Cardiac Monitoring with Textile Capacitive Electrodes in Driving Applications: Characterization of Signal Quality and RR Duration Accuracy
Abstract
1. Introduction
1.1. Background and Motivation
1.2. Capacitive ECG Technology and Use Cases
1.3. Circuit Architectures for cECG
1.4. cECG Electrode Materials and Shapes
1.5. Number of Electrodes and Spatial Positioning
1.6. Unexplored Research Questions
- Design textile electrodes using industrial embroidering methods, a realistic and seamless approach in the context of seat cover design in the automotive industry.
- Integrate pairs of electrodes into the seat back of a car seat in a practical and user-friendly way, as expected for the normal use of a vehicle.
- Integrate the electrodes following very diverse configurations to cover a wide range of situations in terms of placement and form factor.
- For data collection, recruit numerous subjects of varying body sizes, to obtain generalizable results.
- Evaluate the performance of the electrode integration based on known or expected needs in the context of monitoring vital signs while driving, notably noise level and RR duration accuracy.
2. Materials and Methods
2.1. Capacitive Sensing: Principle and Implementation
2.1.1. Design and Prototyping of Textile Electrodes
- A.73.41 with a surface area of 7.62 cm × 7.62 cm (58.06 cm2);
- A.73.42 with a surface area of 11.43 cm × 5.08 cm (58.06 cm2);
- A.73.50 with a surface area of 5.08 cm × 5.08 cm (25.81 cm2).
- A.73.46 with a surface area of 7.62 cm × 7.62 cm (58.06 cm2).
2.1.2. ECG Acquisition Data Pipeline: Hardware and Software
- An instrumentation amplifier with a common-mode rejection of 80 dB and a gain of 100.
- A high-pass filter with a cutoff frequency of 1.3 Hz (blocking the DC component of the cECG) and a fast restore function reducing settling time.
- A low-pass filter with a cutoff frequency of 41 Hz and an additional gain of 5, for a total gain of 500.
- An integrated right leg drive (RLD) amplifier reinjecting the inverted common mode signal into the subject’s body via the RLD bracelet (a standard anti-static wrist strap).
- Display cECG and rECG time series in a graphical user interface—for real-time visual inspection.
- Send a time marker to the driving simulator software and the data synchronizer every two seconds—to serve as reference for time interpolation in the data synchronization software.
- Send cECG data, rECG data, video frame timestamps, and driving simulator time markers to the data synchronization software—to put all the collected information of the study in the same timeframe.
2.2. Experimental Setup
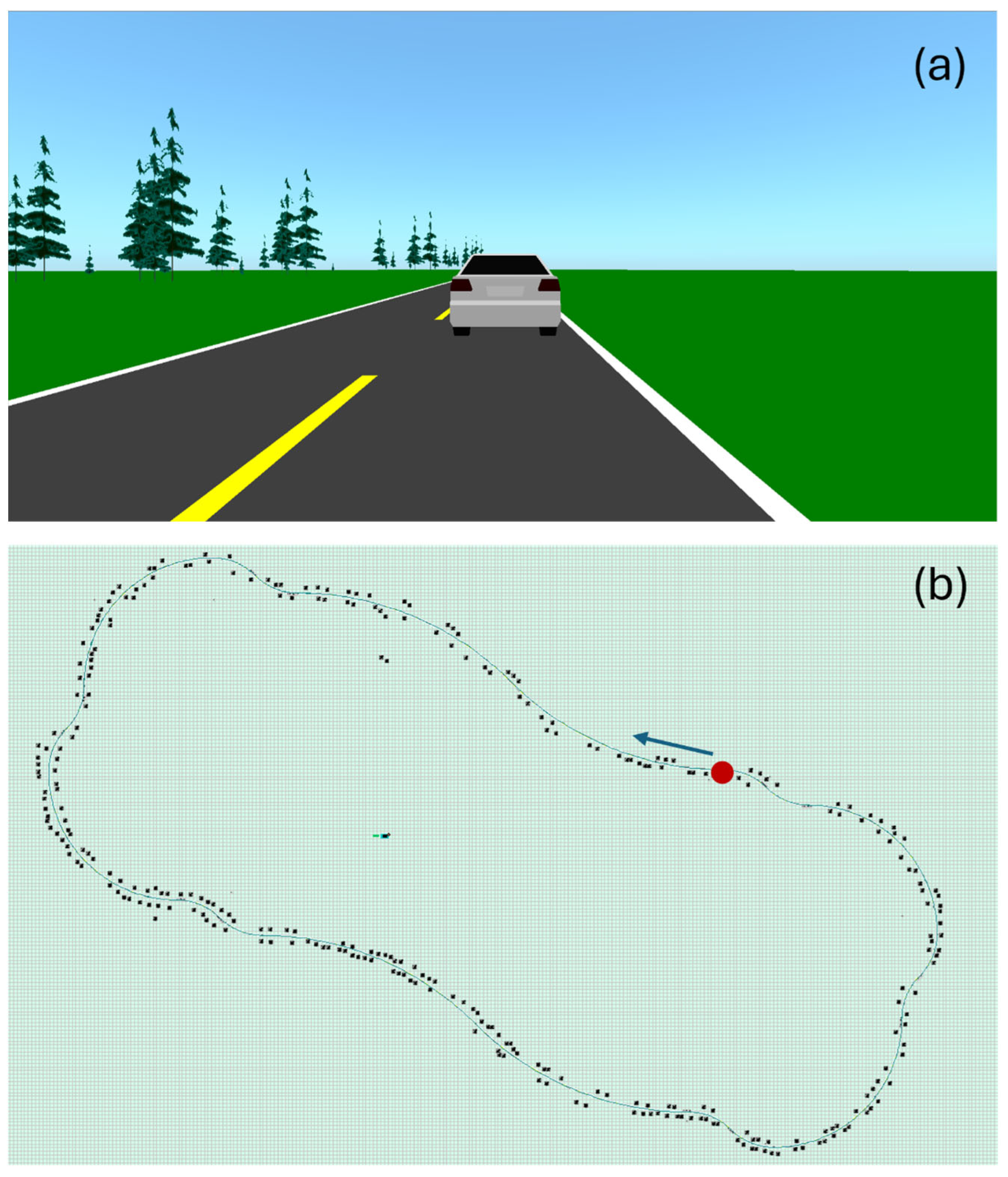
2.2.1. Driving Simulator
- Three 32-inch Samsung 1080p screens by Samsung Group (Suwon, Republic of Korea)—model UN32N5300AFXZC.
- A racing car seat by GTR Simulator (Ontario, CA, USA)—model S105L-BKRD.
- A set consisting of a steering wheel and pedals by Logitech International S.A. (San Jose, CA, USA)—model B016JBE8LU.
- A York driving simulator software by York Computer Technologies Inc. (Kingston, ON, Canada)—version 7.08.24.

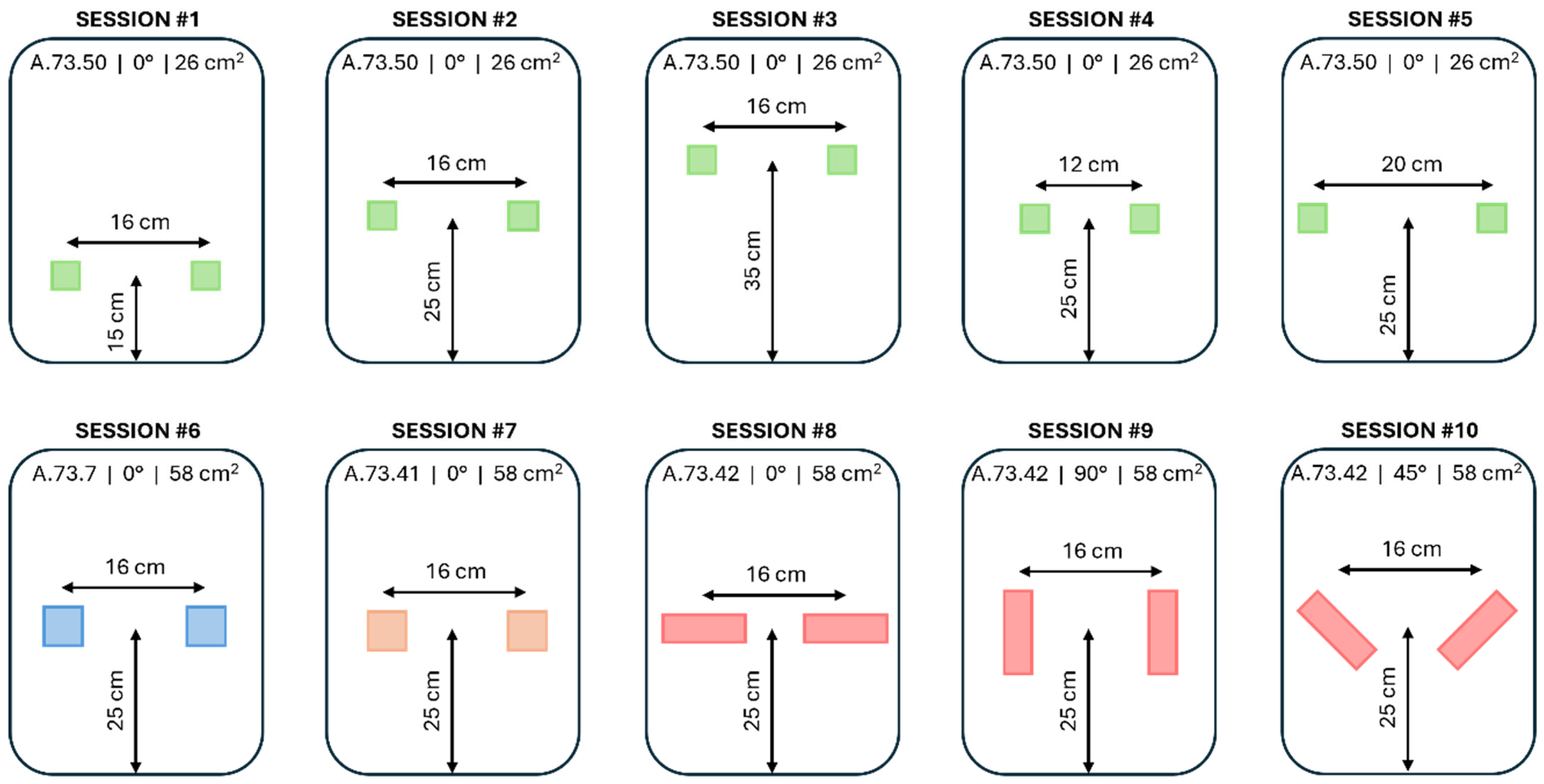
2.2.2. Integration of the Textile Electrodes onto the Driving Seat
2.3. Data Acquisition
2.3.1. Selection of Subjects
2.3.2. Acquisition Protocol
2.4. Data Analysis
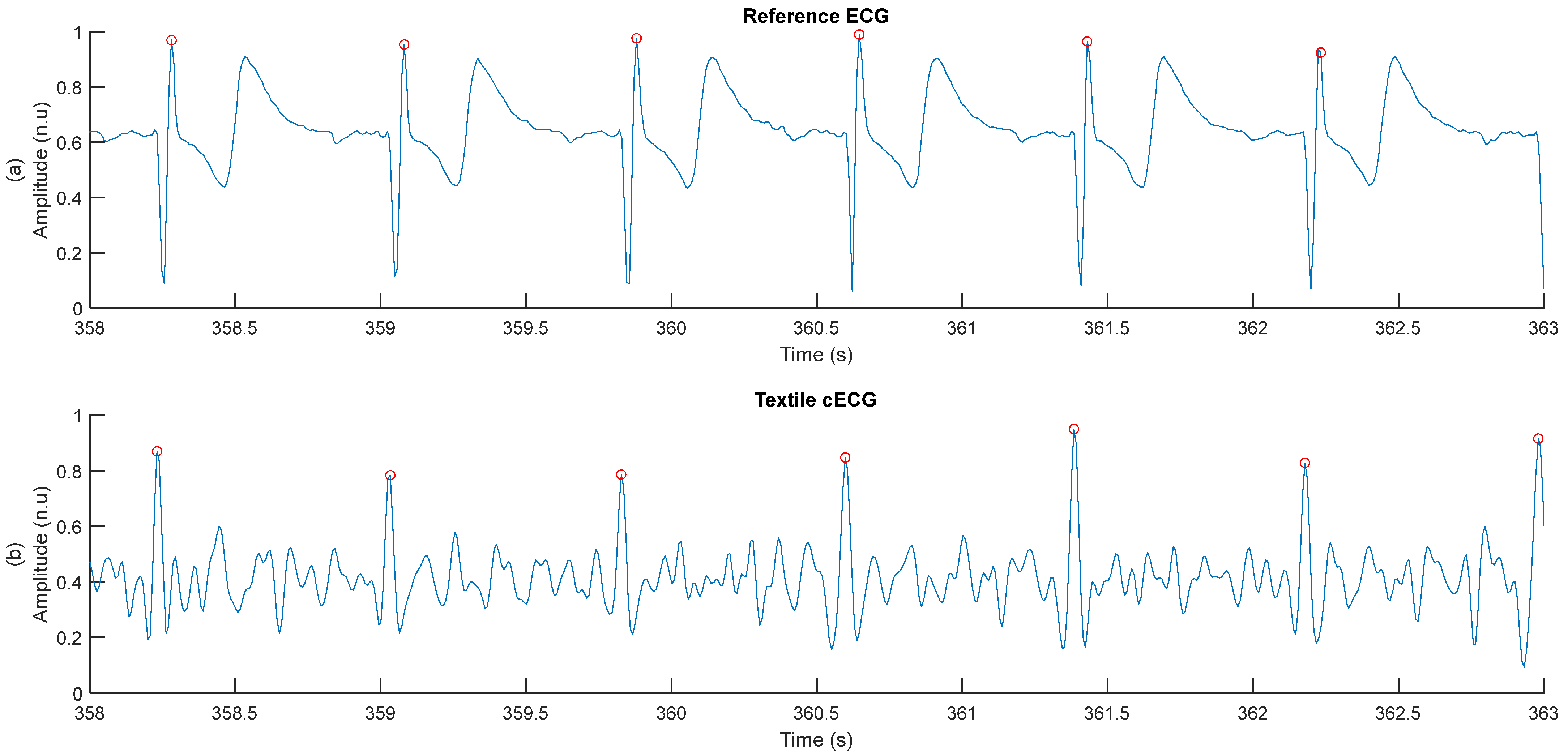
2.4.1. Measurement of cECG RR Durations
- A [5–15 Hz] band-pass filter is applied to enhance the QRS complex and attenuate any other feature of the cECG. To do so, the FFT of the cECG signal is performed. The amplitude of all frequencies outside of the range [5–15 Hz] is put to zero. Then, the inverse FFT of the spectrum is performed to obtain the filtered cECG signal in the time domain.
- The amplitude of the filtered cECG is clipped at the 95th percentile to eliminate the highest amplitudes, but without eliminating the QRS complexes.
- The first derivative of the filtered cECG signal is approximated simply by calculating the difference between cECG points at time t and time t − 1.
- Squaring is applied to all data points of the signal from step 3.
- A moving average filter is applied to the signal from step 4. The filter is implemented with a window of 19 points moving with a step of 10 points. The averaged point is at the center of the window.

2.4.2. Assessment of cECG Signal Quality
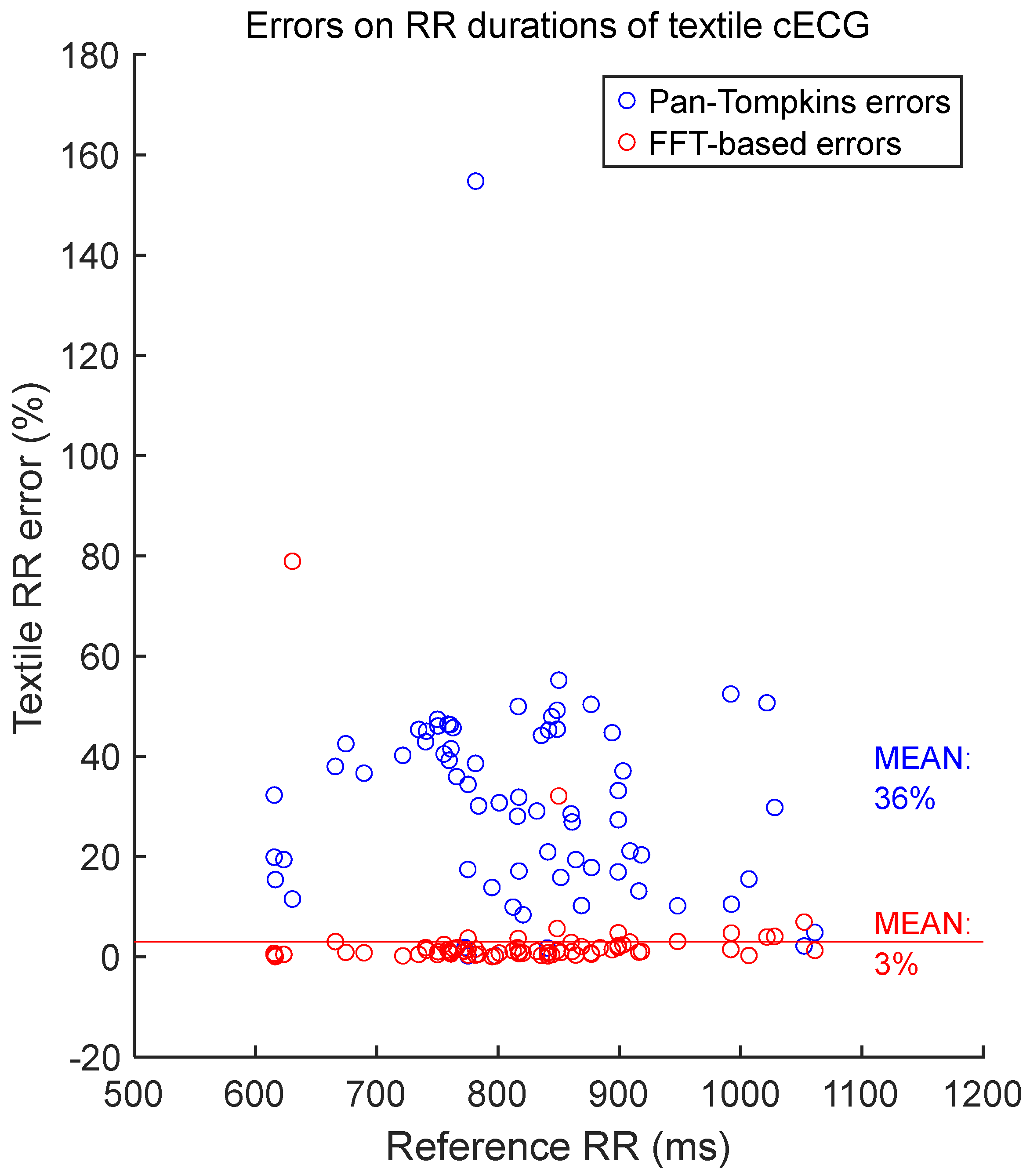
2.4.3. Assessment of the Accuracy of RR Duration Measurements
3. Results
3.1. General Assessment of Signal Quality
3.2. Verification of the FFT-Based Method for Measuring RR Duration
3.3. General Assessment of the Accuracy of RR Durations Measurements
3.4. Relationship Between Signal Quality and Accuracy
3.5. Effect of Electrode Position on Signal Quality
3.6. Effect of Electrode Form Factor on Signal Quality
3.7. Effect of Electrode’s Fabric Type on Signal Quality
4. Discussion
4.1. Summary and Interpretation of the Results
4.2. Originality and Relevance
4.3. Limitations
4.4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A



References
- AlArnaout, Z.; Zaki, C.; Kotb, Y.; AlAkkoumi, M.; Mostafa, N. Exploiting heart rate variability for driver drowsiness detection using wearable sensors and machine learning. Sci. Rep. 2025, 15, 24898. [Google Scholar] [CrossRef]
- Srinivasan, A.G.; Smith, S.S.; Pattinson, C.L.; Mann, D.; Sullivan, K.; Salmon, P.; Soleimanloo, S.S. Heart rate variability as an indicator of fatigue: A structural equation model approach. Transp. Res. Part F Traffic Psychol. Behav. 2024, 103, 420–429. [Google Scholar] [CrossRef]
- Zeng, C.; Zhang, J.; Su, Y.; Li, S.; Wang, Z.; Li, Q.; Wang, W. Driver fatigue detection using heart rate variability features from 2-minute electrocardiogram signals while accounting for sex differences. Sensors 2024, 24, 4316. [Google Scholar] [CrossRef] [PubMed]
- Persson, A.; Jonasson, H.; Fredriksson, I.; Wiklund, U.; Ahlström, C. Heart Rate Variability for Classification of Alert Versus Sleep Deprived Drivers in Real Road Driving Conditions. IEEE Trans. Intell. Transp. Syst. 2020, 22, 3316–3325. [Google Scholar] [CrossRef]
- Transport Canada. Road Safety in Canada—Improve Your Safety; Transport Canada: Ottawa, ON, Canada, 2023. [Google Scholar]
- Bayne, A.; Trivedi, N.; Liotta, M.; Siegfried, A.; Gaspar, J.; Carney, C. Countermeasures to Reduce Drowsy Driving: Results of a Literature Review and Discussions with Experts; Foundation for Traffic Safety: Washington, DC, USA, 2022. [Google Scholar]
- Dagli, R. Driver Fatigue and Road Safety-Implication in an Indian Context. Int. J. Adv. Eng. Technol. 2016, 9, 502. [Google Scholar]
- Tefft, B. Drowsy Driving in Fatal Crashes, United States, 2017–2021; Foundation for Traffic Safety: Washington, DC, USA, 2024. [Google Scholar]
- Leonhardt, S.; Leicht, L.; Teichmann, D. Unobtrusive vital sign monitoring in automotive environments—A review. Sensors 2018, 18, 3080. [Google Scholar] [CrossRef]
- Warnecke, J.M.; Lasenby, J.; Deserno, T.M. Robust in-vehicle heartbeat detection using multimodal signal fusion. Sci. Rep. 2023, 13, 20864. [Google Scholar] [CrossRef]
- Naziyok, T.P.; Zeleke, A.A.; Röhrig, R. Contactless patient monitoring for general wards: A systematic technology review. Explor. Complex. Health Interdiscip. Syst. Approach 2016, 228, 707–711. [Google Scholar]
- Sidikova, M.; Martinek, R.; Kawala-Sterniuk, A.; Ladrova, M.; Jaros, R.; Danys, L.; Simonik, P. Vital sign monitoring in car seats based on electrocardiography, ballistocardiography and seismocardiography: A review. Sensors 2020, 20, 5699. [Google Scholar] [CrossRef]
- D’Angelo, L.T.; Parlow, J.; Spiessl, W.; Hoch, S.; Lüth, T.C. A system for unobtrusive in-car vital parameter acquisition and processing. In Proceedings of the 2010 4th International Conference on Pervasive Computing Technologies for Healthcare, Munich, Germany, 22–25 March 2010; pp. 1–7. [Google Scholar]
- D’Angelo, L.T.; Lüth, T.C. Integrated Systems for Distraction—Free Vital Signs Measurement in Vehicles. Auto Tech Rev. 2012, 1, 34–38. [Google Scholar] [CrossRef]
- Gomez-Clapers, J.; Casanella, R. A fast and easy-to-use ECG acquisition and heart rate monitoring system using a wireless steering wheel. IEEE Sens. J. 2011, 12, 610–616. [Google Scholar] [CrossRef]
- Jeong, I.C.; Lee, D.H.; Park, S.W.; Ko, J.I.; Yoon, H.R. Automobile driver’s stress index provision system that utilizes electrocardiogram. In Proceedings of the 2007 IEEE Intelligent Vehicles Symposium, Istanbul, Turkey, 13–15 June 2007; pp. 652–656. [Google Scholar]
- Jung, S.-J.; Shin, H.-S.; Chung, W.-Y. Driver fatigue and drowsiness monitoring system with embedded electrocardiogram sensor on steering wheel. IET Intell. Transp. Syst. 2014, 8, 43–50. [Google Scholar] [CrossRef]
- Lee, H.B.; Choi, J.M.; Kim, J.S.; Kim, Y.S.; Baek, H.J.; Ryu, M.S.; Sohn, R.H.; Park, K.S. Nonintrusive biosignal measurement system in a vehicle. In Proceedings of the 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007; pp. 2303–2306. [Google Scholar]
- Shin, H.-S.; Jung, S.-J.; Kim, J.-J.; Chung, W.-Y. Real time car driver’s condition monitoring system. In Proceedings of the SENSORS, 2010 IEEE, Waikoloa, HI, USA, 1–4 November 2010; pp. 951–954. [Google Scholar]
- Silva, H.; Lourenço, A.; Fred, A. In-vehicle driver recognition based on hand ECG signals. In Proceedings of the 2012 ACM International Conference on Intelligent User Interfaces, Lisbon, Portugal, 14 February 2012; pp. 25–28. [Google Scholar]
- Baek, H.J.; Lee, H.B.; Kim, J.S.; Choi, J.M.; Kim, K.K.; Park, K.S. Nonintrusive biological signal monitoring in a car to evaluate a driver’s stress and health state. Telemed. E-Health 2009, 15, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, T.; Makikawa, M. ECG monitoring of a car driver using capacitively-coupled electrodes. In Proceedings of the 2008 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Milan, Italy, 25–29 August 2008; pp. 1315–1318. [Google Scholar]
- Xu, X.; Ta, L. A novel driver-friendly ecg monitoring system based on capacitive-coupled electrode. Inf. Technol. J. 2013, 12, 4730. [Google Scholar] [CrossRef]
- Hughes-Riley, T.; Hill-Casey, F.; Oliveira, C.; Shahidi, A.; Hurley, W.; Dias, T. Understanding the design rules for a nonintrusive, textile, heart rate monitoring system. Digit. Med. 2019, 5, 162–169. [Google Scholar] [CrossRef]
- Leicht, L.; Skobel, E.; Knackstedt, C.; Mathissen, M.; Sitter, A.; Wartzek, T.; Möhler, W.; Reith, S.; Leonhardt, S.; Teichmann, D. Capacitive ECG monitoring in cardiac patients during simulated driving. IEEE Trans. Biomed. Eng. 2018, 66, 749–758. [Google Scholar] [CrossRef]
- Ishijima, M. Monitoring of electrocardiograms in bed without utilizing body surface electrodes. IEEE Trans. Biomed. Eng. 1993, 40, 593–594. [Google Scholar] [CrossRef]
- Lim, Y.G.; Kim, K.K.; Park, K.S. ECG recording on a bed during sleep without direct skin-contact. IEEE Trans. Biomed. Eng. 2007, 54, 718–725. [Google Scholar] [CrossRef]
- Ueno, A.; Akabane, Y.; Kato, T.; Hoshino, H.; Kataoka, S.; Ishiyama, Y. Capacitive sensing of electrocardiographic potential through cloth from the dorsal surface of the body in a supine position: A preliminary study. IEEE Trans. Biomed. Eng. 2007, 54, 759–766. [Google Scholar] [CrossRef]
- Wu, K.-F.; Zhang, Y.-T. Contactless and continuous monitoring of heart electric activities through clothes on a sleeping bed. In Proceedings of the 2008 International Conference on Information Technology and Applications in Biomedicine, Shenzhen, China, 30–31 May 2008; pp. 282–285. [Google Scholar]
- Kim, K.K.; Lim, Y.K.; Park, K.S. The electrically noncontacting ECG measurement on the toilet seat using the capacitively-coupled insulated electrodes. In Proceedings of the 26th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Francisco, CA, USA, 31 August–3 September 2004; pp. 2375–2378. [Google Scholar]
- Higashi, D.; Imai, T.; Ueno, A.; Miyashita, O. A wearable capacitive heart-rate monitor for controlling electrically assisted bicycle. In Proceedings of the 2009 International Conference on Electrical Machines and Systems, Tokyo, Japan, 15–18 November 2009; pp. 1–6. [Google Scholar]
- Aleksandrowicz, A.; Leonhardt, S. Wireless and non-contact ECG measurement system–the “Aachen SmartChair”. Acta Polytech. 2007, 47, 4–5. [Google Scholar] [CrossRef]
- Arnrich, B.; Kappeler-Setz, C.; Schumm, J.; Tröster, G. Design, Implementation and Evaluation of a Multimodal Sensor System Integrated into an Airplane Seat. In Sensor Fusion-Foundation and Applications; IntechOpen: London, UK, 2010; p. 159. [Google Scholar]
- Lim, Y.G.; Chung, G.S.; Park, K.S. Capacitive driven-right-leg grounding in indirect-contact ECG measurement. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 1250–1253. [Google Scholar]
- Lim, Y.G.; Kim, K.K.; Park, S. ECG measurement on a chair without conductive contact. IEEE Trans. Biomed. Eng. 2006, 53, 956–959. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Hwang, S.H.; Yoon, H.N.; Lee, W.K.; Park, K.S. Heart rate variability monitoring during sleep based on capacitively coupled textile electrodes on a bed. Sensors 2015, 15, 11295–11311. [Google Scholar] [CrossRef] [PubMed]
- Sirtoli, V.G.; Morelli, L.; Zednik, R.J.; Cowan, G.; Gagnon, G. Motion artifact modeling of capacitive electrodes based on triboelectric nanogenerators. IEEE Trans. Instrum. Meas. 2024, 73, 1–10. [Google Scholar] [CrossRef]
- Chamadiya, B.; Mankodiya, K.; Wagner, M.; Hofmann, U.G. Textile-based, contactless ECG monitoring for non-ICU clinical settings. J. Ambient Intell. Humaniz. Comput. 2013, 4, 791–800. [Google Scholar] [CrossRef]
- Luna-Lozano, P.S.; Pallas-Areny, R. Power-Line Interference in the ECG Obtained with Capacitive Electrodes. In Proceedings of the 5th European Conference of the International Federation for Medical and Biological Engineering, Budapest, Hungary, 14–18 September 2011; Springer: Berlin/Heidelberg, Germany, 2012; pp. 910–913. [Google Scholar]
- Magno, M.; Spagnol, C.; Benini, L.; Popovici, E. A low power wireless node for contact and contactless heart monitoring. Microelectron. J. 2014, 45, 1656–1664. [Google Scholar] [CrossRef]
- Su, P.-C.; Hsueh, Y.-H.; Ke, M.-T.; Chen, J.-J.; Lai, P.-C. Noncontact ECG monitoring by capacitive coupling of textiles in a chair. J. Healthc. Eng. 2021, 2021, 6698567. [Google Scholar] [CrossRef]
- Chamadiya, B.; Heuer, S.; Hofmann, U.; Wagner, M. Towards a capacitively coupled electrocardiography system for car seat integration. In Proceedings of the 4th European Conference of the International Federation for Medical and Biological Engineering, Antwerp, Belgium, 23–27 November 2009; pp. 1217–1221. [Google Scholar]
- Yu, X. Real-Time Nonintrusive Detection of Driver Drowsiness; Center for Transportation Studies; University of Minnesota: Minneapolis, MN, USA, 2009; Report No. CTS 09-15. [Google Scholar]
- Uguz, D.U.; Dettori, R.; Napp, A.; Walter, M.; Marx, N.; Leonhardt, S.; Hoog Antink, C. Car Seats with Capacitive ECG Electrodes Can Detect Cardiac Pacemaker Spikes. Sensors 2020, 20, 6288. [Google Scholar] [CrossRef]
- Walter, M.; Eilebrecht, B.; Wartzek, T.; Leonhardt, S. The smart car seat: Personalized monitoring of vital signs in automotive applications. Pers. Ubiquitous Comput. 2011, 15, 707–715. [Google Scholar] [CrossRef]
- Leicht, L.; Skobel, E.; Mathissen, M.; Leonhardt, S.; Weyer, S.; Wartzek, T.; Reith, S.; Möhler, W.; Teichmann, D. Capacitive ECG recording and beat-to-beat interval estimation after major cardiac event. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 7614–7617. [Google Scholar]
- Eilebrecht, B.; Wartzek, T.; Lem, J.; Vogt, R.; Leonhardt, S. Capacitive electrocardiogram measurement system in the driver seat. ATZ Worldw. Emagazine 2011, 113, 50–55. [Google Scholar] [CrossRef]
- Jung, S.-J.; Shin, H.-S.; Chung, W.-Y. Highly sensitive driver health condition monitoring system using nonintrusive active electrodes. Sens. Actuators B Chem. 2012, 171, 691–698. [Google Scholar] [CrossRef]
- Chamadiya, B.; Heuer, S.; Wagner, M.; Hofmann, U.G. Textile Capacitive Electrocardiography for an Automotive Environment. In Proceedings of the BIODEVICES, Rome, Italy, 26–29 January 2011; pp. 422–425. [Google Scholar]
- Schneider, J.; Koellner, C.; Heuer, S. An approach to automotive ECG measurement validation using a car-integrated test framework. In Proceedings of the 2012 IEEE Intelligent Vehicles Symposium, Alcalá de Henares, Spain, 3–7 June 2012; pp. 950–955. [Google Scholar]
- Wartzek, T.; Eilebrecht, B.; Lem, J.; Lindner, H.-J.; Leonhardt, S.; Walter, M. ECG on the road: Robust and unobtrusive estimation of heart rate. IEEE Trans. Biomed. Eng. 2011, 58, 3112–3120. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, X.B. Capacitive biopotential measurement for electrophysiological signal acquisition: A review. IEEE Sens. J. 2016, 16, 2832–2853. [Google Scholar] [CrossRef]
- Xu, J.; Mitra, S.; Van Hoof, C.; Yazicioglu, R.F.; Makinwa, K.A. Active electrodes for wearable EEG acquisition: Review and electronics design methodology. IEEE Rev. Biomed. Eng. 2017, 10, 187–198. [Google Scholar] [CrossRef]
- Chen, M.; Chun, H.S.; Castro, I.D.; Torfs, T.; Lin, Q.; Van Hoof, C.; Wang, G.; Lian, Y.; Van Helleputte, N. A 400 GΩ input-impedance active electrode for non-contact capacitively coupled ECG acquisition with large linear-input-range and high CM-interference-tolerance. IEEE Trans. Biomed. Circuits Syst. 2019, 13, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, E.; Guerrero, F.; García, P.; Haberman, M. A simple and reproducible capacitive electrode. Med. Eng. Phys. 2016, 38, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Lessard-Tremblay, M.; Weeks, J.; Morelli, L.; Cowan, G.; Gagnon, G.; Zednik, R.J. Contactless capacitive electrocardiography using hybrid flexible printed electrodes. Sensors 2020, 20, 5156. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, A.; Das, D.M.; Shojaei Baghini, M. Adaptive analogue calibration technique to compensate electrode motion artefacts in biopotential recording. IET Circuits Devices Syst. 2020, 14, 327–332. [Google Scholar] [CrossRef]
- Nakamura, H.; Sakajiri, Y.; Ishigami, H.; Ueno, A. A novel analog front end with voltage-dependent input impedance and bandpass amplification for capacitive biopotential measurements. Sensors 2020, 20, 2476. [Google Scholar] [CrossRef]
- Nakamura, H.; Ueno, A. Bootstrapped non-inverting front-end amplifier for capacitive electrocardiogram measurement. In Proceedings of the 2018 IEEE Biomedical Circuits and Systems Conference (BioCAS), Cleveland, OH, USA, 17–19 October 2018; pp. 1–4. [Google Scholar]
- Duverger, J.E.; Bellemin, V.; Renaud Dumoulin, G.-G.; Forcier, P.; Decaens, J.; Gagnon, G.; Saidi, A. Respiratory monitoring with textile inductive electrodes in driving applications: Effect of electrode’s positioning and form factor on signal quality. Sensors 2025, 25, 2035. [Google Scholar] [CrossRef]
- Duverger, J.E.; Bellemin, V.; Forcier, P.; Decaens, J.; Gagnon, G.; Saidi, A. A quantitative method to guide the integration of textile inductive electrodes in automotive applications for respiratory monitoring. Sensors 2024, 24, 7483. [Google Scholar] [CrossRef]
- Khanehshenas, F.; Mazloumi, A.; Nahvi, A.; Nickabadi, A.; Sadeghniiat, K.; Rahimiforoushani, A.; Aghamalizadeh, A. A hybrid approach for driver drowsiness detection utilizing practical data to improve performance system and applicability. Work 2024, 77, 1165–1177. [Google Scholar] [CrossRef]
- Pan, H.; Logan, D.B.; Stephens, A.N.; Payre, W.; Wang, Y.; Peng, Z.; Qin, Y.; Koppel, S. Exploring the effect of driver drowsiness on takeover performance during automated driving: An updated literature review. Accid. Anal. Prev. 2025, 216, 108023. [Google Scholar] [CrossRef]
- Soares, S.; Ferreira, S.; Couto, A. Driving simulator experiments to study drowsiness: A systematic review. Traffic Inj. Prev. 2020, 21, 29–37. [Google Scholar] [CrossRef]
- Thiffault, P.; Bergeron, J. Monotony of road environment and driver fatigue: A simulator study. Accid. Anal. Prev. 2003, 35, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Tompkins, W.J. A Real-Time QRS Detection Algorithm. IEEE Trans. Biomed. Eng. 1985, 32, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Sedghamiz, H. Matlab Implementation of Pan Tompkins ECG QRS Detector. Available online: https://www.researchgate.net/publication/313673153_Matlab_Implementation_of_Pan_Tompkins_ECG_QRS_detector (accessed on 27 May 2024).
- Asl, S.N.; Oehler, M.; Schilling, M. Noise model of capacitive and textile capacitive noncontact electrodes for bioelectric applications. IEEE Trans. Biomed. Circuits Syst. 2018, 12, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Lin, S.-Y.; Chang, W.-Y. Novel stable capacitive electrocardiogram measurement system. Sensors 2021, 21, 3668. [Google Scholar] [CrossRef]
- Kang, Y.; Choi, S.; Koo, C.; Joung, Y. Development and Optimization of Silicon− Dioxide− Coated Capacitive Electrode for Ambulatory ECG Measurement System. Sensors 2022, 22, 8388. [Google Scholar] [CrossRef]
- Dieudonné, M. Electromagnetic hypersensitivity: A critical review of explanatory hypotheses. Environ. Health 2020, 19, 48. [Google Scholar] [CrossRef]
- Heuer, S.; Chiriac, S.; Kirst, M.; Gharbi, A.; Stork, W. Signal quality assessment for capacitive ECG monitoring systems using body-sensor-impedance. In Proceedings of the International Conference on Bio-Inspired Systems and Signal Processing, Marbella, Spain, 2–4 March 2011; pp. 454–458. [Google Scholar]
- Kim, J.H.; Lee, J.-K.; Kim, H.-G.; Kim, K.-B.; Kim, H.R. Possible effects of radiofrequency electromagnetic field exposure on central nerve system. Biomol. Ther. 2018, 27, 265. [Google Scholar] [CrossRef]
- Bujnowski, A.; Kaczmarek, M.; Wtorek, J.; Osinski, K.; Strupinska, D. Estimation of electrode contact in capacitive ECG measurement. In Proceedings of the 2019 12th International Conference on Human System Interaction (HSI), Richmond, VA, USA, 25–27 June 2019; pp. 132–136. [Google Scholar]
- Arquilla, K.; Webb, A.K.; Anderson, A.P. Textile electrocardiogram (ECG) electrodes for wearable health monitoring. Sensors 2020, 20, 1013. [Google Scholar] [CrossRef] [PubMed]
- Etana, B.B.; Malengier, B.; Kwa, T.; Krishnamoorthy, J.; Langenhove, L.V. Evaluation of novel embroidered textile-electrodes made from hybrid Polyamide conductive threads for surface EMG sensing. Sensors 2023, 23, 4397. [Google Scholar] [CrossRef] [PubMed]
- Fink, P.L.; Sayem, A.S.M.; Teay, S.H.; Ahmad, F.; Shahariar, H.; Albarbar, A. Development and wearer trial of ECG-garment with textile-based dry electrodes. Sens. Actuators A Phys. 2021, 328, 112784. [Google Scholar] [CrossRef]
- Sirtoli, V.G.; Granata, S.; Gagnon, G.; Cowan, G.E. Input resistance boosting for capacitive biosignal acquisition electrodes. IEEE Sens. J. 2023, 24, 3004–3014. [Google Scholar] [CrossRef]
- Sirtoli, V.G.; Liamini, M.; Lins, L.T.; Lessard-Tremblay, M.; Cowan, G.E.; Zednik, R.J.; Gagnon, G. Removal of motion artifacts in capacitive electrocardiogram acquisition: A review. IEEE Trans. Biomed. Circuits Syst. 2023, 17, 394–412. [Google Scholar] [CrossRef]
- Ebrahimpour Moghaddam Tasouj, P.; Soysal, G.; Eroğul, O.; Yetkin, S. ECG Signal Analysis for Detection and Diagnosis of Post-Traumatic Stress Disorder: Leveraging Deep Learning and Machine Learning Techniques. Diagnostics 2025, 15, 1414. [Google Scholar] [CrossRef]
- Farzaneh, N.; Ghanbari, H.; Liu, M.; Cao, L.; Ward, K.R.; Ansari, S. A comprehensive comparison of six publicly available algorithms for localization of QRS complex on electrocardiograph. In Proceedings of the 2023 45th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Sydney, Australia, 24–27 July 2023; pp. 1–4. [Google Scholar]
- Liu, F.; Liu, C.; Jiang, X.; Zhang, Z.; Zhang, Y.; Li, J.; Wei, S. Performance analysis of ten common QRS detectors on different ECG application cases. J. Healthc. Eng. 2018, 2018, 9050812. [Google Scholar] [CrossRef]
- Ma, C.; Wang, Z.; Zhao, L.; Long, X.; Vullings, R.; Aarts, R.M.; Li, J.; Liu, C. Deep learning-based signal quality assessment in wearable ECG monitoring. In Proceedings of the 2023 Computing in Cardiology (CinC), Atlanta, GA, USA, 1–4 October 2023; pp. 1–4. [Google Scholar]
- Zidelmal, Z.; Amirou, A.; Adnane, M.; Belouchrani, A. QRS detection based on wavelet coefficients. Comput. Methods Programs Biomed. 2012, 107, 490–496. [Google Scholar] [CrossRef]






















| Subject (#) | Bustline (cm) | Waistline (cm) | Shoulder Line (cm) | Torso Length (cm) | Height (cm) |
|---|---|---|---|---|---|
| 1 | 95 | 92 | 112 | 40 | 182 |
| 2 | 134 | 127 | 139 | 52 | 190 |
| 3 | 98 | 98 | 129 | 39.5 | 185 |
| 4 | 88 | 78 | 104 | 38 | 160 |
| 5 | 91 | 92 | 110 | 41 | 187.5 |
| 6 | 93 | 83 | 110 | 38 | 179 |
| 7 | 101 | 100 | 122.5 | 39 | 179 |
| Segment (#) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Radius (m) | 2000 | 2000 | 400 | 400 | 850 | 400 | 400 | 850 | 400 | 400 |
| Arc (m) | 1531 | 1531 | 306 | 306 | 1202 | 306 | 306 | 1202 | 306 | 306 |
| Direction | Right | Left | Right | Left | Left | Right | Left | Left | Right | Left |
| Segment (#) | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 |
| Radius (m) | 2000 | 2000 | 400 | 400 | 850 | 400 | 400 | 850 | 400 | 400 |
| Arc (m) | 1531 | 1531 | 306 | 306 | 1202 | 306 | 306 | 1202 | 306 | 306 |
| Direction | Right | Left | Right | Left | Left | Right | Left | Left | Right | Left |
| Session (#) | Height (cm) | Spacing (cm) | Angle (°) | Shape | Area (cm2) | Fabric | Electrode |
|---|---|---|---|---|---|---|---|
| 1 | 15 | 16 | 0 | S | 26 | E | A.73.50 |
| 2 | 25 | 16 | 0 | S | 26 | E | A.73.50 |
| 3 | 35 | 16 | 0 | S | 26 | E | A.73.50 |
| 4 | 25 | 12 | 0 | S | 26 | E | A.73.50 |
| 5 | 25 | 20 | 0 | S | 26 | E | A.73.50 |
| 6 | 25 | 16 | 0 | S | 58 | W | A.73.7 |
| 7 | 25 | 16 | 0 | S | 58 | E | A.73.41 |
| 8 | 25 | 16 | 0 | R | 58 | E | A.73.42 |
| 9 | 25 | 16 | 90 | R | 58 | E | A.73.42 |
| 10 | 25 | 16 | 45 | R | 58 | E | A.73.42 |
| Comparison # | Independent Variable | cECG Groups | Acquisition Session # |
|---|---|---|---|
| I | Height | 15 cm | 1 |
| 25 cm | 2 | ||
| 35 cm | 3 | ||
| II | Spacing | 12 cm | 4 |
| 16 cm | 2 | ||
| 20 cm | 5 | ||
| III | Angle | 0° | 8 |
| 45° | 10 | ||
| 90° | 9 | ||
| IV | Shape | Square | 7 |
| Rectangle at 0° | 8 | ||
| Rectangle at 45° | 10 | ||
| Rectangle at 90° | 9 | ||
| V | Size | 26 cm2 | 2 |
| 58 cm2 | 7 | ||
| VI | Fabric | Woven | 6 |
| Embroidered | 7 |
| rECG SQI | cECG SQI | |
|---|---|---|
| Median | 4.29 | 0.69 |
| Mean | 4.4 | 0.78 |
| STD | 1.41 | 0.36 |
| Range | 4.97 | 1.53 |
| N | 70 | 70 |
| p value | 1.7 × 10−21 | |
| Percentage of Data Within ERP Range | |||||
|---|---|---|---|---|---|
| [0, 5] | ]5, 10] | ]10, 15] | ]15, 20] | ]20, ∞[ | |
| Median | 86 | 11 | 0 | 0 | 0 |
| Mean | 81 | 12 | 2 | 1 | 4 |
| STD | 18 | 8 | 4 | 3 | 10 |
| Range | 91 | 34 | 23 | 20 | 49 |
| N | 70 | 70 | 70 | 70 | 70 |
| Height: 15 cm SQI | Height: 25 cm SQI | Height: 35 cm SQI | |
|---|---|---|---|
| Median | 0.65 | 0.67 | 0.80 |
| Mean | 0.61 | 0.72 | 0.88 |
| STD | 0.28 | 0.37 | 0.53 |
| Range | 1.33 | 1.92 | 2.87 |
| N | 245 | 245 | 245 |
| p value |
| ||
| Width: 12 cm SQI | Width: 16 cm SQI | Width: 20 cm SQI | |
|---|---|---|---|
| Median | 0.94 | 0.67 | 1.20 |
| Mean | 0.96 | 0.72 | 1.21 |
| STD | 0.41 | 0.37 | 0.58 |
| Range | 2.11 | 1.92 | 2.82 |
| N | 245 | 245 | 245 |
| p value |
| ||
| Angle: 0° SQI | Angle: 45° SQI | Angle: 90° SQI | |
|---|---|---|---|
| Median | 0.58 | 0.65 | 0.69 |
| Mean | 0.61 | 0.65 | 0.76 |
| STD | 0.40 | 0.39 | 0.47 |
| Range | 2.82 | 1.75 | 2.67 |
| N | 245 | 245 | 245 |
| p value |
| ||
| Square SQI | Rectangle at 0° SQI | Rectangle at 45° SQI | Rectangle at 90° SQI | |
|---|---|---|---|---|
| Median | 0.70 | 0.58 | 0.65 | 0.69 |
| Mean | 0.73 | 0.61 | 0.65 | 0.76 |
| STD | 0.43 | 0.40 | 0.39 | 0.47 |
| Range | 2.07 | 2.82 | 1.75 | 2.67 |
| N | 245 | 245 | 245 | 245 |
| p value |
| |||
| Area: 26 cm2 SQI | Area: 58 cm2 SQI | |
|---|---|---|
| Median | 0.67 | 0.70 |
| Mean | 0.72 | 0.73 |
| STD | 0.37 | 0.43 |
| Range | 1.92 | 2.07 |
| N | 245 | 245 |
| p value | 3.7 × 10−1 | |
| Fabric: Woven SQI | Fabric: Embroidered SQI | |
|---|---|---|
| Median | 0.66 | 0.70 |
| Mean | 0.64 | 0.73 |
| STD | 0.37 | 0.43 |
| Range | 1.78 | 2.07 |
| N | 245 | 245 |
| p value | 2.1 × 10−3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duverger, J.E.; Renaud Dumoulin, G.-G.; Bellemin, V.; Forcier, P.; Decaens, J.; Gagnon, G.; Saidi, A. Cardiac Monitoring with Textile Capacitive Electrodes in Driving Applications: Characterization of Signal Quality and RR Duration Accuracy. Sensors 2025, 25, 6097. https://doi.org/10.3390/s25196097
Duverger JE, Renaud Dumoulin G-G, Bellemin V, Forcier P, Decaens J, Gagnon G, Saidi A. Cardiac Monitoring with Textile Capacitive Electrodes in Driving Applications: Characterization of Signal Quality and RR Duration Accuracy. Sensors. 2025; 25(19):6097. https://doi.org/10.3390/s25196097
Chicago/Turabian StyleDuverger, James Elber, Geordi-Gabriel Renaud Dumoulin, Victor Bellemin, Patricia Forcier, Justine Decaens, Ghyslain Gagnon, and Alireza Saidi. 2025. "Cardiac Monitoring with Textile Capacitive Electrodes in Driving Applications: Characterization of Signal Quality and RR Duration Accuracy" Sensors 25, no. 19: 6097. https://doi.org/10.3390/s25196097
APA StyleDuverger, J. E., Renaud Dumoulin, G.-G., Bellemin, V., Forcier, P., Decaens, J., Gagnon, G., & Saidi, A. (2025). Cardiac Monitoring with Textile Capacitive Electrodes in Driving Applications: Characterization of Signal Quality and RR Duration Accuracy. Sensors, 25(19), 6097. https://doi.org/10.3390/s25196097







