A Systematic Review of Chest-Worn Sensors in Cardiac Assessment: Technologies, Advantages, and Limitations
Abstract
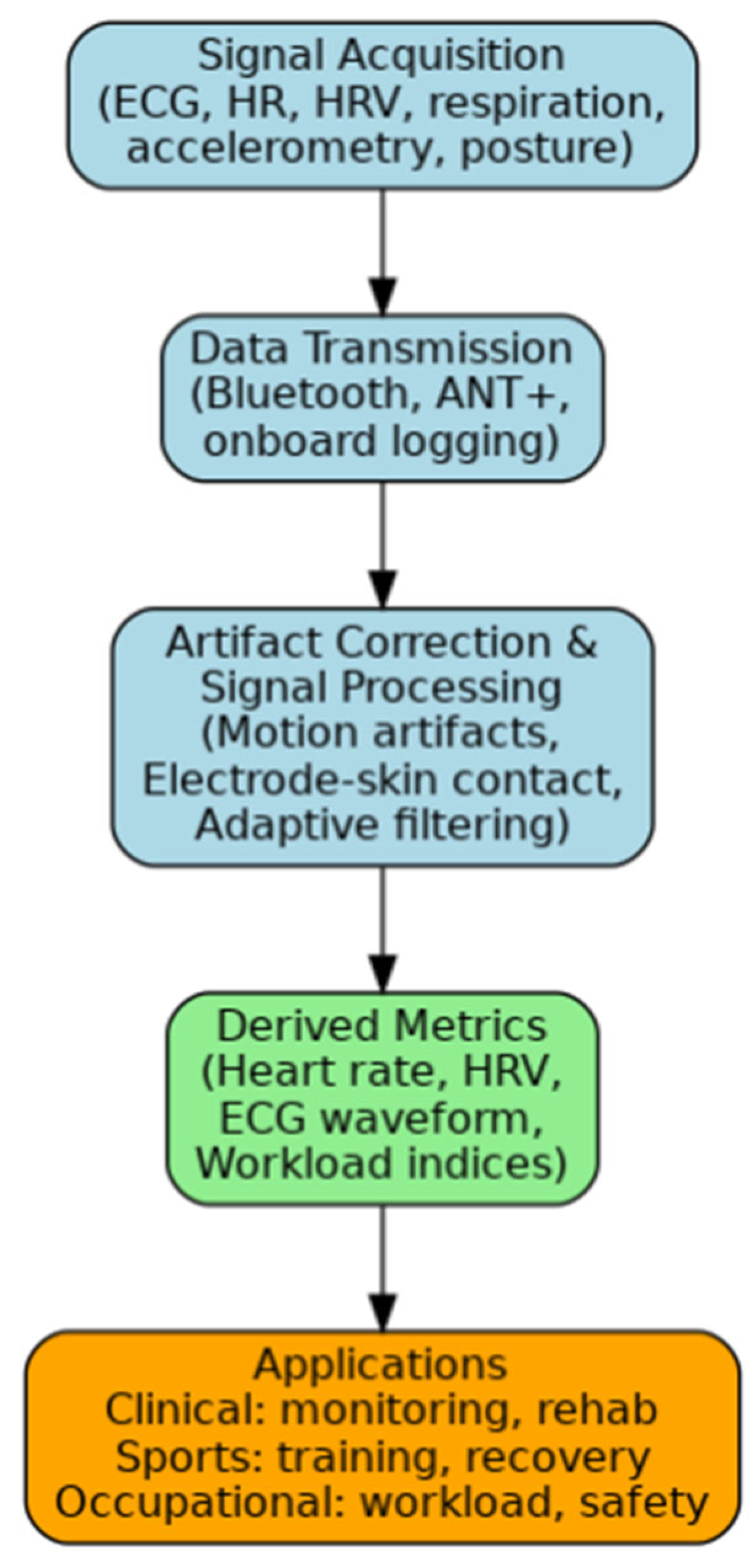
1. Introduction
2. Methodology
2.1. Research Question and Eligibility Criteria
- Population (P): Studies involving individuals across clinical, sporting, or occupational settings who use chest-worn band sensors.
- Intervention (I): The use of chest-worn band sensors for cardiac monitoring.
- Comparison (C): Between chest-worn bands or a chest-worn band compared to the gold standard.
- Outcome (O): Measurement of cardiac physiological parameters and assessment of the advantages and limitations of chest-worn sensors in various contexts.
2.2. Information Sources
2.3. Search Strategy
2.4. Selection Process
2.5. Data Collection Process
2.6. Quality Assessment
2.7. Effect Measures and Synthesis Methods
3. Results
- Device type not aligned (not chest strap-focused): Studies were excluded under this category if they did not involve or focus on chest-strap-based ECG sensors. This included studies examining alternative devices such as patches, belts, wristbands, adhesive tapes, e-tattoos, or general electrode-based systems, as well as those that failed to specify the ECG device model used. Moreover, research that was only dedicated to the parts of a chest strap or comparing different methods of fixing a strap without considering the strap as a system was also excluded because it did not align with the review’s emphasis on holistic ECG monitoring based on chest straps.
- Insufficient attention to cardiac parameters: Although the studies may involve physiological monitoring, the main concern remains focused on other parameters according to the various physiological components (energy expenditure/obesity, sleep/wake classification, training adaptation, or any other non-cardiac parameter). These articles were disqualified since the focus of this review is cardiac-specific metrics (such as heart rate, heart rate variability, and arrhythmias).
- Data availability limitations (no results presented): Some references are methodological proposals, ongoing studies, or conceptual papers that lack experimental data or validation results. Others are guidelines or overviews without original data. These do not provide the empirical evidence required for the evaluation of sensor accuracy or applicability.
- Algorithm-focused without sensor validation: A few studies concentrate on algorithm development or signal processing pipelines (e.g., arrhythmia detection or classification models), without actual validation of chest-strap sensor performance or without specifying the hardware used. Thus, they do not meet the inclusion criteria centred on device-level evaluation.
- Duplicated or covered in other sources: Some studies use methodologies or datasets already validated and detailed in another included article, particularly when marked as “overnight” protocols, where relevant parameters were reported elsewhere.

4. Discussion
4.1. Overview of Chest-Strap Device Usage
4.2. Clinical Applications and Constraints
4.3. Environmental and User Factors
4.4. Technical and Ergonomic Considerations and Limitations
4.5. Quality Assessment
4.6. Main Methodological Limitations of the Review
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| CE | Conformité Européenne |
| ECG | Electrocardiogram |
| FDA | Food and Drug Administration |
| HR | Heart Rate |
| HRV | Heart Rate Variability |
| IBI | Inter-Beat Interval |
| IMU | Inertial Measurement Unit |
| LOAs | Reported Limits of Agreement |
| MAPE | Absolute Mean Errors Below 1% |
| MMAT | Mixed Methods Appraisal Tool |
| OSH | Occupational Safety and Health |
| PPE | Personal Protective Equipment |
| PPG | Photoplethysmography |
| RRIs | RR Intervals |
Appendix A. Comparative Table Summarizing Recent Reviews on Wearable Cardiac Monitoring and Explicitly Highlighting Its Contributions
| Article | Device Focus | Systematic Method (PRISMA) | Contexts Analyzed | Value Added |
| Vermunicht et al., 2025 [15] | Chest-worn ECG strap | Yes | Cardiac patients in clinical care | Exclusive validation on cardiac patients; 24h monitoring outside of exercise; focus on ECG chest straps |
| Jamieson et al., 2025 [14] | Consumer-grade wearables | No - Guide | General health/cardiovascular | Broad device comparison (wrist, chest, patch, etc.); lacks systematic context focus |
| Murray et al., 2025 [18] | General wearables | Yes | Heart failure & pulmonary congestion | Emphasis on clinical diagnostics; sensor type not exclusive |
| Ranjan et al., 2025 [17] | Multiple wearable types | Yes | Clinical, ambulatory, telehealth | Analysis of ECG/PPG in diverse devices; not chest-focused |
| Wang et al., 2024 [19] | Consumer ECG wearables | Yes | Cardiac health monitoring | Focuses on consumer devices and ECG-diagnosing algorithms; strong emphasis on deep learning/CNNs for ECG analysis |
| Dahiya et al., 2024 [16] | Chest, wrist, patch, textile | No - Scoping review | Healthy adults, cardiac patients | Summarizes 12 studies; compares device types (including chest straps, textile shirts), safety, accuracy, minor adverse effects |
Appendix B. Search Strings and Corresponding Results by Database
| Database | Query | Results |
| Pubmed | ("Chest Strap" OR "Thoracic Sensor" OR "Wearable Sensor" OR "Wearable devices" OR "Wearable" OR "Chest-worn" OR "Thoracic band" OR "Chest belt" OR " Chest monitor") | 39,048 |
| ("Physiologic* Data" OR "Heart Rate" OR “HR” OR "Biometric" OR "Heart Rate Monitoring" OR "Biometry" OR "Blood Pressure" OR “Physiologic* signals" OR “Physiological measures” OR “Physiological Parameters” OR “Physiologic* Monitoring" OR "Ambulatory Monitoring" OR "Ambulatory Blood Pressure Monitoring" OR "Ambulatory Electrocardiography" OR "ECG" OR "Ambulatory Electrocardiography Monitoring" OR “Electrocardiogram” OR “Heart Rate Variability” OR “HRV” OR “Cardiac* stress”) | 1,259,126 | |
| #1 AND #2 | 9064 | |
| Plus filters | 682 | |
| Science Direct | (“Chest Based Sensor” OR “Chest Strap” OR “Wearable”) AND (“Heart Rate” OR “Heart Rate Variability” OR “Cardiac Stress” OR “Electrocardiogram”) | 34,704 |
| Plus Filters | 969 | |
| Web of Science | TS= ("Chest Strap" OR "Thoracic Sensor" OR "Wearable Sensor" OR "Wearable devices" OR "Wearable" OR "Chest-worn" OR "Thoracic band" OR "Chest belt" OR " Chest monitor") https://www.webofscience.com/wos/woscc/summary/b927efd4-0e7f-4c16-bd1c-8c58a3149b3c-0162417e45/relevance/1 (accessed on 23 September 2025). | 98,757 |
| TS= ("Physiologic* Data" OR "Heart Rate" OR “HR” OR "Biometric" OR "Heart Rate Monitoring" OR "Biometry" OR "Blood Pressure" OR “Physiologic* signals" OR “Physiological measures” OR “Physiological Parameters” OR “Physiologic* Monitoring" OR "Ambulatory Monitoring" OR "Ambulatory Blood Pressure Monitoring" OR "Ambulatory Electrocardiography" OR "ECG" OR "Ambulatory Electrocardiography Monitoring" OR “Electrocardiogram” OR “Heart Rate Variability” OR “HRV” OR “Cardiac* stress”) https://www.webofscience.com/wos/woscc/summary/8e6ff41b-268f-4dd3-9a5d-1e993fff617e-0162418751/relevance/1 (accessed on 23 September 2025). | 1,127,002 | |
| #1 AND #2 (https://www.webofscience.com/wos/woscc/summary/65cf83c4-e063-4d96-b9b4-3f59d5664cf1-016241a15a/relevance/1 (accessed on 23 September 2025)) | 13,318 | |
| Plus filters (https://www.webofscience.com/wos/woscc/summary/9beed659-2175-48c7-9945-7b63aac1448d-016242107d/relevance/1 (accessed on 23 September 2025)) | 4298 | |
| Scopus | TITLE-ABS-KEY ("Chest Strap" OR "Thoracic Sensor" OR "Wearable Sensor" OR "Wearable devices" OR "Wearable" OR "Chest-worn" OR "Thoracic band" OR "Chest belt" OR " Chest monitor") AND TITLE-ABS-KEY ("Physiologic* Data" OR "Heart Rate" OR “HR” OR "Biometric" OR "Heart Rate Monitoring" OR "Biometry" OR "Blood Pressure" OR “Physiologic* signals" OR “Physiological measures” OR “Physiological Parameters” OR “Physiologic* Monitoring" OR "Ambulatory Monitoring" OR "Ambulatory Blood Pressure Monitoring" OR "Ambulatory Electrocardiography" OR "ECG" OR "Ambulatory Electrocardiography Monitoring" OR “Electrocardiogram” OR “Heart Rate Variability” OR “HRV” OR “Cardiac* stress”) | 21,288 |
| Plus Filters | 5926 |
References
- Seshadri, D.R.; Rowbottom, J.R.; Drummond, C.; Voos, J.E.; Craker, J. A Review of Wearable Technology: Moving beyond the Hype: From Need through Sensor Implementation. In Proceedings of the 2016 8th Cairo International Biomedical Engineering Conference (CIBEC), Cairo, Egypt, 15–17 December 2016; pp. 52–55. [Google Scholar]
- Sawka, M.N.; Friedl, K.E. Emerging Wearable Physiological Monitoring Technologies and Decision Aids for Health and Performance. J. Appl. Physiol. 2018, 124, 430–431. [Google Scholar] [CrossRef] [PubMed]
- Kazanskiy, N.L.; Khonina, S.N.; Butt, M.A. A Review on Flexible Wearables—Recent Developments in Non-Invasive Continuous Health Monitoring. Sens. Actuators A Phys. 2024, 366, 114993. [Google Scholar] [CrossRef]
- Gjoreski, H.; Gams, M. Activity/Posture Recognition Using Wearable Sensors Placed on Different Body Locations. In Proceedings of the Signal and Image Processing and Applications/716: Artificial Intelligence and Soft Computing, Crete, Greece, 22–24 June 2011; ACTA Press: Calgary, AB, Canada, 2011. [Google Scholar]
- Khokhlov, I.; Reznik, L.; Cappos, J.; Bhaskar, R. Design of Activity Recognition Systems with Wearable Sensors. In Proceedings of the 2018 IEEE Sensors Applications Symposium (SAS), Seoul, Republic of Korea, 12–14 March 2018; pp. 1–6. [Google Scholar]
- Angelucci, A.; Camuncoli, F.; Dotti, F.; Bertozzi, F.; Galli, M.; Tarabini, M.; Aliverti, A. Validation of a Body Sensor Network for Cardiorespiratory Monitoring during Dynamic Activities. Biocybern. Biomed. Eng. 2024, 44, 794–803. [Google Scholar] [CrossRef]
- Martín-Escudero, P.; Cabanas, A.M.; Dotor-Castilla, M.L.; Galindo-Canales, M.; Miguel-Tobal, F.; Fernández-Pérez, C.; Fuentes-Ferrer, M.; Giannetti, R. Are Activity Wrist-Worn Devices Accurate for Determining Heart Rate during Intense Exercise? Bioengineering 2023, 10, 254. [Google Scholar] [CrossRef] [PubMed]
- Cosoli, G.; Spinsante, S.; Scalise, L. Wrist-Worn and Chest-Strap Wearable Devices: Systematic Review on Accuracy and Metrological Characteristics. Measurement 2020, 159, 107789. [Google Scholar] [CrossRef]
- Aksüt, G.; Eren, T. Evaluation of Wearable Device Technology in Terms of Health and Safety in Firefighters. Technol. Health Care 2024, 33, 726–736. [Google Scholar] [CrossRef]
- Srikrishnarka, P.; Haapasalo, J.; Hinestroza, J.P.; Sun, Z.; Nonappa. Wearable Sensors for Physiological Condition and Activity Monitoring. Small Sci. 2024, 4, 2300358. [Google Scholar] [CrossRef]
- Chuo, Y.; Marzencki, M.; Hung, B.; Jaggernauth, C.; Tavakolian, K.; Lin, P.; Kaminska, B. Mechanically Flexible Wireless Multisensor Platform for Human Physical Activity and Vitals Monitoring. EEE Trans. Biomed. Circuits Syst. 2010, 4, 281–294. [Google Scholar] [CrossRef]
- Dieffenderfer, J.P.; Goodell, H.; Bent, B.; Beppler, E.; Jayakumar, R.; Yokus, M.; Jur, J.S.; Bozkurt, A.; Peden, D. Wearable Wireless Sensors for Chronic Respiratory Disease Monitoring. In Proceedings of the 2015 IEEE 12th International Conference on Wearable and Implantable Body Sensor Networks (BSN), Cambridge, MA, USA, 9–12 June 2015; pp. 1–6. [Google Scholar]
- Umair, M.; Chalabianloo, N.; Sas, C.; Ersoy, C. HRV and Stress: A Mixed-Methods Approach for Comparison of Wearable Heart Rate Sensors for Biofeedback. IEEE Access 2021, 9, 14005–14024. [Google Scholar] [CrossRef]
- Jamieson, A.; Chico, T.J.A.; Jones, S.; Chaturvedi, N.; Hughes, A.D.; Orini, M. A Guide to Consumer-Grade Wearables in Cardiovascular Clinical Care and Population Health for Non-Experts. NPJ Cardiovasc. Health 2025, 2, 44. [Google Scholar] [CrossRef]
- Vermunicht, P.; Makayed, K.; Buyck, C.; Knaepen, L.; Piedrahita Giraldo, J.S.; Naessens, S.; Hens, W.; Craenenbroeck, E.V.; Laukens, K.; Desteghe, L.; et al. Continuous Heart Rate Measurements in Patients with Cardiac Disease: Device Comparison and Development of a Novel Artefact Removal Procedure. Digit. Health 2025, 11, 20552076251337598. [Google Scholar] [CrossRef]
- Dahiya, E.S.; Kalra, A.M.; Lowe, A.; Anand, G. Wearable Technology for Monitoring Electrocardiograms (ECGs) in Adults: A Scoping Review. Sensors 2024, 24, 1318. [Google Scholar] [CrossRef]
- Ranjan, S.; Baria, D.; Reddy, Y.C. Systematic Review: Wearable Technology for Cardiac Rhythm Monitoring. J. Heart Valve Dis. 2025, 30, 71–77. [Google Scholar]
- Murray, C.P.; Kenny, A.P.; O’Sullivan, N.J.; Murphy, R.T.; Curtain, J.P. Efficacy of Wearable Devices Detecting Pulmonary Congestion in Heart Failure: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2025, 12, 1612545. [Google Scholar] [CrossRef]
- Wang, G.; Shanker, S.; Nag, A.; Lian, Y.; John, D. ECG Biometric Authentication Using Self-Supervised Learning for IoT Edge Sensors. IEEE J. Biomed. Health Inform. 2024, 28, 6606–6618. [Google Scholar] [CrossRef] [PubMed]
- Hong, Q.N.; Fàbregues, S.; Bartlett, G.; Boardman, F.; Cargo, M.; Dagenais, P.; Gagnon, M.-P.; Griffiths, F.; Nicolau, B.; O’Cathain, A.; et al. The Mixed Methods Appraisal Tool (MMAT) Version 2018 for Information Professionals and Researchers. Educ. Inf. 2018, 34, 285–291. [Google Scholar] [CrossRef]
- Bläsing, D.; Buder, A.; Reiser, J.E.; Nisser, M.; Derlien, S.; Vollmer, M. ECG Performance in Simultaneous Recordings of Five Wearable Devices Using a New Morphological Noise-to-Signal Index and Smith-Waterman-Based RR Interval Comparisons. PLoS ONE 2022, 17, e0274994. [Google Scholar] [CrossRef] [PubMed]
- Constantini, K.; Stickford, A.S.L.; Bleich, J.L.; Mannheimer, P.D.; Levine, B.D.; Chapman, R.F. Synchronizing Gait with Cardiac Cycle Phase Alters Heart Rate Response during Running. Med. Sci. Sports Exerc. 2018, 50, 1046–1053. [Google Scholar] [CrossRef]
- Etiwy, M.; Akhrass, Z.; Gillinov, L.; Alashi, A.; Wang, R.; Blackburn, G.; Gillinov, S.M.; Phelan, D.; Gillinov, A.M.; Houghtaling, P.L.; et al. Accuracy of Wearable Heart Rate Monitors in Cardiac Rehabilitation. Cardiovasc. Diagn. Ther. 2019, 9, 262–271. [Google Scholar] [CrossRef]
- Flores, G.; Monteiro, D.; Silva, F.; Duarte-Mendes, P. Heart Rate Variability Activity in Soccer Athletes after a Musculoskeletal Injury. JRM J. Rehabil. Med. 2024, 56, jrm24969. [Google Scholar] [CrossRef]
- Gilgen-Ammann, R.; Schweizer, T.; Wyss, T. RR Interval Signal Quality of a Heart Rate Monitor and an ECG Holter at Rest and during Exercise. Eur. J. Appl. Physiol. 2019, 119, 1525–1532. [Google Scholar] [CrossRef]
- Martín Gómez, R.; Allevard, E.; Kamstra, H.; Cotter, J.; Lamb, P. Validity and Reliability of Movesense HR+ ECG Measurements for High-Intensity Running and Cycling. Sensors 2024, 24, 5713. [Google Scholar] [CrossRef]
- Kuo, M.-H.; Lin, Y.-J.; Huang, W.-W.; Chiang, K.-T.; Tu, M.-Y.; Chu, C.-M.; Lai, C.-Y. G Tolerance Prediction Model Using Mobile Device–Measured Cardiac Force Index for Military Aircrew: Observational Study. JMIR Mhealth Uhealth 2023, 11, e48812. [Google Scholar] [CrossRef] [PubMed]
- Marzano-Felisatti, J.M.; De Lucca, L.; Priego-Quesada, J.I.; Pino-Ortega, J. Heart Rate Measurement Accuracy During Intermittent Efforts Under Laboratory Conditions: A Comparative Analysis Between Chest Straps and Armband. Appl. Sci. 2024, 14, 11872. [Google Scholar] [CrossRef]
- Maza, A.; Goizueta, S.; Llorens, R. Reliability of a Low-Cost Chest Strap to Estimate Short-Term and Ultra-Short-Term Heart Rate Variability Measures in Response to Emotionally Valenced Stimuli. IEEE Sens. J. 2024, 24, 8008–8014. [Google Scholar] [CrossRef]
- Mishra, V.; Pope, G.; Lord, S.; Lewia, S.; Lowens, B.; Caine, K.; Sen, S.; Halter, R.; Kotz, D. Continuous Detection of Physiological Stress with Commodity Hardware. ACM Trans. Comput. Healthc. 2020, 1, 8. [Google Scholar] [CrossRef]
- Montes, J.; Navalta, J.W. Reliability of the Polar T31 Uncoded Heart Rate Monitor in Free Motion and Treadmill Activities. Int. J. Exerc. Sci. 2019, 12, 69–76. [Google Scholar] [CrossRef]
- Nuske, H.J.; Goodwin, M.S.; Kushleyeva, Y.; Forsyth, D.; Pennington, J.W.; Masino, A.J.; Finkel, E.; Bhattacharya, A.; Tan, J.; Tai, H.; et al. Evaluating Commercially Available Wireless Cardiovascular Monitors for Measuring and Transmitting Real-time Physiological Responses in Children with Autism. Autism Res. 2022, 15, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Di Palma, S.; Tonacci, A.; Narzisi, A.; Domenici, C.; Pioggia, G.; Muratori, F.; Billeci, L. Monitoring of Autonomic Response to Sociocognitive Tasks during Treatment in Children with Autism Spectrum Disorders by Wearable Technologies: A Feasibility Study. Comput. Biol. Med. 2017, 85, 143–152. [Google Scholar] [CrossRef]
- Parak, J.; Salonen, M.; Myllymäki, T.; Korhonen, I. Comparison of Heart Rate Monitoring Accuracy between Chest Strap and Vest during Physical Training and Implications on Training Decisions. Sensors 2021, 21, 8411. [Google Scholar] [CrossRef]
- Plews, D.J.; Scott, B.; Altini, M.; Wood, M.; Kilding, A.E.; Laursen, P.B. Comparison of Heart-Rate-Variability Recording with Smartphone Photoplethysmography, Polar H7 Chest Strap, and Electrocardiography. J. Sports Phys. Perform. 2017, 12, 1324–1328. [Google Scholar] [CrossRef]
- Rogers, B.; Schaffarczyk, M.; Clauß, M.; Mourot, L.; Gronwald, T. The Movesense Medical Sensor Chest Belt Device as Single Channel ECG for RR Interval Detection and HRV Analysis during Resting State and Incremental Exercise: A Cross-Sectional Validation Study. Sensors 2022, 22, 2032. [Google Scholar] [CrossRef]
- Romagnoli, S.; Sbrollini, A.; Colaneri, M.; Marcantoni, I.; Morettini, M.; Zitti, G.; Brocchini, M.; Pozzi, M.; Burattini, L. Initial Investigation of Athletes’ Electrocardiograms Acquired by Wearable Sensors during the Pre-Exercise Phase. Open Biomed. Eng. J. 2021, 15, 37–44. [Google Scholar] [CrossRef]
- Saggu, D.K.; Udigala, M.N.; Sarkar, S.; Sathiyamoorthy, A.; Dash, S.; P, V.R.M.; Rajan, V.; Calambur, N. Feasibility of Using Chest Strap and Dry Electrode System for Longer Term Cardiac Arrhythmia Monitoring: Results from a Pilot Observational Study. Indian Pacing Electrophysiol. J. 2024, 24, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Skála, T.; Vícha, M.; Rada, M.; Vácha, J.; Flašík, J.; Táborský, M. Feasibility of Evaluation of Polar H10 Chest-Belt ECG in Patients with a Broad Range of Heart Conditions. Cor Vasa 2022, 64, 411–422. [Google Scholar] [CrossRef]
- Speer, K.E.; Koenig, J.; Telford, R.M.; Olive, L.S.; Mara, J.K.; Semple, S.; Naumovski, N.; Telford, R.D.; McKune, A.J. Relationship between Heart Rate Variability and Body Mass Index: A Cross-Sectional Study of Preschool Children. Prev. Med. Rep. 2021, 24, 101638. [Google Scholar] [CrossRef] [PubMed]
- Van Oost, N.; Masci, F.; Schyvens, A.-M.; Peters, B.; Dirix, H.; Ross, V.; Wets, G.; Verbraecken, J.; Neven, A.; Aerts, J.-M. A New Perspective on Validation of Wearables for Stress Monitoring of Occupational Drivers. In Proceedings of the 2024 46th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 15–19 July 2024; IEEE: Piscataway, NJ, USA, 2024; pp. 1–4. [Google Scholar]
- Vila, G.; Godin, C.; Charbonnier, S.; Campagne, A. Real-Time Quality Index to Control Data Loss in Real-Life Cardiac Monitoring Applications. Sensors 2021, 21, 5357. [Google Scholar] [CrossRef] [PubMed]
- Chow, H.-W.; Yang, C.-C. Accuracy of Optical Heart Rate Sensing Technology in Wearable Fitness Trackers for Young and Older Adults: Validation and Comparison Study. JMIR Mhealth Uhealth 2020, 8, e14707. [Google Scholar] [CrossRef]
- Cosoli, G.; Antognoli, L.; Veroli, V.; Scalise, L. Accuracy and Precision of Wearable Devices for Real-Time Monitoring of Swimming Athletes. Sensors 2022, 22, 4726. [Google Scholar] [CrossRef]
- Higgins, T.R.; Lloyd, T.; Manalansan, K.; McDonald, S.; Thomas, B. Comparisons in Heart Rate Readings between the Bioconnected Wireless Exercise Earpiece and a Polar T31-Coded Chest Strap during a GXT. J. Hum. Sport Exerc. 2018, 13, 530–540. [Google Scholar] [CrossRef]
- Hoevenaars, D.; Yocarini, I.E.; Paraschiakos, S.; Holla, J.F.M.; De Groot, S.; Kraaij, W.; Janssen, T.W.J. Accuracy of Heart Rate Measurement by the Fitbit Charge 2 During Wheelchair Activities in People with Spinal Cord Injury: Instrument Validation Study. JMIR Rehabil. Assist. Technol. 2022, 9, e27637. [Google Scholar] [CrossRef]
- Kuo, J.; Koppel, S.; Charlton, J.L.; Rudin-Brown, C.M. Evaluation of a Video-Based Measure of Driver Heart Rate. J. Saf. Res. 2015, 54, 55.e29-59. [Google Scholar] [CrossRef]
- Liu, Y.; Barker, A.R.; Li, M.; Gu, Y.; Williams, C.A. Validation of Polar Verity Sense for Heart Rate Monitoring during School-Based High-Intensity Interval Training in Adolescents. J. Sports Sci. 2025, 43, 1076–1084. [Google Scholar] [CrossRef]
- Milena, Č.; Romano, C.; De Tommasi, F.; Carassiti, M.; Formica, D.; Schena, E.; Massaroni, C. Linear and Non-Linear Heart Rate Variability Indexes from Heart-Induced Mechanical Signals Recorded with a Skin-Interfaced IMU. Sensors 2023, 23, 1615. [Google Scholar] [CrossRef]
- Navalta, J.W.; Montes, J.; Bodell, N.G.; Salatto, R.W.; Manning, J.W.; DeBeliso, M. Concurrent Heart Rate Validity of Wearable Technology Devices during Trail Running. PLoS ONE 2020, 15, e0238569. [Google Scholar] [CrossRef] [PubMed]
- Navalta, J.W.; Davis, D.W.; Carrier, B.; Malek, E.M.; Vargas, N.; Perdomo Rodriguez, J.; Weyers, B.; Carlos, K.; Peck, M. Validity and Reliability of Wearable Devices during Self-Paced Walking, Jogging and Overground Skipping. Sport Mont 2023, 21, 23–29. [Google Scholar] [CrossRef]
- Romano, C.; Schena, E.; Formica, D.; Massaroni, C. Comparison between Chest-Worn Accelerometer and Gyroscope Performance for Heart Rate and Respiratory Rate Monitoring. Biosensors 2022, 12, 834. [Google Scholar] [CrossRef] [PubMed]
- Coospo Monitor de Frequência Cardíaca com Cinta Torácica Coospo H6—Sensor de Frequência Cardíaca Esportiva. Available online: https://www.coospo.com/pt/products/h6-monitor-de-frequencia-cardiaca-com-cinta-toracica (accessed on 28 June 2025).
- Garmin. HRM-Dual: Especificações. Available online: https://www.garmin.com/pt-PT/p/649059/ (accessed on 11 July 2025).
- Polar Electro Monitores de FC e de Atividade e Computadores de Ciclismo|Polar Portugal. Available online: https://www.polar.com/pt?srsltid=AfmBOorpXfyFMt0gv-DYDB9y9HxNqkilv370vlRwiWebxxKneSzt-_4x (accessed on 28 June 2025).
- Zephyr Technology BioHarness 3.0 User Manual. Available online: https://www.zephyranywhere.com/media/download/bioharness3-user-manual.pdf (accessed on 11 July 2025).
- Movesense Movesense for Health & Medical. Available online: https://www.movesense.com/movesense-for-health-medical/ (accessed on 28 June 2025).
- Khundaqji, H.; Hing, W.; Furness, J.; Climstein, M. Wearable Technology to Inform the Prediction and Diagnosis of Cardiorespiratory Events: A Scoping Review. PeerJ 2021, 9, e12598. [Google Scholar] [CrossRef]
- Ferreira, S.; Rodrigues, F.; Kallio, J.; Coelho, F.; Kyllonen, V.; Rocha, N.; Rodrigues, M.A.; Vildjiounaite, E. From Controlled to Chaotic: Disparities in Laboratory vs Real-World Stress Detection. In Proceedings of the 2024 International Conference on Content-Based Multimedia Indexing (CBMI), Reykjavik, Iceland, 18 September 2024; IEEE: Piscataway, NJ, USA, 2025; pp. 1–7. [Google Scholar]
- Keersmaeker, F.D.; Boxem, R.V.; Pelsser, C.; Sadre, R. The Forest Behind the Tree: Revealing Hidden Smart Home Communication Patterns. arXiv 2025, arXiv:2502.08535. [Google Scholar] [CrossRef]
- Casas, A.; Vicente, A.; Solano-García, L.; Laparra, J. From Concept to Context: Evaluating Medical Device Usability Where It Matters Most. In Health Informatics and Biomedical Engineering Applications; Morales, A., Laparra, J., Kalra, J., Eds.; AHFE Open Access: New York, NY, USA, 2024; Volume 142, pp. 1–11. [Google Scholar] [CrossRef]
- Alkurdi, A.; He, M.; Cerna, J.; Clore, J.; Sowers, R.; Hsiao-Wecksler, E.T.; Hernandez, M.E. Extending Anxiety Detection from Multimodal Wearables in Controlled Conditions to Real-World Environments. Sensors 2025, 25, 1241. [Google Scholar] [CrossRef] [PubMed]
- Sannomia, D.; Ferreira, T.B.; Ferreira, M.d.C. Evaluation of the Use of Accessibility Tools in the Information Technology Industry: A Case Study. In Proceedings of the 2019 IEEE Symposium on Visual Languages and Human-Centric Computing (VL/HCC), Memphis, TN, USA, 14–18 October 2019; pp. 261–263. [Google Scholar]
- Planning, P.; Vignali, C.; Britzelmaier, B. Work in Progress: The Role of Brand Trust in the Individual Decision Process towards the Use of New Technologies: An Empirical Investigation. Psychol. Educ. Rev. 2011, 35, 8–11. [Google Scholar] [CrossRef]
- Nazari, G.; Bobos, P.; MacDermid, J.C.; Sinden, K.E.; Richardson, J.; Tang, A. Psychometric Properties of the Zephyr Bioharness Device: A Systematic Review. BMC Sports Sci. Med. Rehabil. 2018, 10, 6. [Google Scholar] [CrossRef]
- Scardulla, F.; Cosoli, G.; Spinsante, S.; Poli, A.; Iadarola, G.; Pernice, R.; Busacca, A.; Pasta, S.; Scalise, L.; D’Acquisto, L. Photoplethysmograhic Sensors, Potential and Limitations: Is It Time for Regulation? A Comprehensive Review. Measurement 2023, 218, 113150. [Google Scholar] [CrossRef]
- Bent, B.; Goldstein, B.A.; Kibbe, W.A.; Dunn, J.P. Investigating Sources of Inaccuracy in Wearable Optical Heart Rate Sensors. npj Digit. Med. 2020, 3, 18. [Google Scholar] [CrossRef]
- Romagnoli, S.; Ripanti, F.; Morettini, M.; Burattini, L.; Sbrollini, A. Wearable and Portable Devices for Acquisition of Cardiac Signals While Practicing Sport: A Scoping Review. Sensors 2023, 23, 3350. [Google Scholar] [CrossRef]
- Mohamoud, A.; Jensen, J.; Buda, K.G. Consumer-Grade Wearable Cardiac Monitors: What They Do Well, and What Needs Work. Clevel. Clin. J. Med. 2024, 91, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Shcherbina, A.; Enge, M.; Mattsson, C.M.; Waggott, D. Accuracy in Wrist-Worn, Sensor-Based Measurements of Heart Rate and Energy Expenditure in a Diverse Cohort. J. Pers. Med. 2017, 7, 3. [Google Scholar] [CrossRef]
- Valenza, G.; Iozzia, L.; Cerina, L.; Mainardi, L.; Barbieri, R. Assessment of Instantaneous Cardiovascular Dynamics from Video Plethysmography. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Jeju, Republic of Korea, 11–15 July 2017; pp. 1776–1779. [Google Scholar]
- Valenza, G.; Iozzia, L.; Cerina, L.; Mainardi, L.; Barbieri, R. Analysis of Instantaneous Linear, Nonlinear and Complex Cardiovascular Dynamics from Videophotoplethysmography. Methods Inf. Med. 2018, 57, 135–140. [Google Scholar] [CrossRef]
- Masè, M.; Micarelli, A.; Strapazzon, G. Hearables: New Perspectives and Pitfalls of In-Ear Devices for Physiological Monitoring. A Scoping Review. Front. Physiol. 2020, 11, 568886. [Google Scholar] [CrossRef]
- Stutz, J.; Eichenberger, P.; Oetiker, C.; Huber, S.; Hirzel, I.; Spengler, C. Physical Activity Intensity Classification during Activities of Daily Living in Older Adults Using Accelerometers: Is the Ear the New Wrist? Curr. Issues Sport Sci. 2024, 9, 076. [Google Scholar] [CrossRef]
- Kligfield, P.; Gettes, L.S.; Bailey, J.J.; Childers, R.; Deal, B.J.; Hancock, E.W.; Van Herpen, G.; Kors, J.A.; Macfarlane, P.; Mirvis, D.M.; et al. Recommendations for the Standardization and Interpretation of the Electrocardiogram: Part I: The Electrocardiogram and Its Technology: A Scientific Statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society Endorsed by the International Society for Computerized Electrocardiology. Circulation 2007, 115, 1306–1324. [Google Scholar] [CrossRef]
- Kolodziej, M.; Tarnowski, P.; Sawicki, D.J.; Majkowski, A.; Rak, R.J.; Bala, A.; Pluta, A. Fatigue Detection Caused by Office Work with the Use of EOG Signal. IEEE Sens. J. 2020, 20, 15213–15223. [Google Scholar] [CrossRef]
- Sankar S, H.; Krishna Jyothis, V.; Prasad, G.; S, A.; J, A. Cardiovascular Health Prediction Using HRV Parameters: A Comparative Study of Machine Learning Techniques. In Proceedings of the 2024 Control Instrumentation System Conference (CISCON), Manipal, India, 2–3 August 2024; IEEE: Piscataway, NJ, USA, 2024; pp. 1–6. [Google Scholar]
- Tarvainen, M.P.; Niskanen, J.-P.; Lipponen, J.A.; Ranta-aho, P.O.; Karjalainen, P.A. Kubios HRV—Heart Rate Variability Analysis Software. Comput. Methods Programs Biomed. 2014, 113, 210–220. [Google Scholar] [CrossRef]
- Dunn, J.; Runge, R.; Snyder, M. Wearables and the Medical Revolution. Pers. Med. 2018, 15, 429–448. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.D.; Biller-Andorno, N. The Regulatory Gap in Digital Health and Alternative Pathways to Bridge It. Health Policy Technol. 2022, 11, 100663. [Google Scholar] [CrossRef]
- Gaspar, C.; Olkkonen, J.; Passoja, S.; Smolander, M. Paper as Active Layer in Inkjet-Printed Capacitive Humidity Sensors. Sensors 2017, 17, 1464. [Google Scholar] [CrossRef]
- Koohi, H.; Nadernejad, E.; Fathi, M. Employing Sensor Network to Guide Firefighters in Dangerous Area. Int. J. Eng. 2010, 23, 191–202. [Google Scholar]
- Shakeriaski, F.; Ghodrat, M. Challenges and Limitation of Wearable Sensors Used in Firefighters’ Protective Clothing. J. Fire Sci. 2022, 40, 214–245. [Google Scholar] [CrossRef]
- Wang, F.; Yuan, H. Challenges of the Sensor Web for Disaster Management. Int. J. Digit. Earth 2010, 3, 260–279. [Google Scholar] [CrossRef]
- Levin, A.; Gong, S.; Cheng, W. Wearable Smart Bandage-Based Bio-Sensors. Biosensors 2023, 13, 462. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, Y.; Kim, S.; Kim, S.; Yoo, S. Contactless Fatigue Level Diagnosis System Through Multimodal Sensor Data. Bioengineering 2025, 12, 116. [Google Scholar] [CrossRef]
- Evenson, K.R.; Goto, M.M.; Furberg, R.D. Systematic Review of the Validity and Reliability of Consumer-Wearable Activity Trackers. Int. J. Behav. Nutr. Phys. Act. 2015, 12, 159. [Google Scholar] [CrossRef]
- Giggins, O.M.; Muggeridge, D. P1 State of Play of Wearable Devices for the Measurement of Heart Rate: A Systematic Review of the Accuracy of Wrist-Worn Technologies. Heart 2019, 105, A5. [Google Scholar] [CrossRef]
- Khakurel, J.; Porras, J.; Melkas, H.; Fu, B. A Comprehensive Framework of Usability Issues Related to the Wearable Devices. In Convergence of ICT and Smart Devices for Emerging Applications; Paiva, S., Paul, S., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 21–66. ISBN 978-3-030-41368-2. [Google Scholar]
- Schall, M.C.; Sesek, R.F.; Cavuoto, L.A. Barriers to the Adoption of Wearable Sensors in the Workplace: A Survey of Occupational Safety and Health Professionals. Hum. Factors 2018, 60, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, P.; Lee, H.; Kirtley, S.; Collins, G.S. A Systematic Review Showed More Consideration Is Needed When Conducting Nonrandomized Studies of Interventions. J. Clin. Epidemiol. 2020, 117, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
| “Chest Strap” AND | (“Chest Strap” OR “Thoracic Sensor” OR “Wearable Sensor” OR “Wearable devices” OR “Wearable” OR “Chest-worn” OR “Thoracic band” OR “Chest belt” OR “ Chest monitor”) |
| “Physiological Data” | (“Physiologic * Data” OR “Heart Rate” OR “HR” OR “Biometric” OR “Heart Rate Monitoring” OR “Biometry” OR “Blood Pressure” OR “Physiologic * signals” OR “Physiological measures” OR “Physiological Parameters” OR “Physiologic * Monitoring” OR “Ambulatory Monitoring” OR “Ambulatory Blood Pressure Monitoring” OR “Ambulatory Electrocardiography” OR “ECG” OR “Ambulatory Electrocardiography Monitoring” OR “Electrocardiogram” OR “Heart Rate Variability” OR “HRV” OR “Cardiac * stress”) |
| (A) Studies Evaluating Chest Straps. CPM—Cardiac Parameters Measured. | |||||||
|---|---|---|---|---|---|---|---|
| Reference | Population | Study Type | Aim | Sensor (Model and Technology) | Comparison/ Reference Device | CPM | Application Context |
| Bläsing et al. [21] | 13 healthy participants | Comparative experimental study | To compare the usability and data quality of various consumer and professional electrocardiogram (ECG) devices in both research and leisure contexts, by developing two novel approaches: one to assess local noise and waveform disturbances, and another to verify and classify RR intervals (RRIs). | Polar H10 (chest strap, 1000 Hz, Infrared) + Polar RS800 Multi (wrist/storage) | NeXus-10 MKII (chest patch electrodes, 8000 Hz, Bluetooth); eMotion Faros 360° (chest patch electrodes, 1000 Hz, Bluetooth); SOMNOtouch NIBP (chest patch electrodes, 512 Hz, Bluetooth); Hexoskin Hx1 (Shirt, 256 Hz, Bluetooth) | RRI; Heart Rate (HR) | Experimental: treadmill and leisure scenarios |
| Constantini et al. [22] | 10 elite male distance runners | Experimental crossover study with within-subject comparison | To examine the effects of timing foot strikes to the systolic or diastolic phase of the cardiac cycle on heart rate, oxygen consumption, and ventilatory responses in elite distance runners. | Zephyr BioHarness 3.0 (chest strap) | Unpublished values and tolerances collected previously by authors JLB and PDM | HR; ECG waveform; RRI | Sports: treadmill |
| Etiwy et al. [23] | 80 adults enrolled in a Phase II or III CR programme | Cross-sectional comparative validation | To assess the accuracy of four commercially available, optically based wearable heart rate monitors in patients with cardiovascular disease attending a cardiac rehabilitation programme at a tertiary care centre. | Polar H7 (chest strap) | Standard 12-Lead ECG (chest patch electrodes); 2 of these wristband HR monitors: Apple Watch, Fitbit Blaze, Garmin Forerunner 235, TomTom Spark Cardio | HR; ECG waveform | Clinical: treadmill |
| Flores et al. [24] | 15 semi-professional soccer players | Cohort study | To analyze autonomic nervous system adaptations following musculoskeletal injury in athletes by measuring heart rate variability. | Polar H10 (chest strap, Bluetooth); Polar m200 (wristband/storage, Bluetooth) | No comparison device | Heart Rate Variability (HRV); RRI | Occupational (in lab) |
| Gilgen-Ammann, Schweizer, and Wyss [25] | 10 healthy adults (5 male + 5 female) | Laboratory-based comparative validation study | To assess the RR interval signal quality of the medilog® AR12plus Holter monitor and the Polar H10 chest strap at rest and during exercise in healthy individuals, using visual ECG inspection as the reference. | Polar H10 (chest strap, 1 ms) | Medilog® AR12plus Holter (3-lead ECG Holter; chest patch electrodes, 1 ms, signals recovered) | RRI; ECG waveform | Experimental (in lab): sedentary activities, walking with workload, walking and running on treadmill |
| Martín Gómez et al. [26] | 21 healthy adults | Experimental | To evaluate the validity and reliability of Movesense HR + ECG measurements across various exercise modes and intensities, using standard three-lead ECG as the reference, and to compare the performance of the Garmin HRM-Pro against the same criterion. | Movesense HR+ (chest strap, single-channel ECG, 500 Hz, Bluetooth) | ADInstruments (chest patch electrode, standard three-lead ECG, 1000 Hz) + Garmin HRM-Pro with Garmin Fenix 3 watch (chest strap + wristband) | R-R peak; ECG; HR; HRV | Experimental (in lab): treadmill or a cycle ergometer |
| Kuo et al. [27] | 213 military aircrew trainees | Observational study, G tolerance prediction model development | To verify participants’ cardiac performance during walking using the CFI and to develop a formula predicting individual G tolerance in centrifuge training. | Zephyr BioHarness 3.0 (chest strap) | Omron 1100U sphygmomanometer (armband) | HR | Occupational |
| Marzano-Felisatti et al. [28] | 30 physically active males | Experimental | To evaluate the accuracy of two chest straps and one armband during intermittent exercise in laboratory conditions, comparing their performance in effort and recovery phases to identify strengths and limitations of armband heart rate monitoring relative to chest straps. | Garmin HRM-Dual (chest strap, 4 Hz, ANT+); Coospo H6 (chest strap, 4 Hz, ANT+) | Coospo HW807 (armband, 4 Hz, ANT+) | HR | Experimental (in lab): cycle ergometer |
| Maza, Goizueta, and Llorens [29] | 29 healthy participants | Validation | To investigate the reliability of a widely used low-cost chest strap in detecting HRV fluctuations in response to emotionally valenced stimuli, by assessing its similarity and agreement with a five-lead cardiac monitor under short-term and ultra-short-term conditions. | Polar H10 (chest strap, 1 Hz, Bluetooth) | Shimmer3 ECG (four-lead cardiac monitor, 8 Hz, Bluetooth) | HRV | Experimental: emotion recognition research |
| Mishra et al. [30] | 27 university students | Validation and field study using commodity hardware | To evaluate the viability of using a commercially available heart rate monitor (Polar H7) to detect stress, by assessing its performance in both controlled laboratory settings and free-living conditions, as a low-cost alternative to clinical-grade sensors. | Polar H7 (chest strap; 1 Hz, Buetooth) | Biopac MP150 (standard ECG, chest patch electrode, signal recovered); Zephyr HXM (chest strap; 1 Hz, Bluetooth) | HR; HRV; RRI | Experimental (in lab/in loco): laboratory stress induction and real-life (field) monitoring |
| Montes and Navalta [31] | 40 healthy young adults | Test–retest reliability study | To determine the reliability of the T31 heart rate monitor at rest and during motion-based activities, including free movement and treadmill exercise, in both male and female participants. | Polar T31 (chest strap) + Polar CE0537 (wrist/storage) | No direct comparison in this study | HR | Experimental (in lab); fitness, general exercise |
| Nuske et al. [32] | Study 1: 23 adults (typical); Study 2: 32 children with ASD and 23 typically developing children (8–12 yrs) | Two-phase feasibility and validation study (lab setting) | To evaluate the suitability, comfort, and validity of commercially available ambulatory cardiovascular monitors for measuring psychosocial stress in children with and without ASD, by first testing a validation framework in adults and then applying it to children. | Polar H7 (ECG, chest strap, Bluetooth) | Mio Fuse (PPG, wristband, Bluetooth); PulseOn (PPG, wristband, Bluetooth); Biopac MP-150 (standard ECG, chest patch electrode, signal recovered) | HR; HRV | Clinical: ASD stress assessment |
| Di Palma et al. [33] | 5 male children with High-Functioning ASD; diagnosed with ADOS-2 and WISC-IV | Feasibility study (longitudinal, 6 months) | To assess autonomic nervous system responses in children with ASD during therapeutic sessions involving interactive serious games, using wearable technologies to correlate physiological signals with engagement levels and support therapy personalisation. | Shimmer® IFC-CNR wireless ECG chest strap (chest strap, single lead, 200 Hz, Bluetooth) | ELA medical (Holter) | HR | Clinical: therapy with “serious games” for ASD |
| Parak et al. [34] | 25 healthy adults | Validation of form factor for HR/HRV sensors | To compare the accuracy of a chest strap and a vest against a clinical ECG monitor for HR and HRV monitoring, and to analyze the impact of their accuracy on accumulated physiological metrics (Training Impulse (TRIMP), Excess Post-exercise Oxygen Consumption (EPOC), and energy expenditure (EE)) used in training monitoring and planning. | Suunto Movesense ECG (chest strap and sports vest, 125 Hz, Bluetooth) | Bittium Faros (3-lead Holter ECG, 256 Hz) | HR; HRV; RRI | Experimental (in lab): sports training and performance monitoring |
| Plews et al. [35] | 26 healthy individuals (elite, well-trained, and recreational athletes) | Validation study during resting breathing (1 min) | To compare the accuracy and validity of HRV recordings obtained using a PPG smartphone application (HRV4Training) and the Polar H7 chest strap against the gold standard ECG. | HRV4Training smartphone app (photoplethysmography (PPG), video camera, 180 Hz); Polar H7 (chest strap, Bluetooth) | Cosmed Quark T12x (standard 12-lead ECG, chest patch electrode) | HRV | Sports: resting |
| Rogers et al. [36] | 21 physically active adults | Cross-sectional validation study | To evaluate the agreement of the Movesense Medical chest-strap device (single-channel ECG) with a 12-channel ECG system for RR interval detection and selected HRV measures during rest, incremental cycling exercise, and post-exercise recovery. | Movesense Medical (chest strap; single-channel ECG; 512 Hz) | CardioPart 12 Blue (standard 12-lead ECG, 500 Hz) | RRI; HRV | Experimental: rest, ramp test, recovery |
| Romagnoli et al. [37] | 51 healthy Caucasian athletes training 4 ± 1 times/week | Initial observational study for reference value development | To support large-scale prevention of sport-related sudden cardiac death by identifying electrocardiographic features that may serve as reference values in the pre-exercise phase. | Zephyr BioHarness 3.0 (chest strap, 1-lead ECG, 250 Hz, Bluetooth) | Reference values from clinical 12-lead ECG | HR; HRV; ECG waveform | Sports: pre-exercise monitoring |
| Saggu et al. [38] | 34 patients [subgroup A: 20 inpatients (24 h), subgroup B: 14 ambulatory (12 weeks)] | Pilot observational feasibility study | To design and evaluate the feasibility of an investigational external cardiac monitor using a chest strap with single-lead dry electrodes for affordable long-term (3–6 months) cardiac monitoring, assessing its ECG diagnostic quality, patient comfort, and effectiveness in detecting cardiac arrhythmias compared to existing short- and long-term monitoring methods. | Zephyr BioHarness 3.0 + Reveal LINQ™ electronics (chest strap, single lead) | DR220 Holter (chest patch electrodes) | ECG waveform; HR; Inter-Beat Interval (IBI) | Clinical: short- and long-term monitoring |
| Skála et al. [39] | 161 participants: hospitalized patients (54), outpatients (53), healthy controls (54) | Validation | To verify the feasibility of accurate long-term evaluation of all heartbeats on a single-lead ECG by an experienced cardiologist across patients with varying body types, rhythms, and cardiac devices, and to assess the presence of artefacts or noise that may hinder ECG evaluation in different patient groups. | Polar H10 (chest Strap, 1-lead ECG) | Standard 12-lead ECG (chest patch electrodes) | ECG waveform | Clinical: hospitalized and outpatient cardiology |
| Speer et al. [40] | 146 healthy Australian preschool children (3–5 years old) | Cross-sectional study | To investigate the relationship between resting vagally mediated heart rate variability (HRV) and body mass index (BMI) in Australian preschool children aged 3 to 5 years. | Polar H10 (chest strap, Bluetooth 4.0) | Compared to electrocardiographic-derived recordings | HRV | Clinical: children’s relationship between resting vagally mediated HRV and BMI |
| Van Oost et al. [41] | 24 healthy young adults (students) | Validation study in controlled dynamic protocol | To validate the accuracy of commercial wearable devices, including the Zephyr BioHarness 3.0 chest-strap device and six wrist-worn wearables, for heart rate measurement and stress monitoring in road freight drivers under both transient and steady-state conditions. | Zephyr BioHarness 3.0 (chest strap) | CAM-14 module (standard 12-lead ECG, chest patch electrodes) | HR | Occupational: drivers (in lab) |
| Vila et al. [42] | 3 healthy male adults | Field validation and algorithm development | To develop and validate a signal quality index for data loss in IBI signals and assess the accuracy of a wrist-worn sensor against a wearable ECG in real-life conditions. | Zephyr BioHarness 3 (chest strap, 250 Hz, signal recovered) | Empatica E4 (wristband, 64 Hz, signal recovered); Pan-Tompkins ECG-derived IBI as academic reference | HR; IBI; HRV | Occupational (in loco) |
| (B) Reported results from studies evaluating chest straps. | |||||||
| Reference | Reported Results | ||||||
| Bläsing et al. [21] | Accuracy: Not reported (NR); Precision: NR. Device comparison: Polar has the highest percentage of missed beats during P3 (2-back) with 6.6% misclassification, but also the best result in P4 (0.02% misclassification). Polar scores better in phases with higher HR. Most scores were low or of insufficient quality (below 99%: 7 participants), mainly attributable to the 2-back task, but achieved very high scores during the running phase. | ||||||
| Constantini et al. [22] | Accuracy: NR; Precision: NR. Device comparison: Group mean HR was significantly lower during diastolic compared with systolic stepping (p < 0.001); strong correlations were observed between diastolic and systolic stepping for HR, step rate (SR), and step length (p < 0.05, r = 0.95 for all comparisons) | ||||||
| Etiwy et al. [23] | Accuracy: Polar chest strap (rc of 0.99). Among wrist-worn monitors, the Apple Watch performed best with rc = 0.80, followed by the Fitbit Blaze (rc = 0.78), TomTom Spark Cardio (rc =0.76) and Garmin Forerunner 235 (rc = 0.52); Precision: NR. Device comparison: Wrist-worn HR monitors, the Apple Watch, and TomTom Spark Cardio were most accurate, with no statistical difference from ECG (p = 0.62 for TomTom Spark Cardio and p = 0.09 for Apple Watch) | ||||||
| Flores et al. [24] | Accuracy: NR; Precision: NR. Device comparison: Results show differences between T1 and T2 (p ≤ 0.05) in low-frequency power (n.u.) (p = 0.001) and high-frequency power (n.u.) (p = 0.001), in low-frequency/high-frequency ratio (p = 0.001), and in high-frequency power (ms2) (p = 0.017) measures. No statistical differences were found in low-frequency power (ms2) (p = 0.233). The low-frequency power (n.u.) was significantly lower after injury compared with LF power (n.u.) values after full return to play. In high-frequency power, there was a significant difference between the two moments with high values after injury. | ||||||
| Gilgen-Ammann, Schweizer, and Wyss [25] | Accuracy: NR; Precision: NR. Device comparison: RR interval signal qualities of 94.6% and 99.6% were demonstrated for the medilog® AR12plus and the Polar H10. During the high-intensity activities, the RR interval signal quality of the medilog® AR12plus dropped to 89.8%, whereas the Polar H10 maintained a signal quality of 99.4%. The correlation between both systems was high (r = 0.997, p > 0.001). | ||||||
| Martín Gómez et al. [26] | Accuracy: Movesense HR+ mean absolute percentage error (MAPE)= 1%; rC (Lin)= 0,99; Garmin HRM MAPE= 13%; rC (Lin)= 0,32; Precision: Movesense HR+ = 99.6%; Garmin HRM = 87.7%. Device comparison: Bland–Altman analysis compared to the criterion indicated mean differences (SD) in RR’ intervals of 0.23 (22.3) ms for Movesense HR+ at rest and 0.38 (18.7) ms during the incremental test. The mean difference for Garmin HRM-Pro at rest was −8.5 (111.5) ms and 27.7 (128.7) ms for the incremental test. The incremental test correlation was very strong (r = 0.98) between Movesense HR+ and the criterion, and moderate (r = 0.66) for Garmin HRM-Pro. | ||||||
| Kuo et al. [27] | Accuracy: NR; Precision: NR. Device comparison: Walking cardiac force index (WCFI) positively correlated with Relaxed G tolerance (RGT) (r = 0.234; p = 0.001) and straining G tolerance (SGT) (r = 0.256; p < 0.001). RGT = 0.066 × age + 0.043 × (WCFI × 100) − 0.037 × height + 0.015 × SBP − 0.010 × HR + 7.724. SGT = 0.103 × (WCFI × 100) − 0.069 × height + 0.018 × SBP + 15.899. | ||||||
| Marzano-Felisatti et al. [28] | Accuracy: NR; Precision: NR. Device comparison: The ICC (intraclass correlation coefficients) values indicate a strong agreement between the Garmin and Coospo chest straps (ICC = 0.6–1.0). However, lower ICC values between the Coospo Armband and both chest straps (ICC = 0.10–0.77) reflect the higher measurement of discrepancies, particularly during effort stages | ||||||
| Maza, Goizueta, and Llorens [29] | Accuracy: NR; Precision: NR. Device comparison: Signals recorded by both devices were highly correlated with no significant discrepancies between measures; strong to excellent agreement in time-, frequency-, and nonlinear measures | ||||||
| Mishra et al. [30] | Accuracy: NR; Precision: NR. Device comparison: F1-score up to 0.87 (lab) and 0.66 (field); strong correlation with clinical ECG (r > 0.95 for most features) | ||||||
| Montes and Navalta [31] | Accuracy: NR; Precision: NR. Device comparison: Cronbach’s α from 0.90 to 0.99 across all test conditions; all p < 0.001; excellent reliability | ||||||
| Nuske et al. [32] | Accuracy: NR; Precision: NR. Device comparison: HR ↑ and HRV ↓ during stress vs. rest (p < 0.001 for both devices); Sampling Fidelity ≥ 83%; Spike Rate ≤ 13%; η2 > 0.25 for HR and 0.16–0.26 for HRV effects | ||||||
| Di Palma et al. [33] | Accuracy: NR; Precision: NR. Device comparison: Physiological events (↑ HR, ↓ Root Mean Square of Successive Differences (RMSSD), ↓ Respiratory Sinus Arrhythmia (RSA)) correlated with sociocognitive engagement; increased “lower RSA” and “lower RMSSD” events over time; ECG well-tolerated throughout | ||||||
| Parak et al. [34] | Accuracy: Strap = 99,24%; Vest = 84,70%. Precision: NR. Device comparison: Chest strap: HR MAPE = 0.76%, EPOC MAPE = 3.90%, TRIMP MAPE = 0.38%; Vest: HR MAPE = 3.32%, EPOC MAPE = 54.15%, TRIMP MAPE = 8.99%; chest strap more accurate across all measures | ||||||
| Plews et al. [35] | Accuracy: NR; Precision: NR. Device comparison: All differences vs. ECG were “trivial”; technical error of estimate (TEE) coefficient variation (CV) %: PPG GB = 3.8%, Polar H7 NB = 8.6%; correlations r = 0.99–1.00; mean bias < 2.0 ms | ||||||
| Rogers et al. [36] | Accuracy: NR; Precision: NR. Device comparison: High correlations for HRV parameters: Pearson’s r = 0.95–1.00; small bias (e.g., meanRR PRE bias = 0.0 ms, Limits of Agreement (LOAs) ± 1.9 ms); short-term scaling exponent of Detrended Fluctuation Analysis (DFA a1) agreement r ≥ 0.95 | ||||||
| Romagnoli et al. [37] | Accuracy: NR; Precision: NR. Device comparison: Median values reported with interquartile range; significant differences found vs. clinical ECG norms (e.g., ↓ HRV, ↑ QRS duration, ↓ QT interval) | ||||||
| Saggu et al. [38] | Accuracy: NR; Precision: NR. Device comparison: Diagnostic-quality ECG for 76.5% of monitoring; arrhythmia yield: 24% (24 h) and 64% (12 weeks); comfort reported in 94.9% | ||||||
| Skála et al. [39] | Accuracy: NR; Precision: NR. Device comparison: Basic rhythm reliably determined in the majority of patients; 2.16% noise | ||||||
| Speer et al. [40] | Accuracy: NR; Precision: NR. Device comparison: Significant inverse relationship between RMSSD (ln) and BMI (β = −0.06; 95% CI = −0.12–−0.01; p = 0.032) | ||||||
| Van Oost et al. [41] | Accuracy: NR; Precision: NR. Device comparison: Zephyr showed near-perfect accuracy (MAPE and CCC) in dynamic HR; wrist-wearables varied: Fitbits performed best, WHOOP and Withings worst; transitions (HR dynamics) caused a performance drop in all devices | ||||||
| Vila et al. [42] | Accuracy: NR; Precision: NR. Device comparison: Median error for mean HR: 3.2%; RMSSD: 62%; Low Frequency (LF): 25%; High Frequency (HF): 63%. Accuracy improved when no missing samples (0.0%, 27%, and 6.4%, respectively) | ||||||
| Reference | Aim | Population | Sensor (Model and Technology) | Target Device | Reported Results (Reference vs. Target) | CPM | Application Context |
|---|---|---|---|---|---|---|---|
| Chow and Yang [43] | To compare the real-time heart rate (HR) tracking performance of two commercial fitness wearables (photoplethysmography (PPG)-based) in younger versus older adults during moderate physical activity | 20 adults aged 65 years and above (Senior) and 20 adults aged between 20 years and 26 years (Young) | Polar H7 (chest strap) | Xiaomi Mi Band 2 (Xiaomi Corporation) and Garmin Vivosmart HR+ (Garmin International Inc) | The Garmin device produced more reliable and accurate HR readings than the Xiaomi one. The accuracy levels of both devices were negatively correlated with the level of activity intensity. For both devices, the measurement accuracy deteriorated in individuals while cycling. | HR | Experimental (in lab): treadmill, upright stationary bike, and elliptical machine: aerobic training. |
| Cosoli et al. [44] | To evaluate the accuracy and precision of wrist-worn (Polar Vantage V2, Garmin Venu Sq) versus chest-strap (Polar H10) HR monitors during swimming and dry-land activities in expert swimmers. | 10 expert swimmers | Polar H10 (cardiac belt, 130 Hz) | Polar Vantage V2; Garmin Venu Sq | Precision and accuracy worsen in water tests. The metrological performance in terms of accuracy of Polar Vantage V2 is better compared to Garmin Venu Sq. | HR | Occupational/sports/experimental: swimming in different styles (in loco); walking/running on a treadmill (in lab) |
| Higgins et al. [45] | To evaluate the validity of an earpiece HR monitoring device against a previously validated chest-strap HR monitoring device | 15 college students | Polar T31 (chest strap) + Polar FT1 HR monitor (Bluetooth) | Bioconnected wireless exercise earpieces | Device Correlation: Strong overall correlation between earpiece and chest strap (r = 0.97); Meets validity threshold (r ≥ 0.90) for HR monitoring devices. Measurement Accuracy: 521 ± 117 HR data points (earpiece) vs. 517 ± 118 (chest strap). Close overlap in readings for first 350 s of protocol. Max discrepancy: <10 beats per minute (bpm) during walk-to-jog transition. Algorithm Differences: Chest strap showed sudden HR spikes (5 sec averaging); earpiece demonstrated gradual increases (continuous monitoring). | HR | Experimental (in lab) |
| Hoevenaars et al. [46] | To assess the reliability of Fitbit Charge 2’s PPG-based HR monitoring in spinal cord injury (SCI) wheelchair users, investigating the impact of exercise intensity and neurological impairment level on measurement accuracy | 48 participants (38 with SCI and 10 without) | Polar H7 HR Monitor (chest strap, Bluetooth Low Energy) | Fitbit Charge 2 | Overall Accuracy (All Lesions): Mean Absolute Percentage Error (MAPE): 12.99% (outside acceptable ±10% range); Agreement: Moderate (CCC = 0.577). Accuracy by Lesion Level: Non-SCI: 8.09% (within acceptable range); Lesions below T5: 11.16%; Lesions T1–T5: 10.5%; Cervical Lesions (tetraplegia): 20.43% (significantly reduced accuracy); Accuracy by Activity Intensity: Rest: 6.5% (best performance); Moderate Activity: 12.97%; Strength Exercises: 14.2% (worst performance). | HR | Clinical/experimental: rest, wheelchair activities, and a 30 min strength exercise block |
| Kuo et al. [47] | To assess the feasibility of using imaging PPG (IPPG) from in-vehicle face video for HR monitoring during real-world driving, compared to chest-strap measurements. | 10 drivers | Zephyr Bioharness 3.0 (chest strap) | IPPG (camera-based) | 48–75% accuracy in 4/10 participants | HR | Occupational (in loco): drivers |
| Liu et al. [48] | To assess the validity of the Polar Verity Sense (PVS) armband versus the Polar H10 chest strap for HR monitoring during high-intensity interval training (HIIT) in adolescents. | 39 students (7th grade) | Polar H10 (chest strap; Bluetooth) | PVS | Strong agreement between PVS and H10 overall (r = 0.93, mean absolute error (MAE) = 4 bpm, 2.8% error). Slightly reduced accuracy at high intensity (≥80% max HR, r= 0.84). Unaffected by sex, body mass index, waist size, or fitness level. PVS is a valid, practical alternative to chest straps for HIIT monitoring in school settings. | HR | Experimental (in lab): HIIT |
| Milena et al. [49] | To assess the feasibility of deriving HR variability (HRV) metrics from mechanical cardiac signals (recorded via accelerometer and gyroscope) as an alternative to conventional electrical signals (ECG) | 22 healthy subjects | Zephyr Bioharness 3.0 (chest strap, 1-lead ECG, 250 Hz) | Inertial Measurement Unit (IMU) sensor (Xsens DOT) | Gyrocardiogram (GCG) in lying posture showed the highest accuracy; seismocardiogram (SCG) was less reliable than GCG, especially in seated posture. | HRV; R-R peaks | Experimental (in lab): sitting (1) and lying (2) posture |
| Navalta et al. [50] | To determine concurrent heart rate validity during trail running | 21 healthy subjects | Polar H7 (chest strap, 1000 Hz) | Garmin Fenix 5 wristwatch, Jabra Elite Sport earbuds, Motiv ring, Scosche Rhythm+ forearm band, Suunto Spartan Sport watch with accompanying chest strap | Garmin Fenix 5 (MAPE = 13%, Bland–Altman Limits of Agreement (LOA) = −32 to 162, Lin’s Concordance Coefficient (rC) = 0.32), Jabra Elite Sport (MAPE = 23%, LOA = −464 to 503, rC = 0.38), Motiv ring (MAPE = 16%, LOA = −52 to 96, rC = 0.29), Scosche Rhythm+ (MAPE = 6%, LOA = −114 to 120, rC = 0.79), Suunto Spartan Sport (MAPE = 2%, LOA = −62 to 61, rC = 0.96). | HR | Experimental (in lab): The trail runs were out and back with the first 1.61 km in an uphill direction, and the 1.61 return being downhill in nature |
| Navalta et al. [51] | To determine the validity of the PVS optical HR monitor for measuring HR and HRV during rest and exercise, using the Polar H10 chest strap as the criterion device. | 17 healthy adults | Polar H10 (chest strap, 1000 Hz, Bluetooth) | PVS (PPG armband) | PVS HR: r = 0.99 vs. H10; HRV: Intraclass Correlation Coefficient = 0.83, r = 0.84; mean bias: −3.3 ms; all within acceptable limits. Wearable, comfortable, good agreement with the criterion device, wrist/arm placement flexibility | HR, HRV | Experimental (in lab): Exercise monitoring |
| Romano et al. [52] | To compare the performance of accelerometer (ACC) and gyroscope (GYR) sensors (embedded in a single IMU) for simultaneous HR and respiratory rate (RR) monitoring, while evaluating the impact of window length and posture on accuracy | 18 healthy subjects | Zephyr Bioharness 3.0 (chest strap, 250 Hz) | Chest-worn IMU (Shimmer3)—Accelerometer and Gyroscope | HR: 5 s windows yielded the worst agreement with ECG, especially in the standing posture (LOA ~ 12.5–12.8 bpm); 55 s windows showed the best agreement (LOA ~ 3.5–3.7 bpm for SCG and GCG); Other window sizes (15–45 s) showed comparable and stable performance. MAE were similar for SCG and GCG, with SCG differing by no more than 0.53 bpm in the seated posture. | HR | Experimental (in lab) |
| Brand | Polar | Zephyr | Movesense | Garmin | Coospo | Shimmer® | ||
|---|---|---|---|---|---|---|---|---|
| Model | H7 | H10 | T31 | Bioharness 3.0 | Sensor | HRM-Dual | H6 | IFC cnr |
| Dimension | 30 × 20 × 9 mm | 65 × 34 × 10 mm | 28 × 7 mm | 36.6 × 10.6 mm | 62 × 34 × 11 mm | 60 × 33.8 × 12.2 | 50 × 25 × 23 mm | |
| Weight | 100 g | 60 g | 89 g | 9.4 g | 54.4 g | 46.4 g | 30 g | |
| Performance | No recording mode | CPU velocity: 64 MHz; Memory: MB; Recording mode | Coded; No recording mode | Continuous physiological monitoring; ROG status (Red/Orange/Green) alerts; Data logging up to 500+ h; Multiple transmission modes; Recording mode | Nordic Semiconductor nRF52832, 32-bit ARM Cortex-M4, 64 kB RAM, 512 kB FLASH; Recording mode | No recording mode | No recording mode | 12-bit A/D resolution; 200 Hz sampling rate; Recording mode |
| Connectivity | Bluetooth Low Energy (BLE); Analogue; Transmission rate varies with receiving device; Short transmission range (receiving device should be in front of the user, fixed on a belt or pocket) | BLE; Analogue; ANT 2.1; Transmission range: 9000 cm | Analogue 5 kHz | Bluetooth 2.1 + EDR; IEEE 802.15.4 (2.405–2.480 GHz); USB (for charging/configuration) | BLE 4.0/5.0 | ANT; BLE 2 | Bluetooth (10 m); ANT+ (7 m); | Chipcon CC2420 radio transceiver (2.4 GHz), Rufa™ antenna, RN-41 Bluetooth® module; Short-range transmission (up to 30 m); low-power modes for energy efficiency |
| Durability | −10 °C to +50 °C; Water Resistant (WR) | −10 °C to +50 °C; WR 30 m | WR | IP55 water/dust resistant; Operating Temp: −30 °C to +60 °C; Storage Temp: −40 °C to +85 °C | WR 30 m | WR 10 m | +5 °C a + 40 °C, ≤95% Relativity Humidity | Designed for long-term chest wear; comfortable and adaptable to body shape |
| Sensors | Electrodes | Electrodes; Accelerometer | Electrodes | Electrodes (250 Hz); Respiratory (25 Hz); 3-axis accelerometer (100 Hz); Posture detection; Internal temperature sensor | Accelerometer, Gyroscope, Magnetometer, Temperature, Electrodes | Electrodes | Electrodes | ECG with gain of 175; uses low-power CMOS op-amps; cable-free electrode connection |
| Battery | Type: CR 2025; Lifetime: 200 h; Rechargeable: No; Replaceable: yes | Duration: 165 mAh; Type: coin cell; Rechargeable: No; Replaceable: yes | Duration: 2500 h; Non-replaceable. | Rechargeable Lithium-Polymer (3.7 V); 12–24 h (transmit), 35 h (logging); Charging via USB: 3 h full, 1 h to 90% | CR2025 type battery (lithium coin battery), duration varies depending on use, up to several months | CR2032; 3.5 y (1 h a day); Replaceable: yes; Rechargeable: no | Type: CR2032; 300 h; Replaceable: yes; Rechargeable: no | 3 V Li-ion, 280 mAh; transmission: 60 mA; reception: 40 mA; idle: 1.4 mA; sleep: 50 µA |
| Extra features | Discontinued from 2020 | Firmware upgradeable | ROG status logic configurable; Software Development Kit available; Data export in CSV/HEX for analysis (e.g., MATLAB) | Software controllable red LEDs, 3 Mbit EEPROM for storage, API for development, OTA firmware | Includes 2 GB SD Card for onboard storage; SPI via USART1; suitable for wearable applications | |||
| Sensor (Model) | Key Advantages | Key Limitations | Application-Specific Notes/Trade-offs |
|---|---|---|---|
| Polar H7 | High correlation with ECG (r ≈ 0.99); high sampling rate (≥1000 Hz); widely used as reference; Bluetooth connectivity; low cost; real-time data transmission; usable in children | Susceptible to motion artefacts; limited raw data; not FDA/CE approved | Good for low- to moderate-intensity lab or clinical settings; less reliable during high-intensity or dynamic activity |
| Polar H10 | User-friendly; stable electrode contact; high sampling rate (≥1000 Hz); Bluetooth connectivity; affordable; reliable for HRV measures; usable in children; excellent RR interval accuracy | Strap placement sensitivity; discomfort in long-term wear; limited raw data; not CE approved | Strong choice for lab, sports, and clinical research requiring HRV; trade-off between comfort and precision during long sessions |
| Polar T31 | Low cost; lightweight; user-friendly; water-resistant | Lacks advanced HRV/ECG; less accurate at high intensity | Suitable for general exercise and fitness monitoring; not recommended for research or clinical HRV assessment |
| Zephyr BioHarness 3.0 | Multimodal (ECG, HR, respiration, accelerometry); Bluetooth connectivity; field-based; comfortable with low skin irritations; long battery; accurate RR interval; supports cardiac–locomotor entrainment | Bulkier; reduced comfort for long-term wear; signal degradation with sweat | Excellent for field-based occupational or sports monitoring; trade-off between multimodal functionality and comfort; very suitable for stress and physiological studies |
| Movesense HR+ / Medical / ECG | High accuracy (MAPE <1%); Bluetooth connectivity; real-time ECG/HRV transmission; user-friendly | Limited population validation; less accurate at high intensity; strap discomfort when long-term wear | Ideal for research or monitoring intense activity; less validated in children/older adults |
| Garmin HRM-Dual | Affordable; reliable HR during moderate exercise; good agreement with other straps | Less accurate HRV; weaker under in-motion activity | Best for moderate exercise; limited HRV precision; comfort may be preferred over advanced metrics |
| Coospo H6 | Low cost; good HR accuracy | Limited validation; less stable in high-intensity activity | Suitable for general fitness; not ideal for high-intensity sports or HRV research |
| Shimmer® IFC-CNR | Research-grade ECG; comfortable; customizable; Bluetooth connectivity; allows real-time and offline analysis; usable in children; experimental studies | Expensive; less user-friendly; limited general availability | Best for controlled experimental settings; not practical for field or consumer use |
| Reference | SD | S1 | S2 | SD.1 | SD.2 | SD.3 | SD.4 | SD.5 | Level |
|---|---|---|---|---|---|---|---|---|---|
| Bläsing et al. [21] | 3 | Y | Y | N | Y | Y | CT | Y | Medium |
| Constantini et al. [22] | 3 | Y | Y | Y | Y | Y | CT | Y | High |
| Etiwy et al. [23] | 4 | Y | Y | Y | Y | Y | Y | Y | High |
| Flores et al. [24] | 3 | Y | Y | N | Y | Y | N | CT | Medium |
| Gilgen-Ammann, Schweizer, and Wyss [25] | 4 | Y | Y | Y | N | Y | Y | Y | High |
| Martín Gómez et al. [26] | 4 | Y | Y | Y | N | Y | Y | Y | High |
| Kuo et al. [27] | 3 | Y | Y | Y | Y | Y | Y | Y | High |
| Marzano-Felisatti et al. [28] | 3 | Y | Y | N | Y | Y | CT | Y | Medium |
| Maza, Goizueta, and Llorens [29] | 3 | Y | Y | N | Y | Y | CT | Y | Medium |
| Mishra et al. [30] | 3 | Y | Y | N | Y | Y | CT | Y | Medium |
| Montes and Navalta [31] | 3 | Y | Y | N | Y | Y | CT | Y | Medium |
| Nuske et al. [32] | 3 | Y | Y | Y | Y | Y | Y | Y | High |
| Di Palma et al. [33] | 3 | Y | Y | CT | Y | Y | N | Y | Medium |
| Parak et al. [34] | 3 | Y | Y | CT | Y | Y | Y | Y | High |
| Plews et al. [35] | 3 | Y | Y | CT | Y | Y | N | Y | Medium |
| Rogers et al. [36] | 3 | Y | Y | CT | Y | N | N | Y | Low |
| Romagnoli et al. [37] | 4 | Y | Y | Y | N | Y | Y | Y | High |
| Saggu et al. [38] | 3 | Y | Y | CT | Y | N | CT | Y | Low |
| Skála et al. [54] | 3 | Y | Y | Y | Y | Y | Y | Y | High |
| Speer et al. [40] | 3 | Y | Y | Y | Y | Y | Y | CT | High |
| Van Oost et al. [41] | 3 | Y | Y | N | Y | Y | CT | Y | Medium |
| Vila et al. [42] | 3 | Y | Y | N | Y | CT | CT | Y | Low |
| Reference | SD | S1 | S2 | SD.1 | SD.2 | SD.3 | SD.4 | SD.5 | Level |
|---|---|---|---|---|---|---|---|---|---|
| Chow and Yang [43] | 3 | Y | Y | Y | Y | Y | CT | Y | High |
| Cosoli et al. [44] | 3 | Y | Y | N | Y | Y | CT | Y | Medium |
| Higgins et al. [45] | 3 | Y | Y | N | Y | N | CT | Y | Low |
| Hoevenaars et al. [46] | 3 | Y | Y | Y | Y | Y | Y | Y | High |
| Kuo et al. [47] | 3 | Y | Y | N | Y | Y | Y | Y | High |
| Liu et al. [48] | 3 | Y | Y | Y | Y | Y | Y | Y | High |
| Milena et al. [49] | 3 | Y | Y | N | Y | Y | CT | Y | Medium |
| Navalta et al. [50] | 3 | Y | Y | CT | Y | Y | N | Y | Medium |
| Navalta et al. [51] | 3 | Y | Y | CT | Y | Y | CT | Y | Medium |
| Romano et al. [52] | 3 | Y | Y | N | Y | Y | CT | Y | Medium |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, A.; Ferreira, D.F.; Ferreira, S.; Almeida-Antunes, N.; Carvalho, P.; Melo, P.; Rocha, N.; Rodrigues, M.A. A Systematic Review of Chest-Worn Sensors in Cardiac Assessment: Technologies, Advantages, and Limitations. Sensors 2025, 25, 6049. https://doi.org/10.3390/s25196049
Machado A, Ferreira DF, Ferreira S, Almeida-Antunes N, Carvalho P, Melo P, Rocha N, Rodrigues MA. A Systematic Review of Chest-Worn Sensors in Cardiac Assessment: Technologies, Advantages, and Limitations. Sensors. 2025; 25(19):6049. https://doi.org/10.3390/s25196049
Chicago/Turabian StyleMachado, Ana, D. Filipa Ferreira, Simão Ferreira, Natália Almeida-Antunes, Paulo Carvalho, Pedro Melo, Nuno Rocha, and Matilde A. Rodrigues. 2025. "A Systematic Review of Chest-Worn Sensors in Cardiac Assessment: Technologies, Advantages, and Limitations" Sensors 25, no. 19: 6049. https://doi.org/10.3390/s25196049
APA StyleMachado, A., Ferreira, D. F., Ferreira, S., Almeida-Antunes, N., Carvalho, P., Melo, P., Rocha, N., & Rodrigues, M. A. (2025). A Systematic Review of Chest-Worn Sensors in Cardiac Assessment: Technologies, Advantages, and Limitations. Sensors, 25(19), 6049. https://doi.org/10.3390/s25196049









