Smart Total Knee Replacement: Recognition of Activities of Daily Living Using Embedded IMU Sensors and a Novel AI Model in a Cadaveric Proof-of-Concept Study †
Highlights
- Data from dual IMU devices embedded in a knee replacement prosthesis can accurately recognize activities of daily living.
- Enhances the possibility of remote minimally intrusive personalised rehabilitation.
Abstract
1. Introduction
2. Materials and Methods
- -
- Jogging (6 km/h on treadmill);
- -
- Knee bending;
- -
- Sitting down (from standing position);
- -
- Standing up (from seated position);
- -
- Walking (level ground);
- -
- Standing still;
- -
- Stair ascent;
- -
- Stair descent.
3. Data Collection and Processing
3.1. Synchronization of Data from Multiple IMU Devices
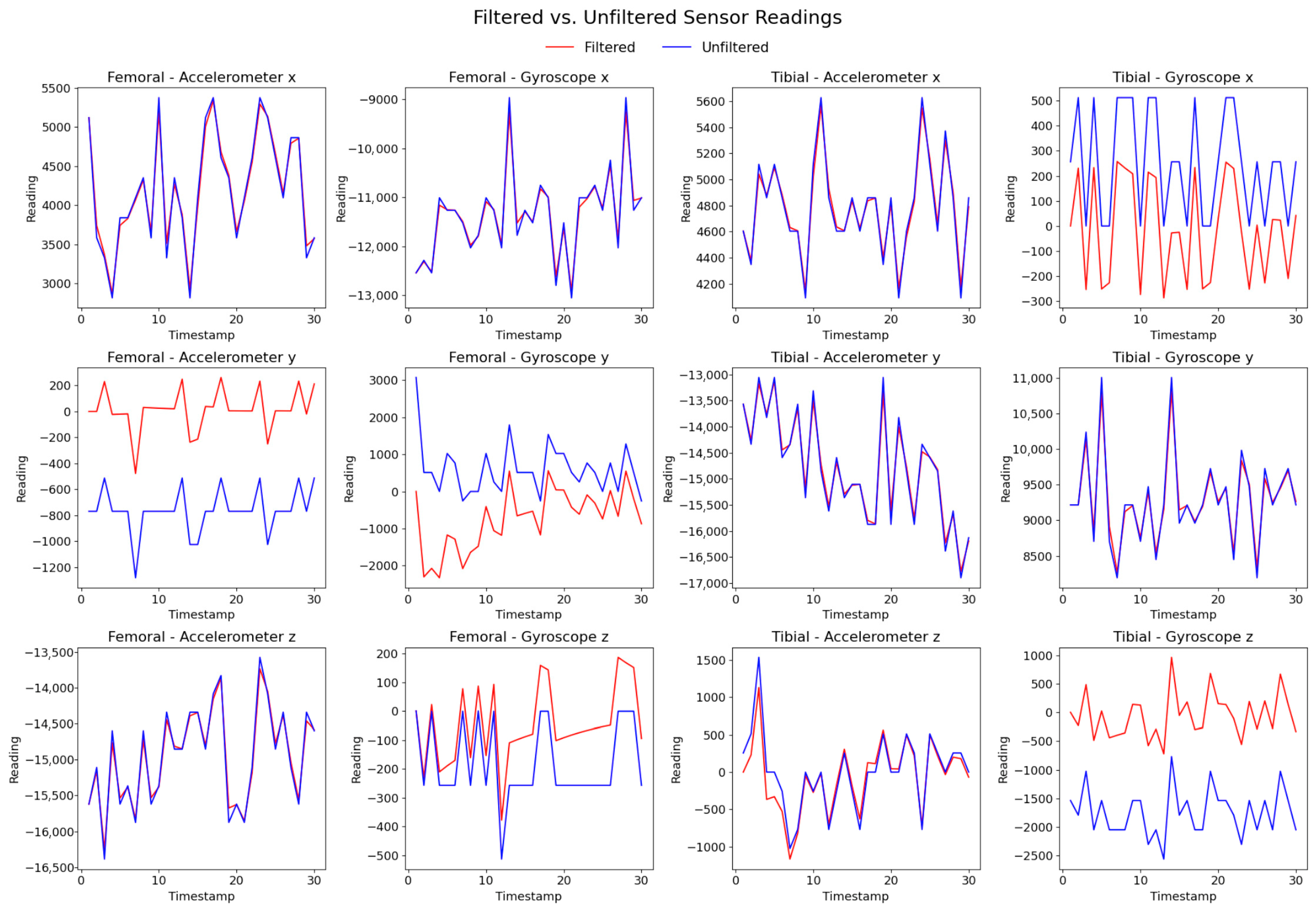
3.2. Data Preprocessing Including Cleanup and Filtering
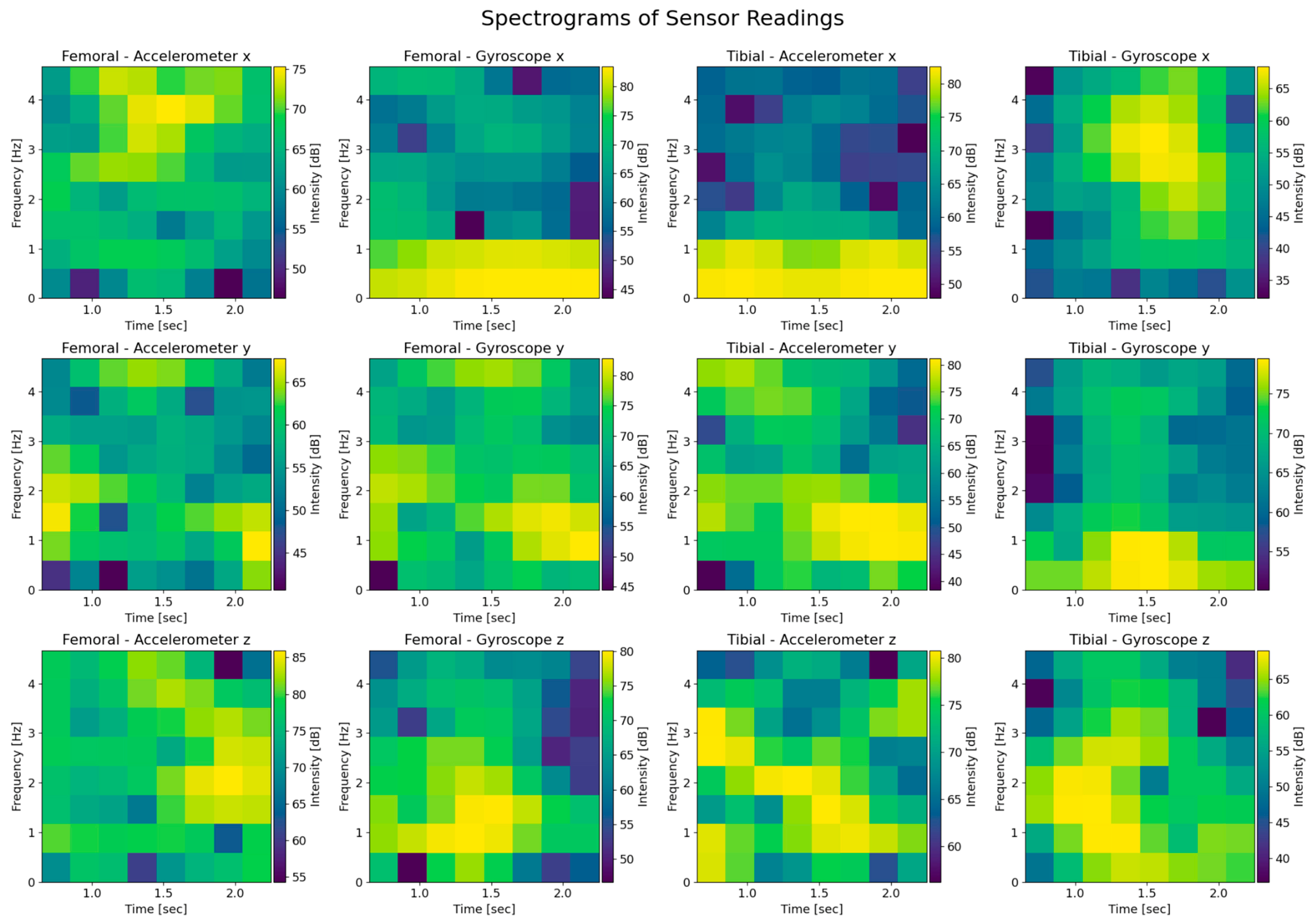
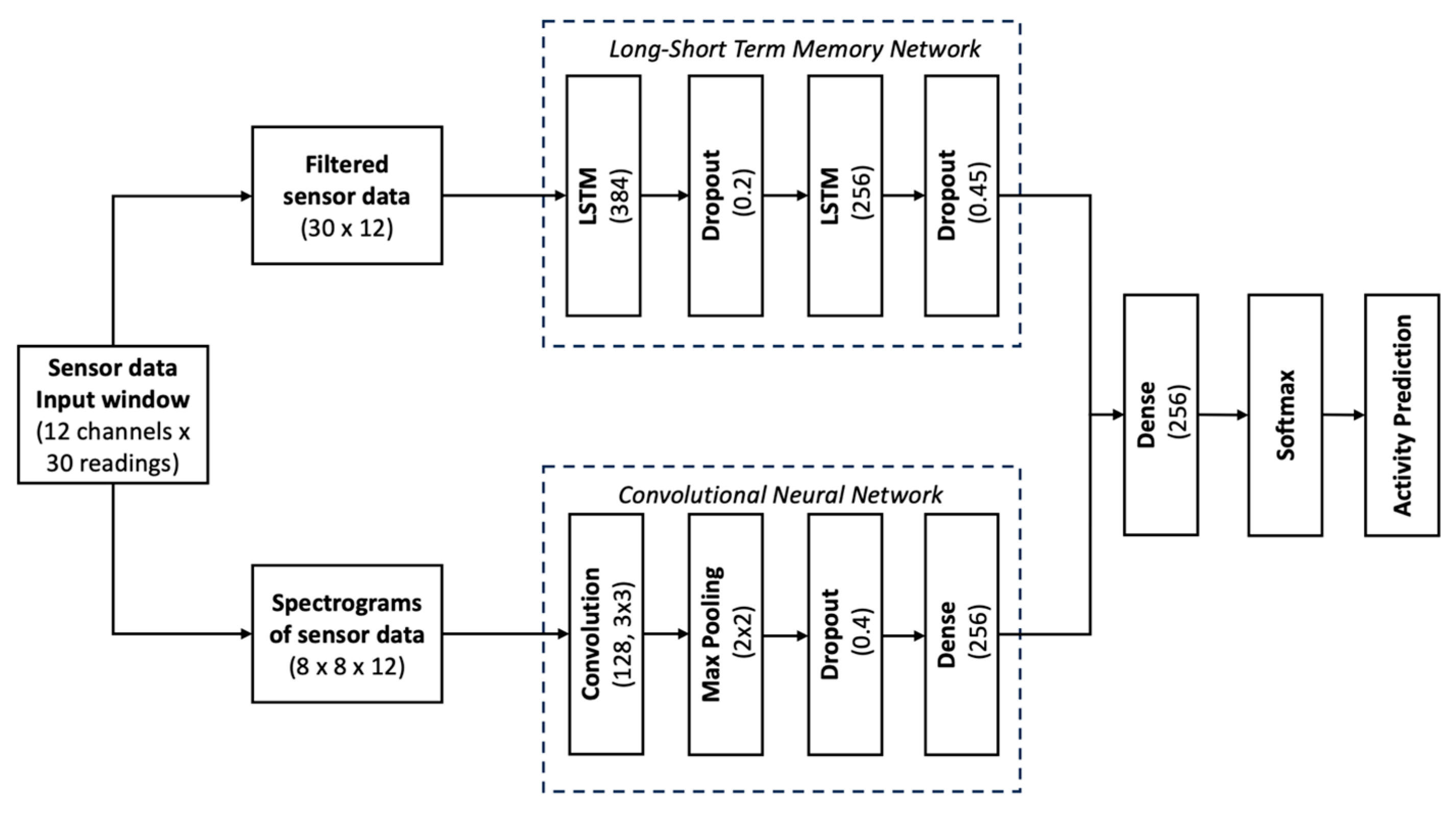
3.3. Artificial Intelligence Model Architecture
4. Results
5. Discussion
6. Conclusions
7. Patents
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adhaye, A.M.; Jolhe, D.A. Gait Measurement Methods: Systematic Review and Comparative Studies. J. Mech. Med. Biol. 2024, 24, 2330002. [Google Scholar] [CrossRef]
- Bini, S.A.; Gillian, N.; Peterson, T.A.; Souza, R.B.; Schultz, B.; Mormul, W.; Cichoń, M.K.; Szczotka, A.B.; Poupyrev, I. Unlocking Gait Analysis Beyond the Gait Lab: High-Fidelity Replication of Knee Kinematics Using Inertial Motion Units and a Convolutional Neural Network. Arthroplast. Today 2025, 33, 101656. [Google Scholar] [CrossRef]
- Motoi, K.; Tanaka, S.; Kuwae, Y.; Yuji, T.; Higashi, Y.; Fujimoto, T.; Yamakoshi, K.I. Evaluation of a Wearable Sensor System Monitoring Posture Changes and Activities for Use in Rehabilitation. J. Robot. Mechatron. 2007, 19, 656–666. [Google Scholar] [CrossRef]
- Constantinescu, D.; Pavlis, W.; Rizzo, M.; Vanden Berge, D.; Barnhill, S.; Hernandez, V.H. The role of commercially available smartphone apps and wearable devices in monitoring patients after total knee arthroplasty: A systematic review. EFORT Open Rev. 2022, 7, 481–490. [Google Scholar] [CrossRef]
- Boekesteijn, R.J.; van Gerven, J.; Geurts, A.C.H.; Smulders, K. Objective gait assessment in individuals with knee osteoarthritis using inertial sensors: A systematic review and meta-analysis. Gait Posture 2022, 98, 109–120. [Google Scholar] [CrossRef]
- Arnold, Y.L.; Wong, D.S.C.M. The global burden of osteoarthritis: Past and future perspectives. Lancet Rheumatol. 2023, 5, e496–e497. [Google Scholar] [CrossRef] [PubMed]
- Pabinger, C.; Lothaller, H.; Geissler, A. Utilization rates of knee-arthroplasty in OECD countries. Osteoarthr. Cartil. 2015, 23, r1664–r1673. [Google Scholar] [CrossRef] [PubMed]
- Hirschmann, M.T.; Friederich, N.F.; Becker, R.; Karlsson, J. Personalised medicine in knee arthroplasty: We need more science! In Knee Surgery, Sports Traumatology, Arthroscopy; John Wiley and Sons Inc: New York, NY, USA, 2019; Volume 27, pp. 1357–1358. [Google Scholar]
- Giesinger, K.; Hamilton, D.F.; Jost, B.; Holzner, B.; Giesinger, J.M. Comparative responsiveness of outcome measures for total knee arthroplasty. Osteoarthr. Cartil. 2014, 22, r184–r189. [Google Scholar] [CrossRef] [PubMed]
- Frimpong, E.; van der Jagt, D.R.; Mokete, L.; Pietrzak, J.; Kaoje, Y.S.; Smith, A.; McVeigh, J.A.; Meiring, R.M. Improvements in Objectively Measured Activity Behaviors Do Not Correlate with Improvements in Patient-Reported Outcome Measures Following Total Knee Arthroplasty. J. Arthroplast. 2020, 35, r712–r719.e4. [Google Scholar] [CrossRef]
- Wu, K.A.; Kugelman, D.N.; Goel, R.K.; Dilbone, E.S.; Ryan, S.P.; Wellman, S.S.; Bolognesi, M.P.; Seyler, T.M. How Do Patient-Reported Outcomes Correlate with Real-Time Objective Measures of Function After Total Knee Arthroplasty? A Prospective Study Using Daily Gait Metrics. J. Arthroplast. 2025, 40, r1711–r1718. [Google Scholar] [CrossRef]
- Wainwright, T.W.; Kehlet, H. Functional recovery following hip and knee arthroplasty: Subjective vs. objective assessment? Acta Orthop. 2022, 93, 739–741. [Google Scholar] [CrossRef]
- Cooper, D.M.; Bhuskute, N.; Walsh, G. Exploring the Impact and Acceptance of Wearable Sensor Technology for Pre- and Postoperative Rehabilitation in Knee Replacement Patients: A U.K.-Based Pilot Study. JBJS Open Access 2022, 7, e21. [Google Scholar] [CrossRef]
- Bolander, R.P.; Mangal, R.K.; Pierce, A.G.; Stulberg, S.D.; D’Apuzzo, M.R.; Hernandez, V.H. Normative Values for Daily Functional Recovery Patterns Following Total Knee Arthroplasty. J. Arthroplast. 2024, 39, r2731–r2736. [Google Scholar] [CrossRef] [PubMed]
- Marchand, R.C.; Taylor, K.B.; Kaczynski, E.C.; Richards, S.; Hutchinson, J.B.; Khodabakhsh, S.; Chapman, R.M. Consistency Is Key: A Secondary Analysis of Wearable Motion Sensor Accuracy Measuring Knee Angles Across Activities of Daily Living Before and After Knee Arthroplasty. Sensors 2025, 25, 3942. [Google Scholar] [CrossRef]
- Cushner, F.D.; Hunter, O.F.; Lee, D.C. How Active Are Our Patients in the First 6 Weeks Following Total Knee Arthroplasty? J. Arthroplast. 2024, 39, rS125–rS129. [Google Scholar] [CrossRef] [PubMed]
- Cushner, F.D.; Yergler, J.D.; Elashoff, B.; Aubin, P.M.; Verta, P.; Scuderi, G.R. Staying Ahead of the Curve: The Case for Recovery Curves in Total Knee Arthroplasty. J. Arthroplast. 2025, 40, r373–r379. [Google Scholar] [CrossRef]
- Prill, R.; Walter, M.; Królikowska, A.; Becker, R. A systematic review of diagnostic accuracy and clinical applications of wearable movement sensors for knee joint rehabilitation. Sensors 2021, 21, 8221. [Google Scholar] [CrossRef] [PubMed]
- Wimmer, M.A.; Nechtow, W.; Schwenke, T.; Moisio, K.C. Knee Flexion and daily activities in patients following total knee replacement: A comparison with ISO standard 14243. Biomed Res. Int. 2015, 2015, 157541. [Google Scholar] [CrossRef]
- Krishnamoorthy, U.; Lakshmipathy, P.; Ramya, M.; Fayek, H.H. Navigating the future of healthcare with innovations and challenges in implantable battery technology for biomedical devices. Discov. Appl. Sci. 2024, 6, 584. [Google Scholar] [CrossRef]
- Vakili, S.; Lanting, B.; Getgood, A.; Willing, R. Comparison of the Kinematics and Laxity of Total Knee Arthroplasty Bearing Designs Stabilized with Specimen-Specific Virtual Ligaments on a Joint Motion Simulator. J. Biomech. Eng. 2024, 146, 081005. [Google Scholar] [CrossRef]
- Vivacqua, T.; Vakili, S.; Willing, R.; Moatshe, G.; Degen, R.; Getgood, A.M. Biomechanical Assessment of Knee Laxity After a Novel Posterolateral Corner Reconstruction Technique. Am. J. Sports Med. 2022, 50, r962–r967. [Google Scholar] [CrossRef]
- Bergmann, G.; Bender, A.; Graichen, F.; Dymke, J.; Rohlmann, A.; Trepczynski, A.; Heller, M.O.; Kutzner, I. Standardized loads acting in knee implants. PLoS ONE 2014, 9, e86035. [Google Scholar] [CrossRef]
- Henke, P.; Ruehrmund, L.; Bader, R.; Kebbach, M. Exploration of the Advanced VIVOTM Joint Simulator: An In-Depth Analysis of Opportunities and Limitations Demonstrated by the Artificial Knee Joint. Bioengineering 2024, 11, 178. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Gerdtman, C.; Lindén, M. Signal quality improvement algorithms for MEMS gyroscope-based human motion analysis systems: A systematic review. Sensors 2018, 18, 1123. [Google Scholar] [CrossRef] [PubMed]
- Özer, İ.; Efe, S.B.; Özbay, H. CNN/Bi-LSTM-based deep learning algorithm for classification of power quality disturbances by using spectrogram images. Int. Trans. Electr. Energy Syst. 2021, 31, e13204. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Y.; Qian, K.; Wu, C. SLNet: A Spectrogram Learning Neural Network for Deep Wireless Sensing. Available online: https://www.usenix.org/conference/nsdi23/presentation/yang-zheng (accessed on 1 June 2025).
- Hill, B.G.; Shah, S.; Moschetti, W.E.; Schilling, P.L. Do Patient-Reported Outcomes Reflect Objective Measures of Function? Implications for Total Knee Arthroplasty. J. Arthroplast. 2023, 38, S162–S168.e3. [Google Scholar] [CrossRef]
- Van der Walt, N.; Salmon, L.J.; Gooden, B.; Lyons, M.C.; O’Sullivan, M.; Martina, K.; Pinczewski, L.A.; Roe, J.P. Feedback From Activity Trackers Improves Daily Step Count After Knee and Hip Arthroplasty: A Randomized Controlled Trial. J. Arthroplast. 2018, 33, r3422–r3428. [Google Scholar] [CrossRef]
- Harding, P.; Holland, A.E.; Delany, C.; Hinman, R.S. Do activity levels increase after total hip and knee arthroplasty? Clin. Orthop. Relat. Res. 2014, 472, r1502–r1511. [Google Scholar] [CrossRef]
- Lebleu, J.; Daniels, K.; Pauwels, A.; Dekimpe, L.; Mapinduzi, J.; Poilvache, H.; Bonnechère, B. Incorporating Wearable Technology for Enhanced Rehabilitation Monitoring after Hip and Knee Replacement. Sensors 2024, 24, 1163. [Google Scholar] [CrossRef]
- Zimmerbiomet. Persona IQ®|Smart Knee Implant Technology for Surgeons. Zimmerbiomet. 2025. Available online: https://www.zimmerbiomet.com (accessed on 1 June 2025).
- Guild, G.N.; Najafi, F.; DeCook, C.A.; Levit, C.; McConnell, M.J.; Bradbury, T.L.; Naylor, B.H. Evaluating Knee Recovery Beyond Patient Reports: A Comparative Study of Smart Implantable Device-Derived Gait Metrics Versus Patient-Reported Outcome Measures in Total Knee Arthroplasty. J. Arthroplast. 2024, 39, r2961–r2969.e1. [Google Scholar] [CrossRef]
- Diaz-Ledezma, C.; Molloy, I.; Nelissen, R.; Mokete, L.; Costantini, J. Is Prescribed Postoperative Physical Therapy Necessary after Routine Primary Total Knee or Total Hip Arthroplasty? J. Arthroplast. 2025, 40, S57–S59. [Google Scholar] [CrossRef] [PubMed]
- Whitebird, R.R.; Solberg, L.I.; Ziegenfuss, J.Y.; Asche, S.E.; Norton, C.K.; Swiontkowski, M.F.; Dehmer, S.P.; Grossman, E.S. Personalized outcomes for hip and knee replacement: The patients point of view. J. Patient Rep. Outcomes 2021, 5, 116. [Google Scholar] [CrossRef] [PubMed]


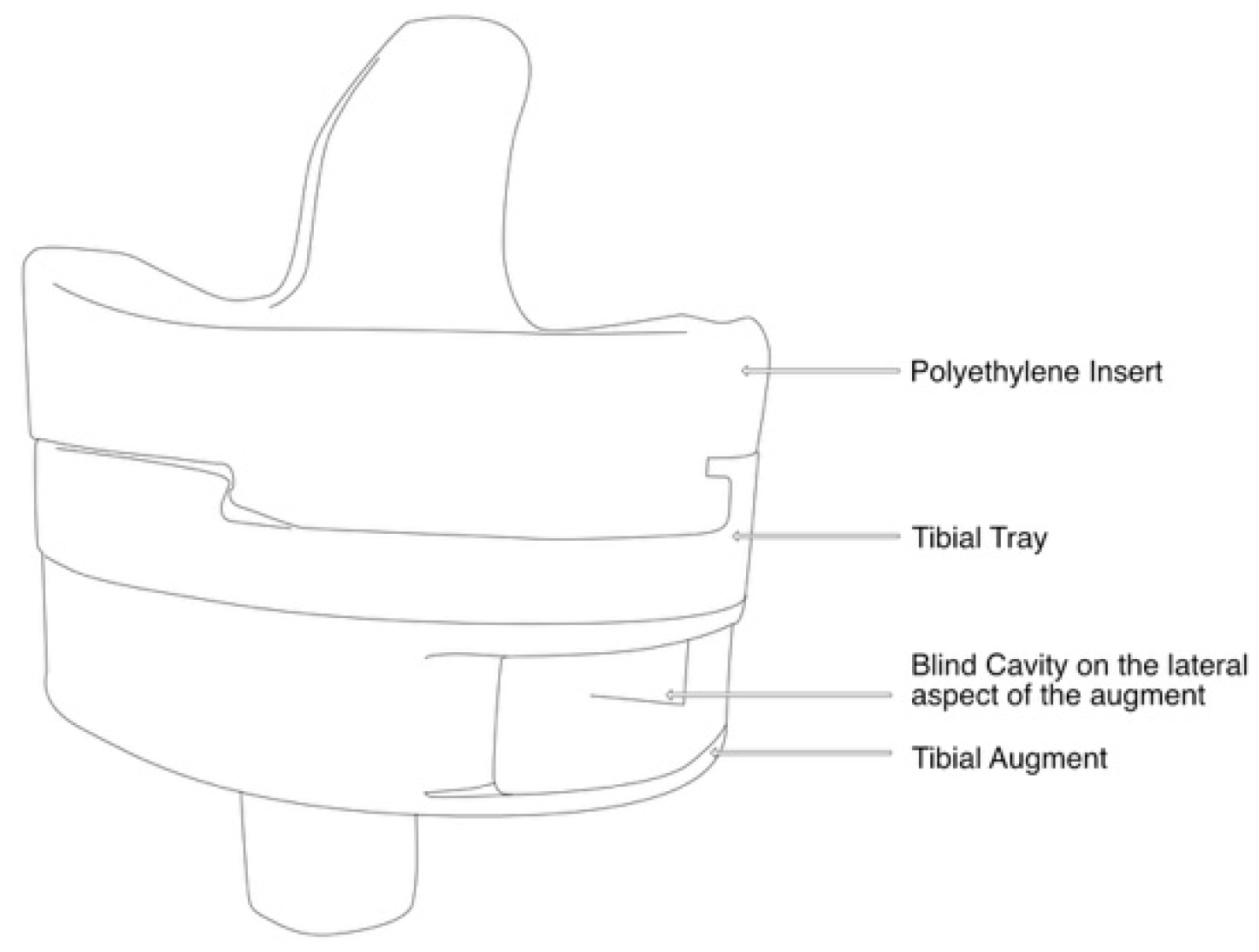
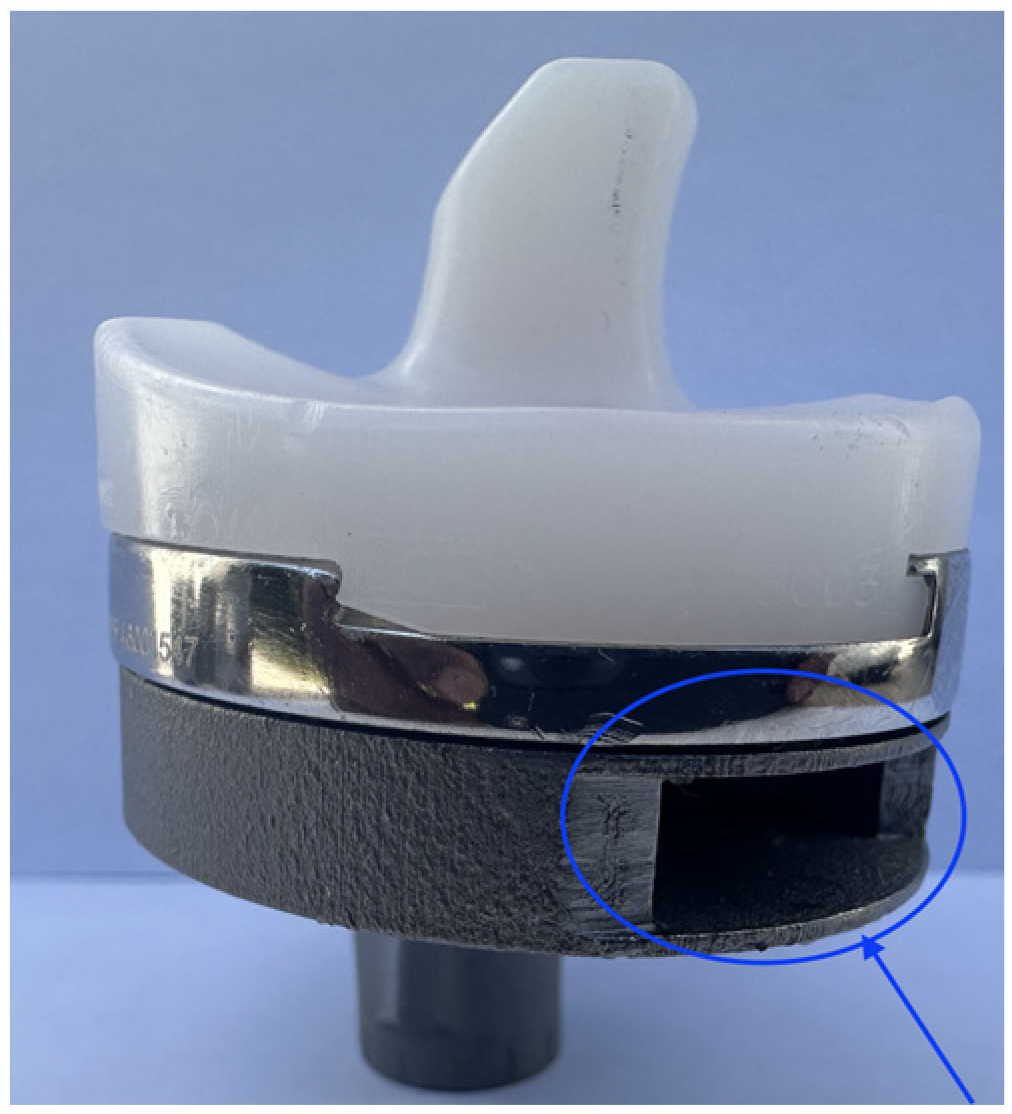



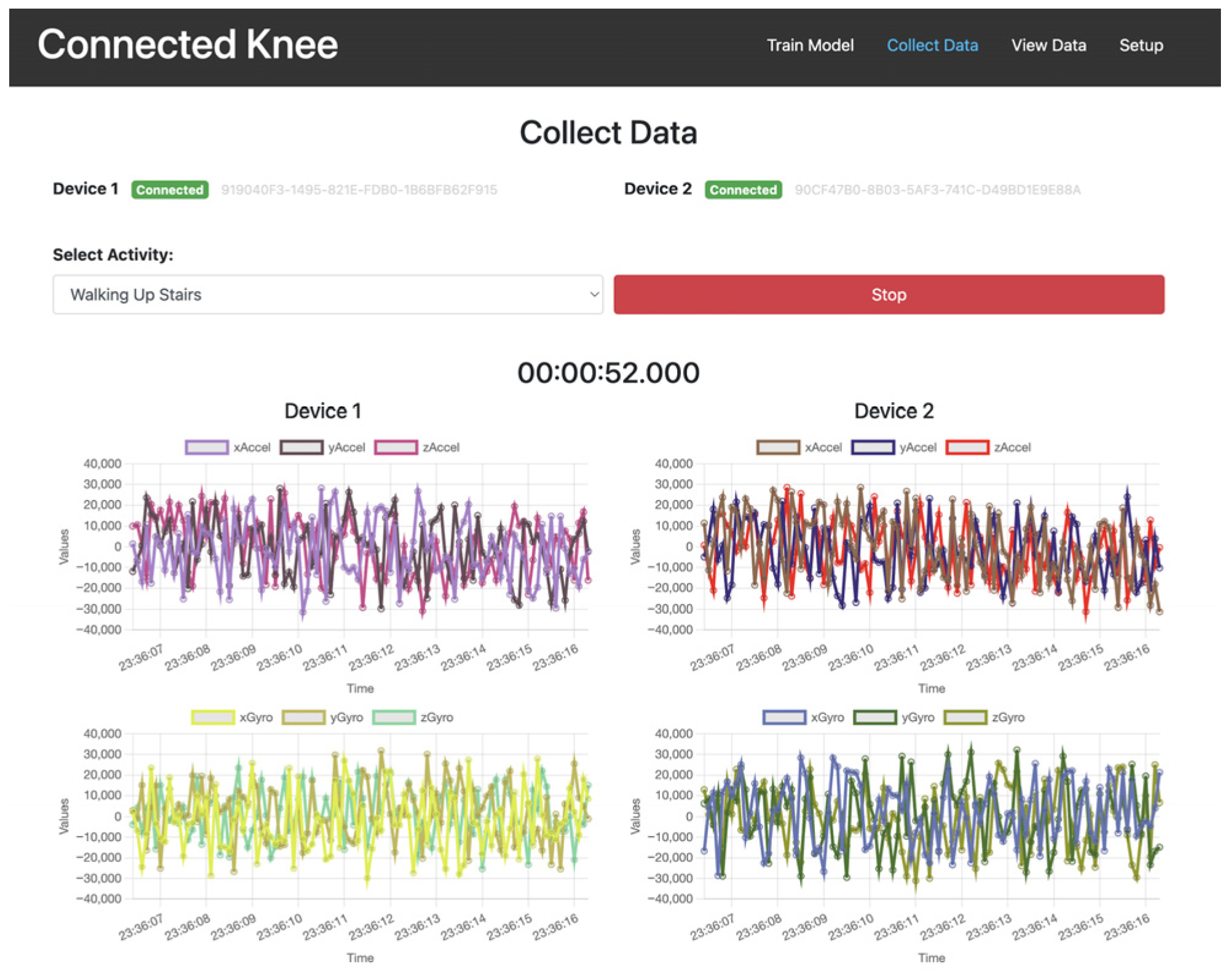
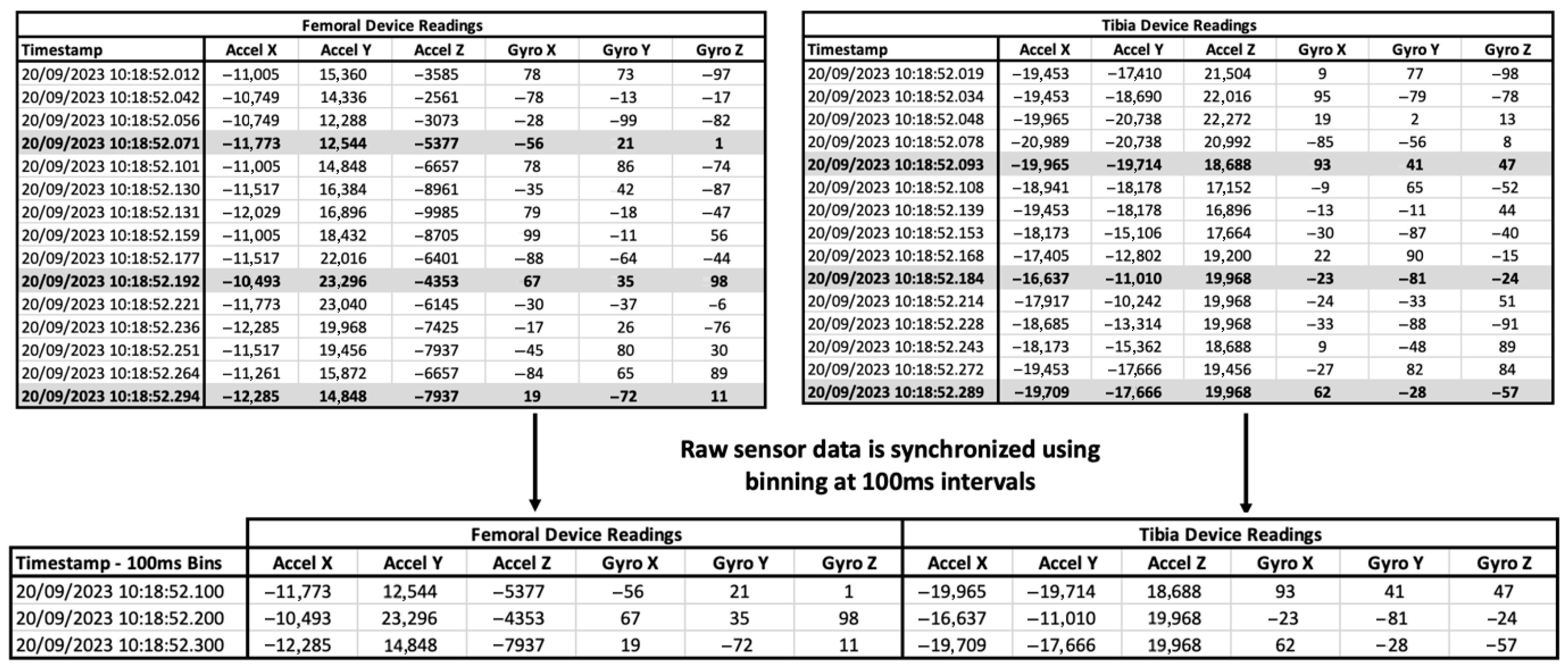

| Motion Simulated Based on Orthoload | Flexion Range of Motion [Degrees] | Tested Rate [Hz] (Original Rate) |
|---|---|---|
| Walking (level ground) | 7–62 | 0.94 (0.47) |
| Jogging (6 km/h on treadmill) | 7–62 | 1.4 (0.7) |
| Stair ascent | 13–94 | 0.56 (0.28) |
| Stair descent | 14–98 | 0.58 (0.29) |
| Sitting down (from standing position) | 6–94 | 0.16 (0.16) |
| Standing up (from seated position) | 6–94 | 0.16 (0.16) |
| Knee bending | 4–98 | 0.13 (0.13) |
| Standing still | 7–13 | 0.1 (0.1) |
| Activity | Precision | Recall | F1 Score | Support |
|---|---|---|---|---|
| Jogging 6 km/h treadmill | 1.00 | 0.80 | 0.89 | 10 |
| Knee bending | 1.00 | 1.00 | 1.00 | 3 |
| Sitting down | 1.00 | 1.00 | 1.00 | 10 |
| Stance | 1.00 | 1.00 | 1.00 | 22 |
| Standing up | 1.00 | 1.00 | 1.00 | 10 |
| Walking | 0.97 | 1.00 | 0.98 | 31 |
| Walking downstairs | 0.95 | 0.88 | 0.91 | 24 |
| Walking upstairs | 0.88 | 0.97 | 0.92 | 31 |
| Accuracy (overall) | — | — | 0.96 | 139 |
| Weighted average | 0.96 | 0.96 | 0.96 | 139 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokete, L.; Conway, A.; Donnelly, E.; Willing, R. Smart Total Knee Replacement: Recognition of Activities of Daily Living Using Embedded IMU Sensors and a Novel AI Model in a Cadaveric Proof-of-Concept Study. Sensors 2025, 25, 6657. https://doi.org/10.3390/s25216657
Mokete L, Conway A, Donnelly E, Willing R. Smart Total Knee Replacement: Recognition of Activities of Daily Living Using Embedded IMU Sensors and a Novel AI Model in a Cadaveric Proof-of-Concept Study. Sensors. 2025; 25(21):6657. https://doi.org/10.3390/s25216657
Chicago/Turabian StyleMokete, Lipalo, Alexander Conway, Emma Donnelly, and Ryan Willing. 2025. "Smart Total Knee Replacement: Recognition of Activities of Daily Living Using Embedded IMU Sensors and a Novel AI Model in a Cadaveric Proof-of-Concept Study" Sensors 25, no. 21: 6657. https://doi.org/10.3390/s25216657
APA StyleMokete, L., Conway, A., Donnelly, E., & Willing, R. (2025). Smart Total Knee Replacement: Recognition of Activities of Daily Living Using Embedded IMU Sensors and a Novel AI Model in a Cadaveric Proof-of-Concept Study. Sensors, 25(21), 6657. https://doi.org/10.3390/s25216657






