IR Spectroscopy as a Diagnostic Tool in the Recycling Process and Evaluation of Recycled Polymeric Materials
Abstract
1. Introduction
- The recycled material is different from virgin polymer: while it may be similar in chemical characteristics and performance, it is effectively a new material.
- Recycled polymers can vary from batch to batch, due to inconsistent and hard-to-control input materials. In some cases, inhomogeneities may be present even from pellet to pellet, or within a single pellet (especially if foreign inclusions are present, such as metal flakes).
- Chemical identification, including additives: especially useful for studying unknown formulations, can achieve quantitative diagnostics through calibration curves based on reference samples of known composition.
- Diagnosis of chemical modifications: important for monitoring degradation during production (e.g., extrusion, molding, spinning) or aging under various environmental conditions.
- Structural characterization: identifying molecular conformation, degree of crystallinity, and structural defects in semi-crystalline polymers.
- Morphological analysis: distinguishing amorphous from crystalline phases, identifying crystalline polymorphs, quantifying their relative fractions, and recognizing polymer chain orientation, as in fibrous materials.
- Thermal transformation analysis: monitoring phase transitions (e.g., melting) and post-treatment effects (annealing, thermal cross-linking) by recording spectra during controlled heating/cooling cycles, thereby reconstructing the material’s thermal history.
2. Materials and Methods
2.1. Materials
2.2. Spectroscopic Characterization
3. Results and Discussion
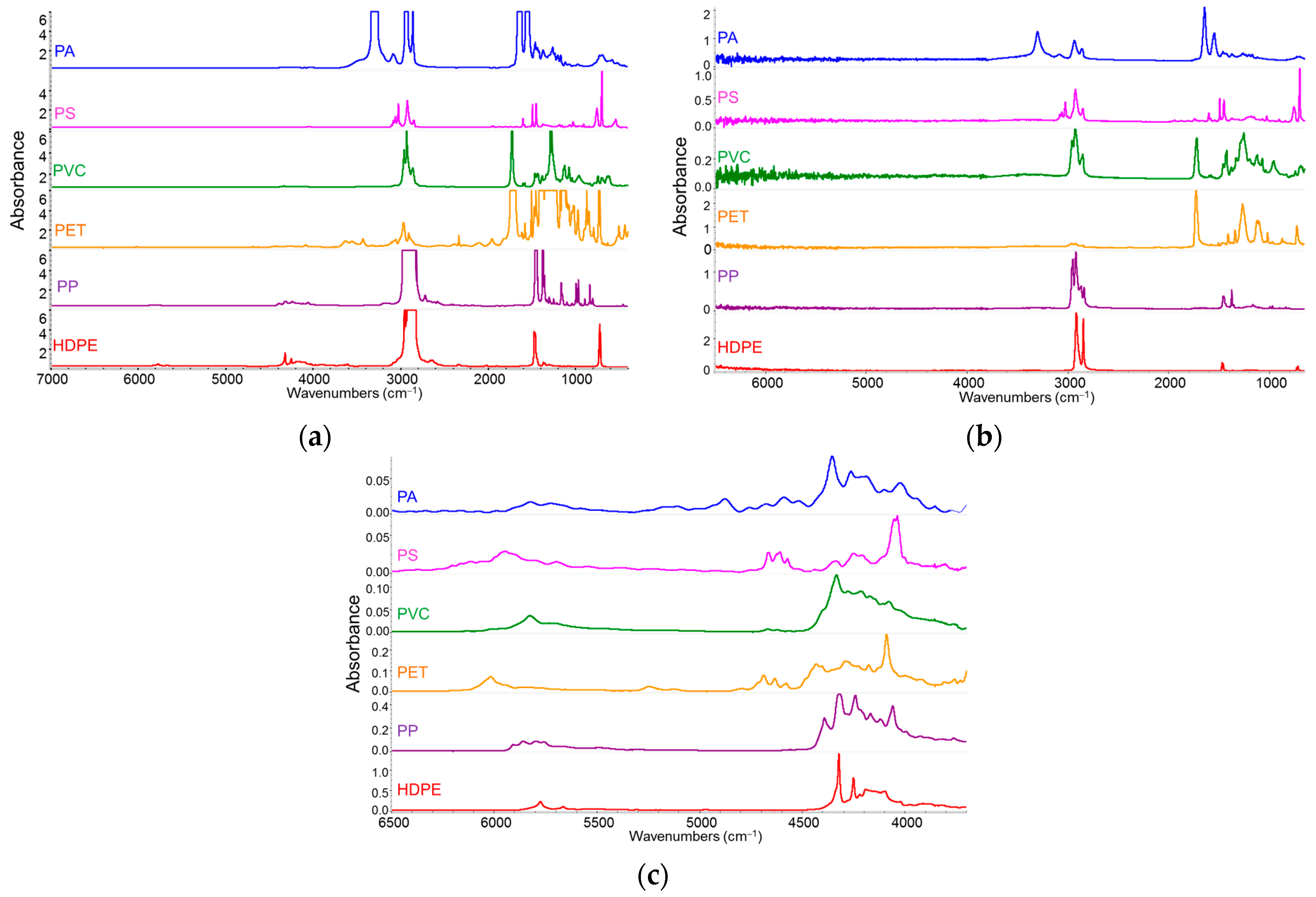
3.1. General Characteristics of Polymeric Materials and Their IR Spectra
- A polymeric material is often a formulation: in addition to the polymer itself, it contains additives that meet processing requirements and ensure the durability of the final product. Additives may provide chemical stability, while fillers can modify mechanical properties (e.g., reinforcing glass fibers) or electrical properties (e.g., graphene particles). Many polymeric materials intended for specific technological applications consist of blends of two or more polymers or copolymers with variable composition. The resulting IR spectrum is therefore a superposition of the spectral responses of all components and cannot perfectly match that of a pure homopolymer or even samples of the same polymer/copolymer that differ in additive type and/or concentration.
- Automated identification (typically using spectral libraries) is an effective and valuable method for determining the polymer family, especially when combined with supervised analysis. This is commonly employed for plastic sorting in recycling processes. However, especially for semi-crystalline polymers, sample preparation can influence morphology [30,31], resulting in spectral differences compared to literature references or database entries. Furthermore, the measurement technique—such as transmission, attenuated total reflectance (ATR), or reflection—can significantly alter the spectral pattern (band shape and intensity), even when spectra are mathematically converted to a common absorbance (or transmittance) scale. Note that absorbance conversion—except in transmission mode—is based on theoretical models and assumptions that are never perfectly met in practice.
- Effective use of spectral libraries often requires data post-processing: baseline correction and subtraction of solvent, substrate, or contamination bands may be necessary. Spectral comparison also requires proper normalization procedures, typically based on normalizing absorbance intensity to a reference band. These processes are not automatic and require expertise, as they may introduce artifacts.
3.2. Techniques and Measurement Setups
- ATR: One of the most convenient techniques for polymeric materials. It detects absorption of the evanescent wave generated at the surface of a high-refractive-index crystal in contact with the sample. Since surface layers often contain additives (e.g., slip agents, release agents), for bulk analysis, it may be necessary to remove the surface layer or section the sample. With special ATR accessories, direct measurements on finished products are also possible.
- Specular reflection: Suitable for polymeric materials with good reflectivity (also depending on surface finish). The sample must be thick enough to prevent the collection of transmitted photons (as in double transmission setups).
- Diffuse Reflectance (DRIFT): Captures diffusely scattered IR radiation over a large solid angle, from which the IR spectrum is obtained. Applicable to samples with high IR scattering (e.g., powders or granular solids), where transmitted IR is negligible and specular reflection is weak.
3.3. Spectral Analysis
3.3.1. Sorting
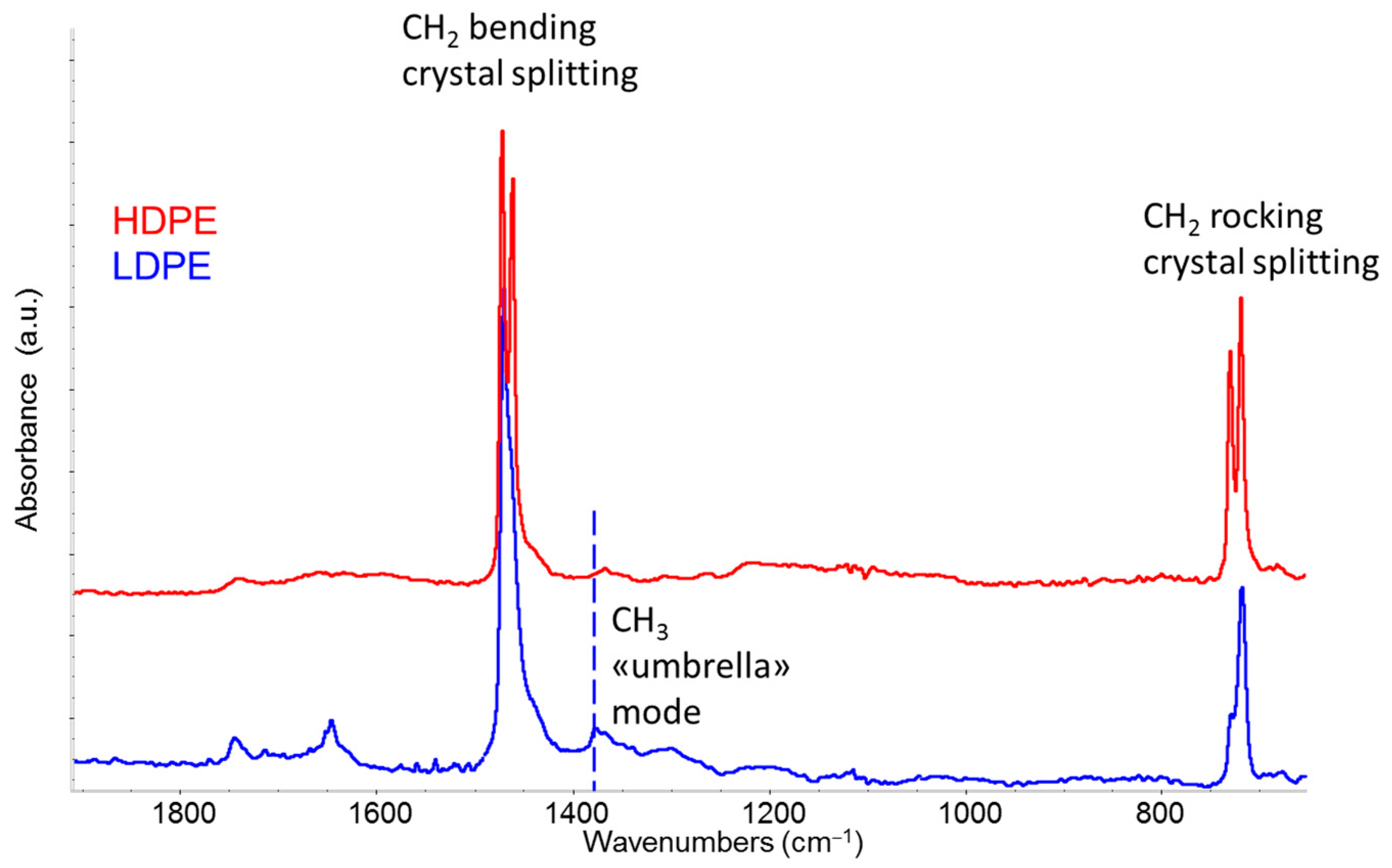
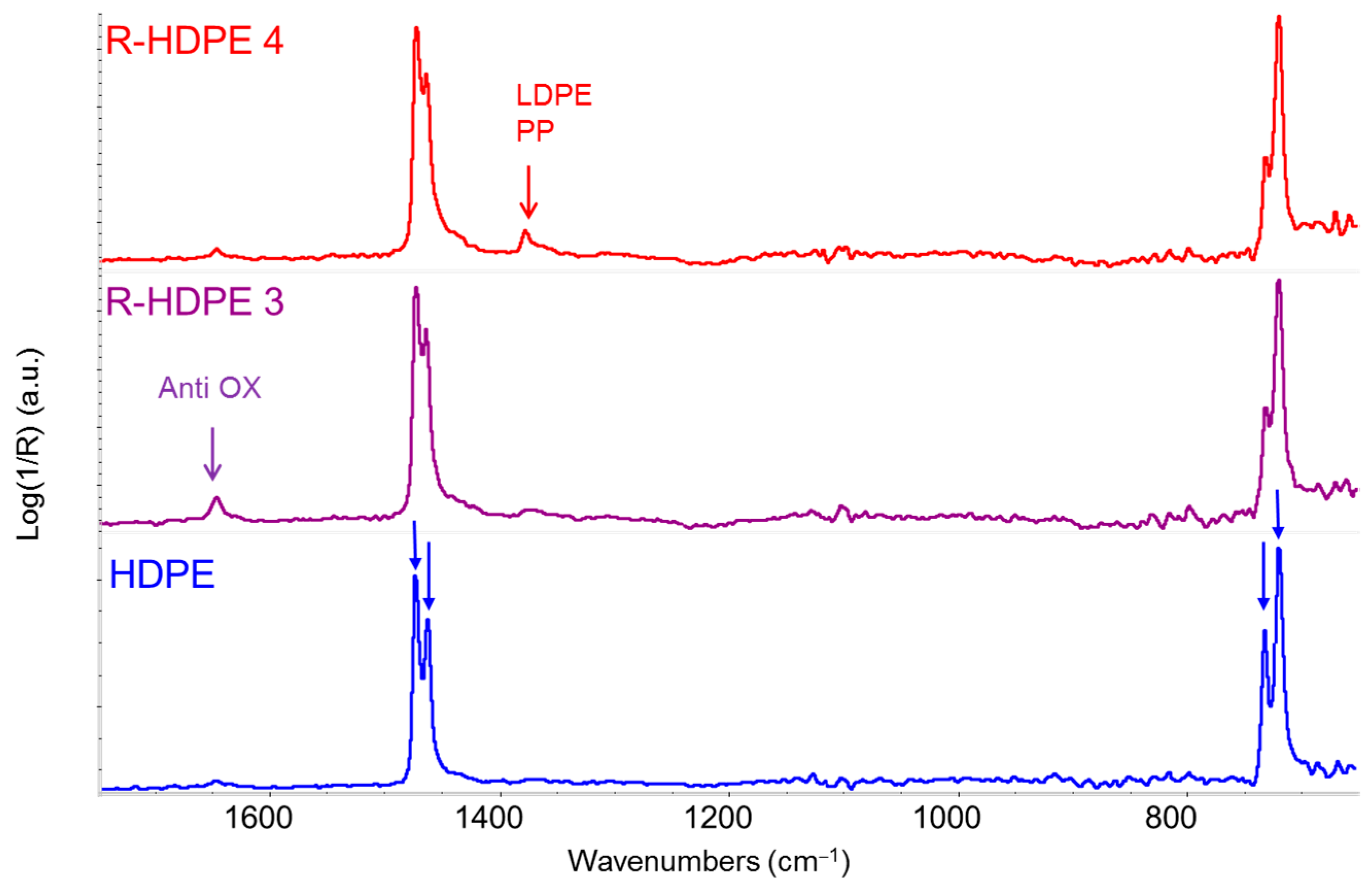
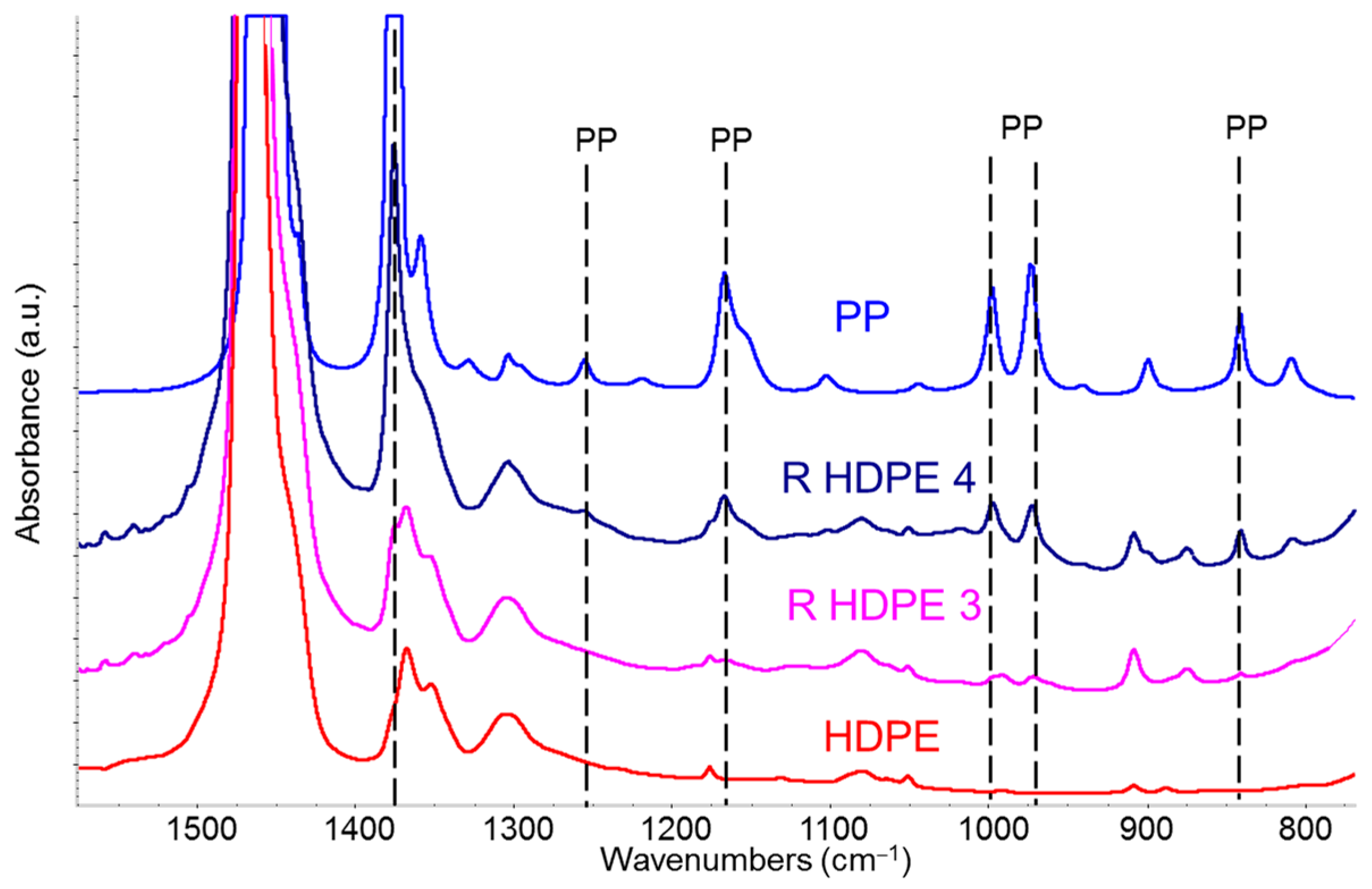
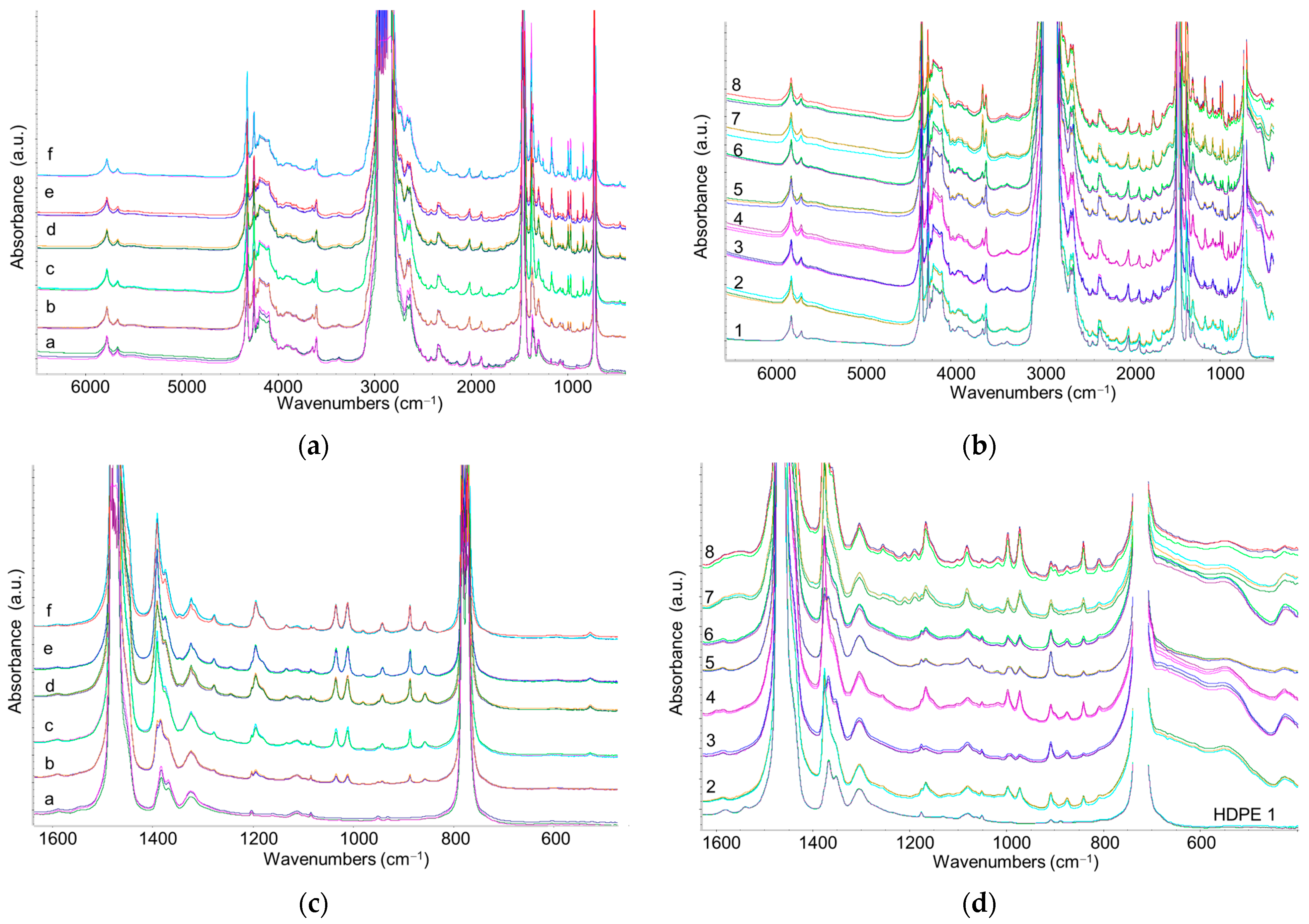
3.3.2. Comparative Analysis of Recycled Polymers: R-HDPE
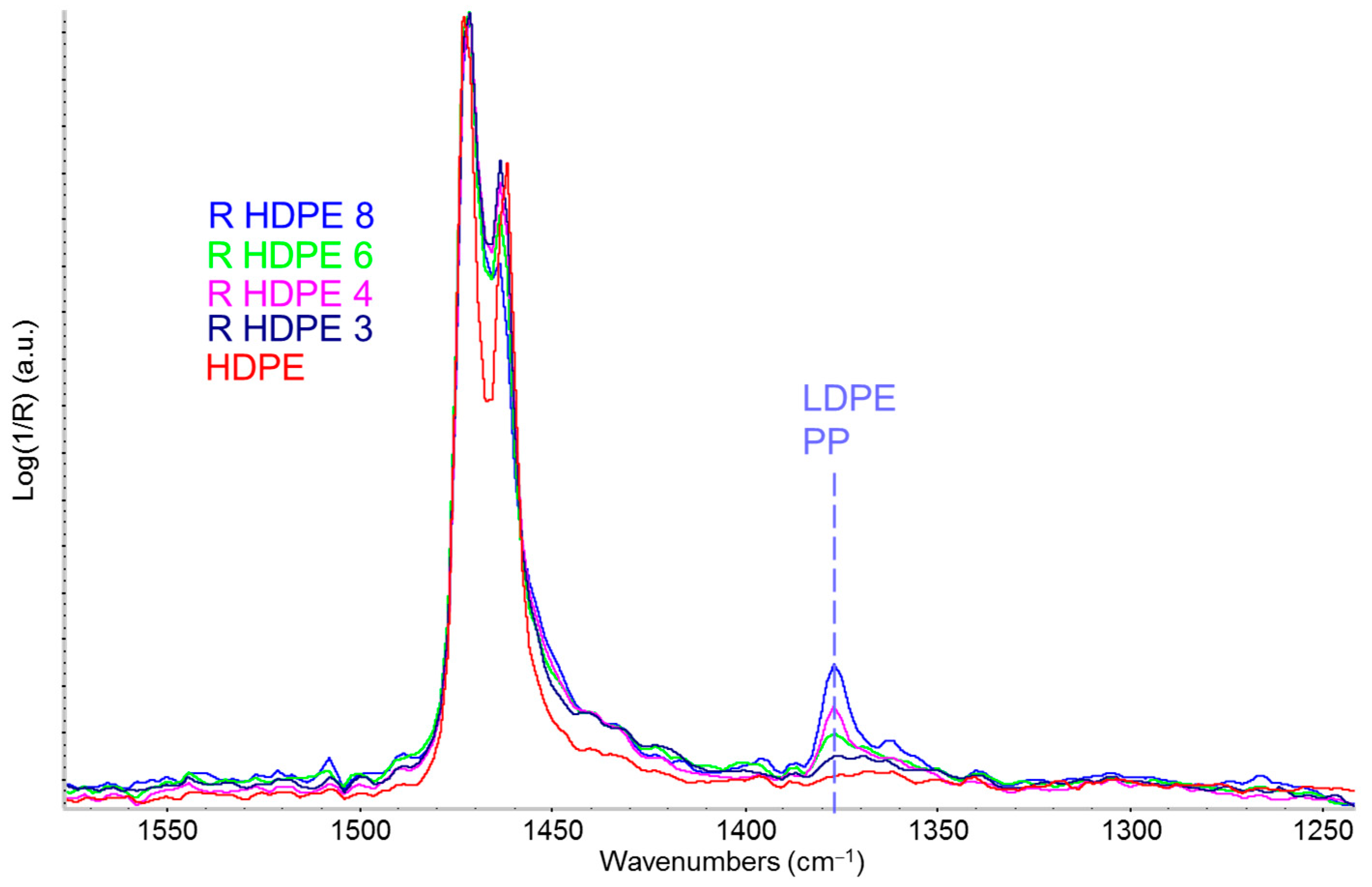
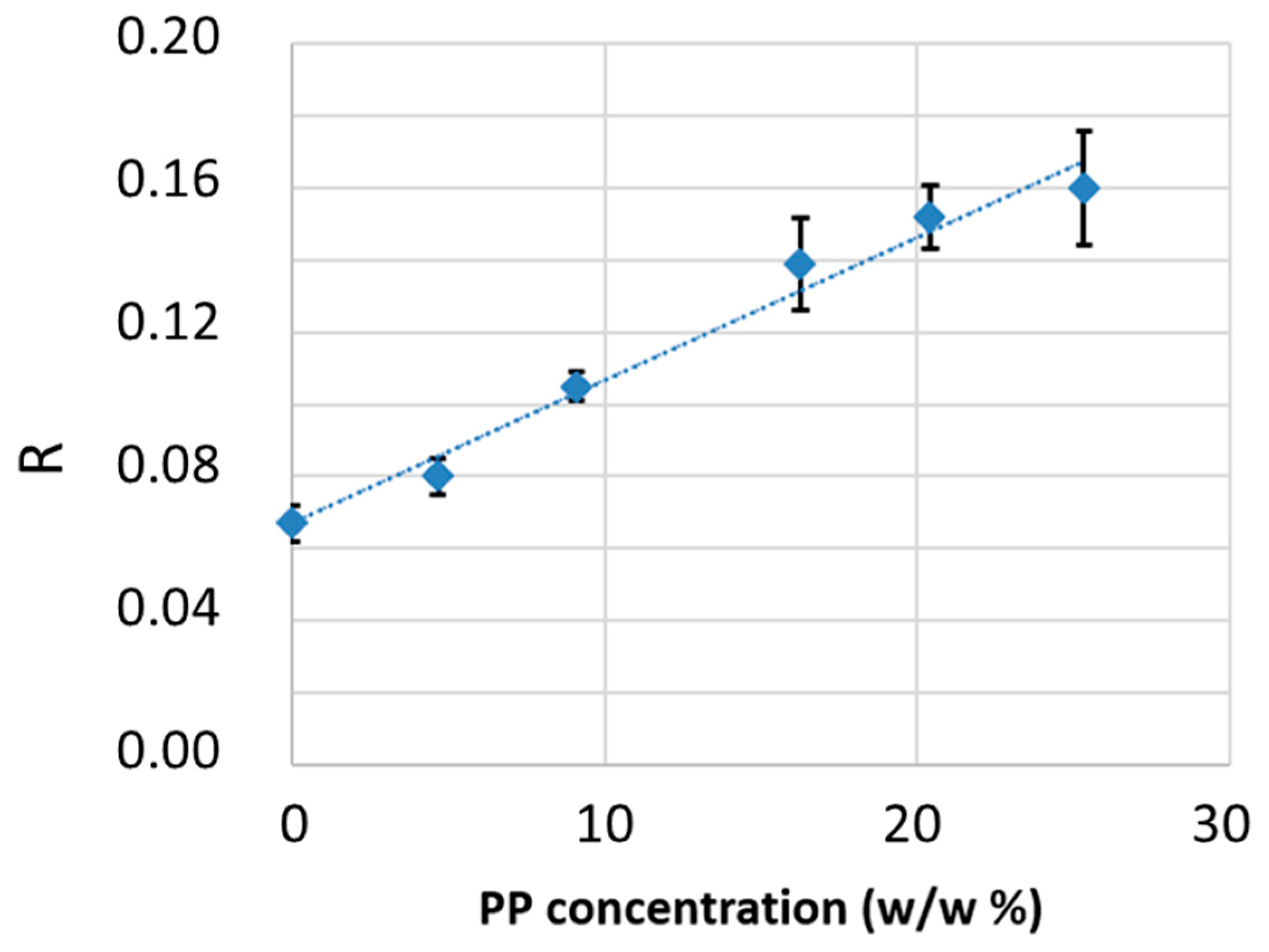
3.3.3. Detection and Quantification of Polypropylene in R-HDPE Samples
- Absorption intensities of overtone and combination bands are much weaker than fundamental transitions, reducing bands saturation effects even in relatively thick films. In fact, thicker samples can enhance the visibility of these weak bands.
- Additives and fillers exhibit very weak absorptions in the NIR, making their contribution negligible compared to the main polymer component. Hence, the obtained information primarily reflects the bulk polymer.
- Transmission mode ensures analysis of the bulk material, preventing overestimation of surface chemical species possibly present on pellets.
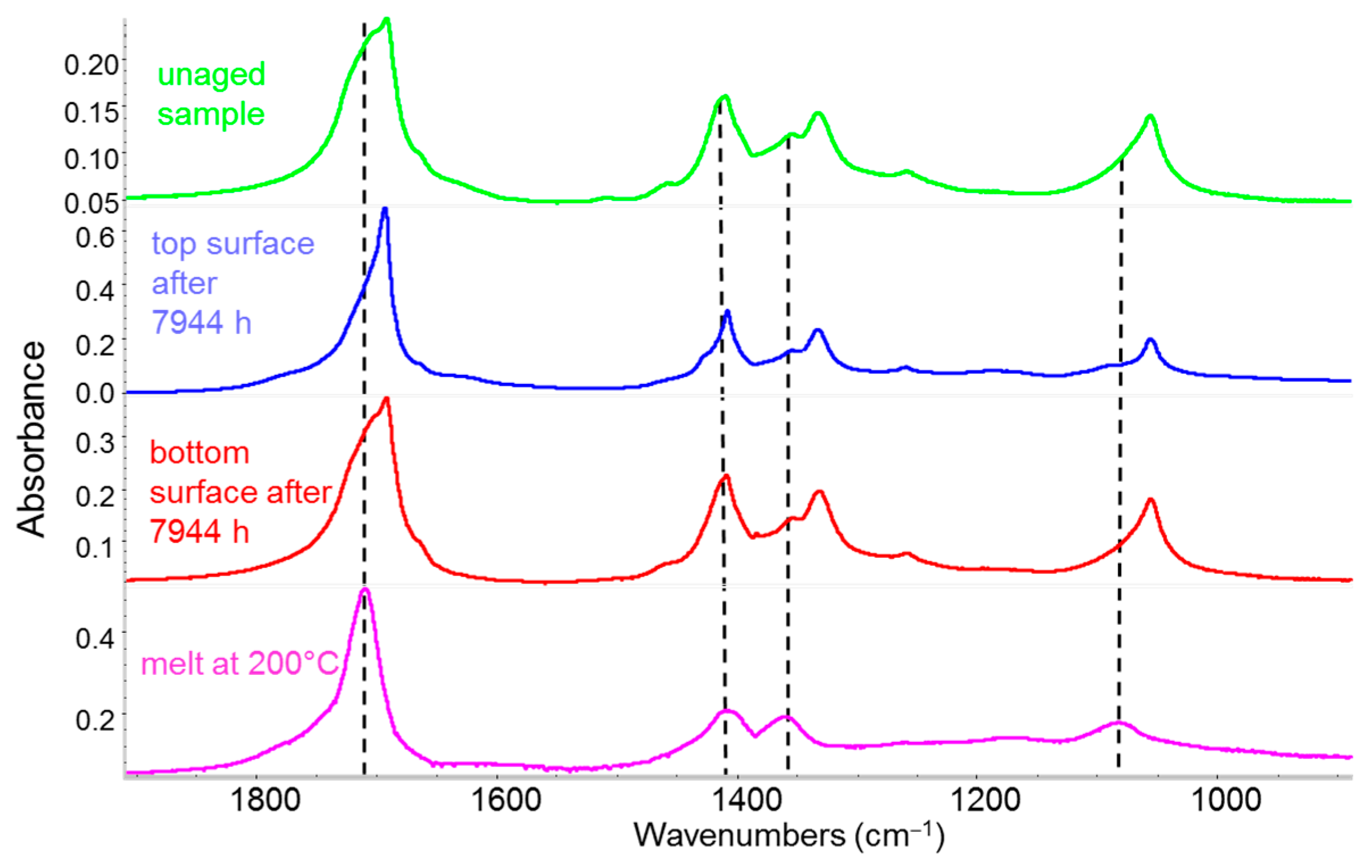
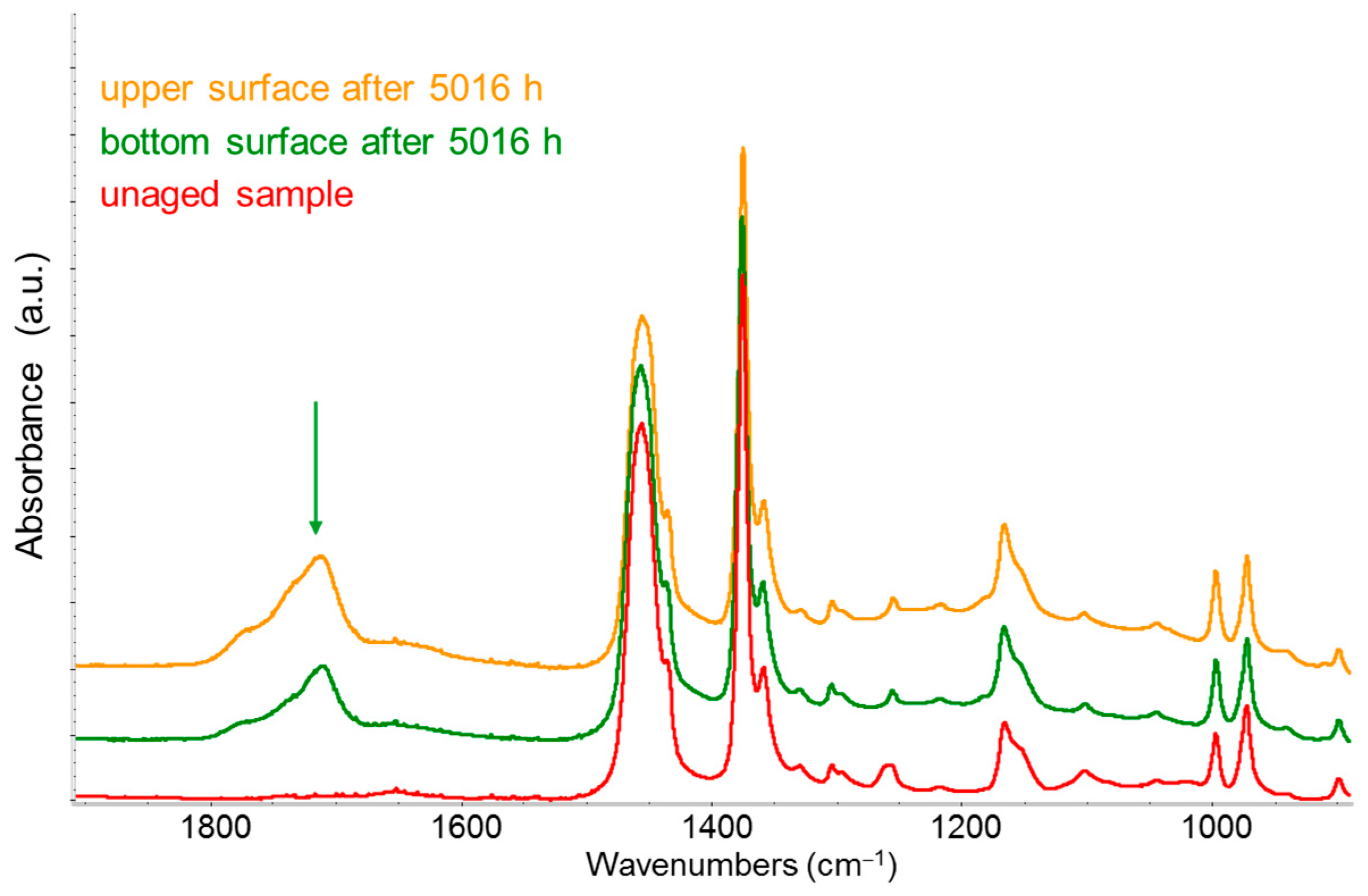
3.3.4. IR Detection of Polymer Degradation
4. Conclusions
- The use of IR for materials characterization provides a detailed description of chemical composition, molecular structure, and morphology. Different materials or issues may require choosing different experimental setups. Standardization of protocols remains a challenge.
- R-materials are different from virgin ones. Available IR techniques allow the identification of several differences between R-polymer materials and virgin polymers.
- Characterization of R-polymers can suggest strategies to bridge the gap and/or develop new structure–property correlations with application perspectives in mind.
- The durability of R-polymers should be evaluated through appropriate accelerated weathering or ageing tests, followed by structural analysis, e.g., using IR.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HDPE | High-Density Polyethylene |
| R-HDPE | Recycled High-Density Polyethylene |
| LDPE | Low-Density Polyethylene |
| PP | Polypropylene (isotactic) |
| ATR | Attenuated Total Reflectance (spectroscopy) |
| NIR | Near-Infrared (spectroscopy) |
References
- Pierri, E.; Egle, L.; Milios, L.; Saveyn, H. EU-Wide End-of-Waste Criteria for Plastic Waste; Publications Office of the European Union: Luxembourg, 2024; p. 775. [Google Scholar]
- Johansson, O. The End-of-Waste for the Transition to Circular Economy: A Legal Review of the European Union Waste 776 Framework Directive. Environ. Policy Law 2023, 53, 167–179. [Google Scholar] [CrossRef]
- Dzoh Fonkou, J.P.; Beggio, G.; Salviulo, G.; Lavagnolo, M.C. Analytical Methods for In-Depth Assessment of Recycled Plastics: A Review. Environments 2025, 12, 154. [Google Scholar] [CrossRef]
- Valerio, O.; Muthuraj, R.; Codou, A. Strategies for polymer to polymer recycling from waste: Current trends and opportunities for improving the circular economy of polymers in South America. Curr. Opin. Green Sustain. Chem. 2020, 25, 100381. [Google Scholar] [CrossRef]
- da Silva, D.J.; Wiebeck, H. Current options for characterizing, sorting, and recycling polymeric waste. Prog. Rubber Plast. Recycl. Technol. 2020, 36, 284–303. [Google Scholar] [CrossRef]
- Lange, J.-P. Managing Plastic Waste—Sorting, Recycling, Disposal, and Product Redesign. ACS Sustain. Chem. Eng. 2021, 9, 15722–15738. [Google Scholar] [CrossRef]
- Natta, G.; Peraldo, M.; Corradini, P. Modificazione mesomorfa smettica del polipropilene isotattico. Atti Accad. Naz. Lincei Rend. Classe Sci. Fis. Mat. Nat. 1959, 26, 14–17. [Google Scholar]
- Zerbi, G. Molecular Vibrations of High Polymers. In Applied Spectroscopy Reviews; Brame, A.D., Ed.; Dekker: New York, NY, USA, 1969; Volume 2, p. 193. [Google Scholar]
- Zbinden, R. Infrared Spectroscopy of High Polymers; Academic Press: New York, NY, USA, 1964. [Google Scholar]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2004. [Google Scholar]
- Shrader, B. Infrared and Raman Spectroscopy; VCH: Weinheim, DE, USA, 1995. [Google Scholar]
- Barnes, A.J.; Orville-Thomas, W.J. (Eds.) Vibrational Spectroscopy-Modern Trends; Elsevier: New York, NY, USA, 1977. [Google Scholar]
- Tasumi, M. Introduction to Experimental Infrared Spectroscopy: Fundamentals and Practical Methods; Wiley: Chichester, UK, 2015. [Google Scholar]
- Günzler, H.; Gremlin, H.-U. IR Spectroscopy. An Introduction; Wiley-VCH: Wenheim, Germany, 2002. [Google Scholar]
- Wilson, E.B.; Decius, J.C.; Cross, P.C. Molecular Vibrations; McGraw-Hill: New York, NY, USA, 1955. [Google Scholar]
- Davies, M. Infra-Red Spectroscopy and Molecular Structure; Elsevier: Amsterdam, NL, USA, 1963. [Google Scholar]
- Califano, S. Vibrational States; John Wiley & Sons: London, UK, 1976. [Google Scholar]
- Barone, V. The virtual multifrequency spectrometer: A new paradigm for spectroscopy. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2016, 6, 86–110. [Google Scholar] [CrossRef]
- Barone, V.; Puzzarini, C. Gas-Phase Computational Spectroscopy: The Challenge of the Molecular Bricks of Life. Annu. Rev. Phys. Chem. 2023, 74, 29–52. [Google Scholar] [CrossRef]
- Dovesi, R.; Orlando, R.; Erba, A.; Zicovich-Wilson, C.M.; Civalleri, B.; Casassa, S.; Maschio, L.; Ferrabone, M.; De La Pierre, M.; D’Arco, P.; et al. CRYSTAL14: A program for the ab initio investigation of crystalline solids. Int. J. Quantum Chem. 2014, 114, 1287–1317. [Google Scholar] [CrossRef]
- Dovesi, R.; Erba, A.; Orlando, R.; Zicovich-Wilson, C.M.; Civalleri, B.; Maschio, L.; Rérat, M.; Casassa, S.; Baima, J.; Salustro, S.; et al. Quantum-mechanical condensed matter simulations with CRYSTAL. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2018, 8, e1360. [Google Scholar] [CrossRef]
- Galimberti, D.; Milani, A.; Maschio, L.; Castiglioni, C. Intermolecular modulation of IR intensities in the solid state. The role of weak interactions in polyethylene crystal: A computational DFT study. J. Chem. Phys. 2016, 145, 144901. [Google Scholar] [CrossRef]
- Milani, A.; Castiglioni, C.; Radice, S. Joint Experimental and Computational Investigation of the Structural and Spectroscopic Properties of Poly(vinylidene fluoride) Polymorphs. J. Phys. Chem. B 2015, 119, 4888–4897. [Google Scholar] [CrossRef]
- Zerbi, G. Vibrational Spectroscopy of Very Large Molecules. In Advances in Infrared and Raman spectroscopy; Clark, R.J.H., Hester, R.E., Eds.; Wiley: Heyden, ID, USA, 1984; Volume 11, p. 301. [Google Scholar]
- Painter, P.C.; Coleman, M.M.; Koening, J.L. The Theory of Vibrational Spectroscopy and its Application to Polymeric Materials; Wiley: Chichester, UK, 1982. [Google Scholar]
- Zerbi, G. Modern Polymer Spectroscopy; Wiley-VCH: Weinheim, UK, 1999. [Google Scholar]
- Everall, N.J.; Chalmers, J.M.; Griffiths, P.R. (Eds.) Vibrational Spectroscopy of Polymers: Principles and Practices; Wiley: New York, NY, USA, 2007. [Google Scholar]
- Castiglioni, C. Theory of Vibrational Spectroscopy of Polymers. In Vibrational Spectroscopy of Polymers: Principles and Practices; Everall, N.J., Chalmers, J.M., Griffiths, P.R., Eds.; Wiley: New York, NY, USA, 2007; pp. 455–483. [Google Scholar]
- Koenig, J.L. Raman Scattering of Synthetic Polymers—A Review. Appl. Spectrosc. Rev. 1971, 4, 233–305. [Google Scholar] [CrossRef]
- Gedde, U.W. Polymer Physics; Springer Science + Business Media, B.V.: Dordrecht, NL, USA, 2001. [Google Scholar]
- Cowie, J.M.G.; Arrighi, V. Polymers: Chemistry and Physics of Modern Materials; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2007. [Google Scholar]
- Karaagac, E.; Jones, M.P.; Koch, T.; Archodoulaki, V.-M. Polypropylene Contamination in Post-Consumer Polyolefin Waste: Characterisation, Consequences and Compatibilisation. Polymers 2021, 13, 2618. [Google Scholar] [CrossRef] [PubMed]
- Karaagac, E.; Jones, M.P.; Koch, T.; Archodoulaki, V.-M. The effect of PP contamination in recycled high-density polyethylene (rPE-HD) from post-consumer bottle waste and their compatibilization with olefin block copolymer (OBC). Waste Manag. 2021, 119, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Hashemnejad, M.; Doshi, A. Quantifying the content of various types of polypropylene in high density polyethylene blends. Polym. Test. 2024, 139, 108577. [Google Scholar] [CrossRef]
- Larsen, Å.G.; Olafsen, K.; Alcock, B. Determining the PE fraction in recycled PP. Polym. Test. 2021, 96, 107058. [Google Scholar] [CrossRef]
- Gallo, R.; Brambilla, L.; Castiglioni, C.; Severini, F. Characterization of naturally weathered polypropylene plates. J. Macromol. Sci. A 2006, 43, 535–554. [Google Scholar] [CrossRef]
- Maria, C. Application of FTIR Spectroscopy in Environmental Studies. In Advanced Aspects of Spectroscopy; InTech: Singapore, 2012. [Google Scholar]
- Nguyen, T.; Shamsabadi, A.A.; Bavarian, M. Coupling ATR-FTIR Spectroscopy with Multivariate Analysis for Polymers Manufacturing and Control of Polymers’ Molecular Weight. Digit. Chem. Eng. 2023, 7, 100089. [Google Scholar] [CrossRef]
- Mwanzia, M.N.; Mugo, W.; Soitah, T.N. Correlation of Mechanical and Optical Properties of Polypropylene Plastic Waste for Application in Composite Panels. J. Agric. Sci. Technol. 2023, 23, 125–130. [Google Scholar] [CrossRef]
- Audy, R.; Enfrin, M.; Boom, Y.J.; Giustozzi, F. Selection of Recycled Waste Plastic for Incorporation in Sustainable Asphalt Pavements: A Novel Multi-Criteria Screening Tool Based on 31 Sources of Plastic. Sci. Total Environ. 2022, 829, 154604. [Google Scholar] [CrossRef]
- Mizushima, M.; Kawamura, T.; Takahashi, K.; Nitta, K. In Situ Near-Infrared Spectroscopic Studies of the Structural Studies of the Structural Changes of Polyethylene During Melting. Polym. J. 2012, 44, 162–166. [Google Scholar] [CrossRef]
- Severini, F.; Gallo, R.; Di Landro, L.; Pegoraro, M.; Brambilla, L.; Tommasini, M.; Castiglioni, C.; Zerbi, G. Chemical and physical modifications of alternating ethylene-carbon monoxide copolymer by outdoor exposure. Polymer 2001, 42, 3609–3625. [Google Scholar] [CrossRef]


| Sample | PP [mg] | HDPE [mg] | ||
|---|---|---|---|---|
| a | 0.0 | 160.5 | 0.00 | 0.067 |
| b | 7.0 | 149.4 | 4.69 | 0.080 |
| c | 13.8 | 151.5 | 9.11 | 0.105 |
| d | 19.6 | 120.5 | 16.27 | 0.139 |
| e | 27.8 | 136.2 | 20.41 | 0.152 |
| f | 26.9 | 106.2 | 25.33 | 0.160 |
| Sample | ||
|---|---|---|
| 8 | 0.124 | 14.25 |
| 4 | 0.107 | 10.00 |
| 7 | 0.089 | 5.50 |
| 3 | 0.08 | 3.25 |
| 6 | 0.078 | 2.75 |
| 2 | 0.077 | 2.50 |
| 5 | 0.073 | 1.50 |
| 1 | 0.068 | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, K.; Brambilla, L.; Castiglioni, C. IR Spectroscopy as a Diagnostic Tool in the Recycling Process and Evaluation of Recycled Polymeric Materials. Sensors 2025, 25, 6205. https://doi.org/10.3390/s25196205
Hu K, Brambilla L, Castiglioni C. IR Spectroscopy as a Diagnostic Tool in the Recycling Process and Evaluation of Recycled Polymeric Materials. Sensors. 2025; 25(19):6205. https://doi.org/10.3390/s25196205
Chicago/Turabian StyleHu, Kaiyue, Luigi Brambilla, and Chiara Castiglioni. 2025. "IR Spectroscopy as a Diagnostic Tool in the Recycling Process and Evaluation of Recycled Polymeric Materials" Sensors 25, no. 19: 6205. https://doi.org/10.3390/s25196205
APA StyleHu, K., Brambilla, L., & Castiglioni, C. (2025). IR Spectroscopy as a Diagnostic Tool in the Recycling Process and Evaluation of Recycled Polymeric Materials. Sensors, 25(19), 6205. https://doi.org/10.3390/s25196205







