Monitoring Sleep and Nightly Recovery with Wrist-Worn Wearables: Links to Training Load and Performance Adaptations
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Sleep and Nightly Recovery
2.4. Subjective Recovery Questions
2.5. Data Analyses
2.6. Statistical Analyses
3. Results
3.1. Training Intervention
3.1.1. Training Characteristics and Training Adaptations
3.1.2. Subjective Recovery
3.1.3. Intervention Effects on Sleep and Nightly Recovery Metrics
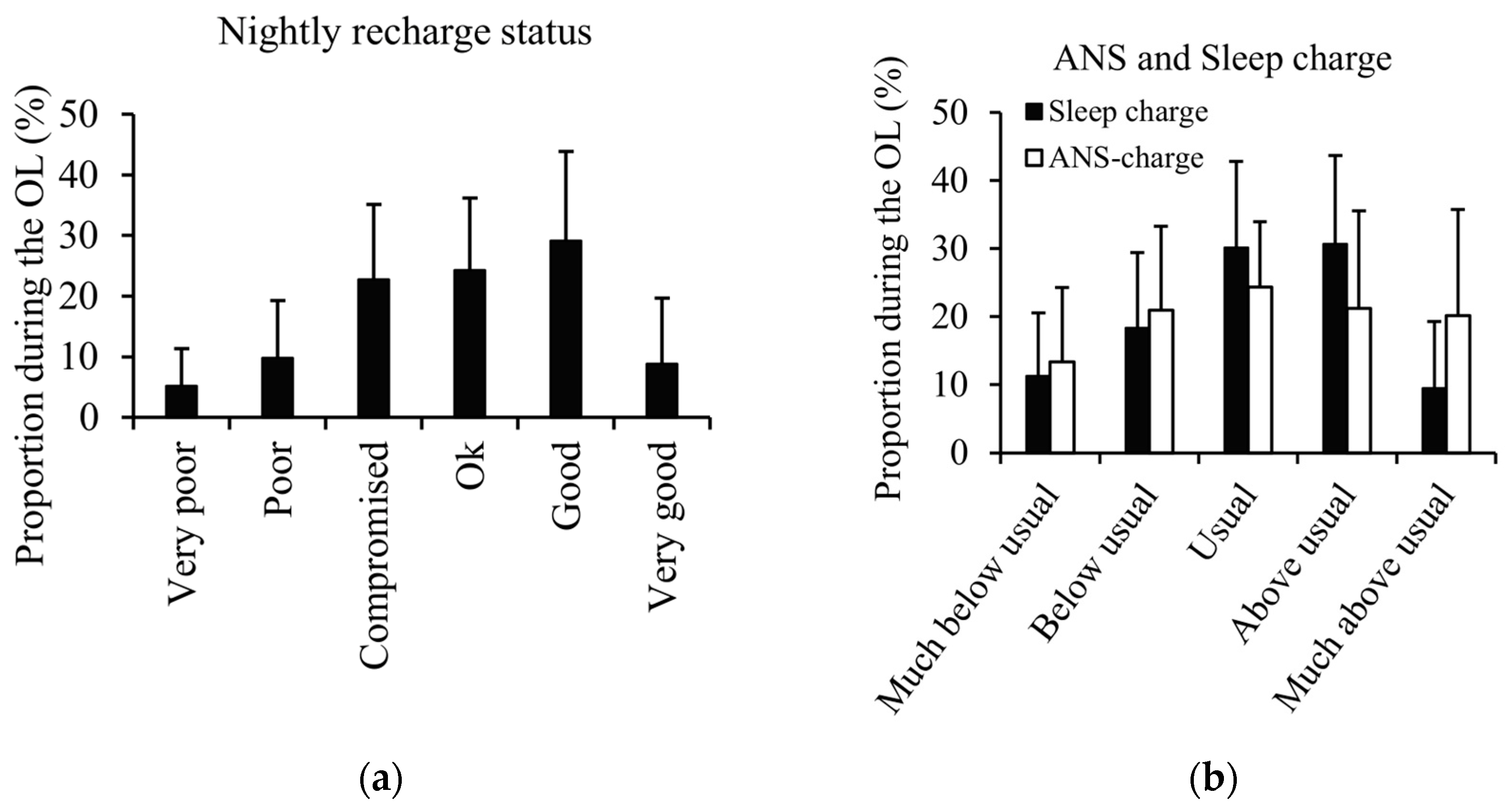
3.1.4. Watch Notifications During the Overload Period
3.2. Associations Between Nightly Recovery Metrics and Running Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newsome, A.; Reed, R.; Sansone, J.; Batrakoulis, A.; McAvoy, C.; Parrott, M. 2024 ACSM Worldwide Fitness Trends: Future Directions of the Health and Fitness Industry. ACSM’s Health Fit. J. 2024, 28, 14–26. [Google Scholar] [CrossRef]
- Butte, N.F.; Ekelund, U.; Westerterp, K.R. Assessing physical activity using wearable monitors: Measures of physical activity. Med. Sci. Sports Exerc. 2012, 44 (Suppl. S1), 5–12. [Google Scholar] [CrossRef] [PubMed]
- Kozey-Keadle, S.; Libertine, A.; Lyden, K.; Staudenmayer, J.; Freedson, P.S. Validation of wearable monitors for assessing sedentary behavior. Med. Sci. Sports Exerc. 2011, 43, 1561–1567. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, M.; Varley, M.C. Wearable Training-Monitoring Technology: Applications, Challenges, and Opportunities. Int. J. Sports Physiol. Perform. 2017, 12 (Suppl. S2), 255–262. [Google Scholar] [CrossRef]
- Düking, P.; Achtzehn, S.; Holmberg, H.C.; Sperlich, B. Integrated Framework of Load Monitoring by a Combination of Smartphone Applications, Wearables and Point-of-Care Testing Provides Feedback that Allows Individual Responsive Adjustments to Activities of Daily Living. Sensors 2018, 18, 1632. [Google Scholar] [CrossRef] [PubMed]
- Driller, M.W.; Dunican, I.C.; Omond, S.E.T.; Boukhris, O.; Stevenson, S.; Lambing, K.; Bender, A.M. Pyjamas, Polysomnography and Professional Athletes: The Role of Sleep Tracking Technology in Sport. Sports 2023, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.H.; Beaumont, C.T.; Strohacker, K. Exploring Regular Exercisers’ Experiences with Readiness/Recovery Scores Produced by Wearable Devices: A Descriptive Qualitative Study. Appl. Psychophysiol. Biofeedback 2024, 49, 395–405. [Google Scholar] [CrossRef]
- Baron, K.G.; Duffecy, J.; Berendsen, M.A.; Cheung Mason, I.; Lattie, E.G.; Manalo, N.C. Feeling validated yet? A scoping review of the use of consumer-targeted wearable and mobile technology to measure and improve sleep. Sleep Med. Rev. 2018, 40, 151–159. [Google Scholar] [CrossRef] [PubMed]
- de Zambotti, M.; Cellini, N.; Goldstone, A.; Colrain, I.M.; Baker, F.C. Wearable Sleep Technology in Clinical and Research Settings. Med. Sci. Sports Exerc. 2019, 51, 1538–1557. [Google Scholar] [CrossRef] [PubMed]
- Boostani, R.; Karimzadeh, F.; Nami, M. A comparative review on sleep stage classification methods in patients and healthy individuals. Comput. Methods Programs Biomed. 2017, 140, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Altini, M.; Kinnunen, H. The Promise of Sleep: A Multi-Sensor Approach for Accurate Sleep Stage Detection Using the Oura Ring. Sensors 2021, 21, 4302. [Google Scholar] [CrossRef] [PubMed]
- Peake, J.M.; Kerr, G.; Sullivan, J.P. A Critical Review of Consumer Wearables, Mobile Applications, and Equipment for Providing Biofeedback, Monitoring Stress, and Sleep in Physically Active Populations. Front. Physiol. 2018, 9, 743. [Google Scholar] [CrossRef]
- Buchheit, M. Monitoring training status with HR measures: Do all roads lead to Rome? Front. Physiol. 2014, 5, 73. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, P.; Yeh, W.H.; Dumont, G.A.; Boivin, D.B. Circadian variation of heart rate variability across sleep stages. Sleep 2013, 36, 1919–1928. [Google Scholar] [CrossRef]
- Nuuttila, O.P.; Seipäjärvi, S.; Kyröläinen, H.; Nummela, A. Reliability and Sensitivity of Nocturnal Heart Rate and Heart-Rate Variability in Monitoring Individual Responses to Training Load. Int. J. Sports Physiol. Perform. 2022, 17, 1296–1303. [Google Scholar] [CrossRef]
- Walsh, N.P.; Halson, S.L.; Sargent, C.; Roach, G.D.; Nédélec, M.; Gupta, L.; Leeder, J.; Fullagar, H.H.; Coutts, A.J.; Edwards, B.J.; et al. Sleep and the athlete: Narrative review and 2021 expert consensus recommendations. Br. J. Sports Med. 2020, 3, bjsports-2020-102025. [Google Scholar] [CrossRef] [PubMed]
- Simpson, N.S.; Gibbs, E.L.; Matheson, G.O. Optimizing sleep to maximize performance: Implications and recommendations for elite athletes. Scand. J. Med. Sci. Sports 2017, 27, 266–274. [Google Scholar] [CrossRef]
- Craven, J.; McCartney, D.; Desbrow, B.; Sabapathy, S.; Bellinger, P.; Roberts, L.; Irwin, C. Effects of Acute Sleep Loss on Physical Performance: A Systematic and Meta-Analytical Review. Sports Med. 2022, 52, 2669–2690. [Google Scholar] [CrossRef] [PubMed]
- Sargent, C.; Lastella, M.; Halson, S.L.; Roach, G.D. How Much Sleep Does an Elite Athlete Need? Int. J. Sports Physiol. Perform. 2021, 16, 1746–1757. [Google Scholar] [CrossRef] [PubMed]
- Fullagar, H.H.K.; Vincent, G.E.; McCullough, M.; Halson, S.; Fowler, P. Sleep and Sport Performance. J. Clin. Neurophysiol. 2023, 40, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Garet, M.; Tournaire, N.; Roche, F. Individual Interdependence between nocturnal ANS activity and performance in swimmers. Med. Sci. Sports Exerc. 2004, 36, 2112–2118. [Google Scholar] [CrossRef] [PubMed]
- Dupuy, O.; Bherer, L.; Audiffren, M.; Bosquet, L. Night and postexercise cardiac autonomic control in functional overreaching. Appl. Physiol. Nutr. Metab. 2013, 38, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.; Svansdottir, S.A.; Dupuy, O.; Louis, J. Does overreaching from endurance-based training impair sleep: A systematic review and meta-analysis. PLoS ONE 2024, 19, e0303748. [Google Scholar] [CrossRef]
- Hausswirth, C.; Louis, J.; Aubry, A.; Bonnet, G.; Duffield, R.; Le Meur, Y. Evidence of disturbed sleep and increased illness in overreached endurance athletes. Med. Sci. Sports Exerc. 2014, 46, 1036–1045. [Google Scholar] [CrossRef] [PubMed]
- Le Meur, Y.; Pichon, A.; Schaal, K.; Schmitt, L.; Louis, J.; Gueneron, J.; Vidal, P.P.; Hausswirth, C. Evidence of parasympathetic hyperactivity in functionally overreached athletes. Med. Sci. Sports Exerc. 2013, 45, 2061–2071. [Google Scholar] [CrossRef] [PubMed]
- Bellenger, C.R.; Karavirta, L.; Thomson, R.L.; Robertson, E.Y.; Davison, K.; Buckley, J.D. Contextualizing Parasympathetic Hyperactivity in Functionally Overreached Athletes With Perceptions of Training Tolerance. Int. J. Sports Physiol. Perform. 2016, 11, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Bourdillon, N.; Yazdani, S.; Nilchian, M.; Mariano, A.; Vesin, J.M.; Millet, G.P. Overload blunts baroreflex only in overreached athletes. J. Sci. Med. Sport 2018, 21, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Lucia, A.; Hoyos, J.; Santalla, A.; Earnest, C.; Chicharro, J.L. Tour de France versus Vuelta a España: Which is harder? Med. Sci. Sports Exerc. 2003, 35, 872–878. [Google Scholar]
- Matomäki, P.; Nuuttila, O.P.; Heinonen, O.J.; Kyröläinen, H.; Nummela, A. How to Equalize High- and Low-Intensity Endurance Exercise Dose. Int. J. Sports Physiol. Perform. 2024, 19, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Nuuttila, O.P.; Nummela, A.; Kyröläinen, H.; Laukkanen, J.; Häkkinen, K. Physiological, Perceptual, and Performance Responses to the 2-Week Block of High- versus Low-Intensity Endurance Training. Med. Sci. Sports Exerc. 2022, 54, 851–860. [Google Scholar] [CrossRef]
- Nuuttila, O.P.; Matomäki, P.; Kyröläinen, H.; Nummela, A. Predicting Running Performance and Adaptations from Intervals at Maximal Sustainable Effort. Int. J. Sports Med. 2023, 44, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Samuels, C.; James, L.; Lawson, D.; Meeuwisse, W. The Athlete Sleep Screening Questionnaire: A new tool for assessing and managing sleep in elite athletes. Br. J. Sports Med. 2016, 50, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Rushall, B.S. A tool for measuring stress tolerance in elite athletes. J. Appl. Sport Psychol. 1990, 2, 51–66. [Google Scholar] [CrossRef]
- Polar Electro, Oy. Polar Recovery Pro [White Paper]. 2019. Available online: https://www.polar.com/img/static/whitepapers/pdf/polar-recovery-pro-white-paper.pdf (accessed on 17 October 2024).
- Nuuttila, O.P.; Uusitalo, A.; Kokkonen, V.P.; Weerarathna, N.; Kyröläinen, H. Monitoring fatigue state with heart rate-based and subjective methods during intensified training in recreational runners. Eur. J. Sport Sci. 2024, 24, 857–869. [Google Scholar] [CrossRef] [PubMed]
- Parent, A.A.; Guadagni, V.; Rawling, J.M.; Poulin, M.J. Performance Evaluation of a New Sport Watch in Sleep Tracking: A Comparison against Overnight Polysomnography in Young Adults. Sensors 2024, 24, 2218. [Google Scholar] [CrossRef] [PubMed]
- Pesonen, A.K.; Kuula, L. The Validity of a New Consumer-Targeted Wrist Device in Sleep Measurement: An Overnight Comparison Against Polysomnography in Children and Adolescents. J. Clin. Sleep Med. 2018, 14, 585–591. [Google Scholar] [CrossRef]
- Nuuttila, O.P.; Korhonen, E.; Laukkanen, J.; Kyröläinen, H. Validity of the Wrist-Worn Polar Vantage V2 to Measure Heart Rate and Heart Rate Variability at Rest. Sensors 2021, 22, 137. [Google Scholar] [CrossRef] [PubMed]
- Polar Electro, Oy. Polar Nightly Recharge [White Paper]. 2019. Available online: https://www.polar.com/img/static/whitepapers/pdf/polar-nightly-recharge-white-paper.pdf (accessed on 17 October 2024).
- Polar Electro, Oy. Polar Sleep Plus Stages [White Paper]. 2024. Available online: https://www.polar.com/img/static/whitepapers/pdf/polar-sleep-plus-stages-white-paper.pdf (accessed on 17 October 2024).
- Saw, A.E.; Main, L.C.; Gastin, P.B. Monitoring the athlete training response: Subjective self-reported measures trump commonly used objective measures: A systematic review. Br. J. Sports Med. 2016, 50, 281–291. [Google Scholar] [CrossRef]
- Manresa-Rocamora, A.; Flatt, A.; Casanova-Lizón, A.; Ballester-Ferrer, J.; Sarabia, J.; Vera-Garcia, F.; Moya-Ramón, M. Heart Rate-Based Indices to Detect Parasympathetic Hyperactivity in Functionally Overreached Athletes. A Meta-Analysis. Scand. J. Med. Sci. Sports 2021, 31, 1164–1182. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Reprint; Psychology Press: New York, NY, USA, 1988; 567p. [Google Scholar]
- Koreki, A.; Sado, M.; Mitsukura, Y.; Tachimori, H.; Kubota, A.; Kanamori, Y.; Uchibori, M.; Usune, S.; Ninomiya, A.; Shirahama, R.; et al. The association between salivary IL-6 and poor sleep quality assessed using Apple watches in stressed workers in Japan. Sci. Rep. 2024, 14, 22620. [Google Scholar] [CrossRef] [PubMed]
- Buchheit, M.; Chivot, A.; Parouty, J.; Mercier, D.; Al Haddad, H.; Laursen, P.B.; Ahmaidi, S. Monitoring endurance running performance using cardiac parasympathetic function. Eur. J. Appl. Physiol. 2010, 108, 1153–1167. [Google Scholar] [CrossRef] [PubMed]
- Nummela, A.; Hynynen, E.; Kaikkonen, P.; Rusko, H. Endurance performance and nocturnal HRV indices. Int. J. Sports Med. 2010, 31, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Plews, D.J.; Laursen, P.B.; Kilding, A.E.; Buchheit, M. Evaluating training adaptation with heart-rate measures: A methodological comparison. Int. J. Sports Physiol. Perform. 2013, 8, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Halson, S.L. Sleep in elite athletes and nutritional interventions to enhance sleep. Sports Med. 2014, 44 (Suppl. S1), 13–23. [Google Scholar] [CrossRef] [PubMed]
- Karemaker, J.M. An introduction into autonomic nervous function. Physiol. Meas. 2017, 38, 89–118. [Google Scholar] [CrossRef] [PubMed]
- Düking, P.; Zinner, C.; Trabelsi, K.; Reed, J.L.; Holmberg, H.C.; Kunz, P.; Sperlich, B. Monitoring and adapting endurance training on the basis of heart rate variability monitored by wearable technologies: A systematic review with meta-analysis. J. Sci. Med. Sport 2021, 24, 1180–1192. [Google Scholar] [CrossRef] [PubMed]
- Nuuttila, O.P.; Nummela, A.; Korhonen, E.; Häkkinen, K.; Kyröläinen, H. Individualized Endurance Training Based on Recovery and Training Status in Recreational Runners. Med. Sci. Sports Exerc. 2022, 54, 1690–1701. [Google Scholar] [CrossRef]
- Cullen, T.; Thomas, G.; Wadley, A.J.; Myers, T. The effects of a single night of complete and partial sleep deprivation on physical and cognitive performance: A Bayesian analysis. J. Sports Sci. 2019, 37, 2726–2734. [Google Scholar] [CrossRef]
- Plews, D.J.; Laursen, P.B.; Le Meur, Y.; Hausswirth, C.; Kilding, A.E.; Buchheit, M. Monitoring training with heart rate-variability: How much compliance is needed for valid assessment? Int. J. Sports Physiol. Perform. 2014, 9, 783–790. [Google Scholar] [CrossRef]
- Mishica, C.; Kyröläinen, H.; Hynynen, E.; Nummela, A.; Holmberg, H.C.; Linnamo, V. Evaluation of nocturnal vs. morning measures of heart rate indices in young athletes. PLoS ONE 2022, 17, e0262333. [Google Scholar] [CrossRef] [PubMed]
- Draganich, C.; Erdal, K. Placebo sleep affects cognitive functioning. J. Exp. Psychol. Learn. Mem. Cogn. 2014, 40, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.L.; Davis, J.E.; Corbett, C.F. Sleep quality: An evolutionary concept analysis. Nurs. Forum 2022, 57, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Mann, T.N.; Lamberts, R.P.; Lambert, M.I. High responders and low responders: Factors associated with individual variation in response to standardized training. Sports Med. 2014, 44, 1113–1124. [Google Scholar] [CrossRef] [PubMed]


| Participants (n = 24) | |
|---|---|
| Sex (females/males) | 14/10 |
| Age (yrs) | 38.6 ± 6.5 |
| Height (cm) | 173.2 ± 9.7 |
| Body mass (kg) | 72.6 ± 14.0 |
| 3000 m time (s) | 13:03 ± 1:56 |
| Description | |
|---|---|
| Sleep time | Time between sleep onset and offset (h). |
| Actual sleep | Time scored asleep between sleep onset and offset/sleep time x 100 (%). |
| Sleep continuity | An estimate of how continuous the sleep was on a scale of 1.0–5.0, where 5.0 reflects uninterrupted sleep. The lower the value the more fragmented the sleep was. |
| Sleep score | A summary parameter that combines sleep time and quality on a scale of 1–100. |
| Sleep charge | Sleep score compared to an individual’s usual level from the past 28 days. |
| Sleep charge notification | Textual feedback on sleep charge on a 5-item scale: much below usual, below usual, usual, above usual, much above usual. |
| HR | Average heart rate from a 4 h period starting at 30 min after detected sleep onset (bpm). |
| RMSSD | Average root mean square of successive differences from a 4 h period starting at 30 min after detected sleep onset (ms). RMSSD is considered as a non-invasive method to assess cardiac parasympathetic nervous system activity. |
| Breathing rate | Average breathing rate as breaths per minute during a 4 h period starting at 30 min after detected sleep onset (bpm). |
| ANS charge | ANS stands for autonomic nervous system. ANS charge combines HR, RMSSD, and breathing rate. It is formed comparing the last night’s values to an individual’s usual level from the past 28 days. The scale is from −10.0 to +10.0. |
T1 | T2 | T3 | ES T1–T2 | ES T2–T3 | ES T1–T3 | |
|---|---|---|---|---|---|---|
| ASSQ | 4.9 ± 2.1 | 4.5 ± 1.8 | 4.8 ± 2.3 | −0.29 (−0.70; 0.12) | 0.15 (−0.26; 0.55) | −0.06 (−0.46; 0.34) |
| Subjective sleep quality (1–5) | 3.3 ± 0.4 | 3.2 ± 0.5 | 3.4 ± 0.5 | −0.16 (−0.56; 0.24) | 0.44 (0.02; 0.86) | 0.16 (−0.24; 0.57) |
| Sleep continuity (1–5) | 3.2 ± 0.6 | 3.1 ± 0.6 | 3.0 ± 0.7 | −0.18 (−0.58; 0.23) | −0.29 (−0.69; 0.12) | −0.52 (−0.94; −0.08) |
| Sleep score (0–100) | 75.9 ± 7.4 | 75.6 ± 7.8 | 75.2 ± 9.0 | −0.08 (−0.48; 0.32) | −0.08 (−0.48; 0.32) | −0.15 (−0.55; 0.25) |
| Sleep time (h) | 7.2 ± 0.8 | 7.1 ± 0.8 | 7.2 ± 0.2 | −0.20 (−0.60; 0.20) | 0.21 (−0.20; 0.61) | 0.00 (−0.40; 0.40) |
| Actual sleep (%) | 93.7 ± 1.6 | 93.6 ± 1.9 | 93.1 ± 3.5 | −0.06 (−0.46; 0.34) | −0.25 (−0.65; 0.16) | −0.26 (−0.66; 0.15) |
| HR (bpm) | 51.4 ± 6.9 | 51.7 ± 7.7 | 50.8 ± 8.6 | 0.10 (−0.31; 0.51) | −0.24 (−0.65; 0.18) | −0.16 (−0.57; 0.25) |
| RMSSD (ms) | 70 ± 22 | 71 ± 24 | 71 ± 23 | 0.10 (−0.31; 0.51) | −0.01 (−0.42; 0.40) | 0.10 (−0.32; 0.50) |
| Breathing rate (bpm) | 13.7 ± 1.0 | 13.7 ± 0.9 | 13.7 ± 0.9 | −0.26 (−0.67; 0.16) | 0.00 (−0.41; 0.41) | −0.28 (−0.69; 0.14) |
| 3000 m Change T1 to T2 | 3000 m Change T1 to Peak | |
|---|---|---|
| Absolute OL results | ||
| Perceived strain (0–2) | 0.24 | 0.27 |
| Perceived muscle soreness (0–2) | 0.14 | 0.20 |
| Subjective sleep quality (1–5) | −0.03 | −0.11 |
| Sleep time (h) | 0.20 | 0.22 |
| Sleep score (1–100) | 0.09 | 0.10 |
| Actual sleep (%) | −0.03 | −0.03 |
| Sleep continuity (1–5) | −0.05 | 0.03 |
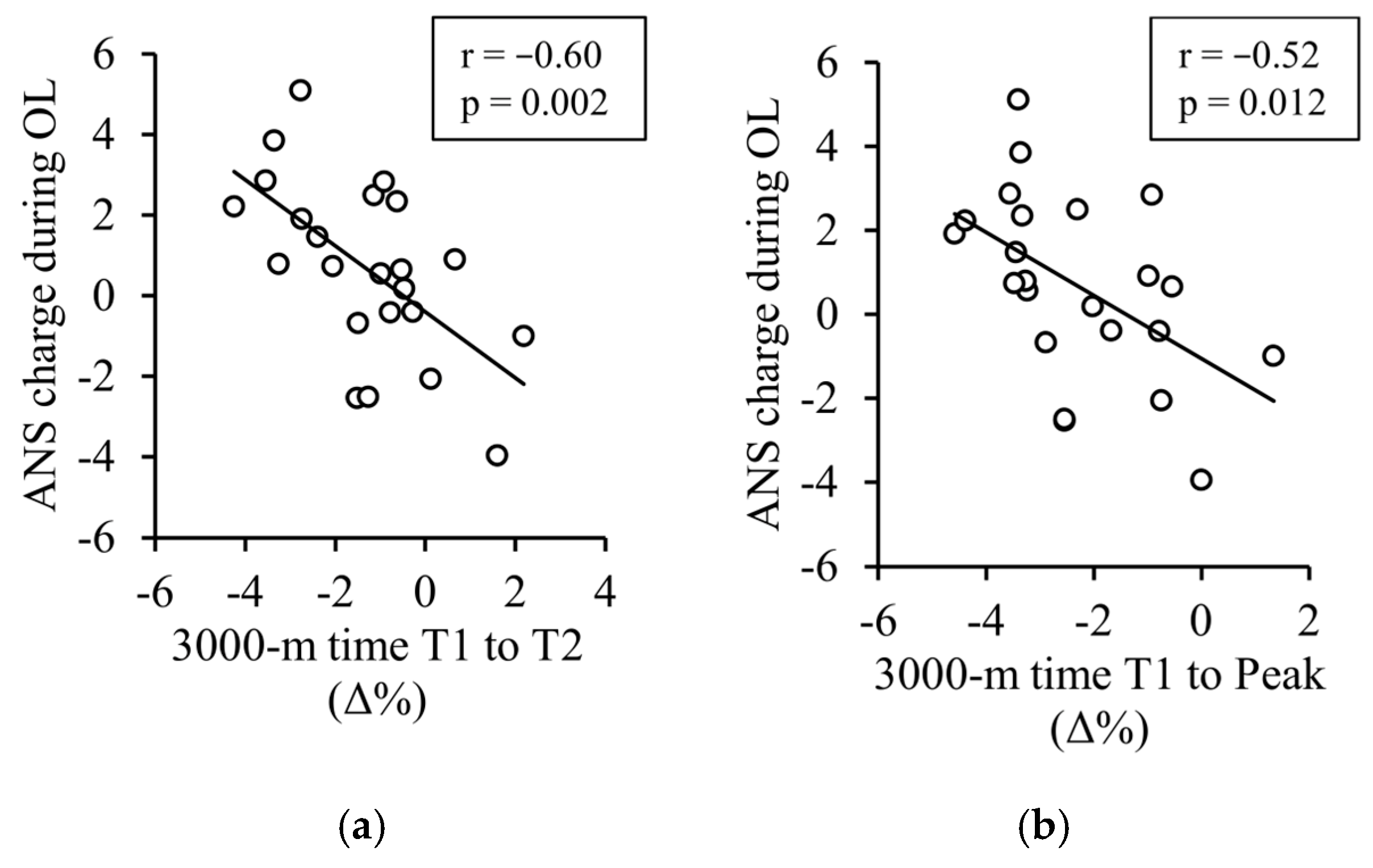
| ANS charge (−10 to 10) | −0.60 ** | −0.52 * |
| Sleep charge (−10 to 10) | 0.21 | 0.01 |
| HR (bpm) | 0.09 | 0.29 |
| RMSSD (ms) | 0.01 | −0.04 |
| Breathing rate (rpm) | 0.39 | 0.52* |
| Change from T1 to T2 | ||
| Perceived strain (0–2) | 0.23 | 0.14 |
| Perceived muscle soreness (0–2) | 0.50 *a | 0.32 a |
| Subjective sleep quality (1–5) | −0.24 | −0.15 |
| Sleep time (h) | 0.14 | 0.16 |
| Sleep score (1–100) | −0.11 | −0.05 |
| Actual sleep (%) | −0.30 | −0.23 |
| Sleep continuity (1–5) | −0.34 | −0.18 |
| HR (bpm) | 0.44 * | 0.49 * |
| RMSSD (ms) | −0.48 * | −0.45 * |
| Breathing rate (bpm) | 0.30 | 0.15 |
| Proportion of negative notifications during the OL | ||
| Nightly recharge status | 0.48 * | 0.36 |
| ANS charge | 0.37 | 0.25 |
| Sleep charge | 0.38 | 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuuttila, O.-P.; Schäfer Olstad, D.; Martinmäki, K.; Uusitalo, A.; Kyröläinen, H. Monitoring Sleep and Nightly Recovery with Wrist-Worn Wearables: Links to Training Load and Performance Adaptations. Sensors 2025, 25, 533. https://doi.org/10.3390/s25020533
Nuuttila O-P, Schäfer Olstad D, Martinmäki K, Uusitalo A, Kyröläinen H. Monitoring Sleep and Nightly Recovery with Wrist-Worn Wearables: Links to Training Load and Performance Adaptations. Sensors. 2025; 25(2):533. https://doi.org/10.3390/s25020533
Chicago/Turabian StyleNuuttila, Olli-Pekka, Daniela Schäfer Olstad, Kaisu Martinmäki, Arja Uusitalo, and Heikki Kyröläinen. 2025. "Monitoring Sleep and Nightly Recovery with Wrist-Worn Wearables: Links to Training Load and Performance Adaptations" Sensors 25, no. 2: 533. https://doi.org/10.3390/s25020533
APA StyleNuuttila, O.-P., Schäfer Olstad, D., Martinmäki, K., Uusitalo, A., & Kyröläinen, H. (2025). Monitoring Sleep and Nightly Recovery with Wrist-Worn Wearables: Links to Training Load and Performance Adaptations. Sensors, 25(2), 533. https://doi.org/10.3390/s25020533






