. Muscle Oxygen Saturation Rates Coincide with Lactate-Based Exercise Thresholds. Eur. J. Appl. Physiol. 2023, 123, 2249–2258. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, A.; Ammann, L.; Gächter, F.; Zibung, M.; Erlacher, D. Muscle Oxygen Saturation Breakpoints Reflect Ventilatory Thresholds in Both Cycling and Running. J. Hum. Kinet. 2022, 83, 87–97. [Google Scholar] [CrossRef]
- Sendra-Pérez, C.; Encarnacion-Martinez, A.; Salvador-Palmer, R.; Murias, J.M.; Priego-Quesada, J.I. Profiles of Muscle-Specific Oxygenation Responses and Thresholds during Graded Cycling Incremental Test. Eur. J. Appl. Physiol. 2024, 125, 237–245. [Google Scholar] [CrossRef]
- Gómez-Carmona, C.D.; Bastida-Castillo, A.; Rojas-Valverde, D.; de la Cruz Sánchez, E.; García-Rubio, J.; Ibáñez, S.J.; Pino-Ortega, J. Lower-Limb Dynamics of Muscle Oxygen Saturation During the Back-Squat Exercise: Effects of Training Load and Effort Level. J. Strength Cond. Res. 2020, 34, 1227. [Google Scholar] [CrossRef]
- Ahmadi, S.; Sinclair, P.J.; Davis, G.M. Muscle Oxygenation after Downhill Walking-Induced Muscle Damage. Clin. Physiol. Funct. Imaging 2008, 28, 55–63. [Google Scholar] [CrossRef]
- Feldmann, A.; Lehmann, R.; Wittmann, F.; Wolf, P.; Baláš, J.; Erlacher, D. Acute Effect of High-Intensity Climbing on Performance and Muscle Oxygenation in Elite Climbers. J. Sci. Sport Exerc. 2022, 4, 145–155. [Google Scholar] [CrossRef]
- Vasquez-Bonilla, A.A.; Brazo-Sayavera, J.; Timón, R.; Olcina, G. Monitoring Muscle Oxygen Asymmetry as a Strategy to Prevent Injuries in Footballers. Res. Q. Exerc. Sport 2023, 94, 609–617. [Google Scholar] [CrossRef]
- Reinpõld, K.; Rannama, I. Oxygen Uptake and Bilaterally Measured Vastus Lateralis Muscle Oxygen Desaturation Kinetics in Well-Trained Endurance Cyclists. J. Funct. Morphol. Kinesiol. 2023, 8, 64. [Google Scholar] [CrossRef]
- Sendra-Pérez, C.; Priego-Quesada, J.I.; Murias, J.M.; Carpes, F.P.; Salvador-Palmer, R.; Encarnación-Martínez, A. Evaluation of Leg Symmetry in Muscle Oxygen Saturation during Submaximal to Maximal Cycling Exercise. Eur. J. Sport Sci. 2025, 25, e12230. [Google Scholar] [CrossRef]
- Matthews, I.R.; Heenan, L.J.; Fisher, K.G.; Flood, E.F.; Wehrman, L.W.; Kirby, B.S.; Wilkins, B.W. Identification of Maximal Steady-State Metabolic Rate by the Change in Muscle Oxygen Saturation. J. Appl. Physiol. 2023, 134, 1349–1358. [Google Scholar] [CrossRef]
- Kirby, B.S.; Clark, D.A.; Bradley, E.M.; Wilkins, B.W. The Balance of Muscle Oxygen Supply and Demand Reveals Critical Metabolic Rate and Predicts Time to Exhaustion. J. Appl. Physiol. 2021, 130, 1915–1927. [Google Scholar] [CrossRef]
- Szucs, B.; Petrekanits, M.; Fekete, M.; Varga, J.T. The Use of Near-Infrared Spectroscopy for the Evaluation of a 4-Week Rehabilitation Program in Patients with COPD. Physiol. Int. 2021, 108, 427–439. [Google Scholar] [CrossRef] [PubMed]
- McGowan, C.J.; Pyne, D.B.; Thompson, K.G.; Raglin, J.S.; Osborne, M.; Rattray, B. Elite Sprint Swimming Performance Is Enhanced by Completion of Additional Warm-up Activities. J. Sports Sci. 2017, 35, 1493–1499. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.S.; McKay, A.K.A.; Kuikman, M.; Ackerman, K.E.; Harris, R.; Elliott-Sale, K.J.; Stellingwerff, T.; Burke, L.M. Auditing the Representation of Female Versus Male Athletes in Sports Science and Sports Medicine Research: Evidence-Based Performance Supplements. Nutrients 2022, 14, 953. [Google Scholar] [CrossRef] [PubMed]
- Dominelli, P.B.; Molgat-Seon, Y.; Griesdale, D.E.G.; Peters, C.M.; Blouin, J.-S.; Sekhon, M.; Dominelli, G.S.; Henderson, W.R.; Foster, G.E.; Romer, L.M.; et al. Exercise-Induced Quadriceps Muscle Fatigue in Men and Women: Effects of Arterial Oxygen Content and Respiratory Muscle Work. J. Physiol. 2017, 595, 5227–5244. [Google Scholar] [CrossRef]
- Yogev, A.; Arnold, J.; Nelson, H.; Clarke, D.C.; Guenette, J.A.; Sporer, B.C.; Koehle, M.S. The Effect of Severe Intensity Bouts on Muscle Oxygen Saturation Responses in Trained Cyclists. Front. Sports Act. Living 2023, 5, 1086227. [Google Scholar] [CrossRef]
- Tam, E.; Bertucco, M.; Capelli, C. The Slow Component of Oxygen Uptake of Insulated Muscular Groups Measured with NIRS during Intermittent Isometric Contractions in Humans. Physiol. Rep. 2025, 13, e70491. [Google Scholar] [CrossRef]
- Fennell, C.R.J.; Mauger, A.R.; Hopker, J.G. Reproducibility of NIRS-Derived Mitochondrial Oxidative Capacity in Highly Active Older Adults. Exp. Gerontol. 2023, 175, 112156. [Google Scholar] [CrossRef]
- McLay, K.M.; Nederveen, J.P.; Pogliaghi, S.; Paterson, D.H.; Murias, J.M. Repeatability of Vascular Responsiveness Measures Derived from Near-Infrared Spectroscopy. Physiol. Rep. 2016, 4, e12772. [Google Scholar] [CrossRef]
- Stöggl, T.; Born, D.-P. Near Infrared Spectroscopy for Muscle Specific Analysis of Intensity and Fatigue during Cross-Country Skiing Competition—A Case Report. Sensors 2021, 21, 2535. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.; Hamilton, D.K.; Cooper, C.E. Muscle Oxygen Changes Following Sprint Interval Cycling Training in Elite Field Hockey Players. PLoS ONE 2015, 10, e0120338. [Google Scholar] [CrossRef] [PubMed]
- Kounalakis, S.N.; Bayios, I.A.; Koskolou, M.D.; Geladas, N.D. Anaerobic Capacity of the Upper Arms in Top-Level Team Handball Players. Int. J. Sports Physiol. Perform. 2008, 3, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.G. Oxygen Regulation in Biological Systems. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R673–R678. [Google Scholar] [CrossRef]
- Caen, K.; Bourgois, J.G.; Stassijns, E.; Boone, J. A Longitudinal Study on the Interchangeable Use of Whole-Body and Local Exercise Thresholds in Cycling. Eur. J. Appl. Physiol. 2022, 122, 1657–1670. [Google Scholar] [CrossRef]
- Caen, K.; Boone, J. Response to Goulding et al. (2022): Are Whole-Body and Local Thresholds Mechanistically Linked? Eur. J. Appl. Physiol. 2023, 123, 421–422. [Google Scholar] [CrossRef]
- Goulding, R.P.; Marwood, S.; Lei, T.-H.; Okushima, D.; Poole, D.C.; Barstow, T.J.; Kondo, N.; Koga, S. Time to Retire the Notion That Local and Whole-Body Exercise Thresholds Are Mechanistically Linked? Eur. J. Appl. Physiol. 2023, 123, 419–420. [Google Scholar] [CrossRef]
- Bonilla, A.V.; González-Custodio, A.; Timón, R.; Cardenosa, A.; Camacho-Cardenosa, M.; Olcina, G. Training Zones through Muscle Oxygen Saturation during a Graded Exercise Test in Cyclists and Triathletes. Biol. Sport 2022, 40, 439–448. [Google Scholar] [CrossRef]
- Born, D.-P.; Stöggl, T.; Swarén, M.; Björklund, G. Near-Infrared Spectroscopy: More Accurate Than Heart Rate for Monitoring Intensity in Running in Hilly Terrain. Int. J. Sports Physiol. Perform. 2017, 12, 440–447. [Google Scholar] [CrossRef]
- Murias, J.M.; Keir, D.A.; Spencer, M.D.; Paterson, D.H. Sex-Related Differences in Muscle Deoxygenation during Ramp Incremental Exercise. Respir. Physiol. Neurobiol. 2013, 189, 530–536. [Google Scholar] [CrossRef]
- Niemeijer, V.M.; Jansen, J.P.; van Dijk, T.; Spee, R.F.; Meijer, E.J.; Kemps, H.M.C.; Wijn, P.F.F. The Influence of Adipose Tissue on Spatially Resolved Near-Infrared Spectroscopy Derived Skeletal Muscle Oxygenation: The Extent of the Problem. Physiol. Meas. 2017, 38, 539–554. [Google Scholar] [CrossRef]
- Sendra-Pérez, C.; Priego-Quesada, J.I.; Salvador-Palmer, R.; Murias, J.M.; Encarnacion-Martinez, A. Sex-Related Differences in Profiles of Muscle Oxygen Saturation of Different Muscles in Trained Cyclists during Graded Cycling Exercise. J. Appl. Physiol. 2023, 135, 1092–1101. [Google Scholar] [CrossRef]
| Muscle | Studies (N) |
|---|---|
| Vastus lateralis | 138 |
| Flexor fingers | 20 |
| Gastrocnemius medialis | 20 |
| Biceps brachii | 12 |
| Brachioradialis | 12 |
| Intercostal | 9 |
| Rectus femoris | 7 |
| Triceps brachii | 6 |
| Latissimus dorsi | 5 |
| Deltoid | 3 |
| Tibialis anterior | 2 |
| Biceps femoris | 2 |
| Trunk extensor | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sendra-Pérez, C.; Encarnación-Martínez, A.; Priego-Quesada, J.I. Critical Narrative Review of the Applications of Near-Infrared Spectroscopy Technology in Sports Science. Sensors 2025, 25, 6798. https://doi.org/10.3390/s25216798
Sendra-Pérez C, Encarnación-Martínez A, Priego-Quesada JI. Critical Narrative Review of the Applications of Near-Infrared Spectroscopy Technology in Sports Science. Sensors. 2025; 25(21):6798. https://doi.org/10.3390/s25216798
Chicago/Turabian StyleSendra-Pérez, Carlos, Alberto Encarnación-Martínez, and Jose I. Priego-Quesada. 2025. "Critical Narrative Review of the Applications of Near-Infrared Spectroscopy Technology in Sports Science" Sensors 25, no. 21: 6798. https://doi.org/10.3390/s25216798
APA StyleSendra-Pérez, C., Encarnación-Martínez, A., & Priego-Quesada, J. I. (2025). Critical Narrative Review of the Applications of Near-Infrared Spectroscopy Technology in Sports Science. Sensors, 25(21), 6798. https://doi.org/10.3390/s25216798








