Gait Variability and Spatiotemporal Parameters During Emotion-Induced Walking: Assessment with Inertial Measurement Units
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Emotion Induction Preparation
2.3. Experimental Procedures
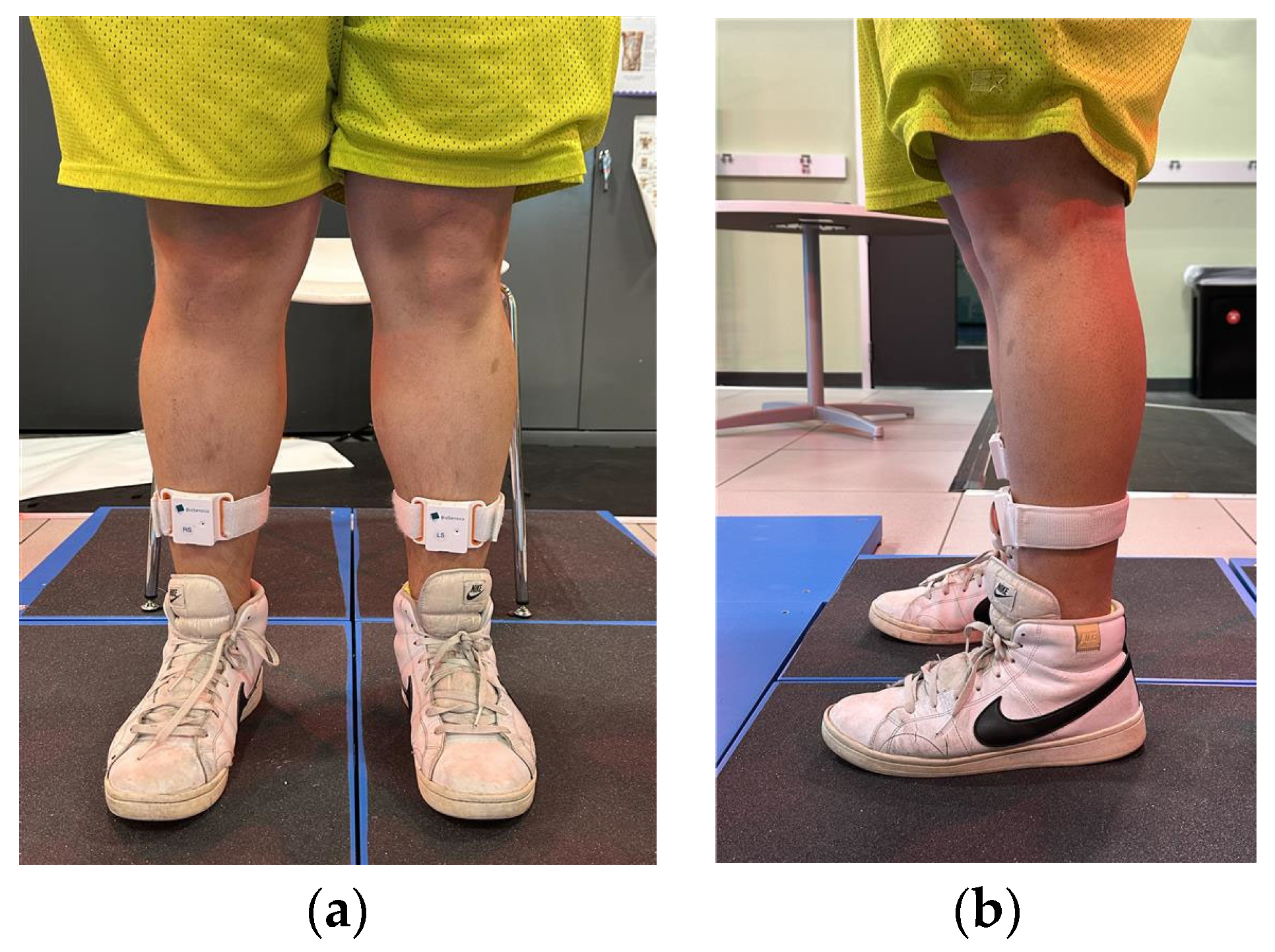
2.4. Data Analysis
3. Results
3.1. Participant Characteristics and Emotion Manipulation Check
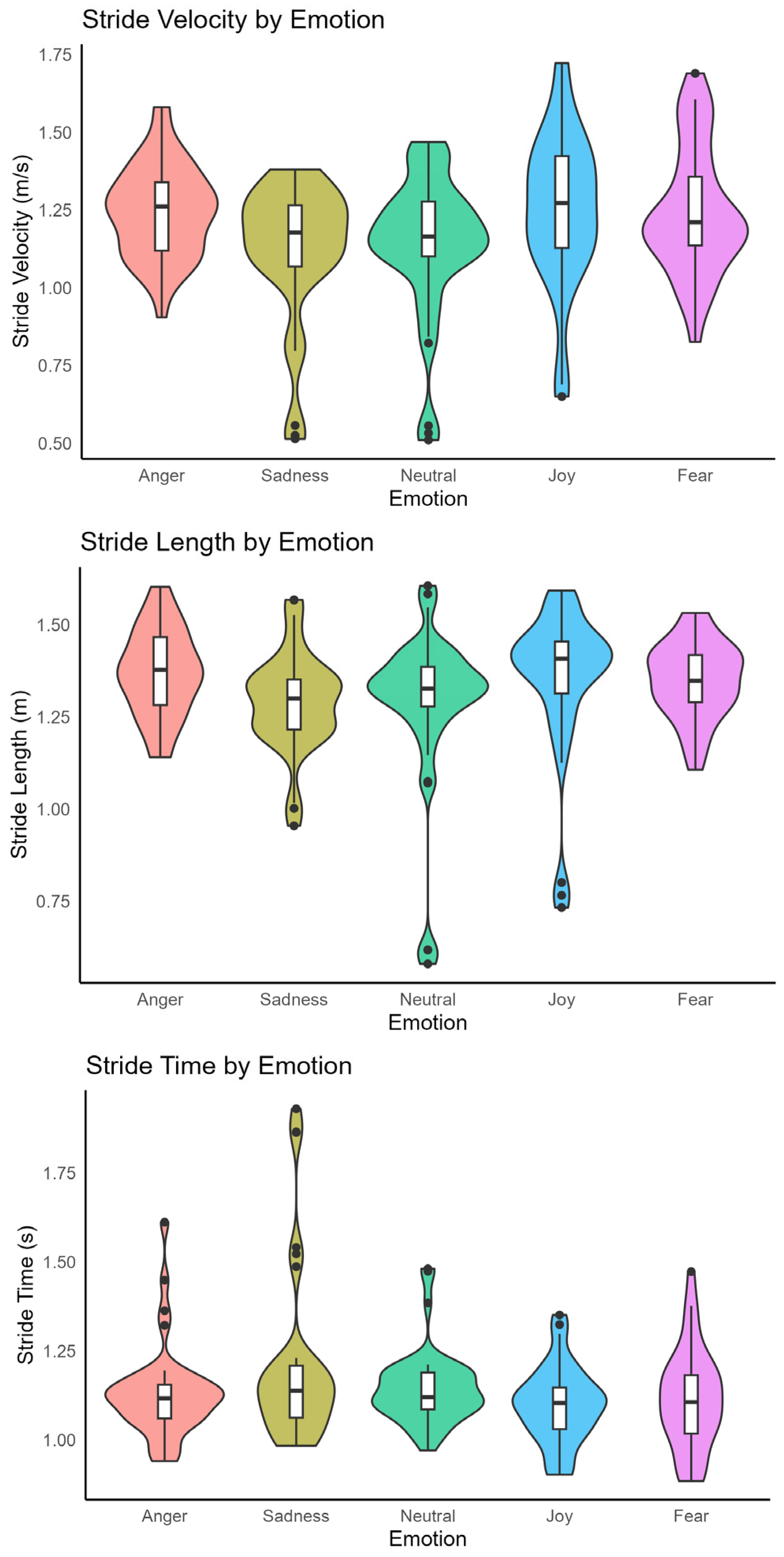
3.2. Spatiotemporal Gait Parameters
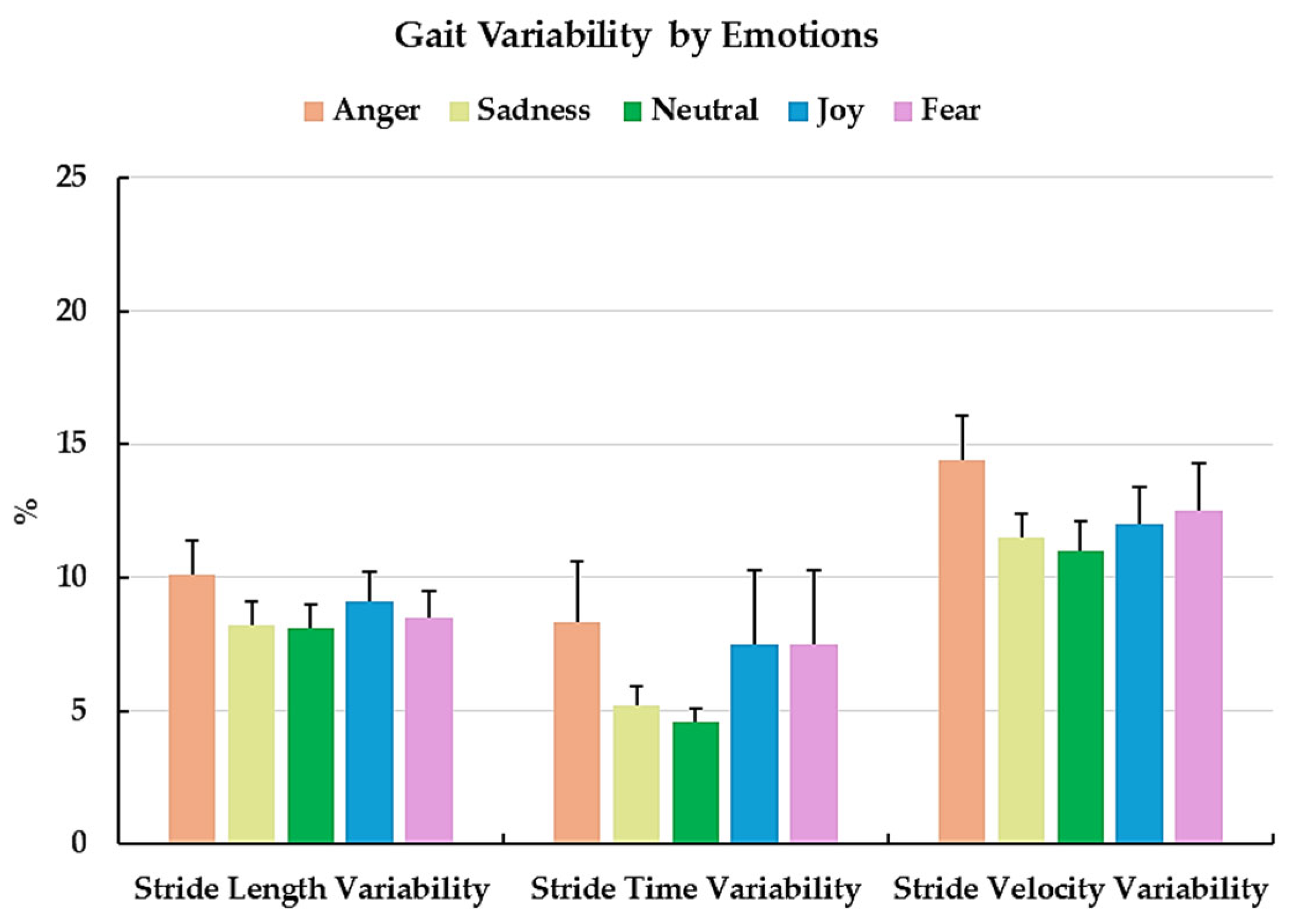
3.3. Gait Variability
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Autobiographical Memories Worksheet
- (a)
- … Where you were:
- (b)
- … Who you were with:
- (c)
- … What caused the feeling/what was it about?
- (a)
- … Where you were:
- (b)
- … Who you were with:
- (c)
- … What caused the feeling/what was it about?
- (a)
- … Where you were:
- (b)
- … Who you were with:
- (c)
- … What caused the feeling/what was it about?
- (a)
- … Where you were:
- (b)
- … Who you were with:
- (c)
- … What caused the feeling/what was it about?
- (a)
- … Where you were:
- (b)
- … Who you were with:
- (c)
- … What caused the feeling/what was it about?
Appendix B
Feeling Questionnaire
References
- Montepare, J.M.; Goldstein, S.B.; Clausen, A. The identification of emotions from gait information. J. Nonverbal Behav. 1987, 11, 33–42. [Google Scholar] [CrossRef]
- Wallbott, H.G. Bodily expression of emotion. Eur. J. Soc. Psychol. 1998, 28, 879–896. [Google Scholar] [CrossRef]
- Fawver, B.; Hass, C.J.; Park, K.D.; Janelle, C.M. Autobiographically recalled emotional states impact forward gait initiation as a function of motivational direction. Emotion 2014, 14, 1125. [Google Scholar] [CrossRef]
- Fawver, B.; Hass, C.J.; Coombes, S.A.; Trapp, S.K.; Janelle, C.M. Recalling fearful memories modifies approach and avoidance behavior based on spatial context. Emotion 2022, 22, 430. [Google Scholar] [CrossRef]
- Park, K.S.; Hass, C.J.; Fawver, B.; Lee, H.; Janelle, C.M. Emotional states influence forward gait during music listening based on familiarity with music selections. Hum. Mov. Sci. 2019, 66, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Frijda, N.H. Emotion, cognitive structure, and action tendency. Cogn. Emot. 1987, 1, 115–143. [Google Scholar] [CrossRef]
- Kang, G.E.; Gross, M.M. The effect of emotion on movement smoothness during gait in healthy young adults. J. Biomech. 2016, 49, 4022–4027. [Google Scholar] [CrossRef]
- Barliya, A.; Omlor, L.; Giese, M.A.; Berthoz, A.; Flash, T. Expression of emotion in the kinematics of locomotion. Exp. Brain Res. 2013, 225, 159–176. [Google Scholar] [CrossRef]
- Hausdorff, J.M. Gait variability: Methods, modeling and meaning. J. Neuroeng. Rehabil. 2005, 2, 19. [Google Scholar] [CrossRef]
- Moon, Y.; Sung, J.; An, R.; Hernandez, M.E.; Sosnoff, J.J. Gait variability in people with neurological disorders: A systematic review and meta-analysis. Hum. Mov. Sci. 2016, 47, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Gross, M.M.; Crane, E.A.; Fredrickson, B.L. Effort-shape and kinematic assessment of bodily expression of emotion during gait. Hum. Mov. Sci. 2012, 31, 202–221. [Google Scholar] [CrossRef]
- Naugle, K.M.; Hass, C.J.; Joyner, J.; Coombes, S.A.; Janelle, C.M. Emotional state affects the initiation of forward gait. Emotion 2011, 11, 267. [Google Scholar] [CrossRef] [PubMed]
- Naugle, K.M.; Hass, C.J.; Bowers, D.; Janelle, C.M. Emotional state affects gait initiation in individuals with Parkinson’s disease. Cogn. Affect. Behav. Neurosci. 2012, 12, 207–219. [Google Scholar] [CrossRef]
- Kang, G.E.; Mickey, B.J.; McInnis, M.G.; Krembs, B.S.; Gross, M.M. Motor behavior characteristics in various phases of bipolar disorder revealed through biomechanical analysis: Quantitative measures of activity and energy variables during gait and sit-to-walk. Psychiatry Res. 2018, 269, 93–101. [Google Scholar] [CrossRef]
- Mancini, M.; Chiari, L.; Holmstrom, L.; Salarian, A.; Horak, F.B. Validity and reliability of an IMU-based method to detect APAs prior to gait initiation. Gait Posture 2016, 43, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Washabaugh, E.P.; Kalyanaraman, T.; Adamczyk, P.G.; Claflin, E.S.; Krishnan, C. Validity and repeatability of inertial measurement units for measuring gait parameters. Gait Posture 2017, 55, 87–93. [Google Scholar] [CrossRef]
- Scattolini, M.; Tigrini, A.; Verdini, F.; Burattini, L.; Fioretti, S.; Mengarelli, A. Inertial Sensing for Human Motion Analysis: Enabling Sensor-to-Body Calibration Through an Anatomical and Functional Combined Approach. IEEE Trans. Neural Syst. Rehabil. Eng. 2025, 33, 1853–1862. [Google Scholar] [CrossRef]
- Lee, P.H.; Macfarlane, D.J.; Lam, T.H.; Stewart, S.M. Validity of the international physical activity questionnaire short form (IPAQ-SF): A systematic review. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 115. [Google Scholar] [CrossRef]
- Kang, G.E.; Gross, M.M. Emotional influences on sit-to-walk in healthy young adults. Hum. Mov. Sci. 2015, 40, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Najafi, B.; Khan, T.; Wrobel, J. Laboratory in a box: Wearable sensors and its advantages for gait analysis. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 6507–6510. [Google Scholar]
- Aminian, K.; Najafi, B.; Büla, C.; Leyvraz, P.-F.; Robert, P. Spatio-temporal parameters of gait measured by an ambulatory system using miniature gyroscopes. J. Biomech. 2002, 35, 689–699. [Google Scholar] [CrossRef]
- Kang, G.E.; Yang, J.; Najafi, B. Does the presence of cognitive impairment exacerbate the risk of falls in people with peripheral neuropathy? An application of body-worn inertial sensors to measure gait variability. Sensors 2020, 20, 1328. [Google Scholar] [CrossRef]
- Najafi, B.; Helbostad, J.L.; Moe-Nilssen, R.; Zijlstra, W.; Aminian, K. Does walking strategy in older people change as a function of walking distance? Gait Posture 2009, 29, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Najafi, B.; Khan, T.; Fleischer, A.; Wrobel, J. The impact of footwear and walking distance on gait stability in diabetic patients with peripheral neuropathy. J. Am. Podiatr. Med. Assoc. 2013, 103, 165–173. [Google Scholar] [CrossRef]
- Benson, L.C.; Räisänen, A.M.; Clermont, C.A.; Ferber, R. Is this the real life, or is this just laboratory? A scoping review of IMU-based running gait analysis. Sensors 2022, 22, 1722. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Fischer, E.; Tunca, C.; Brahms, C.M.; Ersoy, C.; Granacher, U.; Arnrich, B. How we found our imu: Guidelines to IMU selection and a comparison of seven IMUs for pervasive healthcare applications. Sensors 2020, 20, 4090. [Google Scholar] [CrossRef] [PubMed]
- Murri, M.B.; Triolo, F.; Coni, A.; Tacconi, C.; Nerozzi, E.; Escelsior, A.; Respino, M.; Neviani, F.; Bertolotti, M.; Bertakis, K. Instrumental assessment of balance and gait in depression: A systematic review. Psychiatry Res. 2020, 284, 112687. [Google Scholar] [CrossRef]
- Ayoubi, F.; Launay, C.P.; Annweiler, C.; Beauchet, O. Fear of falling and gait variability in older adults: A systematic review and meta-analysis. J. Am. Med. Dir. Assoc. 2015, 16, 14–19. [Google Scholar] [CrossRef]
- Avanzino, L.; Lagravinese, G.; Abbruzzese, G.; Pelosin, E. Relationships between gait and emotion in Parkinson’s disease: A narrative review. Gait Posture 2018, 65, 57–64. [Google Scholar] [CrossRef]
- Kushioka, J.; Sun, R.; Zhang, W.; Muaremi, A.; Leutheuser, H.; Odonkor, C.A.; Smuck, M. Gait variability to phenotype common Orthopedic gait impairments using wearable sensors. Sensors 2022, 22, 9301. [Google Scholar] [CrossRef]
- Hendriks, M.M.; Vos-van der Hulst, M.; Weijs, R.W.; van Lotringen, J.H.; Geurts, A.C.; Keijsers, N.L. Using sensor technology to measure gait capacity and gait performance in rehabilitation inpatients with neurological disorders. Sensors 2022, 22, 8387. [Google Scholar] [CrossRef]
- Tunca, C.; Pehlivan, N.; Ak, N.; Arnrich, B.; Salur, G.; Ersoy, C. Inertial sensor-based robust gait analysis in non-hospital settings for neurological disorders. Sensors 2017, 17, 825. [Google Scholar] [CrossRef] [PubMed]
- Stout, A.; Mabbun, K.; Waldon, K.V.; Alvarez, M.; Nguyen, S.; Barcellano, M.; Cuenca, S.; Tang, C.-F.; Naik, A.D.; Kang, G.E. Effects of cognitive dual-tasking on biomechanics and muscle activity during gait initiation and sit-to-walk in young adults. PLoS ONE 2025, 20, e0328677. [Google Scholar] [CrossRef] [PubMed]
| Measures | Outcomes (mean ± std) |
|---|---|
| Demographic | |
| N | 14 (number of females = 9) |
| Age, years | 20.3 ± 2.2 |
| Height, m | 1.7 ± 0.1 |
| Weight, kg | 63.6 ± 8.3 |
| Body-Mass Index, kg/m2 | 22.8 ± 2.6 |
| Physical Activity Level | |
| Vigorous Activity (Days) | 3.1 ± 2.2 |
| Vigorous (Minutes) | 55.7 ± 54.1 |
| Moderate (Days) | 2.1 ± 2.4 |
| Moderate (Minutes) | 34.3 ± 42.2 |
| Walking (Days) | 4.5 ± 2.8 |
| Walking (Minutes) | 84.3 ± 91.3 |
| Sitting (Hours per Day) | 5.7 ± 2.6 |
| Sitting (Minutes per day) | 342.9 ± 153.7 |
| Target Emotions | Mood Intensity | ||||
|---|---|---|---|---|---|
| Angry | Sad | Joyful | Fearful | Neutral | |
| Angry | 2.8 ± 0.7 | 0.6 ± 1.0 | 0.0 ± 0.0 | 0.5 ± 0.9 | 0.0 ± 0.0 |
| Sad | 0.9 ± 1.2 | 3.2 ± 0.7 | 0.1 ± 0.4 | 0.9 ± 1.3 | 0.1 ± 0.3 |
| Joyful | 0.2 ± 0.6 | 0.1 ± 0.3 | 3.3 ± 0.7 | 0.0 ± 0.2 | 0.3 ± 0.6 |
| Fearful | 0.3 ± 0.7 | 0.9 ± 1.0 | 0.0 ± 0.2 | 3.0 ± 0.7 | 0.0 ± 0.2 |
| Neutral | 0.1 ± 0.4 | 0.3 ± 0.5 | 0.2 ± 0.4 | 0.1 ± 0.4 | 3.2 ± 1.2 |
| Angry | Sad | Neutral | Joyful | Fearful | p-Value | Adjusted p | Effect Size (95% CI) | |
|---|---|---|---|---|---|---|---|---|
| Cadence | 108.7 ± 8.7 | 103.1 ± 15.3 | 105.8 ± 8.8 A* | 110.3 ± 10.5 S* | 108.4 ± 13.0 | 0.0015 | 0.002 | W = 0.31 (0.23, 0.55) |
| Swing (Left) | 40.3 ± 2.2 | 39.5 ± 3.0 | 40.1 ± 2.4 | 40.3 ± 1.9 | 40.0 ± 2.4 | 0.480 | 0.554 | W = 0.06 (0.02, 0.30) |
| Swing (Right) | 41.2 ± 1.5 | 40.6 ± 2.7 | 41.0 ± 1.5 | 41.3 ± 1.6 | 41.4 ± 1.5 | 0.183 | 0.343 | W = 0.11 (0.03, 0.45) |
| Stance (Left) | 59.7 ± 2.2 | 60.5 ± 3.0 | 59.9 ± 2.4 | 59.7 ± 1.9 | 60.0 ± 2.4 | 0.480 | 0.554 | W = 0.06 (0.02, 0.29) |
| Stance (Right) | 58.8 ± 1.5 | 59.4 ± 2.7 | 59.0 ± 1.5 | 58.7 ± 1.6 | 58.6 ± 1.5 | 0.183 | 0.342 | W = 0.11 (0.03, 0.42) |
| DLS (Initial Right) | 10.0 ± 2.7 | 10.5 ± 3.4 | 10.2 ± 2.3 | 9.8 ± 2.1 | 9.9 ± 2.4 | 0.878 | 0.878 | W = 0.02 (0.01, 0.24) |
| DLS (Terminal Left) | 8.4 ± 1.4 | 9.4 ± 2.2 | 8.6 ± 1.8 | 8.6 ± 1.5 | 8.7 ± 1.6 | 0.063 | 0.410 | η2p = 0.16 ([0.05, 0.41) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarez, M.; Stout, A.; Fisanick, L.; Tang, C.-F.; Wilson, D.G.; Gray, L.; Logan, B.; Kang, G.E. Gait Variability and Spatiotemporal Parameters During Emotion-Induced Walking: Assessment with Inertial Measurement Units. Sensors 2025, 25, 6222. https://doi.org/10.3390/s25196222
Alvarez M, Stout A, Fisanick L, Tang C-F, Wilson DG, Gray L, Logan B, Kang GE. Gait Variability and Spatiotemporal Parameters During Emotion-Induced Walking: Assessment with Inertial Measurement Units. Sensors. 2025; 25(19):6222. https://doi.org/10.3390/s25196222
Chicago/Turabian StyleAlvarez, Marvin, Angeloh Stout, Luke Fisanick, Chuan-Fa Tang, David George Wilson, Leslie Gray, Breanne Logan, and Gu Eon Kang. 2025. "Gait Variability and Spatiotemporal Parameters During Emotion-Induced Walking: Assessment with Inertial Measurement Units" Sensors 25, no. 19: 6222. https://doi.org/10.3390/s25196222
APA StyleAlvarez, M., Stout, A., Fisanick, L., Tang, C.-F., Wilson, D. G., Gray, L., Logan, B., & Kang, G. E. (2025). Gait Variability and Spatiotemporal Parameters During Emotion-Induced Walking: Assessment with Inertial Measurement Units. Sensors, 25(19), 6222. https://doi.org/10.3390/s25196222







