A Multimodal System for Comprehensive Cardiovascular Monitoring Using ECG, PCG, and PPG Signal Fusion
Abstract
1. Introduction
2. Methods
2.1. The Proposed Vital-Sign Monitoring System
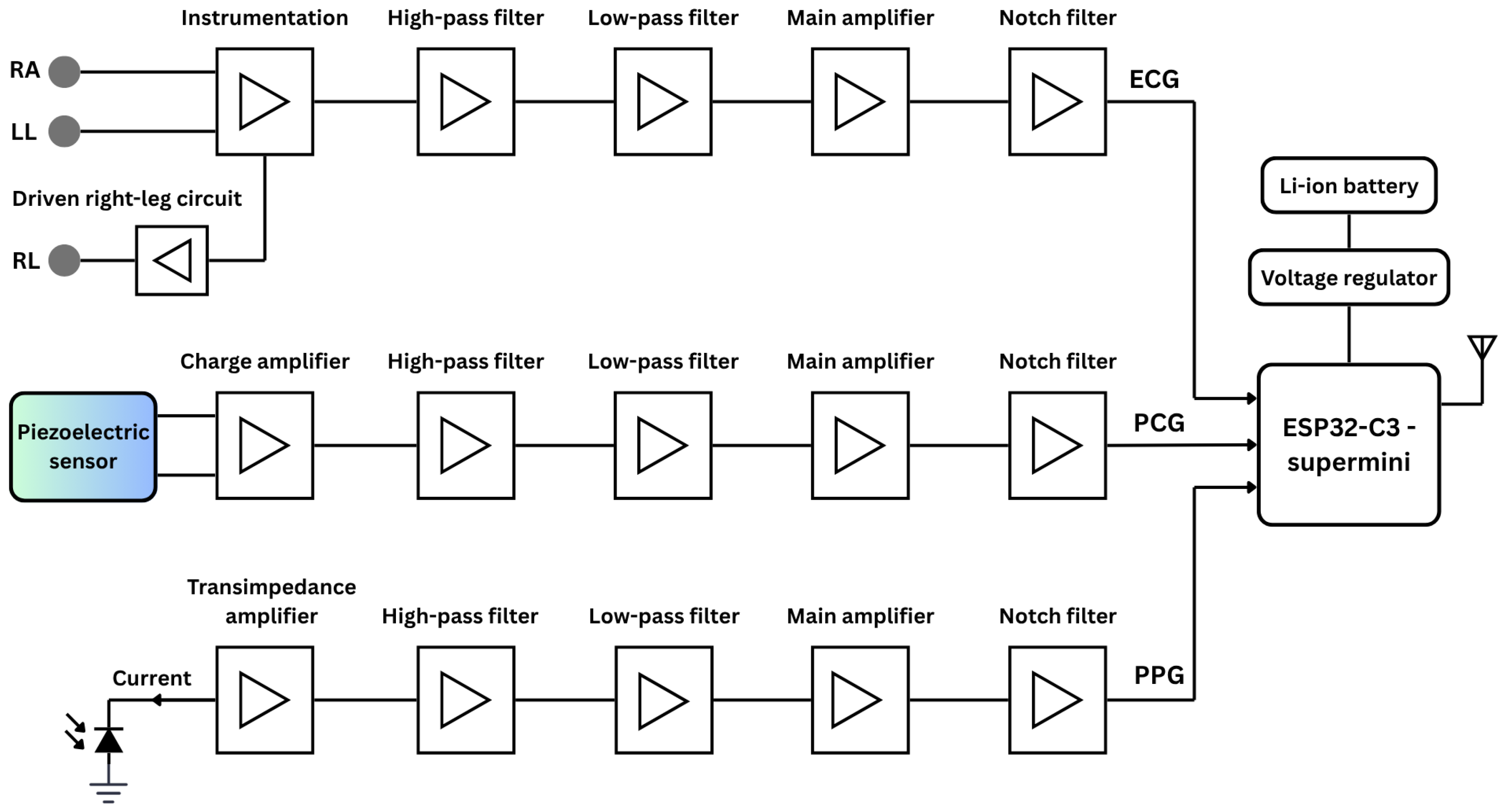
2.1.1. Simplified Circuitry
ECG Circuitry
PCG Circuitry
PPG Circuitry
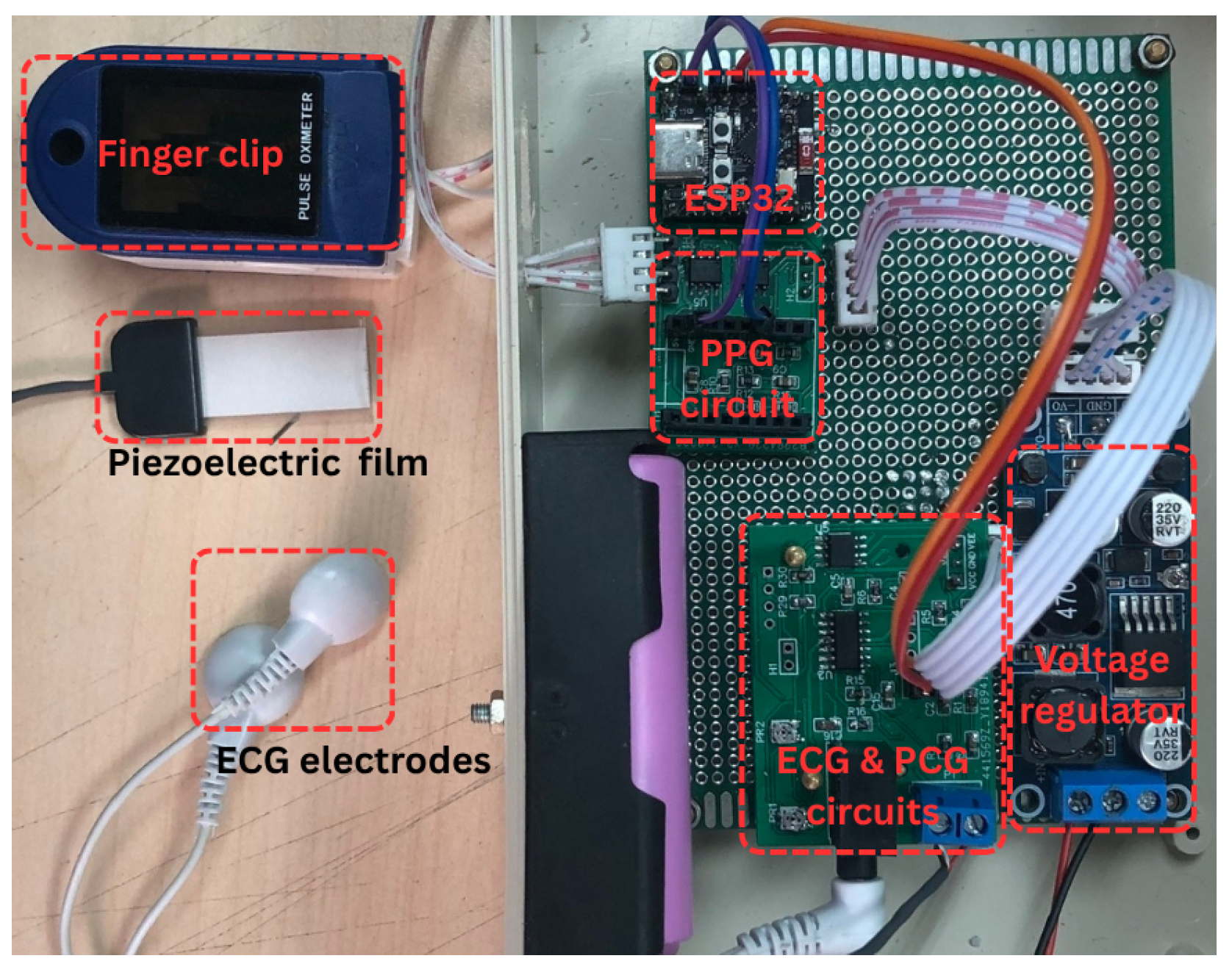
2.1.2. Photograph of the Proposed System Prototype
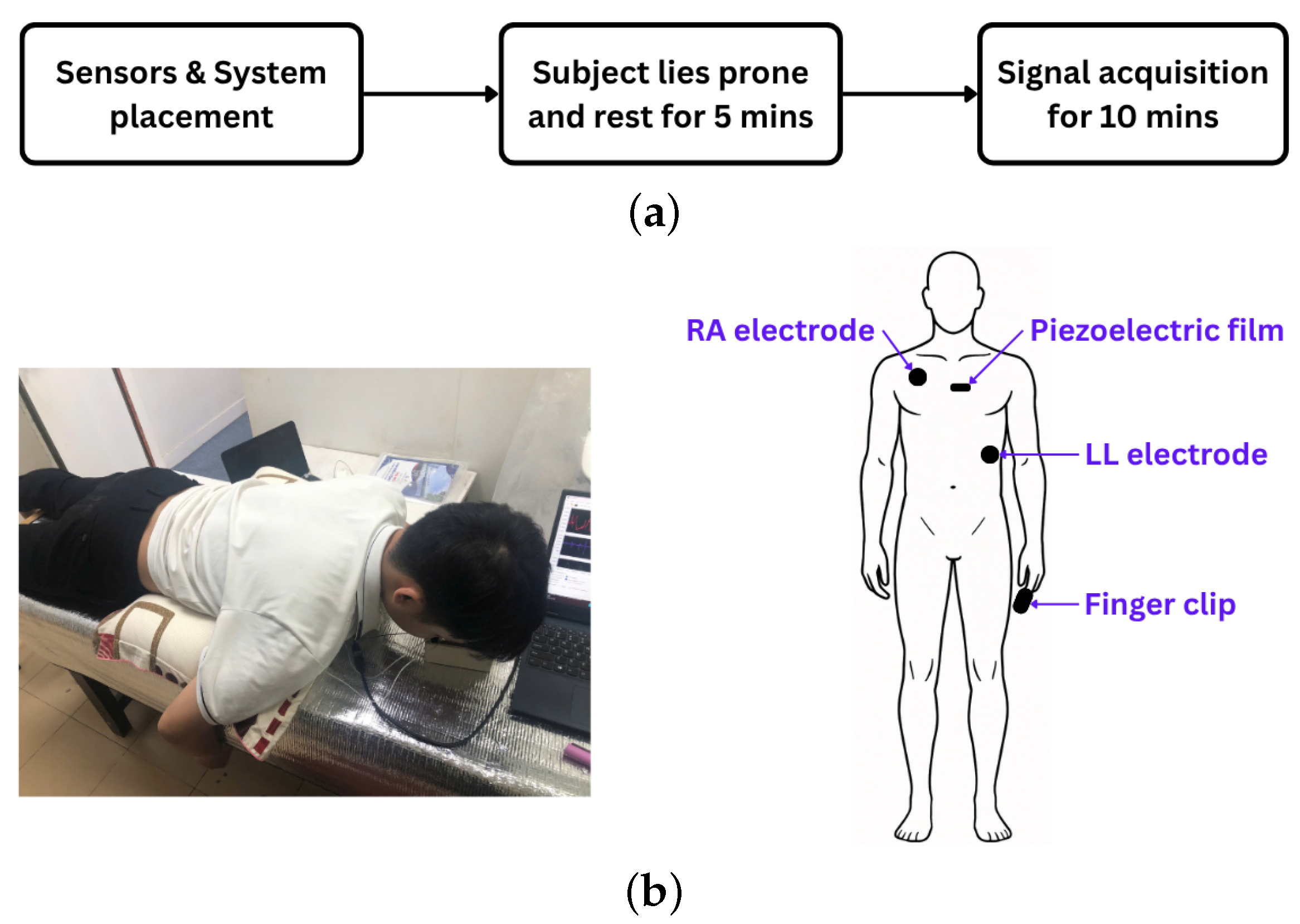
2.2. Experimental Protocol
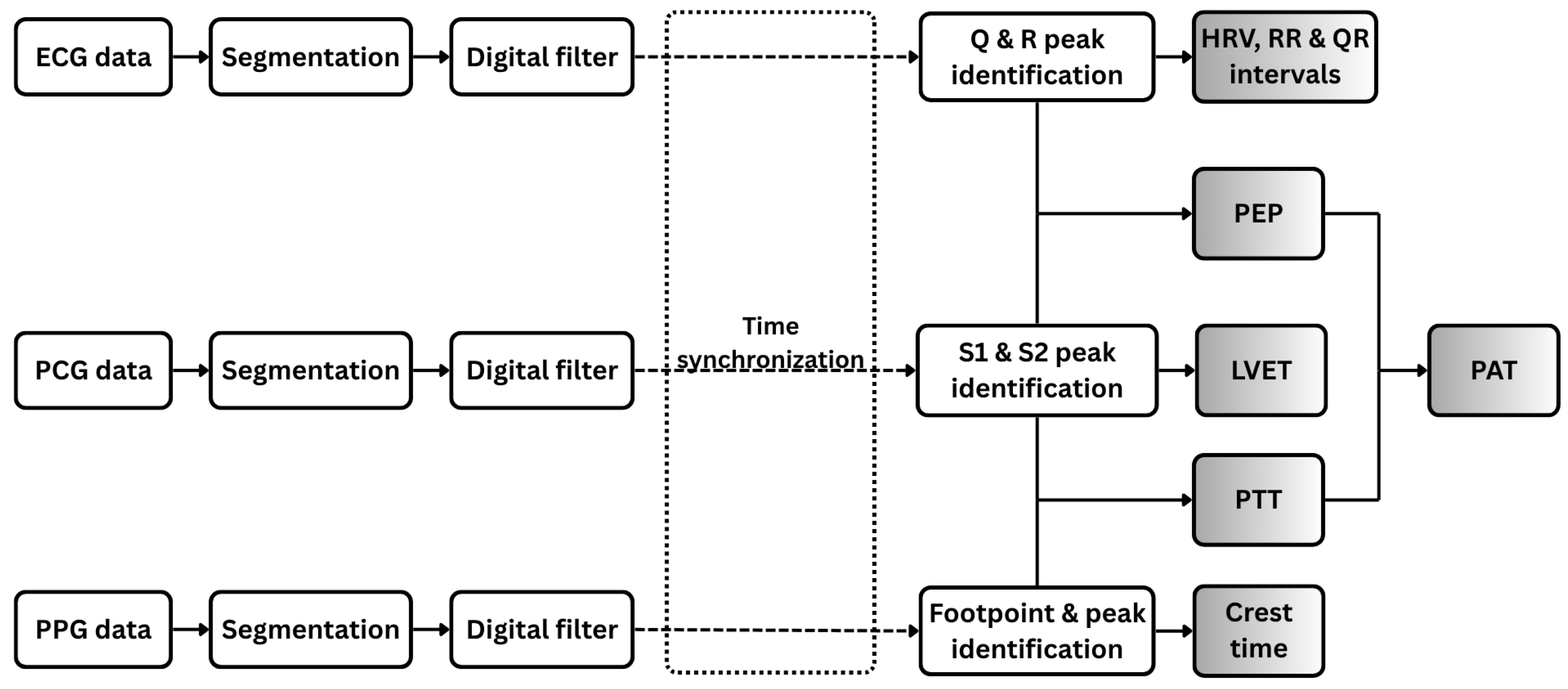
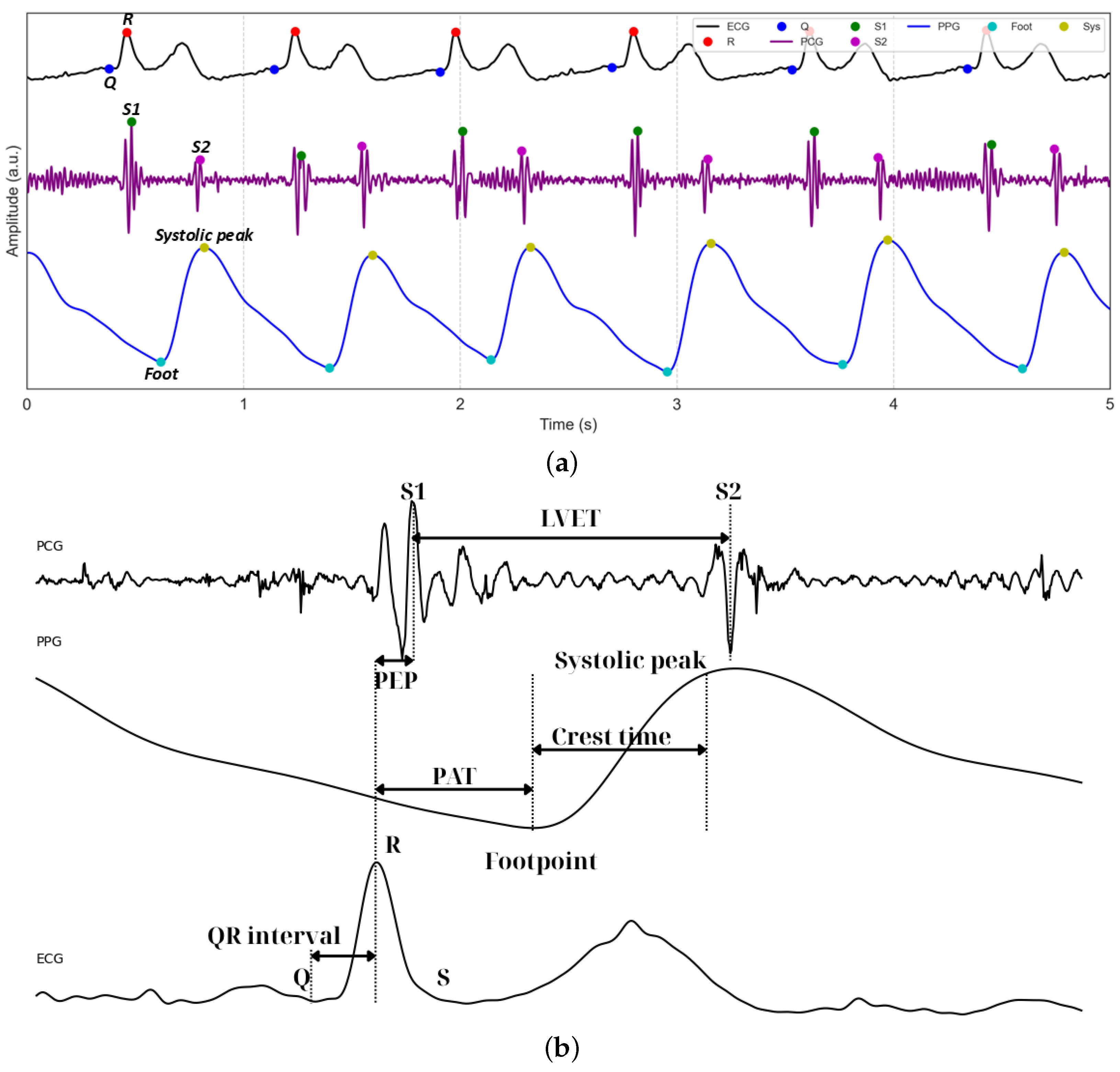
2.3. Signal Processing
2.3.1. ECG Signal Processing and Feature Extraction
2.3.2. PCG Signal Processing and Feature Extraction
2.3.3. PPG Signal Processing and Feature Extraction
2.3.4. Fused Feature Extraction
3. Results and Discussions
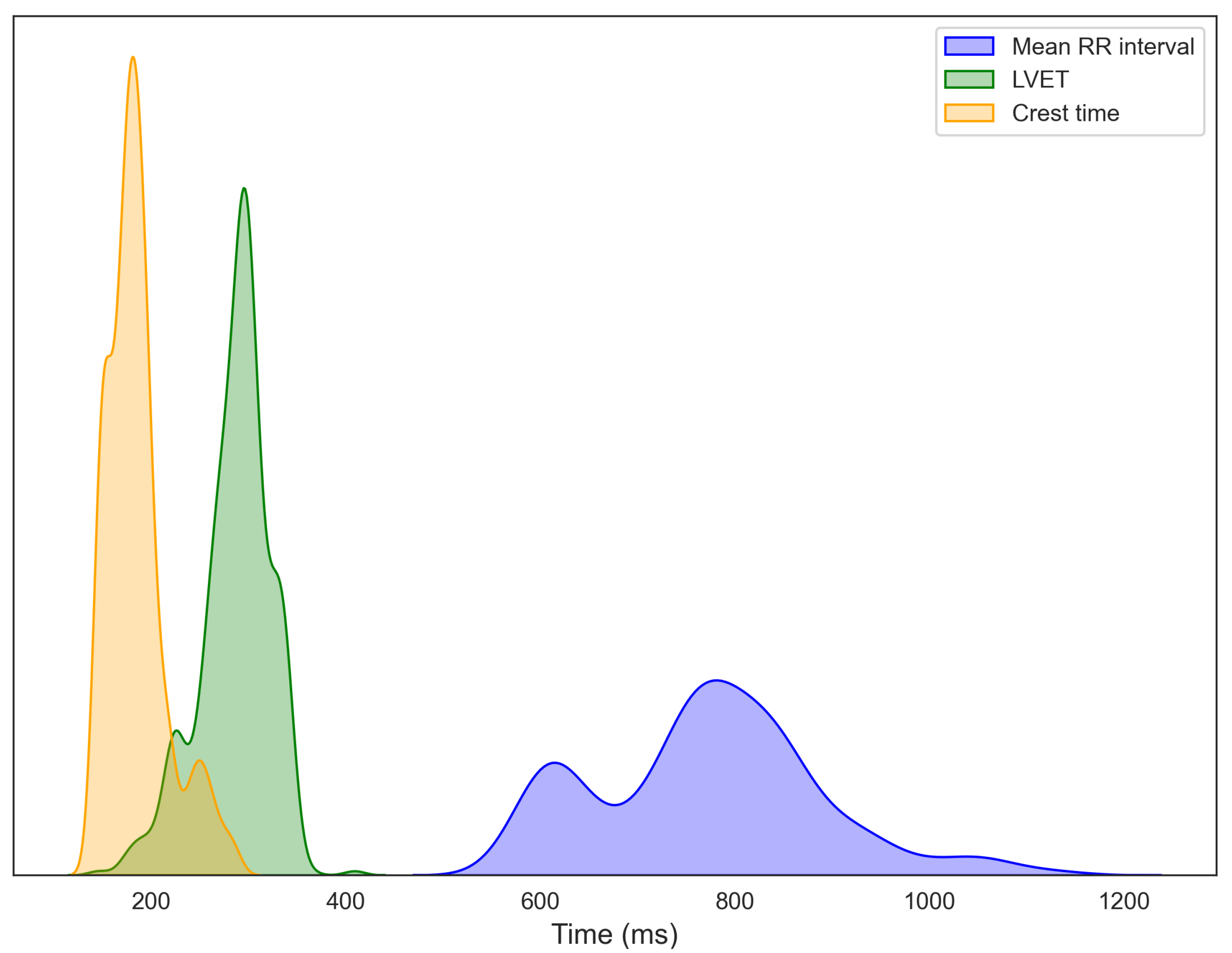
3.1. Data Visualization
3.2. BMI and Age Associations with Vital Signs
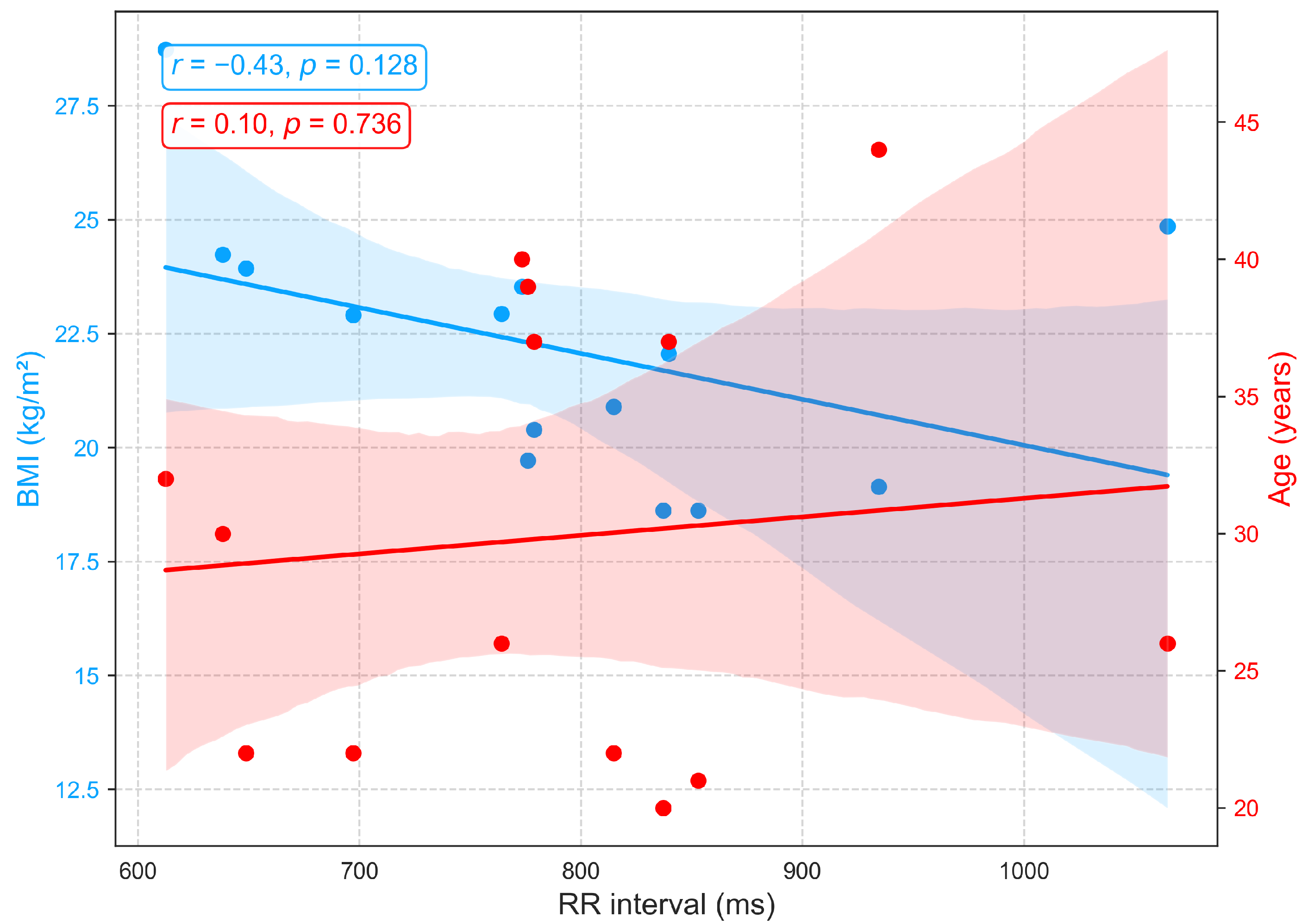
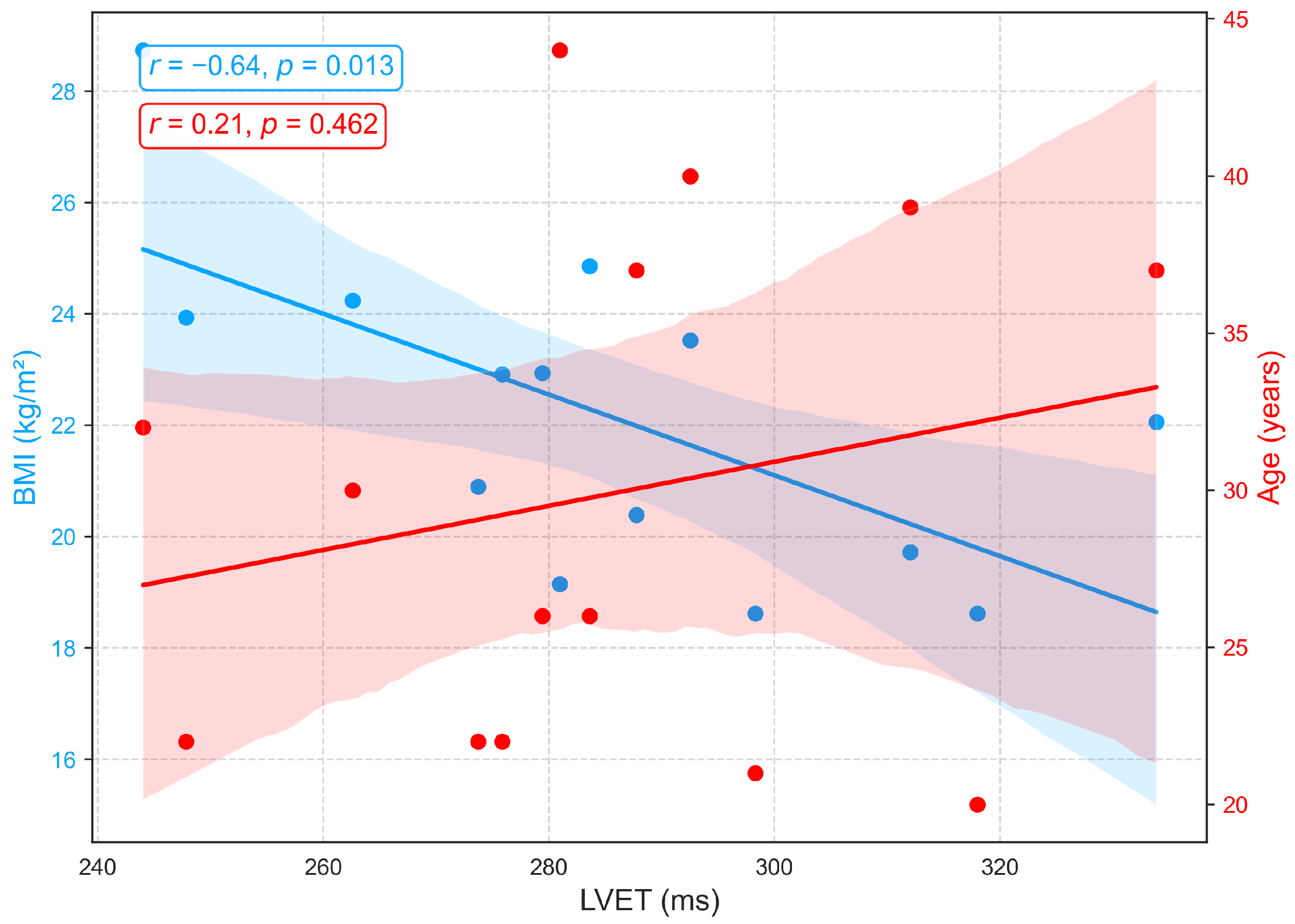
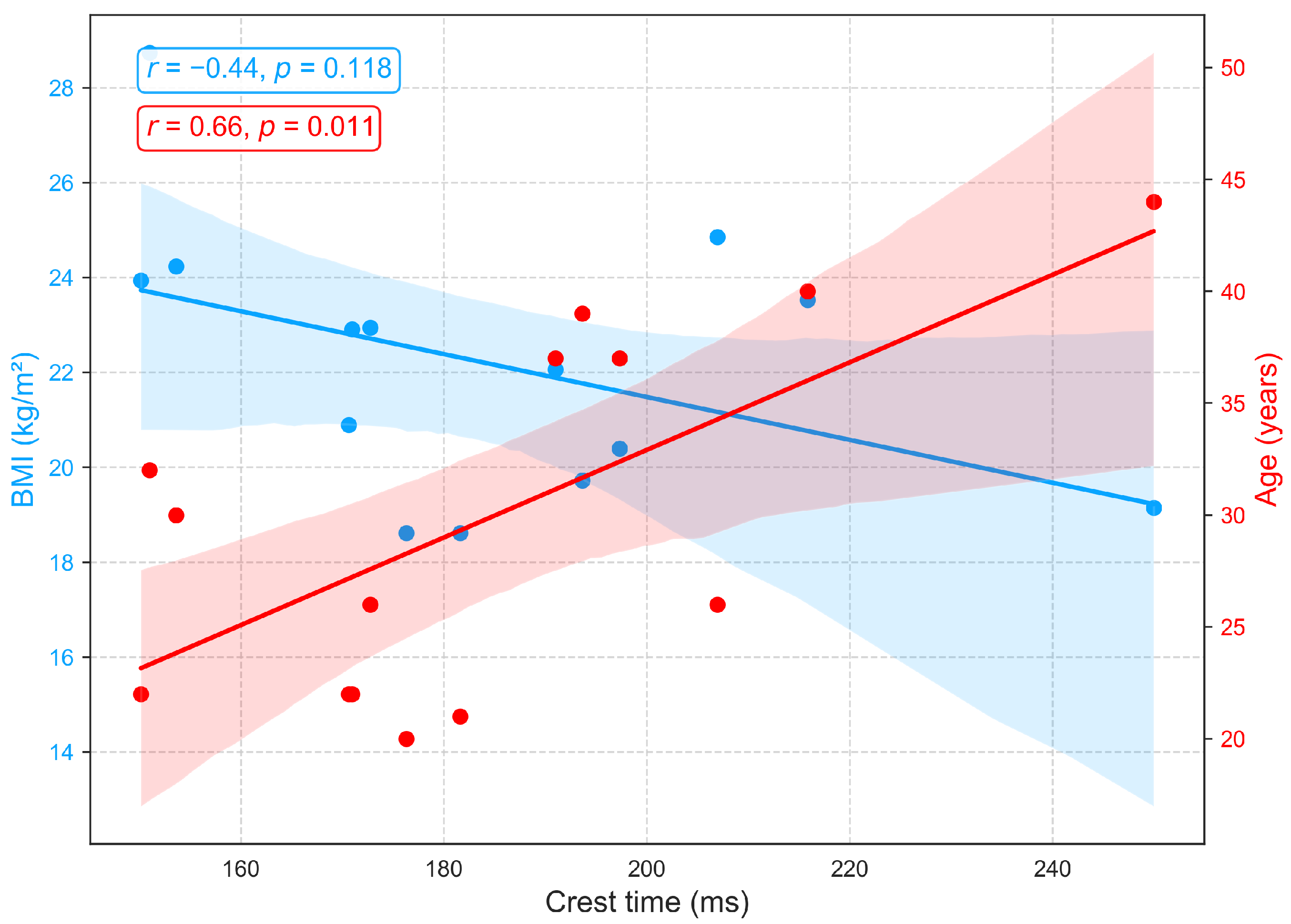
3.2.1. Relationships of BMI and Age with Primary Cardiovascular Features: RRI, LVET, and Crest Time
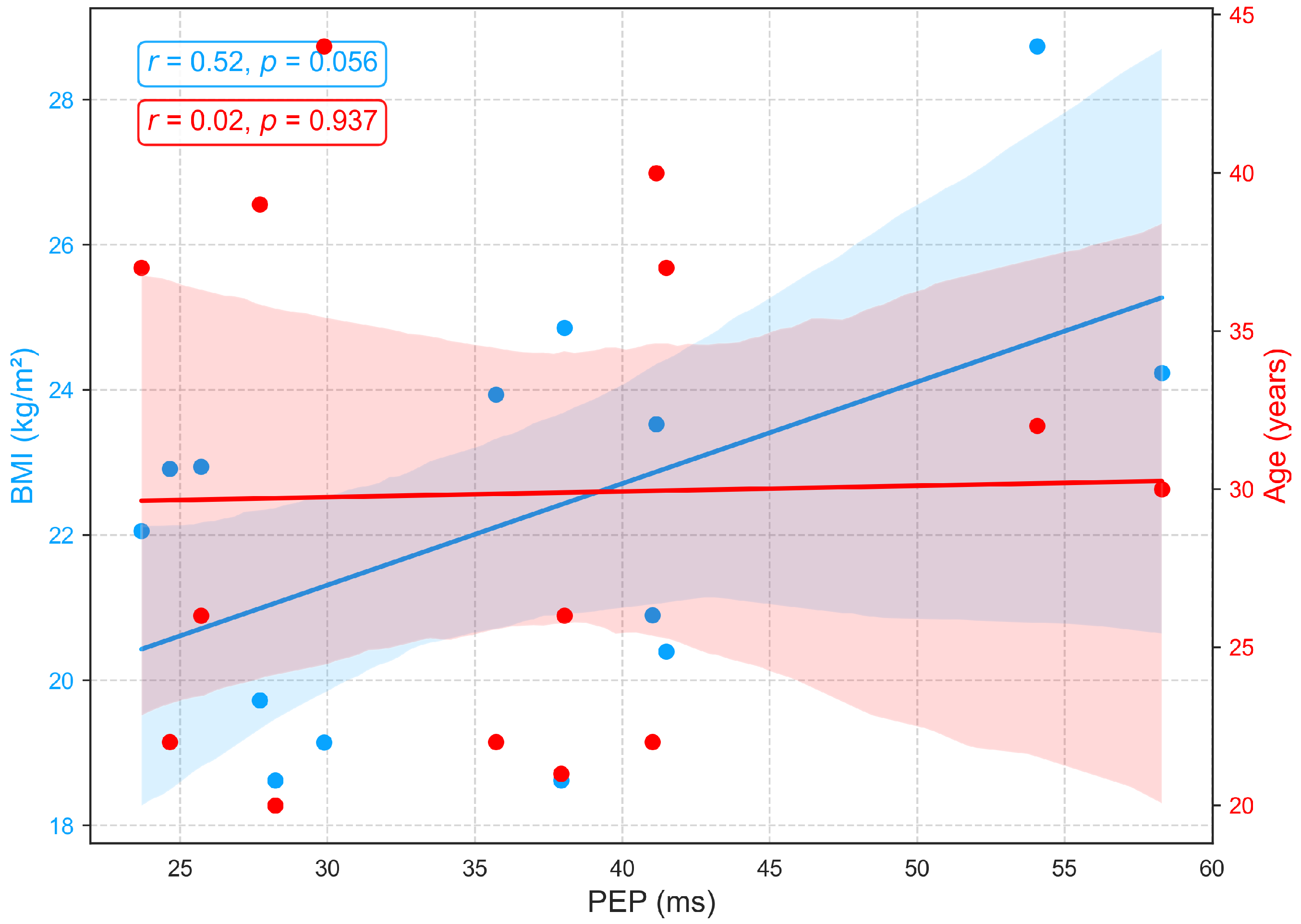
3.2.2. Relationships of BMI and Age with PEP
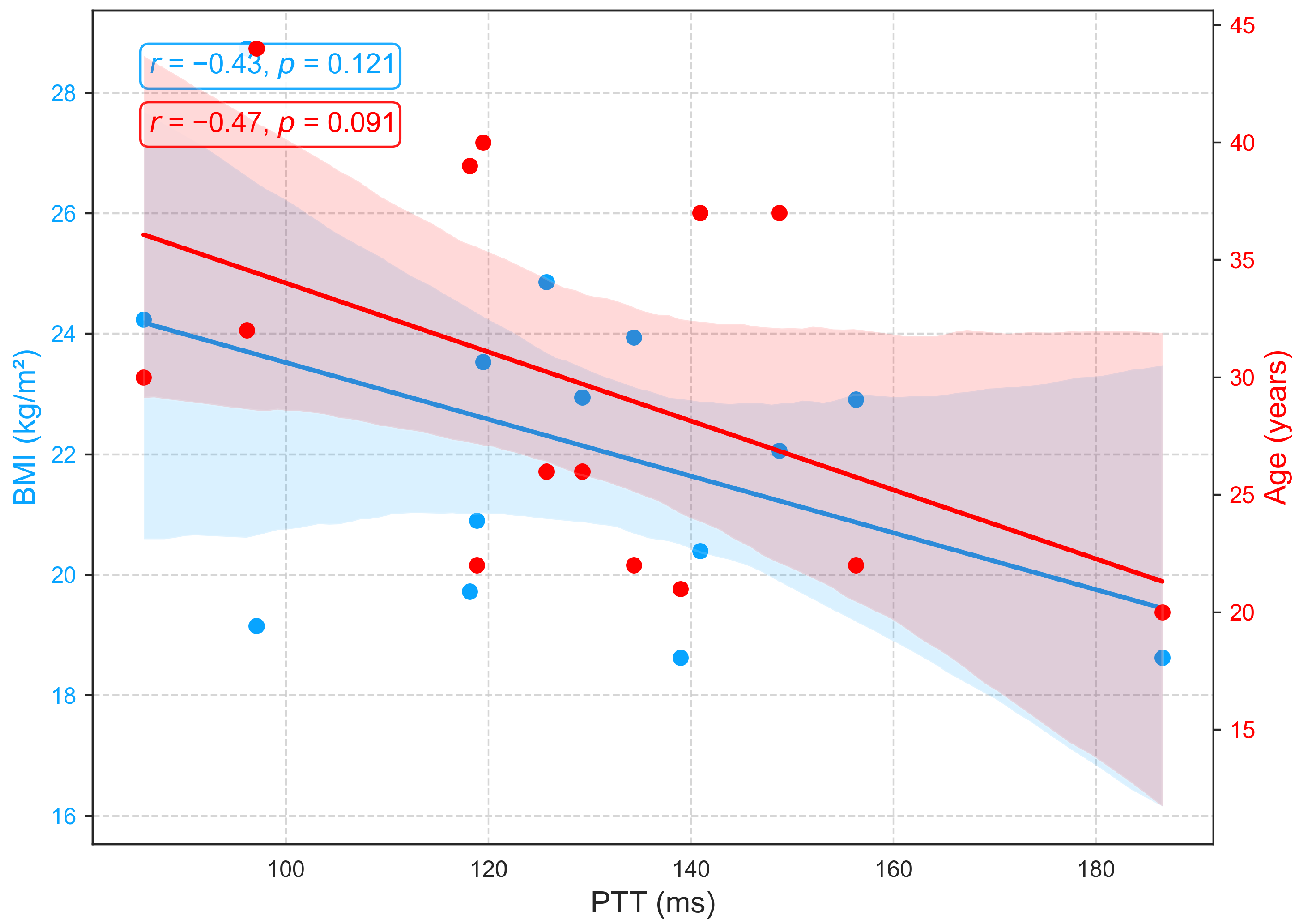
3.2.3. Relationships of BMI and Age with PTT
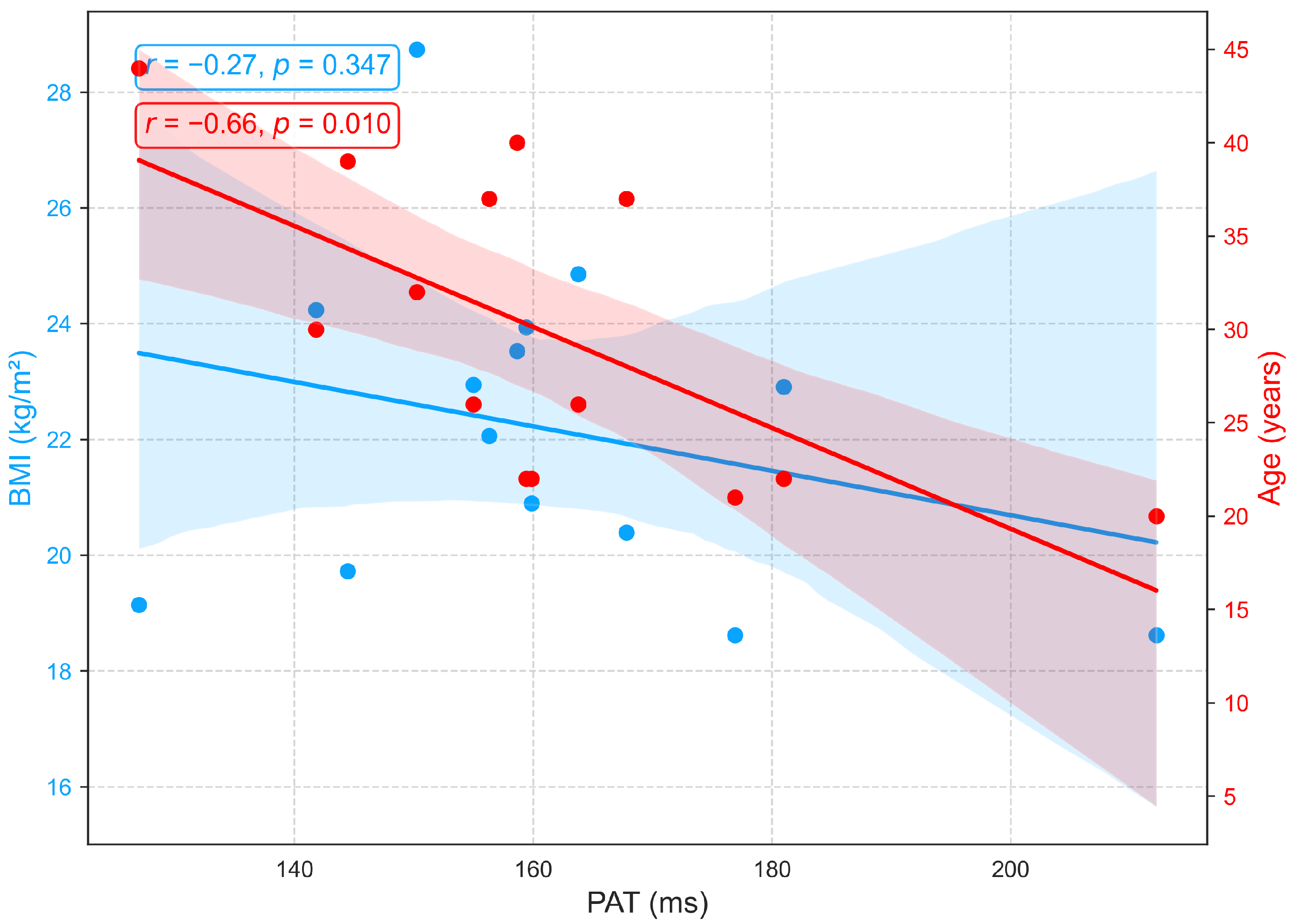
3.2.4. Relationships of BMI and Age with PAT
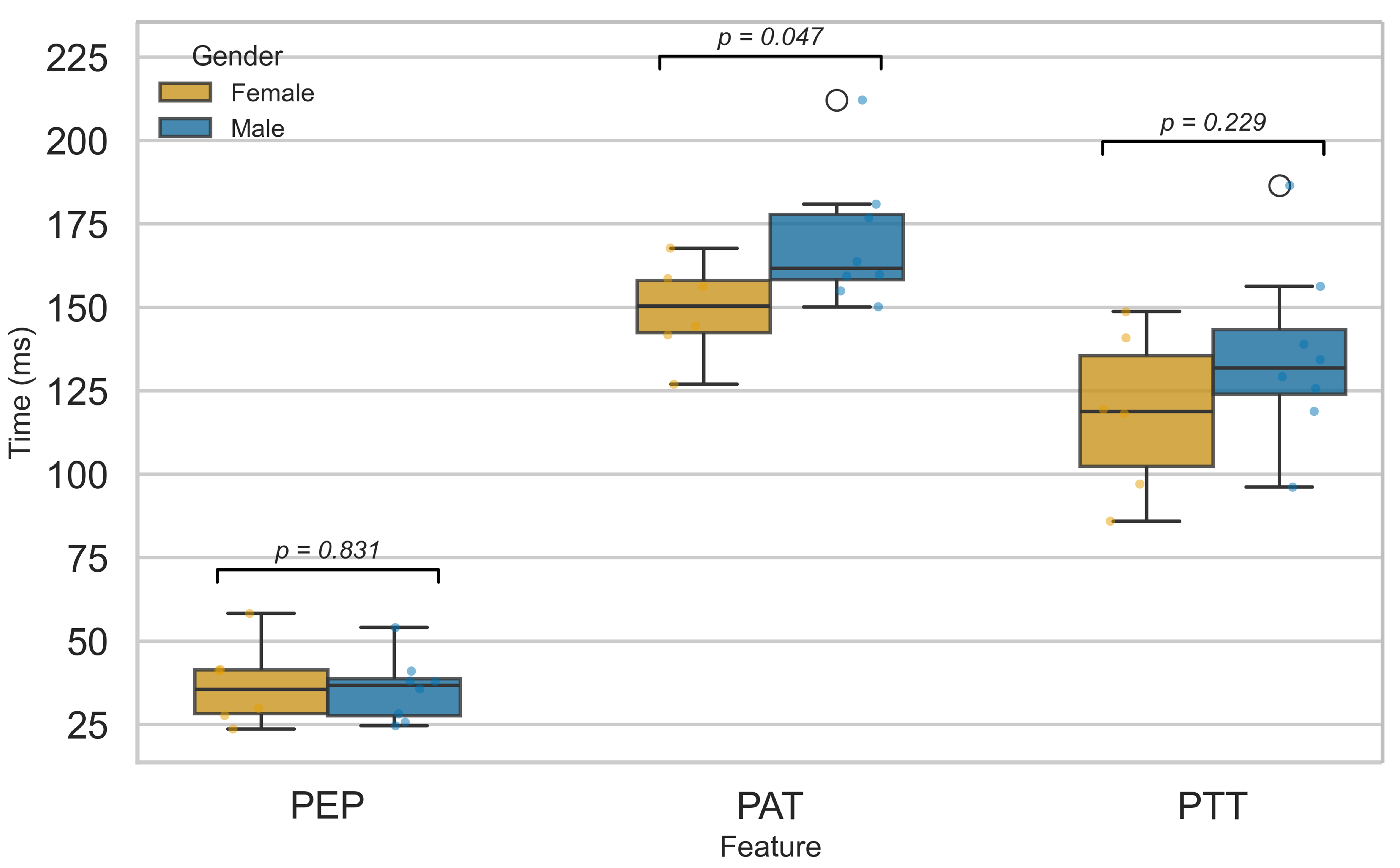
3.2.5. Influence of Sex on Cardiovascular Function
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Velasquez, G.D.D.S.; Borna, S.; Maniaci, M.J.; Coffey, J.D.; Haider, C.R.; Demaerschalk, B.M.; Forte, A.J. Economic perspective of the use of wearables in health care: A systematic review. Mayo Clin. Proc. Digit. Health 2024, 2, 299–317. [Google Scholar] [CrossRef]
- Pedroso, A.F.; Khera, R. Leveraging AI-enhanced digital health with consumer devices for scalable cardiovascular screening, prediction, and monitoring. npj Cardiovasc. Health 2025, 2, 34. [Google Scholar] [CrossRef]
- Grant, J.K.; Javaid, A.; Carrick, R.T.; Koester, M.; Kassamali, A.A.; Kim, C.H.; Isakadze, N.; Wu, K.C.; Blaha, M.J.; Whelton, S.P.; et al. Digital health innovation and artificial intelligence in cardiovascular care: A case-based review. npj Cardiovasc. Health 2024, 1, 26. [Google Scholar] [CrossRef]
- Tejedor, J.; García, C.A.; Márquez, D.G.; Raya, R.; Otero, A. Multiple physiological signals fusion techniques for improving heartbeat detection: A review. Sensors 2019, 19, 4708. [Google Scholar] [CrossRef]
- Liu, G.; Li, Y.; Chen, L.; Jiang, J.; Tian, J.; Feng, P. Design and validation of a novel multiple sites signal acquisition and analysis system based on pressure stimulation for human cardiovascular information. Sci. Rep. 2025, 15, 13392. [Google Scholar] [CrossRef]
- Zang, J.; An, Q.; Li, B.; Zhang, Z.; Gao, L.; Xue, C. A novel wearable device integrating ECG and PCG for cardiac health monitoring. Microsyst. Nanoeng. 2025, 11, 7. [Google Scholar] [CrossRef]
- Sun, C.; Liu, X.; Liu, C.; Wang, X.; Liu, Y.; Zhao, S.; Zhang, M. Enhanced cad detection using novel multi-modal learning: Integration of ecg, pcg, and coupling signals. Bioengineering 2024, 11, 1093. [Google Scholar] [CrossRef]
- Sun, C.; Liu, C.; Wang, X.; Liu, Y.; Zhao, S. Coronary artery disease detection based on a novel multi-modal deep-coding method using ECG and PCG signals. Sensors 2024, 24, 6939. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Yan, B.P.; Zhang, Y.T.; Liu, J.; Zhao, N.; Tsang, H.K. Pulse transit time based continuous cuffless blood pressure estimation: A new extension and a comprehensive evaluation. Sci. Rep. 2017, 7, 11554. [Google Scholar] [CrossRef] [PubMed]
- Mol, A.; Meskers, C.G.; Niehof, S.P.; Maier, A.B.; van Wezel, R.J. Pulse transit time as a proxy for vasoconstriction in younger and older adults. Exp. Gerontol. 2020, 135, 110938. [Google Scholar] [CrossRef] [PubMed]
- Ferizoli, R.; Karimpour, P.; May, J.M.; Kyriacou, P.A. Arterial stiffness assessment using PPG feature extraction and significance testing in an in vitro cardiovascular system. Sci. Rep. 2024, 14, 2024. [Google Scholar] [CrossRef]
- Benetos, A.; Waeber, B.; Izzo, J.; Mitchell, G.; Resnick, L.; Asmar, R.; Safar, M. Influence of age, risk factors, and cardiovascular and renal disease on arterial stiffness: Clinical applications. Am. J. Hypertens. 2002, 15, 1101–1108. [Google Scholar] [CrossRef]
- Mitchell, G. Arterial stiffness in aging: Does it have a place in clinical practice? Recent advances in hypertension. Hypertension 2021, 77, 768–780. [Google Scholar] [CrossRef]
- Kim, H.; Shin, J.; Kim, B.; Kang, J.; Lee, H.; Sung, K. Age-related annual changes in arterial stiffness in healthy adults: Insights from a large Korean cohort study. Atherosclerosis 2024, 398, 118592. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.L.; Lim, W.H.; Seo, J.B.; Kim, S.H.; Zo, J.H.; Kim, M.A. Association between body mass index and arterial stiffness. Cardiometab. Syndr. J. 2022, 2, 49–57. [Google Scholar] [CrossRef]
- Logan, J.; Kang, H.; Kim, S.; Duprez, D.; Kwon, Y.; Jacobs, D., Jr.; Forbang, N.; Lobo, J.; Sohn, M. Association of obesity with arterial stiffness: The Multi-Ethnic Study of Atherosclerosis (MESA). Vasc. Med. 2020, 25, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yan, Y.; Yang, X.; Li, S.; Bazzano, L.; He, J.; Chen, W. Long-term burden of higher body mass index and adult arterial stiffness are linked predominantly through elevated blood pressure. Hypertension 2019, 73, 229–234. [Google Scholar] [CrossRef]
- Li, J.; Ke, L.; Du, Q.; Ding, X.; Chen, X. Research on the classification of ECG and PCG signals based on BiLSTM-GoogLeNet-DS. Appl. Sci. 2022, 12, 11762. [Google Scholar] [CrossRef]
- Golden, D.P.; Wolthuis, R.A.; Hoffler, G.W. A spectral analysis of the normal resting electrocardiogram. IEEE Trans. Biomed. Eng. 2007, BME-20, 366–372. [Google Scholar] [CrossRef]
- Zhang, X.; Durand, L.; Senhadji, L.; Lee, H.C.; Coatrieux, J.L. Time-frequency scaling transformation of the phonocardiogram based of the matching pursuit method. IEEE Trans. Biomed. Eng. 1998, 45, 972–979. [Google Scholar] [CrossRef][Green Version]
- Park, J.; Seok, H.S.; Kim, S.S.; Shin, H. Photoplethysmogram analysis and applications: An integrative review. Front. Physiol. 2022, 12, 808451. [Google Scholar] [CrossRef]
- Council, E.S.; Redon, J.; Narkiewicz, K.; Nilsson, P.M.; Burnier, M.; Viigimaa, M.; Ambrosioni, E.; Coca, A.; Olsen, M.H.; Schmieder, R.E. 2013 ESH/ESC Guidelines for the management of arterial hypertension. Arter. Hypertens. 2013, 17, 69–168. [Google Scholar]
- Makowski, D.; Pham, T.; Lau, Z.J.; Brammer, J.C.; Lespinasse, F.; Pham, H.; Schölzel, C.; Chen, S.A. NeuroKit2: A Python toolbox for neurophysiological signal processing. Behav. Res. Methods 2021, 53, 1689–1696. [Google Scholar] [CrossRef]
- Cherif, L.H.; Debbal, S.M.; Bereksi-Reguig, F. Choice of the wavelet analyzing in the phonocardiogram signal analysis using the discrete and the packet wavelet transform. Expert Syst. Appl. 2010, 37, 913–918. [Google Scholar] [CrossRef]
- Alhakak, A.S.; Teerlink, J.R.; Lindenfeld, J.; Böhm, M.; Rosano, G.M.; Biering-Sørensen, T. The significance of left ventricular ejection time in heart failure with reduced ejection fraction. Eur. J. Heart Fail. 2021, 23, 541–551. [Google Scholar] [CrossRef]
- Sirkiä, J.P.; Panula, T.; Kaisti, M. Investigating the impact of contact pressure on photoplethysmograms. Biomed. Eng. Adv. 2024, 7, 100123. [Google Scholar] [CrossRef]
- Lin, W.Y.; Chou, W.C.; Chang, P.C.; Chou, C.C.; Wen, M.S.; Ho, M.Y.; Lee, W.C.; Hsieh, M.J.; Lin, C.C.; Tsai, T.H.; et al. Identification of location specific feature points in a cardiac cycle using a novel seismocardiogram spectrum system. IEEE J. Biomed. Health Inform. 2016, 22, 442–449. [Google Scholar] [CrossRef]
- Gurev, V.; Tavakolian, K.; Constantino, J.; Kaminska, B.; Blaber, A.P.; Trayanova, N.A. Mechanisms underlying isovolumic contraction and ejection peaks in seismocardiogram morphology. J. Med. Biol. Eng. 2012, 32, 103. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.S.; Firouzmand, M.; Charmi, M.; Hemmati, M.; Moghadam, M.; Ghorbani, Y. Blood pressure estimation from appropriate and inappropriate PPG signals using A whole-based method. Biomed. Signal Process. Control 2019, 47, 196–206. [Google Scholar] [CrossRef]
- van Velzen, M.H.; Loeve, A.J.; Niehof, S.P.; Mik, E.G. Increasing accuracy of pulse transit time measurements by automated elimination of distorted photoplethysmography waves. Med. Biol. Eng. Comput. 2017, 55, 1989–2000. [Google Scholar] [CrossRef]
- Finnegan, E.; Davidson, S.; Harford, M.; Jorge, J.; Watkinson, P.; Young, D.; Tarassenko, L.; Villarroel, M. Pulse arrival time as a surrogate of blood pressure. Sci. Rep. 2021, 11, 22767. [Google Scholar] [CrossRef]
- Zhou, Z.B.; Cui, T.R.; Li, D.; Jian, J.M.; Li, Z.; Ji, S.R.; Li, X.; Xu, J.D.; Liu, H.F.; Yang, Y.; et al. Wearable continuous blood pressure monitoring devices based on pulse wave transit time and pulse arrival time: A review. Materials 2023, 16, 2133. [Google Scholar] [CrossRef] [PubMed]
- Pikkujamsa, S.M.; Makikallio, T.H.; Airaksinen, K.J.; Huikuri, H.V. Determinants and interindividual variation of RR interval dynamics in healthy middle-aged subjects. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H1400–H1406. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Taylor, J.A.; Carr, D.L.; Myers, C.W.; Eckberg, D.L. Mechanisms underlying very-low-frequency RR-interval oscillations in humans. Circulation 1998, 98, 547–555. [Google Scholar] [CrossRef]
- Schneider, M.; Kraemmer, M.M.; Weber, B.; Schwerdtfeger, A.R. Life events are associated with elevated heart rate and reduced heart complexity to acute psychological stress. Biol. Psychol. 2021, 163, 108116. [Google Scholar] [CrossRef]
- Kim, H.G.; Cheon, E.J.; Bai, D.S.; Lee, Y.H.; Koo, B.H. Stress and heart rate variability: A meta-analysis and review of the literature. Psychiatry Investig. 2018, 15, 235. [Google Scholar] [CrossRef]
- Alhakak, A.S.; Olsen, F.J.; Skaarup, K.G.; Lassen, M.C.H.; Johansen, N.D.; Jørgensen, P.G.; Abildgaard, U.; Jensen, G.B.; Schnohr, P.; Søgaard, P.; et al. Age-and sex-based normal reference ranges of the cardiac time intervals: The Copenhagen City Heart Study. Clin. Res. Cardiol. 2025, 114, 430–442. [Google Scholar] [CrossRef] [PubMed]
- Elgendi, M. On the analysis of fingertip photoplethysmogram signals. Curr. Cardiol. Rev. 2012, 8, 14–25. [Google Scholar] [CrossRef]
- Charlton, P.H.; Celka, P.; Farukh, B.; Chowienczyk, P.; Alastruey, J. Assessing mental stress from the photoplethysmogram: A numerical study. Physiol. Meas. 2018, 39, 054001. [Google Scholar] [CrossRef]
- Avram, R.; Tison, G.H.; Aschbacher, K.; Kuhar, P.; Vittinghoff, E.; Butzner, M.; Runge, R.; Wu, N.; Pletcher, M.J.; Marcus, G.M.; et al. Real-world heart rate norms in the Health eHeart study. NPJ Digit. Med. 2019, 2, 58. [Google Scholar] [CrossRef]
- Tomar, A.; Ahluwalia, H.; Isser, H.S.; Gulati, S.; Kumar, P.; Yadav, I. Analysis of ventricular repolarization parameters and heart rate variability in obesity: A comparative study. Sci. Rep. 2024, 14, 25855. [Google Scholar] [CrossRef]
- Willems, J.L.; Roelandt, J.O.S.; De Geest, H.; Kesteloot, H.; Joossens, J.V. The left ventricular ejection time in elderly subjects. Circulation 1970, 42, 37–42. [Google Scholar] [CrossRef]
- Alhakak, A.S.; Olsen, F.J.; Skaarup, K.G.; Lassen, M.C.H.; Johansen, N.D.; Espersen, C.; Abildgaard, U.; Jensen, G.B.; Schnohr, P.; Marott, J.L.; et al. Changes in cardiac time intervals over a decade and the risk of incident heart failure: The Copenhagen City Heart Study. Int. J. Cardiol. 2023, 386, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Romano, M.; Carella, G.; Cotecchia, M.R.; Di Maro, T.; Indolfi, C.; Ferro, G.; Chiariello, M. Abnormal systolic time intervals in obesity and their relationship with the amount of overweight. Am. Heart J. 1986, 112, 356–360. [Google Scholar] [CrossRef]
- Blomstrand, P.; Sjöblom, P.; Nilsson, M.; Wijkman, M.; Engvall, M.; Länne, T.; Nyström, F.H.; Östgren, C.J.; Engvall, J. Overweight and obesity impair left ventricular systolic function as measured by left ventricular ejection fraction and global longitudinal strain. Cardiovasc. Diabetol. 2018, 17, 113. [Google Scholar] [CrossRef] [PubMed]
- Antali, F.; Kulin, D.; Kulin, S.; Miklós, Z. Evaluation of the Age Dependence of Conventional and Novel Photoplethysmography Parameters. Artery Res. 2025, 31, 5. [Google Scholar] [CrossRef]
- Yousef, Q.; Reaz, M.B.I.; Ali, M.A.M. The analysis of PPG morphology: Investigating the effects of aging on arterial compliance. Meas. Sci. Rev. 2012, 12, 266. [Google Scholar] [CrossRef]
- Kortekaas, M.C.; van Velzen, M.H.; Grüne, F.; Niehof, S.P.; Stolker, R.J.; Huygen, F.J. Small intra-individual variability of the pre-ejection period justifies the use of pulse transit time as approximation of the vascular transit. PLoS ONE 2018, 13, e0204105. [Google Scholar] [CrossRef]
- Wiley, C.R.; Pourmand, V.; Thayer, J.F.; Williams, D.P. A close examination of the use of systolic time intervals in the calculation of impedance derived cardiac autonomic balance and regulation. Front. Neurosci. 2021, 15, 625276. [Google Scholar] [CrossRef]
- Chen, S.C.; Chang, J.M.; Liu, W.C.; Tsai, J.C.; Chen, L.I.; Lin, M.Y.; Hsu, P.C.; Lin, T.H.; Su, H.M.; Hwang, S.J.; et al. Significant correlation between ratio of brachial pre-ejection period to ejection time and left ventricular ejection fraction and mass index in patients with chronic kidney disease. Nephrol. Dial. Transplant. 2011, 26, 1895–1902. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marshall, A.G.; Neikirk, K.; Afolabi, J.; Mwesigwa, N.; Shao, B.; Kirabo, A.; Reddy, A.K.; Hinton, A., Jr. Update on the use of pulse wave velocity to measure age-related vascular changes. Curr. Hypertens. Rep. 2024, 26, 131–140. [Google Scholar] [CrossRef]
- Chen, Y.S.; Lu, W.A.; Hsu, L.Y.; Kuo, C.D. Determinants of hand pulse wave velocity and hand pulse transit time in healthy adults. Sci. Rep. 2024, 14, 10144. [Google Scholar] [CrossRef]
- Wang, Y.; Miao, F.; An, Q.; Liu, Z.; Chen, C.; Li, Y. Wearable multimodal vital sign monitoring sensor with fully integrated analog front end. IEEE Sens. J. 2022, 22, 13462–13471. [Google Scholar] [CrossRef]
- Pilz, N.; Patzak, A.; Bothe, T.L. The pre-ejection period is a highly stress dependent parameter of paramount importance for pulse-wave-velocity based applications. Front. Cardiovasc. Med. 2023, 10, 1138356. [Google Scholar] [CrossRef] [PubMed]
- Prabhavathi, K.; Selvi, K.; Poornima, K.N.; Sarvanan, A. Role of biological sex in normal cardiac function and in its disease outcome—A review. J. Clin. Diagn. Res. 2014, 8, BE01. [Google Scholar] [CrossRef] [PubMed]
- Hnatkova, K.; Šišáková, M.; Smetana, P.; Toman, O.; Huster, K.M.; Novotný, T.; Schmidt, G.; Malik, M. Sex differences in heart rate responses to postural provocations. Int. J. Cardiol. 2019, 297, 126–134. [Google Scholar] [CrossRef]
- Hiteshi, A.K.; Li, D.; Gao, Y.; Chen, A.; Flores, F.; Mao, S.S.; Budoff, M.J. Gender differences in coronary artery diameter are not related to body habitus or left ventricular mass. Clin. Cardiol. 2014, 37, 605–609. [Google Scholar] [CrossRef] [PubMed]
- DuPont, J.J.; Kenney, R.M.; Patel, A.R.; Jaffe, I.Z. Sex differences in mechanisms of arterial stiffnes. Br. J. Pharmacol. 2019, 176, 4208–4225. [Google Scholar] [CrossRef]
- Kittnar, O. Selected sex related differences in pathophysiology of cardiovascular system. Physiol. Res. 2019, 69, 21. [Google Scholar] [CrossRef] [PubMed]
- Dehghanojamahalleh, S.; Kaya, M. Sex-related differences in photoplethysmography signals measured from finger and toe. IEEE J. Transl. Eng. Health Med. 2019, 7, 1900607. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, K.T.; Tran, T.N.; Huynh, D.N.; Le, N.K.; Le, C.D.; Mai, H.X.; Huynh, Q.L.; Nguyen, T.H. A Multimodal System for Comprehensive Cardiovascular Monitoring Using ECG, PCG, and PPG Signal Fusion. Sensors 2025, 25, 6708. https://doi.org/10.3390/s25216708
Tran KT, Tran TN, Huynh DN, Le NK, Le CD, Mai HX, Huynh QL, Nguyen TH. A Multimodal System for Comprehensive Cardiovascular Monitoring Using ECG, PCG, and PPG Signal Fusion. Sensors. 2025; 25(21):6708. https://doi.org/10.3390/s25216708
Chicago/Turabian StyleTran, Khang Thanh, Thao Nguyen Tran, Dang Nguyen Huynh, Nguyen Khoa Le, Cao Dang Le, Huu Xuan Mai, Quang Linh Huynh, and Trung Hau Nguyen. 2025. "A Multimodal System for Comprehensive Cardiovascular Monitoring Using ECG, PCG, and PPG Signal Fusion" Sensors 25, no. 21: 6708. https://doi.org/10.3390/s25216708
APA StyleTran, K. T., Tran, T. N., Huynh, D. N., Le, N. K., Le, C. D., Mai, H. X., Huynh, Q. L., & Nguyen, T. H. (2025). A Multimodal System for Comprehensive Cardiovascular Monitoring Using ECG, PCG, and PPG Signal Fusion. Sensors, 25(21), 6708. https://doi.org/10.3390/s25216708





