Passive Brain–Computer Interface Using Textile-Based Electroencephalography
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. EEG Systems
2.3. EEG Acquisition and Preprocessing
2.4. Standard Power Analysis
2.5. Passive Brain–Computer Interface
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- George, L.; Lécuyer, A. Passive Brain–Computer Interfaces. In Guide to Brain-Computer Music Interfacing; Miranda, E.R., Castet, J., Eds.; Springer: London, UK, 2014; pp. 297–308. [Google Scholar] [CrossRef]
- Zander, T.O.; Kothe, C. Towards passive brain–computer interfaces: Applying brain–computer interface technology to human–machine systems in general. J. Neural Eng. 2011, 8, 025005. [Google Scholar] [CrossRef]
- Christensen, J.C.; Estepp, J.R.; Wilson, G.F.; Russell, C.A. The effects of day-to-day variability of physiological data on operator functional state classification. NeuroImage 2012, 59, 57–63. [Google Scholar] [CrossRef]
- Johannesen, J.K.; Bi, J.; Jiang, R.; Kenney, J.G.; Chen, C.-M.A. Machine learning identification of EEG features predicting working memory performance in schizophrenia and healthy adults. Neuropsychiatr. Electrophysiol. 2016, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Lotte, F.; Girolami, M.; Lécuyer, A. Classifying EEG for brain computer interfaces using Gaussian processes. Pattern Recognit. Lett. 2008, 29, 354–359. [Google Scholar] [CrossRef]
- Jamunadevi, C.; Ragupathy, P.; Sritha, P.; Pandikumar, S.; Deepa, S. Performance Analysis of Random Forest Classifier in Extracting Features from the EEG signal. In Proceedings of the 2022 International Conference on Advanced Computing Technologies and Applications (ICACTA), Coimbatore, India, 4–5 March 2022; pp. 1–5. [Google Scholar] [CrossRef]
- Timofeeva, A.Y.; Murtazina, M.S. Feature Selection for EEG Data Based on Logistic Regression. In Proceedings of the 2021 XV International Scientific-Technical Conference on Actual Problems Of Electronic Instrument Engineering (APEIE), Novosibirsk, Russian, 19–21 November 2021; IEEE: New York, NY, USA, 2021; pp. 604–609. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, S.; Xu, Z.; Wang, P.; Wu, X.; Zhang, D. Cognitive Workload Recognition Using EEG Signals and Machine Learning: A Review. IEEE Trans. Cogn. Dev. Syst. 2022, 14, 799–818. [Google Scholar] [CrossRef]
- Weelden, E.; E van Beek, C.W.; Alimardani, M.; Wiltshire, T.J.; Ledegang, W.D.; Groen, E.L.; Louwerse, M.M. A Passive Brain-Computer Interface for Predicting Pilot Workload in Virtual Reality Flight Training. In Proceedings of the 2024 IEEE 4th International Conference on Human-Machine Systems (ICHMS), Toronto, ON, Canada, 15–17 May 2024; IEEE: New York, NY, USA, 2024; pp. 1–6. [Google Scholar] [CrossRef]
- Dehais, F.; Ladouce, S.; Darmet, L.; Nong, T.-V.; Ferraro, G.; Tresols, J.T.; Velut, S.; Labedan, P. Dual Passive Reactive Brain-Computer Interface: A Novel Approach to Human-Machine Symbiosis. Front. Neuroergonomics 2022, 3, 824780. [Google Scholar] [CrossRef]
- Hinss, M.F.; Vitale, V.M.; Brock, A.; Roy, R. EEG-based performance estimation during a realistic drone piloting task. In Proceedings of the Graz BCI—9th Graz Brain-Computer Interface Conference 2024, Graz, Austria, 9–12 September 2024. [Google Scholar]
- National Research Council Canada; Law, A.; Ellis, K.; Hajra, S.; Jennings, S. An Evaluation of Pilot Electroencephalographic Activity during a Helicopter Tracking Task. In Proceedings of the Vertical Flight Society 76th Annual Forum, Virtual, 10 October 2020; pp. 1–8. [Google Scholar] [CrossRef]
- Morales, J.M.; Ruiz-Rabelo, J.F.; Diaz-Piedra, C.; Di Stasi, L.L. Detecting Mental Workload in Surgical Teams Using a Wearable Single-Channel Electroencephalographic Device. J. Surg. Educ. 2019, 76, 1107–1115. [Google Scholar] [CrossRef]
- Kamrud, A.; Borghetti, B.; Kabban, C.S.; Miller, M. Generalized Deep Learning EEG Models for Cross-Participant and Cross-Task Detection of the Vigilance Decrement in Sustained Attention Tasks. Sensors 2021, 21, 5617. [Google Scholar] [CrossRef]
- Löfhede, J.; Seoane, F.; Thordstein, M. Soft textile electrodes for EEG monitoring. In Proceedings of the 10th IEEE International Conference on Information Technology and Applications in Biomedicine, Corfu, Greece, 3–5 November 2010; pp. 1–4. [Google Scholar] [CrossRef]
- Löfhede, J.; Seoane, F.; Thordstein, M. Textile Electrodes for EEG Recording—A Pilot Study. Sensors 2012, 12, 16907–16919. [Google Scholar] [CrossRef]
- Acar, G.; Ozturk, O.; Golparvar, A.J.; Elboshra, T.A.; Böhringer, K.; Yapici, M.K. Wearable and Flexible Textile Electrodes for Biopotential Signal Monitoring: A review. Electronics 2019, 8, 479. [Google Scholar] [CrossRef]
- Golparvar, A.; Ozturk, O.; Yapici, M.K. Gel-Free Wearable Electroencephalography (EEG) with Soft Graphene Textiles. In Proceedings of the 2021 IEEE Sensors, Sydney, Australia, 31 October–3 November 2021; pp. 1–4. [Google Scholar] [CrossRef]
- Arnin, J.; Anopas, D.; Horapong, M.; Triponyuwasi, P.; Yamsa-ard, T.; Iampetch, S.; Wongsawat, Y. Wireless-based portable EEG-EOG monitoring for real time drowsiness detection. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 4977–4980. [Google Scholar] [CrossRef]
- Hou, X.; Liu, Y.; Lim, W.L.; Lan, Z.; Sourina, O.; Mueller-Wittig, W. CogniMeter: EEG-Based Brain States Monitoring. In Transactions on Computational Science XXVIII: Special Issue on Cyberworlds and Cybersecurity; Gavrilova, M.L., Tan, C.J.K., Sourin, A., Eds.; Springer: Berlin, Heidelberg, 2016; pp. 108–126. [Google Scholar] [CrossRef]
- Young, M.S.; Brookhuis, K.A.; Wickens, C.D.; Hancock, P.A. State of science: Mental workload in ergonomics. Ergonomics 2015, 58, 1–17. [Google Scholar] [CrossRef]
- Saeed, S.M.U.; Anwar, S.M.; Majid, M.; Bhatti, A.M. Psychological stress measurement using low cost single channel EEG headset. In Proceedings of the 2015 IEEE International Symposium on Signal Processing and Information Technology (ISSPIT), Abu Dhabi, United Arab Emirates, 7–10 December 2015; pp. 581–585. [Google Scholar] [CrossRef]
- Page, C.; Liu, C.; Meltzer, J.; Hajra, S. Blink-Related Oscillations Provide Naturalistic Assessments of Brain Function and Cognitive Workload within Complex Real-World Multitasking Environments. Sensors 2024, 24, 1082. [Google Scholar] [CrossRef] [PubMed]
- Barry, R.J.; Clarke, A.R.; Johnstone, S.J.; Magee, C.A.; Rushby, J.A. EEG differences between eyes-closed and eyes-open resting conditions. Clin. Neurophysiol. 2007, 118, 2765–2773. [Google Scholar] [CrossRef] [PubMed]
- Mattiev, J.; Sajovic, J.; Drevenšek, G.; Rogelj, P. Assessment of Model Accuracy in Eyes Open and Closed EEG Data: Effect of Data Pre-Processing and Validation Methods. Bioengineering 2022, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- López-Larraz, E.; Escolano, C.; Robledo-Menéndez, A.; Morlas, L.; Alda, A.; Minguez, J. A garment that measures brain activity: Proof of concept of an EEG sensor layer fully implemented with smart textiles. Front. Hum. Neurosci. 2023, 17, 1135153. [Google Scholar] [CrossRef]
- Pwelch. Available online: https://www.mathworks.com/help/signal/ref/pwelch.html?searchHighlight=pwelch&s_tid=srchtitle_support_results_1_pwelch (accessed on 14 March 2025).
- Hajra, S.G.; Liu, C.C.; Song, X.; Fickling, S.D.; Cheung, T.P.L.; D’Arcy, R.C.N. Multimodal characterization of the semantic N400 response within a rapid evaluation brain vital sign framework. J. Transl. Med. 2018, 16, 151. [Google Scholar] [CrossRef]
- Hartoyo, A.; Cadusch, P.J.; Liley, D.T.J.; Hicks, D.G. Inferring a simple mechanism for alpha-blocking by fitting a neural population model to EEG spectra. PLoS Comput. Biol. 2020, 16, e1007662. [Google Scholar] [CrossRef]
- Volavka, J.; Matoušek, M.; Roubíček, J. Mental arithmetic and eye opening. An EEG frequency analysis and GSR study. Electroencephalogr. Clin. Neurophysiol. 1967, 22, 174–176. [Google Scholar] [CrossRef]
- Ristanović, D.; Martinović, Ž.J.; Jovanović, V. Topography of visual EEG reactivity in school-age children. Brain Dev. 1999, 21, 236–243. [Google Scholar] [CrossRef]
- Widagdo, M.M.; Pierson, J.M.; Helme, R.D. Age-Related Changes in Qeeg During Cognitive Tasks. Int. J. Neurosci. 1998, 95, 63–75. [Google Scholar] [CrossRef]
- Asada, H.; Fukuda, Y.; Tsunoda, S.; Yamaguchi, M.; Tonoike, M. Frontal midline theta rhythms reflect alternative activation of prefrontal cortex and anterior cingulate cortex in humans. Neurosci. Lett. 1999, 274, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Ray, W.J.; Cole, H.W. EEG Alpha Activity Reflects Attentional Demands, and Beta Activity Reflects Emotional and Cognitive Processes. Science 1985, 228, 750–752. [Google Scholar] [CrossRef] [PubMed]
- Gevins, A.; Smith, M.E.; McEvoy, L.; Yu, D. High-Resolution EEG Mapping of Cortical Activation Related to Working Memory: Effects of Task Difficulty, Type of Processing, and Practice. | Cerebral Cortex | Oxford Academic. Oxford Academic. Available online: https://academic.oup.com/cercor/article-abstract/7/4/374/344267 (accessed on 22 July 2025).
- Klimesch, W. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Res. Rev. 1999, 29, 169–195. [Google Scholar] [CrossRef] [PubMed]
- Ziccardi, A.; Van Benthem, K.; Liu, C.C.; Herdman, C.M.; Hajra, S.G. Towards ubiquitous and nonintrusive measurements of brain function in the real world: Assessing blink-related oscillations during simulated flight using portable low-cost EEG. Front. Neurosci. 2024, 17, 1286854. [Google Scholar] [CrossRef]
- Gopan, K.G.; Sinha, N.; Babu, J.D. Statistical feature analysis for EEG baseline classification: Eyes Open vs. Eyes Closed. In Proceedings of the 2016 IEEE Region 10 Conference (TENCON), Singapore, 22–25 November 2016; pp. 2466–2469. [Google Scholar] [CrossRef]
- Qayyum, A.; Razzak, I.; Mumtaz, W. Hybrid Deep Shallow Network for Assessment of Depression Using Electroencephalogram Signals. In Neural Information Processing; Springer: Cham, Switzerland, 2020; pp. 245–257. [Google Scholar] [CrossRef]
- Kara, Ş.; Ergin, S. A statistical feature extraction in wavelet domain for movement classification: A case study for eyes open, eyes closed, and open/closed fist tasks. Ejovoc 2018, 8, 158–162. Available online: https://dergipark.org.tr/tr/pub/ejovoc/issue/41199/498001 (accessed on 22 July 2025).
- Antoniou, E.; Bozios, P.; Christou, V.; Tzimourta, K.D.; Kalafatakis, K.; Tsipouras, M.G.; Giannakeas, N.; Tzallas, A.T. EEG-Based Eye Movement Recognition Using Brain–Computer Interface and Random Forests. Sensors 2021, 21, 2339. [Google Scholar] [CrossRef]
- Hajra, S.G.; Gopinath, S.; Liu, C.C.; Pawlowski, G.; Fickling, S.D.; Song, X.; D’Arcy, R.C. Enabling event-related potential assessments using low-density electrode arrays: A new technique for denoising individual channel EEG data. In Proceedings of the 2020 IEEE International IOT, Electronics and Mechatronics Conference (IEMTRONICS), Vancouver, BC, Canada, 9–12 September 2020; pp. 1–7. [Google Scholar] [CrossRef]
- Liu, C.C.; Hajra, S.G.; Fickling, S.D.; Pawlowski, G.; Song, X.; D’Arcy, R.C.N. Novel Signal Processing Technique for Capture and Isolation of Blink-Related Oscillations Using a Low-Density Electrode Array for Bedside Evaluation of Consciousness. IEEE Trans. Biomed. Eng. 2020, 67, 453–463. [Google Scholar] [CrossRef]
- Fickling, S.D.; Bollinger, F.H.; Gurm, S.; Pawlowski, G.; Liu, C.C.; Hajra, S.G.; Song, X.; D’ARcy, R.C.N. Distant Sensor Prediction of Event-Related Potentials. IEEE Trans. Biomed. Eng. 2020, 67, 2916–2924. [Google Scholar] [CrossRef]
- Acampora, E.; Ghosh Hajra, S.; Liu, C.C. Measuring Blink-Related Brainwaves Using Low-Density Electroencephalography with Textile Electrodes for Real-World Applications. Sensors 2025, 25, 4486. [Google Scholar] [CrossRef]
- Sattari, S.; Kenny, R.; Liu, C.C.; Hajra, S.G.; Dumont, G.A.; Virji-Babul, N. Blink-related EEG oscillations are neurophysiological indicators of subconcussive head impacts in female soccer players: A preliminary study. Front. Hum. Neurosci. 2023, 17, 1208498. [Google Scholar] [CrossRef]
- Ghosh Hajra, S.; Meltzer, J.A.; Keerthi, P.; Pappas, C.; Sekuler, A.B.; Cam-CAN Group; Liu, C.C. Spontaneous blinking and brain health in aging: Large-scale evaluation of blink-related oscillations across the lifespan. Front. Aging Neurosci. 2025, 16, 1473178. [Google Scholar] [CrossRef]
- Hajra, S.G.; Liu, C.; Law, A. Neural responses to spontaneous blinking capture differences in working memory load: Assessing blink related oscillations with N-back task. In Proceedings of the Neuroergonomics Conference, Virtual, 11–16 September 2021. [Google Scholar]
- Jurcak, V.; Tsuzuki, D.; Dan, I. 10/20, 10/10, and 10/5 systems revisited: Their validity as relative head-surface-based positioning systems. NeuroImage 2007, 34, 1600–1611. [Google Scholar] [CrossRef]




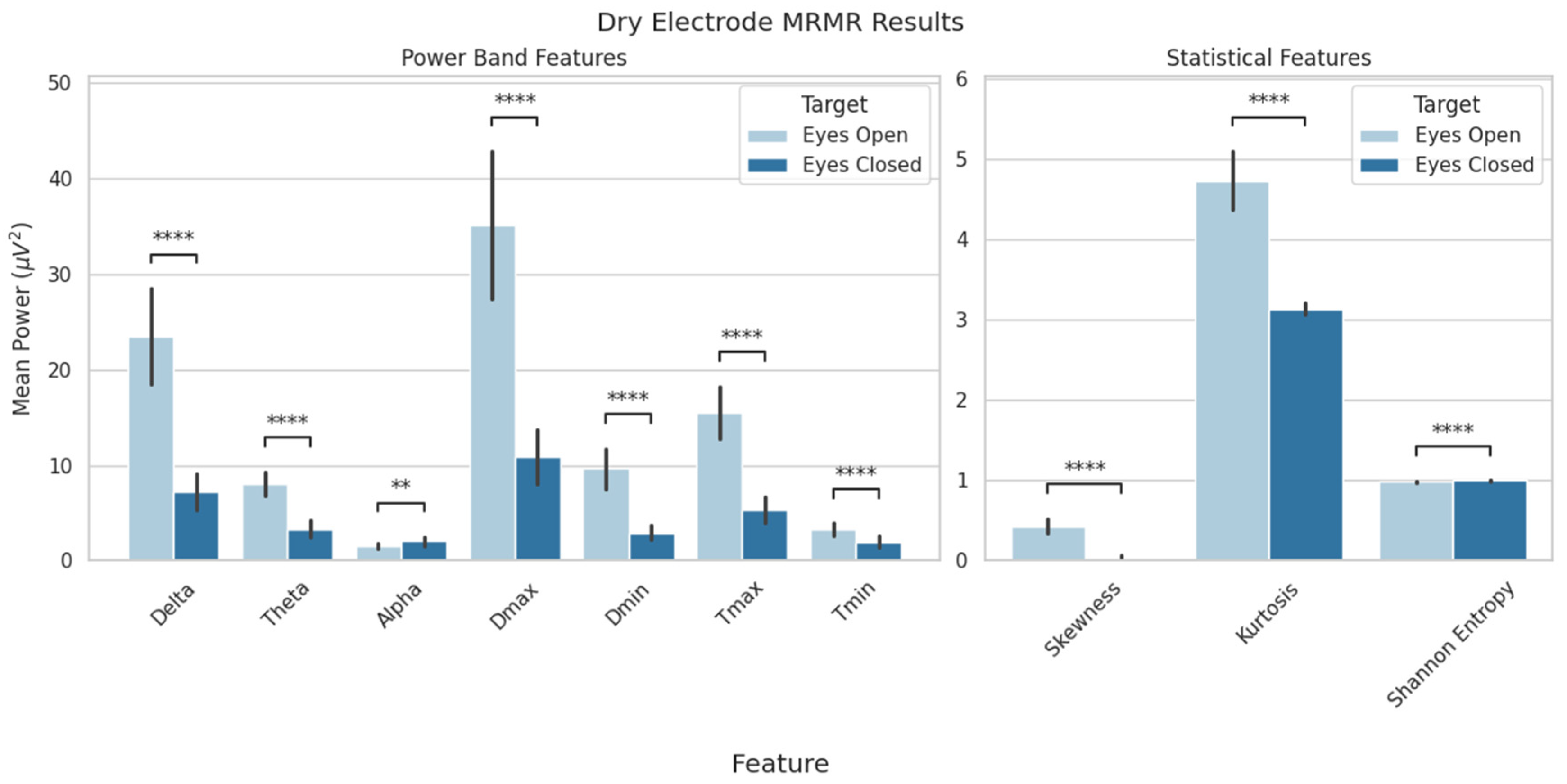
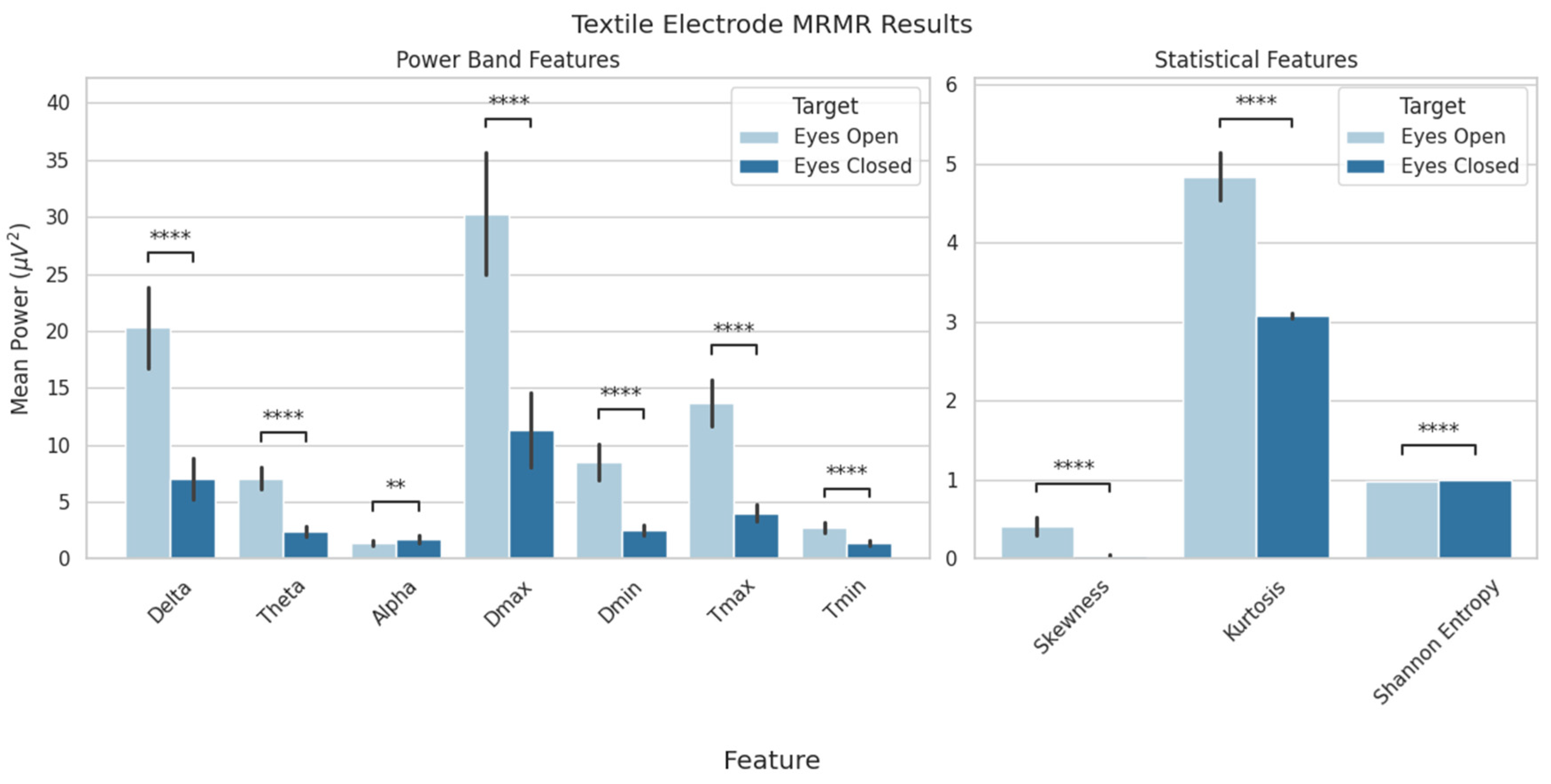
| Model Selection Results | |||||
|---|---|---|---|---|---|
| Model | Metric | ||||
| Accuracy | Precision | Recall | F1 | Fit Time | |
| Dry | |||||
| lSVM | 0.91 ± 0.10 | 0.90 ± 0.13 | 0.95 ± 0.11 | 0.92 ± 0.09 | 0.21 ± 0.10 ms |
| gSVM | 0.75 ± 0.08 | 0.78 ± 0.16 | 0.78 ± 0.18 | 0.75 ± 0.09 | 0.08 ± 0.02 ms |
| KNN | 0.88 ± 0.10 | 0.95 ± 0.12 | 0.82 ± 0.21 | 0.86 ± 0.12 | 0.06 ± 0.01 ms |
| RF | 0.96 ± 0.08 | 0.97 ± 0.11 | 0.97 ± 0.08 | 0.97 ± 0.07 | 5.29 ± 0.09 ms |
| LR | 0.88 ± 0.08 | 0.88 ± 0.13 | 0.90 ± 0.13 | 0.88 ± 0.08 | 0.42 ± 0.05 ms |
| Textile | |||||
| lSVM | 0.95 ± 0.09 | 0.95 ± 0.12 | 0.97 ± 0.08 | 0.95 ± 0.08 | 0.14 ± 0.02 ms |
| gSVM | 0.84 ± 0.16 | 0.86 ± 0.15 | 0.80 ± 0.20 | 0.82 ± 0.17 | 0.08 ± 0.01 ms |
| KNN | 0.89 ± 0.11 | 0.89 ± 0.12 | 0.90 ± 0.13 | 0.89 ± 0.11 | 0.08 ± 0.01 ms |
| RF | 0.97 ± 0.05 | 0.98 ± 0.06 | 0.97 ± 0.08 | 0.97 ± 0.05 | 5.21 ± 0.08 ms |
| LR | 0.94 ± 0.09 | 0.93 ± 0.12 | 0.97 ± 0.08 | 0.94 ± 0.08 | 0.40 ± 0.06 ms |
| Dry/Textile | |||||
| lSVM | 0.95 ± 0.02 | 0.90 ± 0.03 | 1.00 ± 0.00 | 0.95 ± 0.01 | 0.22 ± 0.09 ms |
| gSVM | 0.76 ± 0.04 | 0.73 ± 0.07 | 0.82 ± 0.03 | 0.77 ± 0.03 | 0.08 ± 0.01 ms |
| KNN | 0.82 ± 0.03 | 0.89 ± 0.04 | 0.74 ± 0.03 | 0.81 ± 0.03 | 0.06 ± 0.00 ms |
| RF | 0.94 ± 0.02 | 0.92 ± 0.03 | 0.96 ± 0.02 | 0.94 ± 0.01 | 5.38 ± 0.25 ms |
| LR | 0.91 ± 0.01 | 0.85 ± 0.01 | 1.00 ± 0.00 | 0.92 ± 0.01 | 0.44 ± 0.06 ms |
| Classification Results | |||||
|---|---|---|---|---|---|
| Model | Scoring Metrics | ||||
| Accuracy | Precision | Recall | F1 | Fit Time | |
| Dry | 0.91 ± 0.10 | 0.90 ± 0.13 | 0.95 ± 0.11 | 0.92 ± 0.09 | 0.21 ± 0.10 ms |
| Textile | 0.75 ± 0.08 | 0.78 ± 0.16 | 0.78 ± 0.18 | 0.75 ± 0.09 | 0.08 ± 0.02 ms |
| Dry/Textile | 0.88 ± 0.08 | 0.88 ± 0.13 | 0.90 ± 0.13 | 0.88 ± 0.08 | 0.42 ± 0.05 ms |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anzalone, A.; Acampora, E.; Liu, C.; Hajra, S.G. Passive Brain–Computer Interface Using Textile-Based Electroencephalography. Sensors 2025, 25, 6080. https://doi.org/10.3390/s25196080
Anzalone A, Acampora E, Liu C, Hajra SG. Passive Brain–Computer Interface Using Textile-Based Electroencephalography. Sensors. 2025; 25(19):6080. https://doi.org/10.3390/s25196080
Chicago/Turabian StyleAnzalone, Alec, Emily Acampora, Careesa Liu, and Sujoy Ghosh Hajra. 2025. "Passive Brain–Computer Interface Using Textile-Based Electroencephalography" Sensors 25, no. 19: 6080. https://doi.org/10.3390/s25196080
APA StyleAnzalone, A., Acampora, E., Liu, C., & Hajra, S. G. (2025). Passive Brain–Computer Interface Using Textile-Based Electroencephalography. Sensors, 25(19), 6080. https://doi.org/10.3390/s25196080






