Accuracy of an Overnight Axillary-Temperature Sensor for Ovulation Detection: Validation in 194 Cycles
Abstract
Highlights
- An axillary skin temperature sensor (Tempdrop) can accurately determine the timing of ovulation.
- It provides a clear temperature curve.
- It is an easy and effective alternative to urine ovulation tests for detecting ovulation.
- It can be used to evaluate the quality of the luteal phase.
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Study Design
2.3. Inclusion Criteria
2.4. Estimated Day of Ovulation
2.4.1. Reference Estimated Day of Ovulation
2.4.2. Armband Sensor Estimated Day of Ovulation
- Training phase
- Testing phase
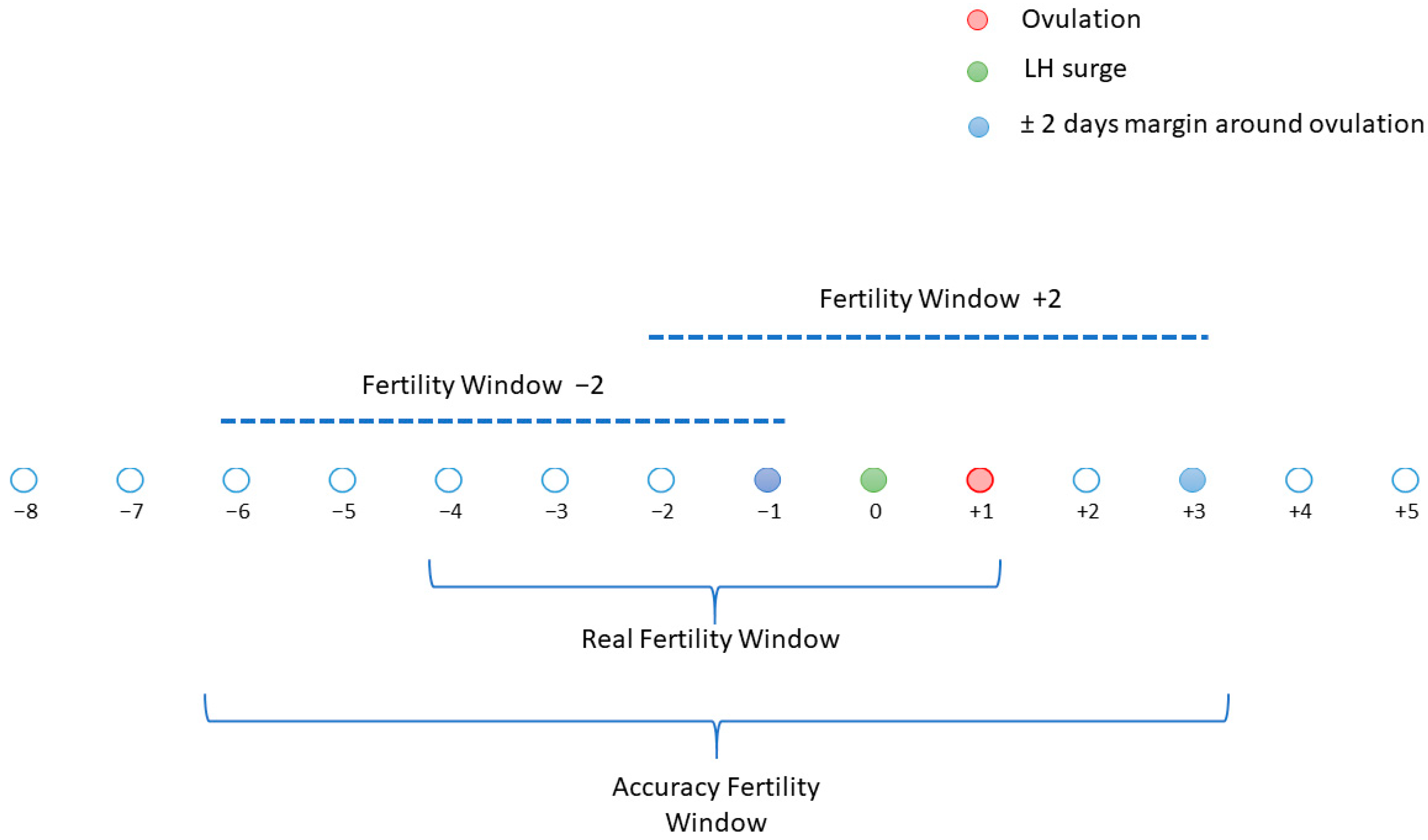
2.5. Peri-Ovulatory Period and Fertility Window
2.5.1. Peri-Ovulatory Period
2.5.2. Fertility Window
2.6. Statistical Analysis
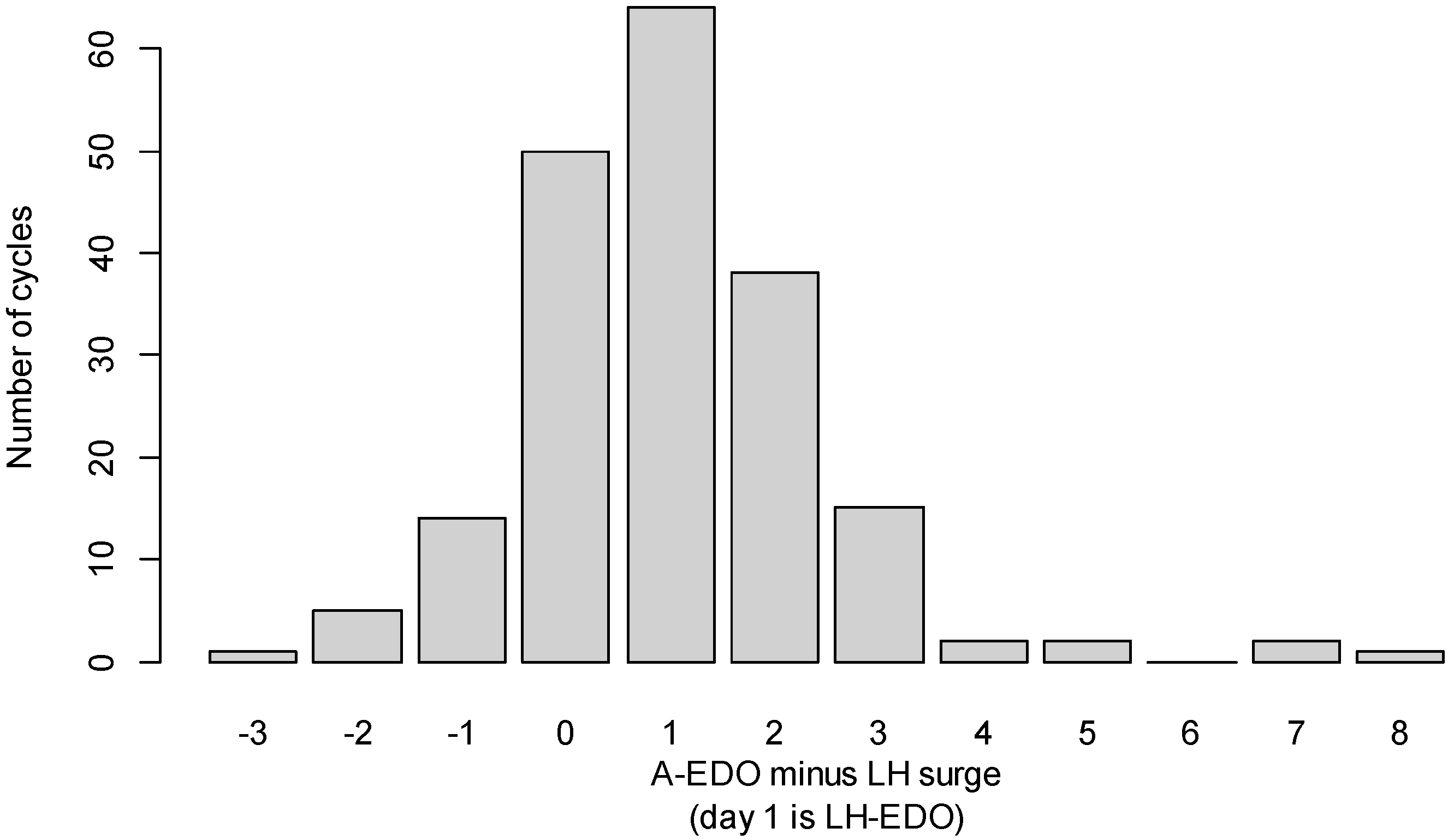
2.6.1. Comparison of A-EDO with the LH-EDO
2.6.2. Performance Metrics for Detecting Fertile Days
- Some definitions:
- Performance metrics
3. Results
3.1. Comparison of the A-EDO and the LH-EDO
3.2. Results of Performance Metrics for Detecting Fertile Days
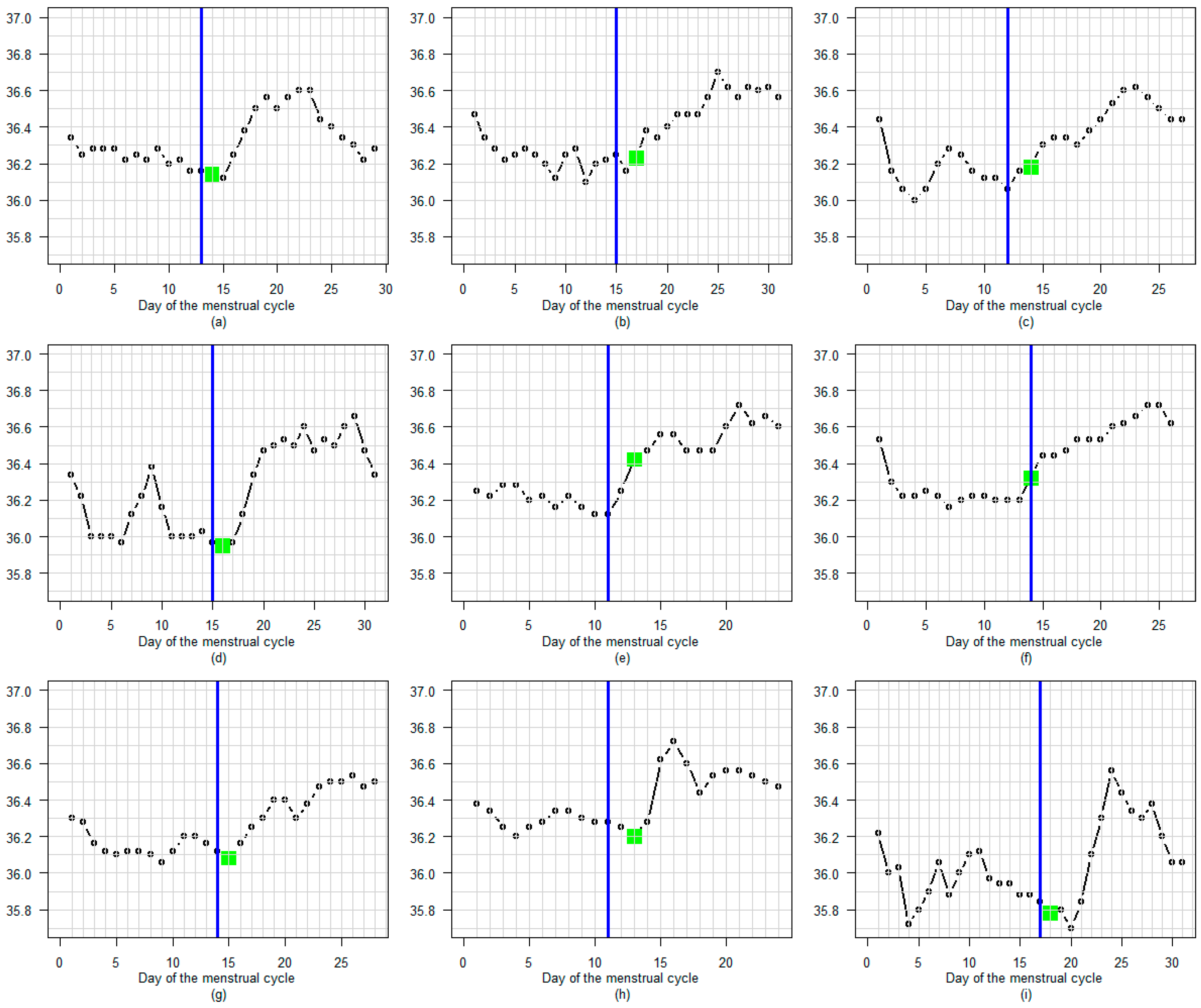
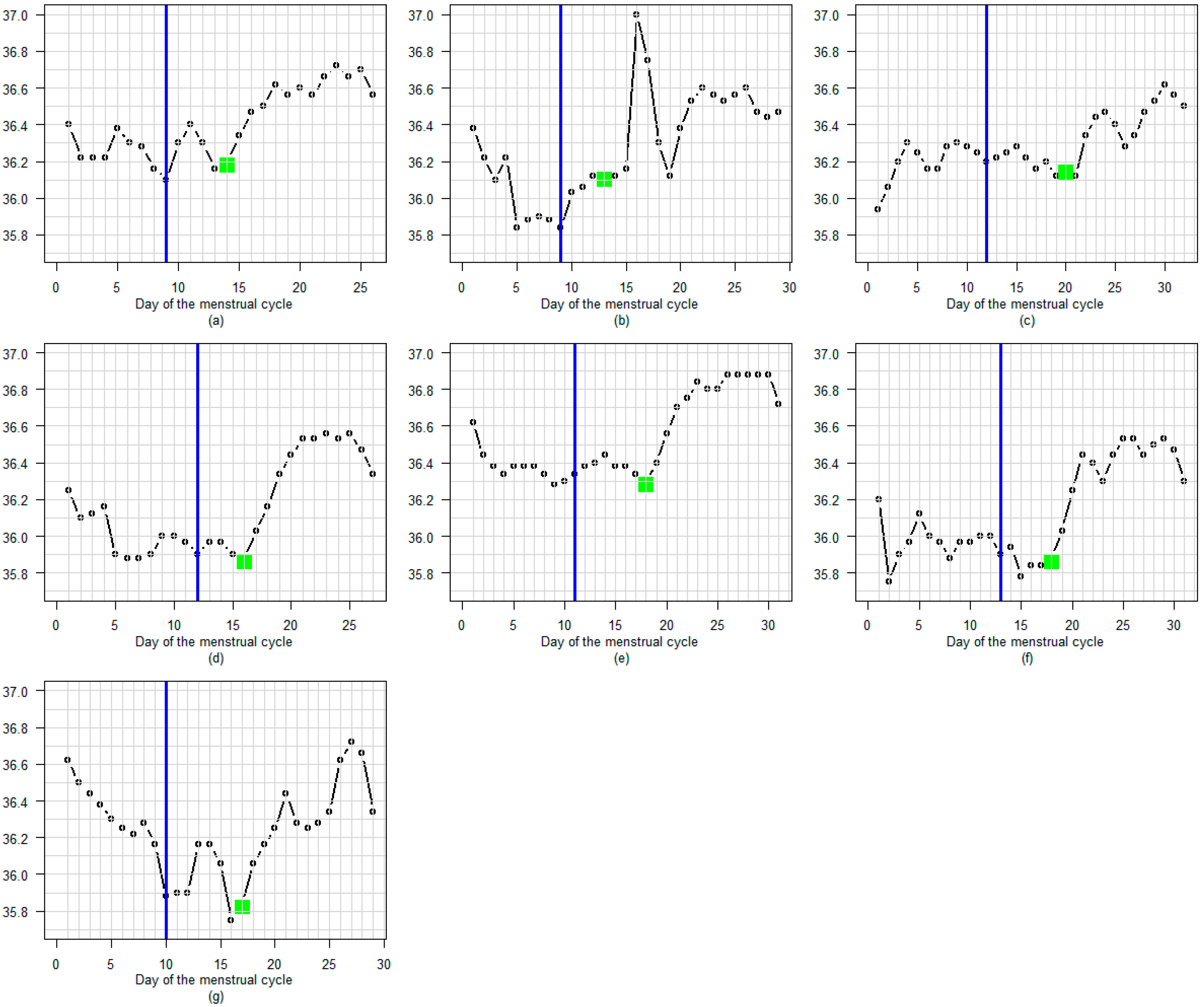
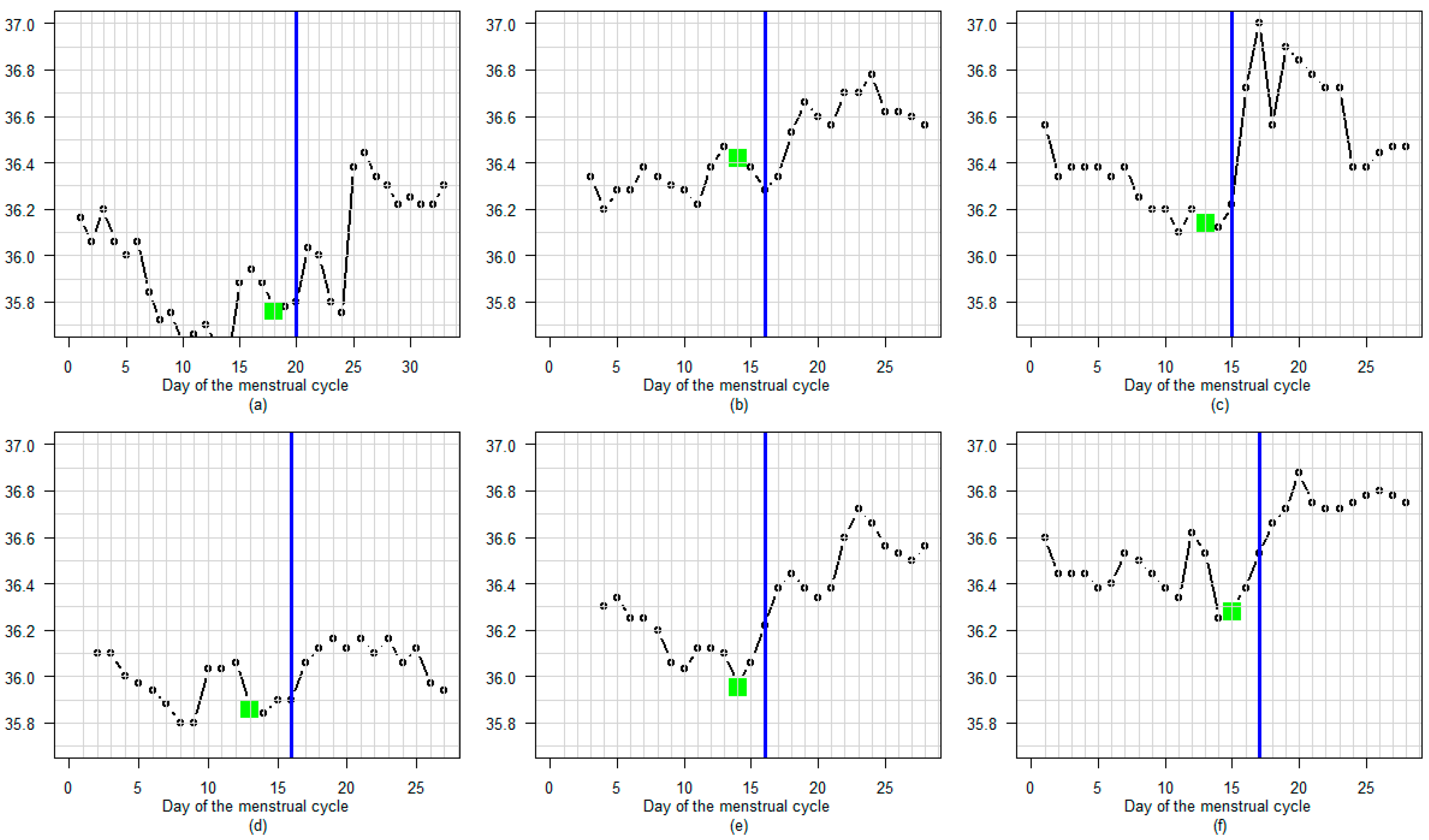
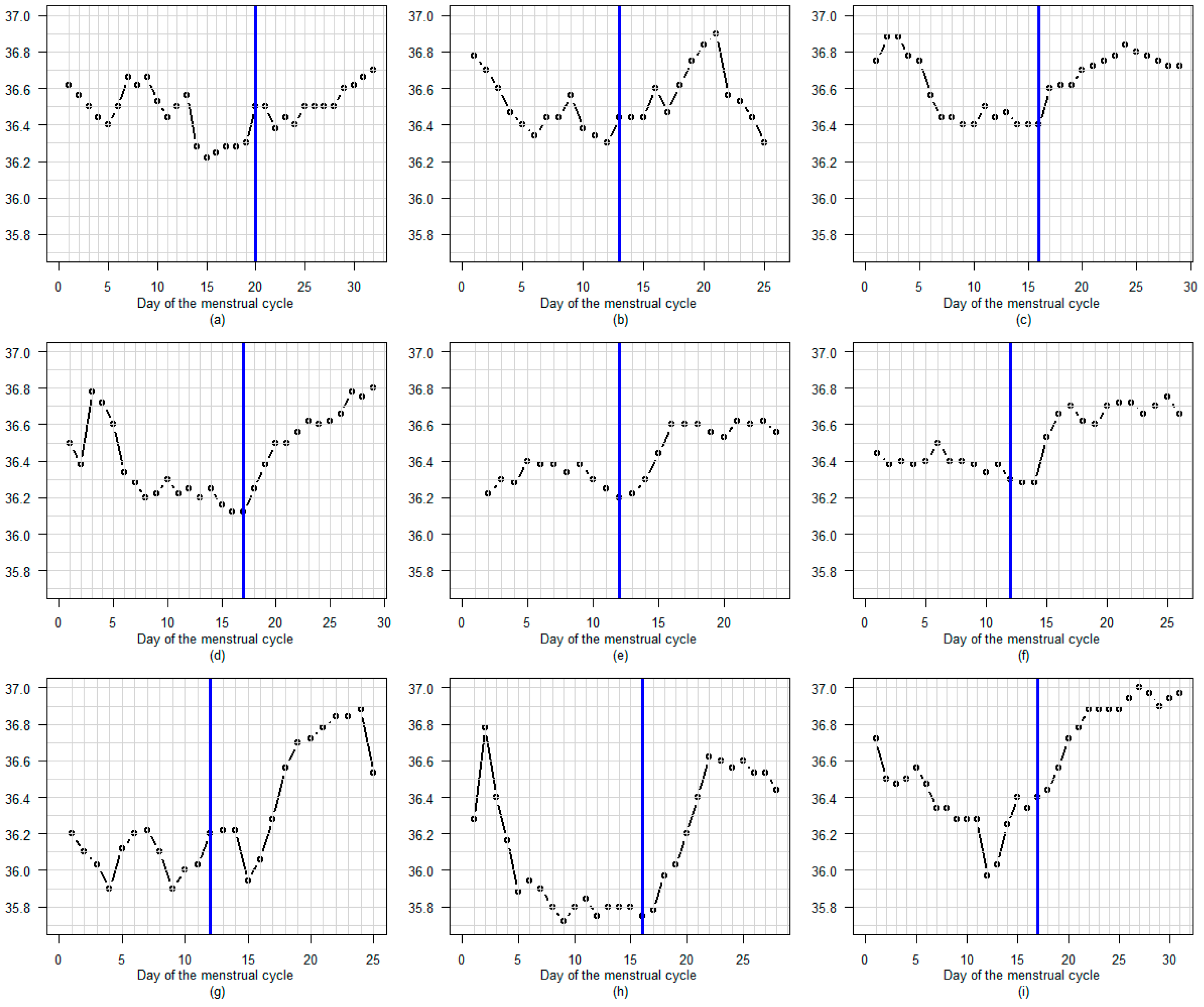
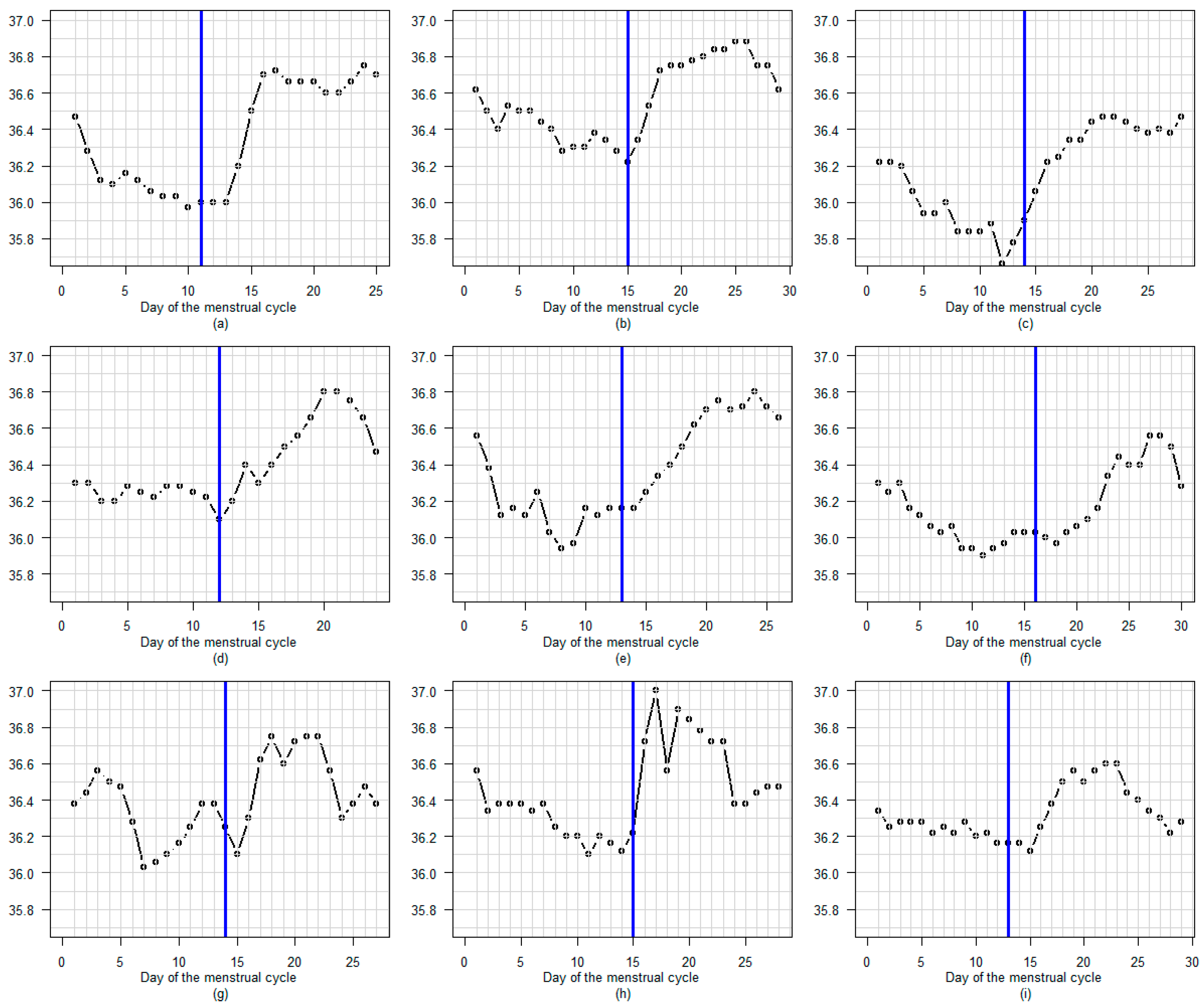
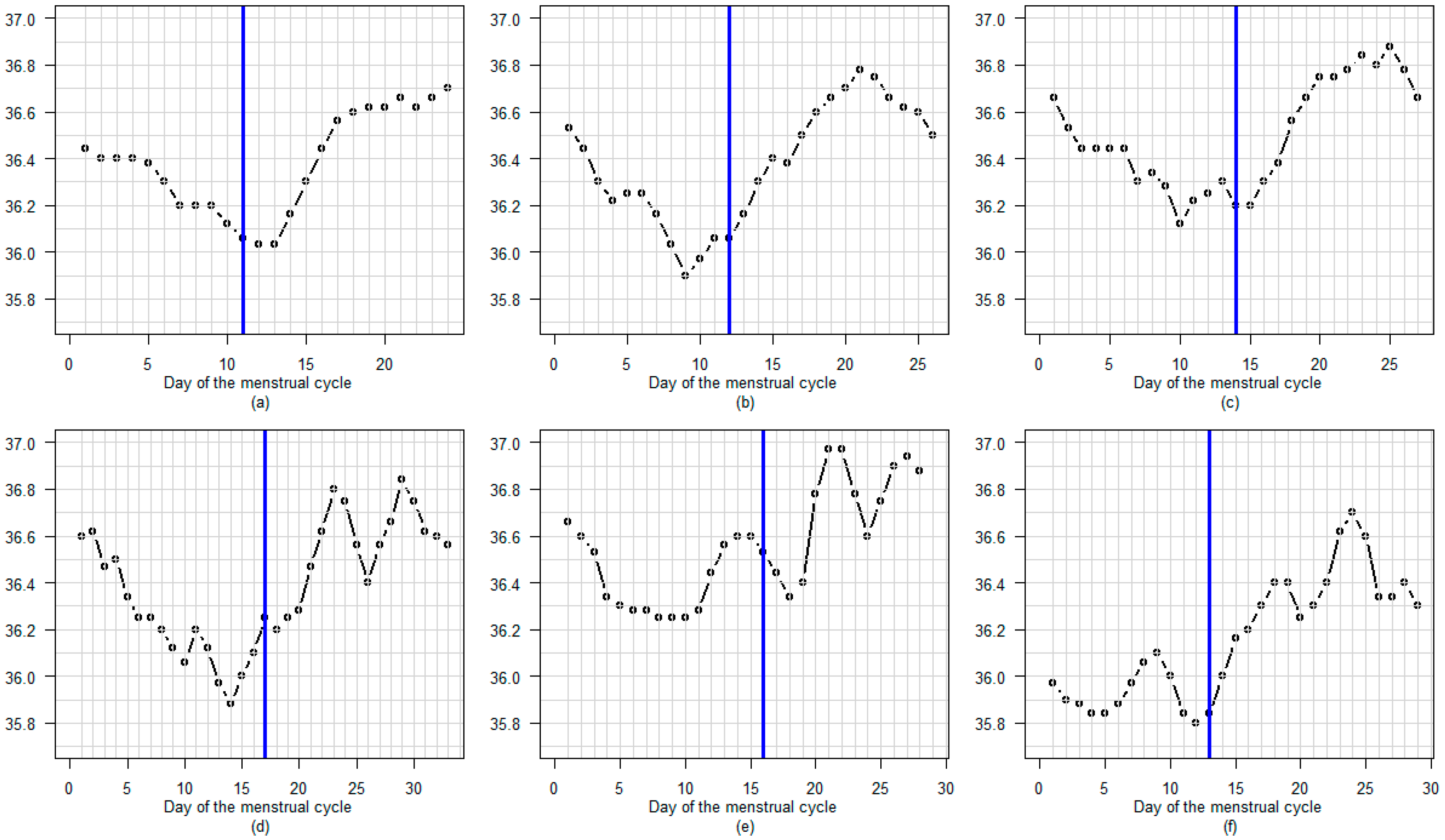
3.3. BBT Profile During the Luteal Phase
3.3.1. Amplitude
3.3.2. Profile
3.3.3. Regular or Fluctuating
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BBT | Basal Body Temperature |
| IRB | Institutional Review Board |
| LH | Luteinizing Hormone |
| A-EDO | Armband-Estimated Day of Ovulation |
| 1D CNN | One-Dimension Convolutional Neural Network |
| s.d. | Standard deviation |
| TP | True Positive |
| TN | True Negative |
| FP | False Positive |
| FN | False Negative |
References
- Su, H.W.; Yi, Y.C.; Wei, T.Y.; Chang, T.C.; Cheng, C.M. Detection of Ovulation, a Review of Currently Available Methods. Bioeng. Transl. Med. 2017, 16, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; She, X.; Cao, J.; Zhang, Y.; Li, Y.; Song, P.X.K. Detection and Prediction of Ovulation from Body Temperature Measured by an In-Ear Wearable Thermometer. IEEE Trans. Biomed. Eng. 2020, 67, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Regidor, P.-A.; Kaczmarczyk, M.; Schiweck, E.; Goeckenjan-Festag, M.; Alexander, H. Identification and Prediction of the Fertile Window with a New Web-Based Medical Device Using a Vaginal Biosensor for Measuring the Circadian and Circamensual Core Body Temperature. Gynecol. Endocrinol. 2018, 34, 256–260. [Google Scholar] [CrossRef]
- Wang, Y.; Park, J.; Zhang, C.Y.; Jukic, A.M.Z.; Baird, D.D.; Coull, B.A.; Hauser, R.; Mahalingaiah, S.; Zhang, S.; Curry, C.L. Performance of Algorithms Using Wrist Temperature for Retrospective Ovulation Day Estimate and next Menses Start Day Prediction: A Prospective Cohort Study. Hum. Reprod. 2025, 40, 469–478. [Google Scholar] [CrossRef]
- Niggli, A.; Rothenbühler, M.; Sachs, M.; Leeners, B. Can Wrist-Worn Medical Devices Correctly Identify Ovulation? Sensors 2023, 23, 9730. [Google Scholar] [CrossRef]
- Thigpen, N.; Patel, S.; Zhang, X. Oura Ring as a Tool for Ovulation Detection: Validation Analysis. J. Med. Internet Res. 2025, 27, e60667. [Google Scholar] [CrossRef]
- Wark, J.D.; Henningham, L.; Gorelik, A.; Jayasinghe, Y.; Hartley, S.; Garland, S.M. Basal Temperature Measurement Using a Multi-Sensor Armband in Australian Young Women: A Comparative Observational Study. JMIR Mhealth Uhealth 2015, 3, e94. [Google Scholar] [CrossRef]
- Uchida, Y.; Izumizaki, M. The Use of Wearable Devices for Predicting Biphasic Basal Body Temperature to Estimate the Date of Ovulation in Women. J. Therm. Biol. 2022, 108, 103290. [Google Scholar] [CrossRef]
- Goodale, B.M.; Shilaih, M.; Falco, L.; Dammeier, F.; Hamvas, G.; Leeners, B. Wearable Sensors Reveal Menses-Driven Changes in Physiology and Enable Prediction of the Fertile Window: Observational Study. J. Med. Internet Res. 2019, 21, e13404. [Google Scholar] [CrossRef] [PubMed]
- Vollman, R.F. The Menstrual Cycle. Major Probl. Obstet. Gynecol. 1977, 7, 1–193. [Google Scholar]
- Écochard, R.; Leiva, R.; Bouchard, T.; Boehringer, H.; Iwaz, J.; Plotton, I. Descriptive Analysis of the Relationship between Progesterone and Basal Body Temperature across the Menstrual Cycle. Steroids 2022, 178, 108964. [Google Scholar] [CrossRef]
- Yu, J.-L.; Su, Y.-F.; Zhang, C.; Jin, L.; Lin, X.-H.; Chen, L.-T.; Huang, H.-F.; Wu, Y.-T. Tracking of Menstrual Cycles and Prediction of the Fertile Window via Measurements of Basal Body Temperature and Heart Rate as Well as Machine-Learning Algorithms. Reprod. Biol. Endocrinol. 2022, 20, 118. [Google Scholar] [CrossRef]
- Masuda, H.; Okada, S.; Shiozawa, N.; Sakaue, Y.; Manno, M.; Makikawa, M.; Isaka, T. Machine Learning Model for Menstrual Cycle Phase Classification and Ovulation Day Detection Based on Sleeping Heart Rate under Free-Living Conditions. Comput. Biol. Med. 2025, 187, 109705. [Google Scholar] [CrossRef] [PubMed]
- Kilungeja, G.; Graham, K.; Liu, X.; Nasseri, M. Machine Learning-Based Menstrual Phase Identification Using Wearable Device Data. npj Women’s Health 2025, 3, 29. [Google Scholar] [CrossRef]
- Wilcox, A.J. The Timing of the “Fertile Window” in the Menstrual Cycle: Day Specific Estimates from a Prospective Study. BMJ 2000, 321, 1259–1262. [Google Scholar] [CrossRef]
- Mu, Q.; Fehring, R.J. A Comparison of Two Hormonal Fertility Monitoring Systems for Ovulation Detection: A Pilot Study. Medicina 2023, 59, 400. [Google Scholar] [CrossRef]
- Ecochard, R.; Boehringer, H.; Rabilloud, M.; Marret, H. Chronological Aspects of Ultrasonic, Hormonal, and Other Indirect Indices of Ovulation. BJOG 2001, 108, 822–829. [Google Scholar] [CrossRef]
- Hilgers, T.W.; Bailey, A.J. Natural Family Planning. II. Basal Body Temperature and Estimated Time of Ovulation. Obstet. Gynecol. 1980, 55, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Templeton, A.A.; Penney, G.C.; Lees, M.M. Relation between the Luteinizing Hormone Peak, the Nadir of the Basal Body Temperature and the Cervical Mucus Score. BJOG 1982, 89, 985–988. [Google Scholar] [CrossRef] [PubMed]
- de Mouzon, J.; Testart, J.; Lefevre, B.; Pouly, J.-L.; Frydman, R. Time Relationships between Basal Body Temperature and Ovulation or Plasma Progestins. Fertil. Steril. 1984, 41, 254–259. [Google Scholar] [CrossRef]
- Quagliarello, J.; Arny, M. Inaccuracy of Basal Body Temperature Charts in Predicting Urinary Luteinizing Hormone Surges. Fertil. Steril. 1986, 45, 334–337. [Google Scholar] [CrossRef]
- Wetzels, L.C.G.; Hoogland, H.J.; de Haan, J. Basal Body Temperature as a Method of Ovulation Detection: Comparison with Ultrasonographical Findings. Gynecol. Obstet. Investig. 1982, 13, 235–240. [Google Scholar] [CrossRef]
- Leader, A.; Wiseman, D.; Taylor, P.J. The Prediction of Ovulation: A Comparison of the Basal Body Temperature Graph, Cervical Mucus Score, and Real-Time Pelvic Ultrasonography. Fertil. Steril. 1985, 43, 385–388. [Google Scholar] [CrossRef]
- Doring, G.K. Basal Temperature Measurement for the Determination of Fertile & Infertile Days of Women. Dtsch. Gesundheitsw. 1957, 12, 422–425. [Google Scholar]
- Gross, B.A. Natural Family Planning Indicators of Ovulation. Clin. Reprod. Fertil. 1987, 5, 91–117. [Google Scholar] [PubMed]
- Morris, N.; Underwood, L.; Easterling, W., Jr. Temporal Relationship between Basal Body Temperature Nadir and Luteinizing Hormone Surge in Normal Women. Fertil. Steril. 1976, 27, 780–783. [Google Scholar] [CrossRef] [PubMed]
- Matthews, C.D.; Broom, T.J.; Black, T.; Tansing, J. Optimal Features of Basal Body Temperature Recordings Associated with Conceptional Cycles. Int. J. Fertil. 1980, 25, 318–320. [Google Scholar]
- Abdullah, S.; Bouchard, T.; Leiva, R.; Boehringer, H.; Iwaz, J.; Ecochard, R. Distinct Urinary Progesterone Metabolite Profiles during the Luteal Phase. Horm. Mol. Biol. Clin. Investig. 2023, 44, 137–144. [Google Scholar] [CrossRef]
- Sitruk-Ware, L.R.; Sterkers, N.; Mowszowicz, I.; Mauvais-Jarvis, P. Inadequate Corpus Luteal Function in Women with Benign Breast Diseases. J. Clin. Endocrinol. Metab. 1977, 44, 771–774. [Google Scholar] [CrossRef] [PubMed]
- Practice Committees of the American Society for Reproductive Medicine and the Society for Reproductive Endocrinology and Infertility. Diagnosis and Treatment of Luteal Phase Deficiency: A Committee Opinion. Fertil. Steril. 2021, 115, 1416–1423. [Google Scholar] [CrossRef]
- Wallach, E.E.; McNeely, M.J.; Soules, M.R. The Diagnosis of Luteal Phase Deficiency: A Critical Review. Fertil. Steril. 1988, 50, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Goeckenjan, M.; Schiwek, E.; Wimberger, P. Continuous Body Temperature Monitoring to Improve the Diagnosis of Female Infertility. Geburtshilfe Frauenheilkd. 2020, 80, 702–712. [Google Scholar] [CrossRef]
- He, H. Diagnosis of Basal Body Temperature, Serum Progesterone and Endometrial Biopsy for Luteal Phase Defect. Zhonghua Fu Chan Ke Za Zhi 1993, 28, 82–123. [Google Scholar]
- Turner, J.V.; McLindon, L.A.; Turner, D.V.; Alefsen, Y.; Ecochard, R. Relationship Between Steroid Hormone Profile and Premenstrual Syndrome in Women Consulting for Infertility or Recurrent Miscarriage. Reprod. Sci. 2024, 31, 736–745. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, B.; Wu, G.; Yang, J. Exploration of the Value of Progesterone and Progesterone/Estradiol Ratio on the HCG Trigger Day in Predicting Pregnancy Outcomes of PCOS Patients Undergoing IVF/ICSI: A Retrospective Cohort Study. Reprod. Biol. Endocrinol. 2021, 19, 184. [Google Scholar] [CrossRef] [PubMed]
- Mesen, T.B.; Young, S.L. Progesterone and the Luteal Phase. Obstet. Gynecol. Clin. N. Am. 2015, 42, 135–151. [Google Scholar] [CrossRef]
- Schliep, K.C.; Mumford, S.L.; Hammoud, A.O.; Stanford, J.B.; Kissell, K.A.; Sjaarda, L.A.; Perkins, N.J.; Ahrens, K.A.; Wactawski-Wende, J.; Mendola, P.; et al. Luteal Phase Deficiency in Regularly Menstruating Women: Prevalence and Overlap in Identification Based on Clinical and Biochemical Diagnostic Criteria. J. Clin. Endocrinol. Metab. 2014, 99, E1007–E1014. [Google Scholar] [CrossRef] [PubMed]
| Performance Metrics | Numbers | Percentage (95% Confidence Interval) |
|---|---|---|
| True positive days | 1138 | |
| True negative days | 4180 | |
| False positive days | 38 | |
| False negative days | 38 | |
| Sensitivity | 1138/(1138 + 38) | 96.8% (95.6; 97.7) |
| Specificity | 4180/(4180 + 38) | 99.1% (98.8; 99.4) |
| Accuracy | (1138 + 4180)/5394 | 98.6% (98.2; 98.9) |
| Positive predictive value | 1138/(1138 + 38) | 96.8% (95.6; 97.7) |
| Negative predictive value | 4180/(4180 + 38) | 99.1% (98.8; 99.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shpaichler, Y.; Thompson, A.; Fromager, B.; Vardi, M.; Ecochard, R. Accuracy of an Overnight Axillary-Temperature Sensor for Ovulation Detection: Validation in 194 Cycles. Sensors 2025, 25, 6327. https://doi.org/10.3390/s25206327
Shpaichler Y, Thompson A, Fromager B, Vardi M, Ecochard R. Accuracy of an Overnight Axillary-Temperature Sensor for Ovulation Detection: Validation in 194 Cycles. Sensors. 2025; 25(20):6327. https://doi.org/10.3390/s25206327
Chicago/Turabian StyleShpaichler, Yaniv, Alicia Thompson, Benedicte Fromager, Michael Vardi, and Rene Ecochard. 2025. "Accuracy of an Overnight Axillary-Temperature Sensor for Ovulation Detection: Validation in 194 Cycles" Sensors 25, no. 20: 6327. https://doi.org/10.3390/s25206327
APA StyleShpaichler, Y., Thompson, A., Fromager, B., Vardi, M., & Ecochard, R. (2025). Accuracy of an Overnight Axillary-Temperature Sensor for Ovulation Detection: Validation in 194 Cycles. Sensors, 25(20), 6327. https://doi.org/10.3390/s25206327






