AI-Based Severity Classification of Dementia Using Gait Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Application of ML for Dementia Classification
2.2. Participants
- Healthy Control: CDR = 0, with normal cognitive function and daily living abilities.
- MCI: CDR = 0.5, with mild cognitive decline but no significant impairment in daily life.
- Mild Dementia: CDR = 1, with clear cognitive decline and mild impairment in daily activities.
- Moderate Dementia: CDR = 2, with moderate to severe cognitive decline and impairment in daily functioning.
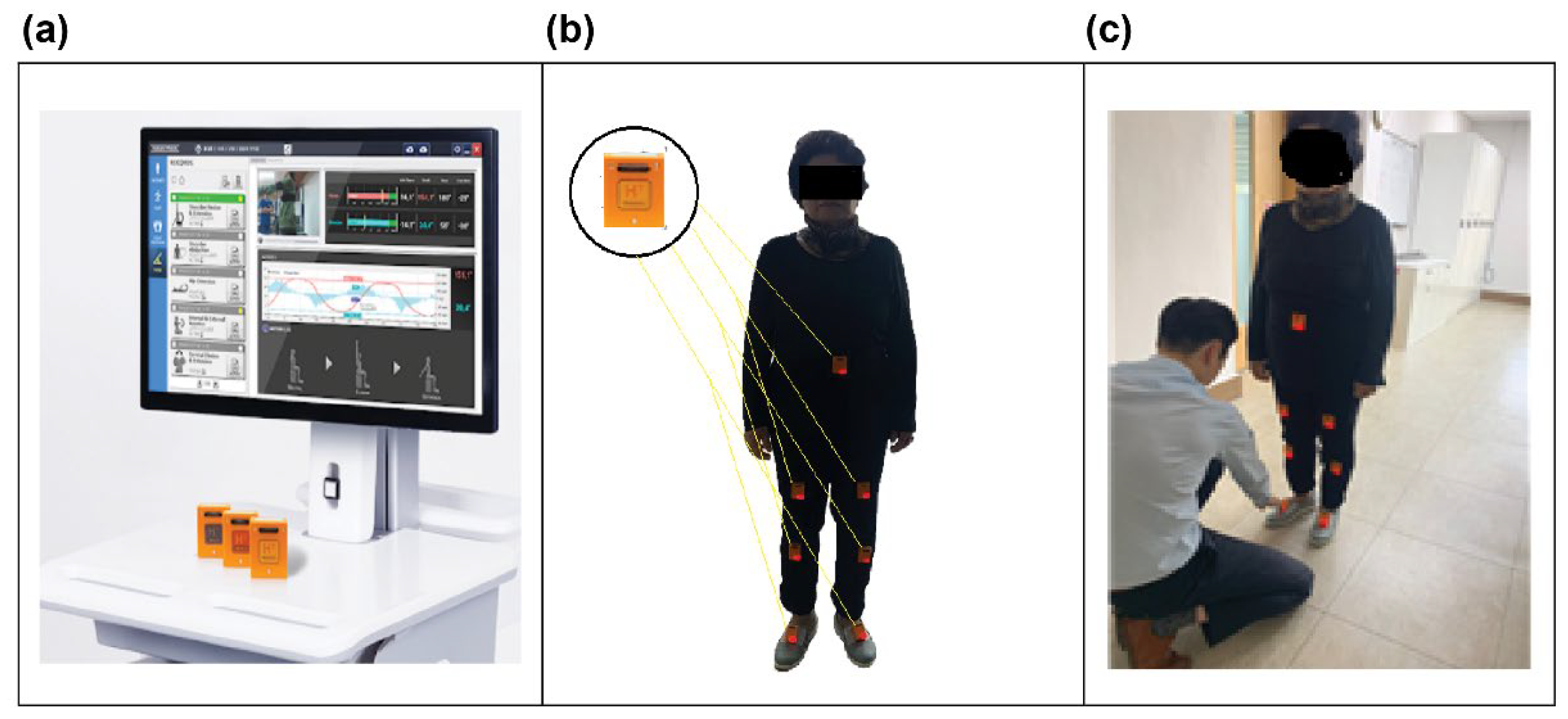
2.3. Gait Analysis
- An 8 m walkway was placed on a level, non-slip floor within a controlled laboratory environment.
- Participants wore comfortable clothing and athletic footwear for all trials.
- Participants were instructed to walk at a comfortable pace as usual while focusing their gaze approximately 5 m ahead to maintain natural gait patterns.
- A one-minute seated rest was provided between trials to minimize fatigue and ensure consistent performance across the eight trials.
- All testing sessions were conducted during the same time of day (morning hours, 9:00–12:00) to control for potential circadian rhythm effects on motor performance.
2.4. Data Preprocessing and Feature Extraction
2.4.1. Signal Filtering and Noise Reduction
2.4.2. Gait Event Detection
2.4.3. Spatiotemporal Parameter Calculation
- Gait cycle time: Time interval between consecutive heel strikes of the same foot;
- Walking velocity: Step length divided by step time;
- Cadence: Number of steps per minute (60/step time);
- Stance and swing phase percentages: Calculated as percentages of total gait cycle time.
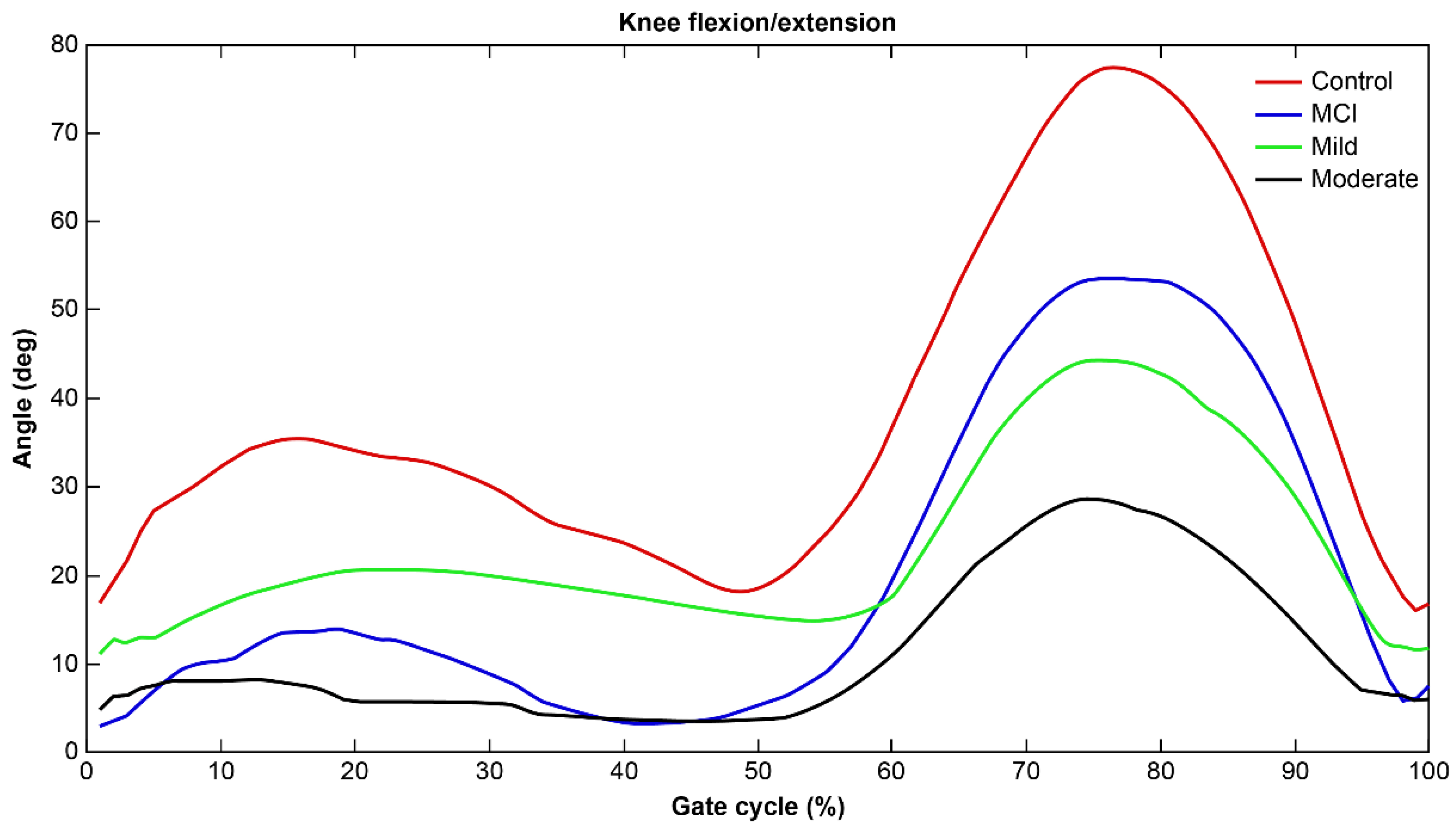
2.4.4. Joint Angle Calculation
2.5. Statistical Analysis
3. Results
3.1. Kruskal–Wallis Test: Differences in Gait Parameters of Each Group
3.2. Logistic Regression Analysis
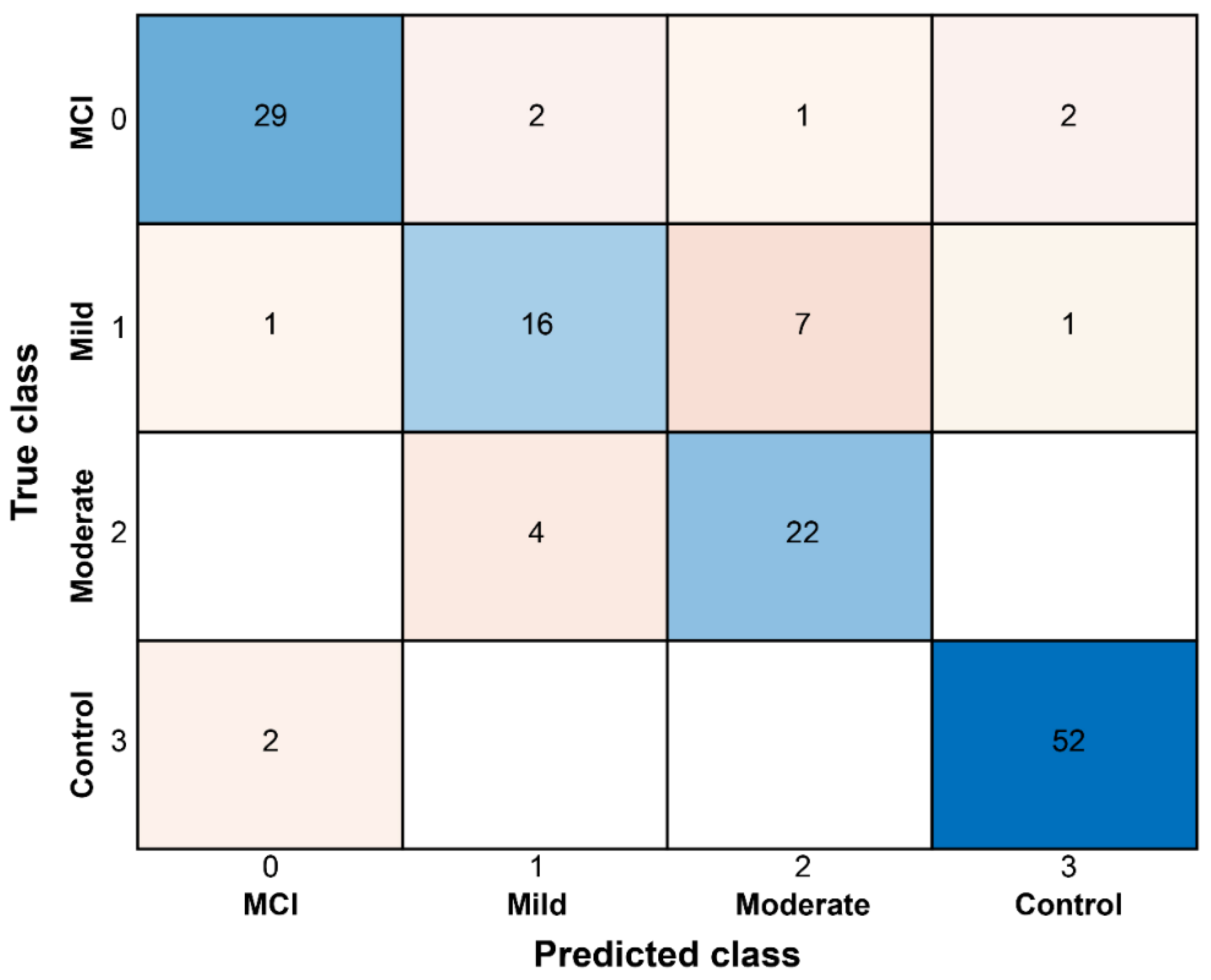
3.3. Classification of Dementia Severity Using AI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Błaszczyk, J.W. Pathogenesis of dementia. Int. J. Mol. Sci. 2022, 24, 543. [Google Scholar] [CrossRef]
- Nichols, E.; Steinmetz, J.D.; Vollset, S.E. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar] [CrossRef]
- Kalaria, R.N.; Maestre, G.E.; Arizaga, R.; Friedland, R.P.; Galasko, D.; Hall, K.; Luchsinger, J.A.; Ogunniyi, A.; Perry, E.K.; Potocnik, F.; et al. Alzheimer’s disease and vascular dementia in developing countries: Prevalence, management, and risk factors. Lancet Neurol. 2008, 7, 812–826. [Google Scholar] [CrossRef]
- Luc, T.V.; Anh, N.T.; Huong, N.T.T. Lower limb strength and associated factors among older patients with dementia. Tap Chi Nghien Cuu Hoc 2024, 177, 39–47. [Google Scholar] [CrossRef]
- Sanders, L.M.J.; Hortobágyi, T.; Karssemeijer, E.G.A.; Van der Zee, E.A.; Scherder, E.J.A.; van Heuvelen, M.J.G. Effects of low- and high-intensity physical exercise on physical and cognitive function in older persons with dementia: A randomized controlled trial. Alzheimer’s Res. Ther. 2020, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, L.H.; Gavett, B.E.; Volkers, K.M.; Blankevoort, C.G.; Scherder, E.J.; Jefferson, A.L.; Steinberg, E.; Nair, A.; Green, R.C.; Stern, R.A. Lower-extremity function in cognitively healthy aging, mild cognitive impairment, and Alzheimer’s disease. Arch. Phys. Med. Rehabil. 2010, 91, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Peel, N.M.; Alapatt, L.J.; Jones, L.V.; Hubbard, R.E. The association between gait speed and cognitive status in community-dwelling older people: A systematic review and meta-analysis. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 943–948. [Google Scholar] [CrossRef]
- Seo, K.; Takayanagi, N.; Sudo, M.; Yamashiro, Y.; Chiba, I.; Makino, K.; Lee, S.; Niki, Y.; Shimada, H. Association between daily gait speed patterns and cognitive impairment in community-dwelling older adults. Sci. Rep. 2023, 13, 2783. [Google Scholar] [CrossRef]
- Knapstad, M.K.; Naterstad, I.; Bogen, B. The association between cognitive impairment, gait speed, and Walk ratio. Front. Aging Neurosci. 2023, 15, 1092990. [Google Scholar] [CrossRef]
- Collyer, T.A.; Murray, A.M.; Woods, R.L.; Storey, E.; Chong, T.T.J.; Ryan, J.; Orchard, S.G.; Brodtmann, A.; Srikanth, V.K.; Shah, R.C.; et al. Association of dual decline in cognition and gait speed with risk of dementia in older adults. JAMA Netw. Open 2022, 5, e2214647. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, S.; Li, Y.; Yu, Y.; Lu, X.; Wang, D. Spatiotemporal, kinematic and kinetic characteristics of gait in children with genu valgus. Chin. J. Tissue Eng. Res. 2021, 25, 2303. [Google Scholar]
- Hulleck, A.A.; Menoth Mohan, D.; Abdallah, N.; El Rich, M.; Khalaf, K. Present and future of gait assessment in clinical practice: Towards the application of novel trends and technologies. Front. Med. Technol. 2022, 4, 901331. [Google Scholar] [CrossRef] [PubMed]
- Duhamel, A.; Bourriez, J.L.; Devos, P.; Krystkowiak, P.; Destée, A.; Derambure, P.; Defebvre, L. Statistical tools for clinical gait analysis. Gait Posture 2004, 20, 204–212. [Google Scholar] [CrossRef]
- Lee, M.; Roan, M.; Smith, B.; Lockhart, T.E. Gait analysis to classify external load conditions using linear discriminant analysis. Hum. Mov. Sci. 2009, 28, 226–235. [Google Scholar] [CrossRef]
- Prisco, G.; Pirozzi, M.A.; Santone, A.; Esposito, F.; Cesarelli, M.; Amato, F.; Donisi, L. Validity of wearable inertial sensors for gait analysis: A systematic review. Diagnostics 2024, 15, 36. [Google Scholar] [CrossRef]
- Juganavar, A.; Joshi, A.; Shegekar, T. Navigating Early Alzheimer’s Diagnosis: A Comprehensive Review of Diagnostic Innovations. Cureus 2023, 15, e44937. [Google Scholar] [CrossRef]
- Liu, Z.; Maiti, T.; Bender, A.R. A role for prior knowledge in statistical classification of the transition from MCI to Alzheimer’s disease. arXiv 2020, arXiv:2012.00538. [Google Scholar]
- Seifallahi, M.; Galvin, J.E.; Ghoraani, B. Curve walking reveals more gait impairments in older adults with mild cognitive impairment than straight walking: A Kinect camera-based study. J. Alzheimer’s Dis. Rep. 2024, 8, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Al-Hammadi, M.; Fleyeh, H.; Åberg, A.C.; Halvorsen, K.; Thomas, I. Machine learning approaches for dementia detection through speech and gait analysis: A systematic literature review. J. Alzheimer’s Dis. 2024, 100, 1–27. [Google Scholar] [CrossRef]
- Harris, E.J.; Khoo, I.-H.; Demircan, E. A survey of human gait-based artificial intelligence applications. Front. Robot. AI 2021, 8, 749274. [Google Scholar] [CrossRef]
- Tang, Y.M.; Fei, B.N.; Li, X.; Zhao, J.; Zhang, W.; Qin, G.Y.; Hu, M.; Ding, J.; Wang, X. Association between gait features assessed by artificial intelligent system and cognitive function decline in patients with silent cerebrovascular disease: Study protocol of a multicenter prospective cohort study (ACCURATE-2). BMC Neurol. 2022, 22, 240. [Google Scholar] [CrossRef]
- Lyall, D.M.; Kormilitzin, A.; Lancaster, C.; Sousa, J.; Petermann-Rocha, F.; Buckley, C.; Harshfield, E.L.; Iveson, M.H.; Madan, C.R.; McArdle, R.; et al. Artificial intelligence for dementia—Applied models and digital health. Alzheimer’s Dement. 2023, 19, 5872–5884. [Google Scholar] [CrossRef]
- Čepukaitytė, G.; Newton, C.; Chan, D. Early detection of diseases causing dementia using digital navigation and gait measures: A systematic review of evidence. Alzheimer’s Dement. 2024, 20, 3054–3073. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Park, J.H.; Jang, S.H.; Cho, J. Novel method of classification in knee osteoarthritis: Machine learning application versus logistic regression model. Ann. Rehabil. Med. 2020, 44, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, L.; Su, S.; Watsford, M.; Wood, L.M.; Duffield, R. Inertial Sensor Estimation of Initial and Terminal Contact during In-Field Running. Sensors 2022, 22, 4812. [Google Scholar] [CrossRef] [PubMed]
- Gillain, S.; Warzee, E.; Lekeu, F.; Wojtasik, V.; Maquet, D.; Croisier, J.L.; Salmon, E.; Petermans, J. The value of instrumental gait analysis in elderly healthy, MCI or Alzheimer’s disease subjects and a comparison with other clinical tests used in single and dual-task conditions. Ann. Phys. Rehabil. Med. 2009, 52, 453–474. [Google Scholar] [CrossRef]
- Valkanova, V.; Ebmeier, K.P. What can gait tell us about dementia? Review of epidemiological and neuropsychological evidence. Gait Posture 2017, 53, 215–223. [Google Scholar] [CrossRef]
- Beauchet, O.; Annweiler, C.; Callisaya, M.L.; De Cock, A.M.; Helbostad, J.L.; Kressig, R.W.; Srikanth, V.; Steinmetz, J.P.; Blumen, H.M.; Verghese, J.; et al. Poor gait performance and prediction of dementia: Results from a meta-analysis. J. Am. Med. Dir. Assoc. 2016, 17, 482–490. [Google Scholar] [CrossRef]
- Vichianin, Y.; Khummongkol, A.; Chiewvit, P.; Raksthaput, A.; Chaichanettee, S.; Aoonkaew, N.; Senanarong, V. Accuracy of Support-Vector Machines for Diagnosis of Alzheimer’s Disease, Using Volume of Brain Obtained by Structural MRI at Siriraj Hospital. Front. Neurol. 2021, 12, 640696. [Google Scholar] [CrossRef] [PubMed]
- Mathotaarachchi, S.; Pascoal, T.A.; Shin, M.; Benedet, A.L.; Kang, M.S.; Beaudry, T.; Fonov, V.S.; Gauthier, S.; Rosa-Neto, P.; Alzheimer’s Disease Neuroimaging Initiative. Identifying individuals with incipient dementia using machine learning and amyloid imaging. Neurobiol. Aging 2017, 59, 80–90. [Google Scholar] [CrossRef]
- Zhong, Q.; Ali, N.; Gao, Y.; Wu, H.; Wu, X.; Sun, C.; Ma, J.; Thabane, L.; Xiao, M.; Zhou, Q.; et al. Gait Kinematic and Kinetic Characteristics of Older Adults with Mild Cognitive Impairment and Subjective Cognitive Decline: A Cross-Sectional Study. Front. Aging Neurosci. 2021, 13, 664558. [Google Scholar] [CrossRef] [PubMed]
- Bovonsunthonchai, S.; Vachalathiti, R.; Hiengkaew, V.; Bryant, M.S.; Richards, J.; Senanarong, V. Quantitative gait analysis in mild cognitive impairment, dementia, and cognitively intact individuals: A cross-sectional case-control study. BMC Geriatr. 2022, 22, 767. [Google Scholar] [CrossRef]
- International Medical Device Regulators Forum. Software as a Medical Device (SaMD): Clinical Evaluation; IMDRF/SaMD WG/N41FINAL:2017; IMDRF Research: Triangle Park, NC, USA, 2017; Available online: https://www.imdrf.org/sites/default/files/docs/imdrf/final/technical/imdrf-tech-170921-samd-n41-clinical-evaluation_1.pdf (accessed on 15 September 2025).
- Goldenholz, D.M.; Kuhn, A.; Austermuehle, A.; Cervenka, M.C.; Theodore, W.H.; Wasade, V.S. Sample Size Analysis for Machine Learning Clinical Validation Studies. Biomedicines 2023, 11, 685. [Google Scholar] [CrossRef]
- Riley, R.D.; Collins, G.S.; Ensor, J.; Archer, L.; Booth, S.; Hooft, L.; Altman, D.G.; Reitsma, J.B.; Snell, K.I.E. Evaluation of clinical prediction models (part 3): Calculating sample size and power for external validation studies. BMJ 2024, 384, e074821. [Google Scholar] [CrossRef]
- Mazzà, C.; Alcock, L.; Aminian, K.; Becker, C.; Bertuletti, S.; Bonci, T.; Brown, P.; Brozgol, M.; Buckley, E.; Carsin, A.E.; et al. Technical validation of real-world monitoring of gait: A multicentric observational study. BMJ Open 2021, 11, e050785. [Google Scholar] [CrossRef]
- McArdle, R.; Del Din, S.; Donaghy, P.; Galna, B.; Thomas, A.J.; Rochester, L. The Impact of Environment on Gait Assessment: Considerations from Real-World Gait Analysis in Dementia Subtypes. Sensors 2021, 21, 813. [Google Scholar] [CrossRef]
- Shah, V.V.; McNames, J.; Mancini, M.; Carlson-Kuhta, P.; Nutt, J.G.; El-Gohary, M.; Curtze, C.; Horak, F.B. Laboratory versus daily life gait characteristics in patients with multiple sclerosis, Parkinson’s disease, and matched controls. J. Neuroeng. Rehabil. 2020, 17, 159. [Google Scholar] [CrossRef] [PubMed]
- Boyer, K.A.; Johnson, R.T.; Banks, J.J.; Jewell, C.; Hafer, J.F. Systematic review and meta-analysis of gait mechanics in young and older adults. Exp. Gerontol. 2017, 95, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.G.; Dingwell, J.B. Effects of walking speed, strength and range of motion on gait stability in healthy older adults. J. Biomech. 2008, 41, 2899–2905. [Google Scholar] [CrossRef]
- Yuan, H. Toward real-world deployment of machine learning for health care: External validation, continual monitoring, and randomized clinical trials. Health Care Sci. 2024, 3, 360–364. [Google Scholar] [CrossRef]
- Lindh-Rengifo, M.; Jonasson, S.B.; Ullén, S.; Palmqvist, S.; van Westen, D.; Stomrud, E.; Mattsson-Carlgren, N.; Nilsson, M.H.; Hansson, O. Effects of Brain Pathologies on Spatiotemporal Gait Parameters in Patients with Mild Cognitive Impairment. J. Alzheimer’s Dis. 2023, 96, 1417–1428. [Google Scholar] [CrossRef] [PubMed]
- Seifallahi, M.; Galvin, J.E.; Ghoraani, B. Detection of mild cognitive impairment using various types of gait tests and machine learning. Front. Neurol. 2024, 15, 1354092. [Google Scholar] [CrossRef] [PubMed]
- Iulita, M.F.; Streel, E.; Harrison, J. Digital biomarkers: Redefining clinical outcomes and the concept of meaningful change. Alzheimer’s Dement. 2025, 21, e70114. [Google Scholar] [CrossRef] [PubMed]


| Group Number | 1 | 2 | 3 | 4 | ||
|---|---|---|---|---|---|---|
| Group | Total | Healthy Control | MCI | Mild Dementia | Moderate Dementia | p-Value |
| n = 139 | n = 54 | n = 34 | n = 25 | n = 26 | ||
| Age (years) | 75.0 (72.0–83.0) | 74.0 (72.0–75.0) | 75.5 (72.0–82.0) | 84.0 (77.0–86.0) | 86.0 (76.0–89.0) | <0.0001 *†: b,c,d,e |
| Gender (male) | 49 (35.25) | 20 (37.04) | 15 (44.12) | 4 (16.0) | 10 (38.46) | 0.13 |
| Gender (female) | 90 (64.75) | 34 (62.96) | 19 (55.88) | 21 (84.0) | 16 (61.54) | |
| Height (cm) | 157.0 (150.0–165.0) | 158.5 (153.0–165.0) | 159.0 (152.0–165.0) | 150.0 (147.0–158.0) | 155.0 (147.0–170.0) | 0.0035 *†: b,d |
| Weight (kg), Mean ± SD | 58.7 ± 10.2 | 60.6 ± 9.8 | 62.6 ± 9.8 | 54.3 ± 7.8 | 54.1 ± 10.8 | 0.0006 *†: e |
| BMI (kg/m2) | 23.1 (21.7– 25.5) | 23.2 (22.1–24.8) | 24.1 (22.7–26.0) | 22.8 (21.5–26.0) | 21.7 (19.0–23.8) | 0.0087 *†: e |
| Parameter | Total (n = 139) | Healthy Control (n = 54) | MCI (n = 34) | Mild Dementia (n = 25) | Moderate Dementia (n = 26) | p-Value |
|---|---|---|---|---|---|---|
| Gait cycle time (sec) | 1.3 (1.1–1.5) | 1.1 (1.1–1.1) | 1.3 (1.2–1.3) | 1.5 (1.3–1.5) | 1.6 (1.5–1.7) | <0.0001 *†: a,b,c,d,e,f |
| Stance phase (%) | 62.3 (60.2–64.3) | 61.8 (60.8–62.9) | 63.7 (60.9–65.7) | 60.6 (58.0–63.9) | 63.9 (61.4–67.5) | 0.0166 |
| Swing phase (%) | 37.7 (35.7–39.7) | 38.2 (37.1–39.2) | 36.6 (34.3–39.2) | 39.5 (36.2–42.0) | 36.1 (32.5–38.6) | 0.0166 |
| Velocity (m/s) | 1.0 (0.7–1.2) | 1.2 (1.2–1.3) | 1.0 (0.9–1.1) | 0.7 (0.6–0.8) | 0.6 (0.4–0.6) | <0.0001 *†: a,b,c,d,e,f |
| Cadence (steps/min) (mean ± SD) | 100.1 ± 12.2 | 110.4 ± 6.1 | 99.2 ± 4.6 | 89.8 ± 7.9 | 83.8 ± 9.1 | <0.0001 *†: a,b,c,d,e |
| Initial double support (%), (mean ± SD) | 12.5 ± 3.6 | 12.1 ± 2.3 | 12.6 ± 4.1 | 11.5 ± 3.8 | 13.7 ± 4.2 | 0.0576 |
| Single support (%) | 37.2 (35.3–39.1) | 37.5 (36.3–38.7) | 36.6 (35.3–40.3) | 37.7 (35.8–40.0) | 36.0 (33.6–38.1) | 0.2343 |
| Terminal double support (%) | 12.5 (10.2–14.4) | 12.3 (10.7–13.5) | 13.0 (9.3–14.6) | 11.6 (9.0–13.6) | 13.5 (11.9–16.9) | 0.0471 |
| Hip joint angle (deg) | 42.0 (33.4–47.1) | 47.8 (45.0–51.1) | 42.4 (38.1–44.8) | 33.3 (27.3–36.7) | 28.6 (26.0–35.2) | <0.0001 *†: a,b,c,d,e |
| Knee joint angle (deg) | 48.0 (39.4–54.2) | 53.9 (51.5–56.6) | 48.1 (44.6–54.4) | 39.4 (35.6–40.3) | 38.9 (35.4–39.9) | <0.0001 *†: a,b,c,d,e |
| Ankle joint angle (deg) (mean ± SD) | 27.0 ± 7.0 | 32.1 ± 3.7 | 23.8 ± 4.2 | 23.9 ± 5.6 | 23.5 ± 9.3 | <0.0001 *†: a,b,c |
| Univariable Analysis | Multivariable Analysis | |||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | R-Square | Max-Rescaled R-Square | |
| Knee joint angle (deg) | 1.269 (1.172–1.374) | <0.0001 | 1.098 (0.905–1.332) | 0.0076 | 0.5745 | 0.7787 |
| Hip joint angle (deg) | 1.510 (1.304–1.749) | <0.0001 | 1.244 (1.030–1.503) | 0.023 | ||
| Classifier | Accuracy (%) | Precision (PPV) (%) | Recall (Sensitivity) (%) | Specificity (%) | F1-Score (%) | AUC | NPV (%) |
|---|---|---|---|---|---|---|---|
| SVM | 86.33 (%) | 90.62 (%) | 85.30 (%) | 91.47 (%) | 87.89 (%) | 0.924 | 88.75 (%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, G.; Cho, J.; Choi, H.; Kim, Y.; Kim, G.-D.; Jang, S.-H. AI-Based Severity Classification of Dementia Using Gait Analysis. Sensors 2025, 25, 6083. https://doi.org/10.3390/s25196083
Moon G, Cho J, Choi H, Kim Y, Kim G-D, Jang S-H. AI-Based Severity Classification of Dementia Using Gait Analysis. Sensors. 2025; 25(19):6083. https://doi.org/10.3390/s25196083
Chicago/Turabian StyleMoon, Gangmin, Jaesung Cho, Hojin Choi, Yunjin Kim, Gun-Do Kim, and Seong-Ho Jang. 2025. "AI-Based Severity Classification of Dementia Using Gait Analysis" Sensors 25, no. 19: 6083. https://doi.org/10.3390/s25196083
APA StyleMoon, G., Cho, J., Choi, H., Kim, Y., Kim, G.-D., & Jang, S.-H. (2025). AI-Based Severity Classification of Dementia Using Gait Analysis. Sensors, 25(19), 6083. https://doi.org/10.3390/s25196083






