Monte Carlo Simulation of the Effect of Melanin Concentration on Light–Tissue Interactions in Reflectance Pulse Oximetry
Abstract
1. Introduction
| Method | Advantages | Limitations | Relevance to Study |
|---|---|---|---|
| Monte Carlo simulation (MCS) | Highly accurate for modeling complex tissue structures and heterogeneous tissue properties [13]. Capable of accounting for scattering, absorption, and tissue heterogeneity, providing a statistically detailed simulation of light transport and interaction [14]. | Can be computationally expensive, requiring significant computational resources and time [15]. | Provides high accuracy and reproducible outcomes by running a very high number of photon iterations, making it ideal for studying their stochastic nature in light–tissue interactions as pigmentation changes. |
| Finite element method (FEM) | Suitable for solving complex systems particularly in laser-based applications [16,17]. | Less accurate in modelling light scattering and absorption compared to MCS [18]. | FEM can be less suited for pulse oximetry applications to model the complex scattering events in tissues such as skin. |
| Finite different method (FDM) | Useful for solving the light diffusion equation in simple tissue models and to achieve a balance between accuracy and computational efficiency [19]. | Assumes constant tissue properties, limiting its application in heterogeneous tissues like skin [20]. | FDM is more suited for simpler models of tissue and may be faster for initial simulations but lacks the precision needed for accurately modelling light absorption and scattering in tissues like skin with varying pigmentation. |
| Diffusion approximation | Computationally efficient for modelling light transport in scattering media especially in deep tissue [21]. | Less accurate for tissues with high scattering properties [22]. | Diffusion approximation is better suited for modelling light in deep tissues rather than superficial layers, which is the main focus of this study. Like FEM, it is also not ideal for predicting radiative transport in turbid media such as the human finger. |
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kang, H.S.; Exworthy, M. Wearing the Future—Wearables to Empower Users to Take Greater Responsibility for Their Health and Care: Scoping Review. JMIR mHealth uHealth 2022, 10, e35684. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.J.; Fernandes, C.I.; Rammal, H.G.; Veiga, P.M. Wearable technology and consumer interaction: A systematic review and research agenda. Comput. Hum. Behav. 2021, 118, 106710. [Google Scholar] [CrossRef]
- Scardulla, F.; Cosoli, G.; Spinsante, S.; Poli, A.; Iadarola, G.; Pernice, R.; Busacca, A.; Pasta, S.; Scalise, L.; D’Acquisto, L. Photoplethysmograhic sensors, potential and limitations: Is it time for regulation? A comprehensive review. Measurement 2023, 218, 113150. [Google Scholar] [CrossRef]
- Hosseini, M.M.; Hosseini, S.T.M.; Qayumi, K.; Hosseinzadeh, S.; Tabar, S.S.S. Smartwatches in healthcare medicine: Assistance and monitoring; a scoping review. BMC Med. Inform. Decis. Mak. 2023, 23, 248. [Google Scholar] [CrossRef]
- Singh, S.; Bennett, M.R.; Chen, C.; Shin, S.; Ghanbari, H.; Nelson, B.W. Impact of Skin Pigmentation on Pulse Oximetry Blood Oxygenation and Wearable Pulse Rate Accuracy: Systematic Review and Meta-Analysis. J. Med. Internet Res. 2024, 26, e62769. [Google Scholar] [CrossRef]
- Al-Halawani, R.; Charlton, P.H.; Qassem, M.; Kyriacou, P.A. A review of the effect of skin pigmentation on pulse oximeter accuracy. Physiol. Meas. 2023, 44, 05TR01. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Huang, Z.; Lui, H.; Hamzavi, I.; McLean, D.I.; Xie, S.; Zeng, H. Monte Carlo simulation of cutaneous reflectance and fluorescence measurements—The effect of melanin contents and localization. J. Photochem. Photobiol. B Biol. 2007, 86, 219–226. [Google Scholar] [CrossRef]
- Cook, P.D.; Bixler, J.N.; Thomas, R.J.; Early, E.A. Prediction of tissue optical properties using the Monte Carlo modeling of photon transport in turbid media and integrating spheres. OSA Contin. 2020, 3, 1456–1476. [Google Scholar] [CrossRef]
- Chatterjee, S.; Kyriacou, P.A. Monte Carlo Analysis of Optical Interactions in Reflectance and Transmittance Finger Photoplethysmography. Sensors 2019, 19, 789. [Google Scholar] [CrossRef]
- Keller, M.D.; Harrison-Smith, B.; Patil, C.; Arefin, M.S. Skin colour affects the accuracy of medical oxygen sensors. Nature 2022, 610, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Bolic, M. Simulating the Effects of Melanin and Air Gap Depth on the Accuracy of Reflectance Pulse Oximeters. In Proceedings of the 16th International Joint Conference on Biomedical Engineering Systems and Technologies, Lisbon, Portuga, 16–18 February 2023; SCITEPRESS—Science and Technology Publications: Setúbal, Portugal, 2023; pp. 64–71. [Google Scholar] [CrossRef]
- Jung, H.; Kim, D.; Lee, W.; Seo, H.; Seo, J.; Choi, J.; Joo, E.Y. Performance evaluation of a wrist-worn reflectance pulse oximeter during sleep. Sleep Health 2022, 8, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Liu, Q. Review of Monte Carlo modeling of light transport in tissues. J. Biomed. Opt. 2013, 18, 050902. [Google Scholar] [CrossRef] [PubMed]
- Krasnikov, I.; Seteikin, A.; Roth, B. Advances in the simulation of light–tissue interactions in biomedical engineering. Biomed. Eng. Lett. 2019, 9, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, E.; Pandey, V.; Mourelatos, Z. Managing the Computational Cost in a Monte Carlo Simulation by Considering the Value of Information. In Proceedings of the SAE 2012 World Congress & Exhibition, Detroit, MI, USA, 24–26 April 2012; SAE International: Warrendale, PA, USA, 2012. [Google Scholar] [CrossRef]
- Klanecek, Z.; Hren, R.; Simončič, U.; Muc, B.T.; Lukač, M.; Milanič, M. Finite Element Method (FEM) Modeling of Laser-Tissue Interaction during Hair Removal. Appl. Sci. 2023, 13, 8553. [Google Scholar] [CrossRef]
- Kim, H.-J.; Um, S.-H.; Kang, Y.G.; Shin, M.; Jeon, H.; Kim, B.-M.; Lee, D.; Yoon, K. Laser–tissue interaction simulation considering skin-specific data to predict photothermal damage lesions during laser irradiation. J. Comput. Des. Eng. 2023, 10, 947–958. [Google Scholar] [CrossRef]
- Jiang, J.; Ren, W.; Isler, H.; Kalyanov, A.; Lindner, S.; Aldo, D.C.M.; Rudin, M.; Wolf, M. Validation and Comparison of Monte Carlo and Finite Element Method in Forward Modeling for Near Infrared Optical Tomography. In Oxygen Transport to Tissue XLI; Ryu, P.-D., LaManna, J.C., Harrison, D.K., Lee, S.-S., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 307–313. [Google Scholar] [CrossRef]
- Ortega-Quijano, N.; Romanov, O.G.; Fanjul-Vélez, F.; Salas-García, I.; Tolstik, A.L.; Arce-Diego, J.L. Numerical modeling of light propagation in biological tissues: Time-resolved 3D simulations based on light diffusion model and FDTD solution of Maxwell’s equations. In Proceedings of the European Conference on Biomedical Optics, Munich, Germany, 22–26 May 2011; Optica Publishing Group: Washington, DC, USA, 2011; p. 80881. [Google Scholar] [CrossRef]
- Hielscher, A.H.; Alcouffe, R.E.; Barbour, R.L. Comparison of finite-difference transport and diffusion calculations for photon migration in homogeneous and heterogeneous tissues. Phys. Med. Biol. 1998, 43, 1285–1302. [Google Scholar] [CrossRef]
- Zhao, Y.; Raghuram, A.; Kim, H.K.; Hielscher, A.H.; Robinson, J.T.; Veeraraghavan, A. High Resolution, Deep Imaging Using Confocal Time-of-flight Diffuse Optical Tomography. IEEE Trans. Pattern Anal. Mach. Intell. 2021, 43, 2206–2219. [Google Scholar] [CrossRef] [PubMed]
- Venugopalan, V.; You, J.S.; Tromberg, B.J. Radiative transport in the diffusion approximation: An extension for highly absorbing media and small source-detector separations. Phys. Rev. E 1998, 58, 2395–2407. [Google Scholar] [CrossRef]
- Al-Halawani, R.; Qassem, M.; Kyriacou, P.A. Monte Carlo simulation of the effect of melanin concentration on light–tissue interactions for transmittance pulse oximetry measurement. J. Biomed. Opt. 2024, 29, S33305. [Google Scholar] [CrossRef] [PubMed]
- Hickey, M.; Kyriacou, P.A. Optimal spacing between transmitting and receiving optical fibres in reflectance pulse oximetry. J. Phys. Conf. Ser. 2007, 85, 012030. [Google Scholar] [CrossRef]
- Kanellis, V.G. A review of melanin sensor devices. Biophys. Rev. 2019, 11, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, J.M.; Wall, R.T.; Zhou, G.X.; Walker, E.C. Multilayer model of photon diffusion in skin. J. Opt. Soc. Am. A 1990, 7, 2141–2153. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.; Vasudevan, S.; Bhusal, A.; Weininger, S.; Chen, Y.; Pfefer, J. Modeling light-tissue interactions in pulse oximetry: Effect of device design and skin pigmentation. In Proceedings of the Design and Quality for Biomedical Technologies XVII, San Francisco, CA, USA, 27 January–1 February 2024; SPIE: St Bellingham, WA, USA, 2024; p. 1283302. [Google Scholar] [CrossRef]
- Rafl, J.; Bachman, T.E.; Rafl-Huttova, V.; Walzel, S.; Rozanek, M. Commercial smartwatch with pulse oximeter detects short-time hypoxemia as well as standard medical-grade device: Validation study. Digit. Health 2022, 8, 20552076221132127. [Google Scholar] [CrossRef] [PubMed]
- Cabanas, A.M.; Martín-Escudero, P.; Shelley, K.H. Improving pulse oximetry accuracy in dark-skinned patients: Technical aspects and current regulations. Br. J. Anaesth. 2023, 131, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Feiner, J.R.; Severinghaus, J.W.; Bickler, P.E. Dark skin decreases the accuracy of pulse oximeters at low oxygen saturation: The effects of oximeter probe type and gender. Anesth. Analg. 2007, 105, S18–S23. [Google Scholar] [CrossRef]

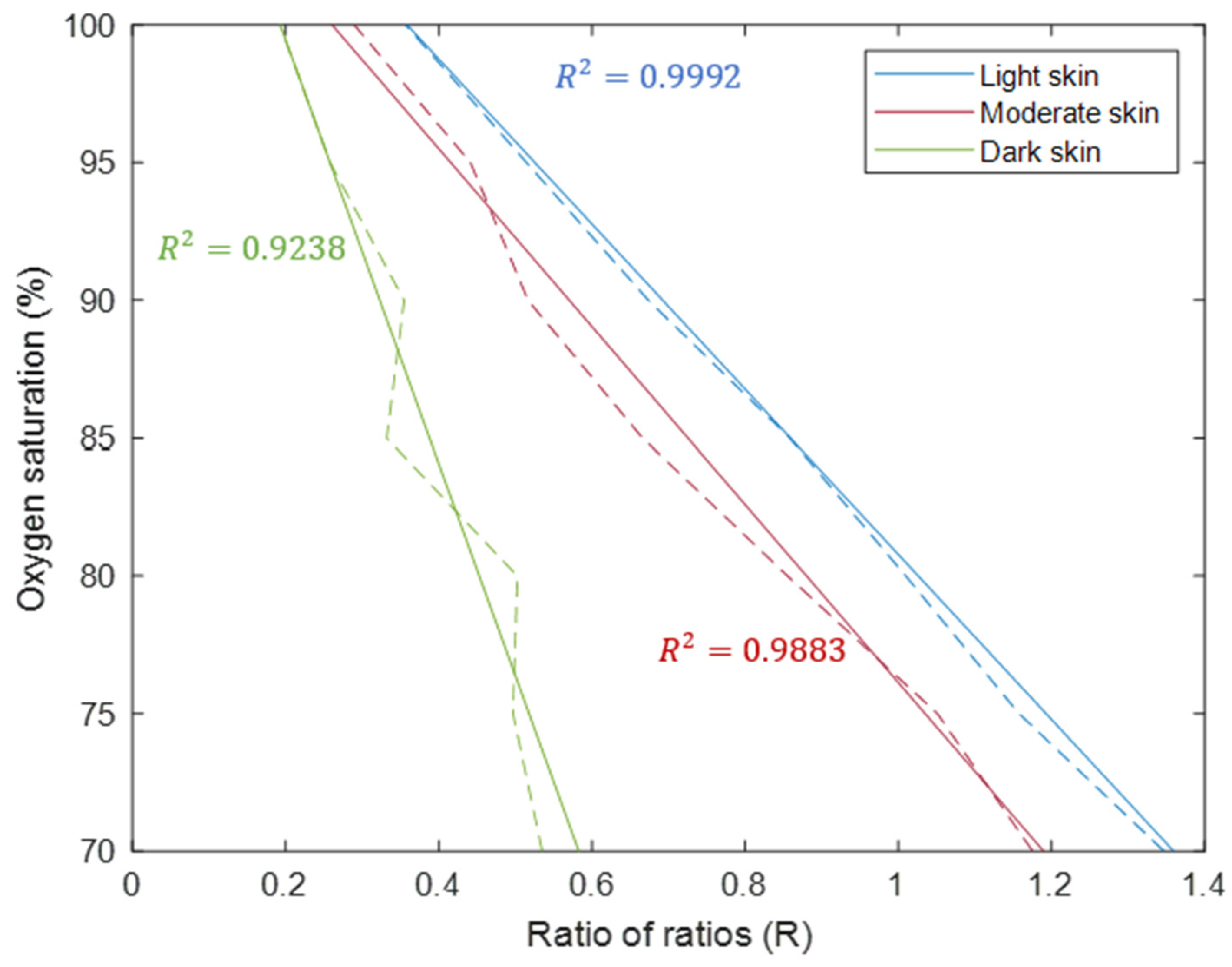


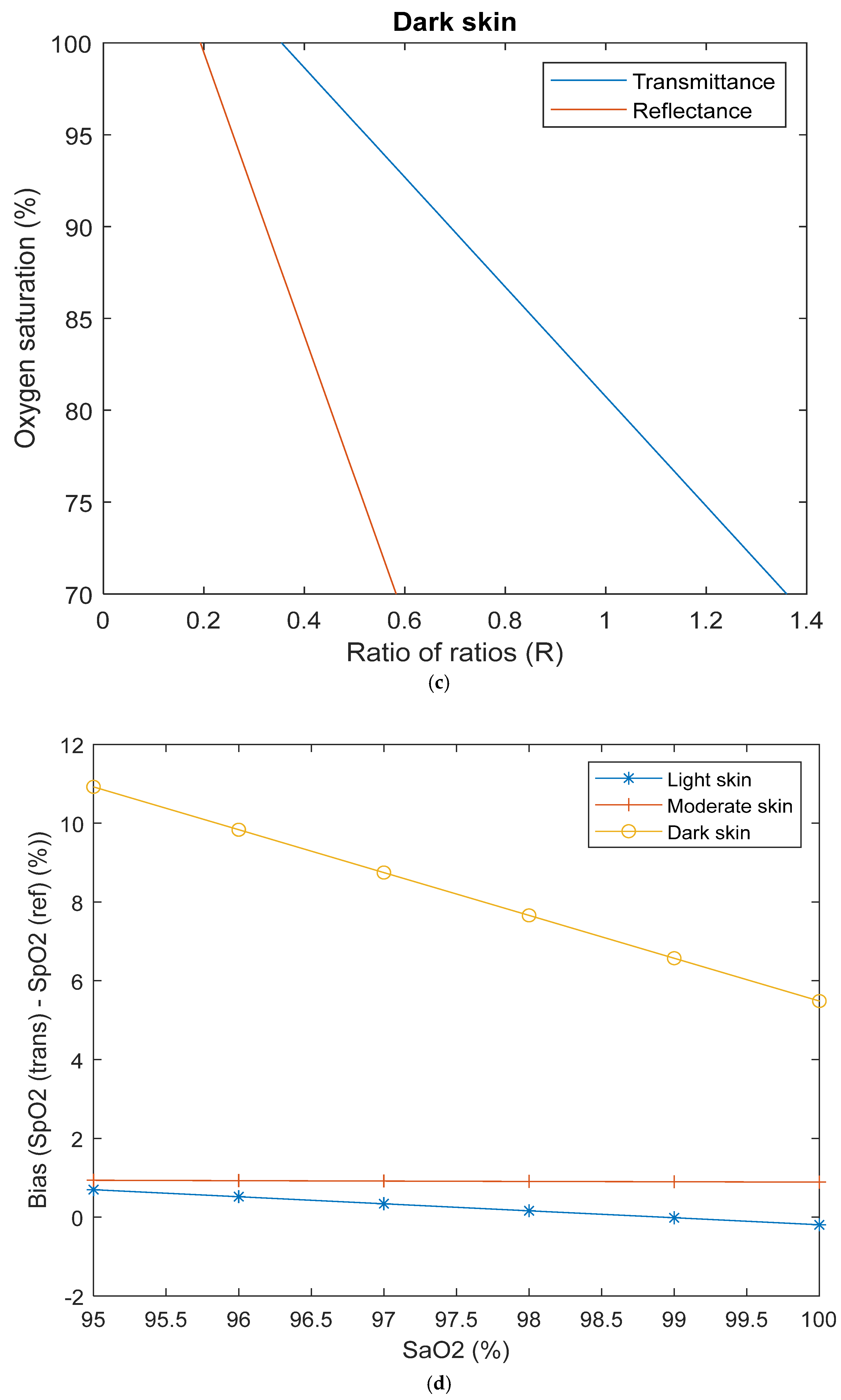
| Transmittance | Reflectance | |
|---|---|---|
| Light skin | SaO2 = 109 − 25.44 × R | SaO2 = 110.8 − 29.98 × R |
| Moderate skin | SaO2 = 109.2 − 32.05 × R | SaO2 = 108.4 − 32.3 × R |
| Dark skin | SaO2 = 110.6 − 49.33 × R | SaO2 = 114.9 − 76.99 × R |
| Commercial | SpO2 = 110 − 25 × R | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Halawani, R.; Qassem, M.; Kyriacou, P.A. Monte Carlo Simulation of the Effect of Melanin Concentration on Light–Tissue Interactions in Reflectance Pulse Oximetry. Sensors 2025, 25, 559. https://doi.org/10.3390/s25020559
Al-Halawani R, Qassem M, Kyriacou PA. Monte Carlo Simulation of the Effect of Melanin Concentration on Light–Tissue Interactions in Reflectance Pulse Oximetry. Sensors. 2025; 25(2):559. https://doi.org/10.3390/s25020559
Chicago/Turabian StyleAl-Halawani, Raghda, Meha Qassem, and Panicos A. Kyriacou. 2025. "Monte Carlo Simulation of the Effect of Melanin Concentration on Light–Tissue Interactions in Reflectance Pulse Oximetry" Sensors 25, no. 2: 559. https://doi.org/10.3390/s25020559
APA StyleAl-Halawani, R., Qassem, M., & Kyriacou, P. A. (2025). Monte Carlo Simulation of the Effect of Melanin Concentration on Light–Tissue Interactions in Reflectance Pulse Oximetry. Sensors, 25(2), 559. https://doi.org/10.3390/s25020559










