Effects of Seat Vibration on Biometric Signals and Postural Stability in a Simulated Autonomous Driving Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
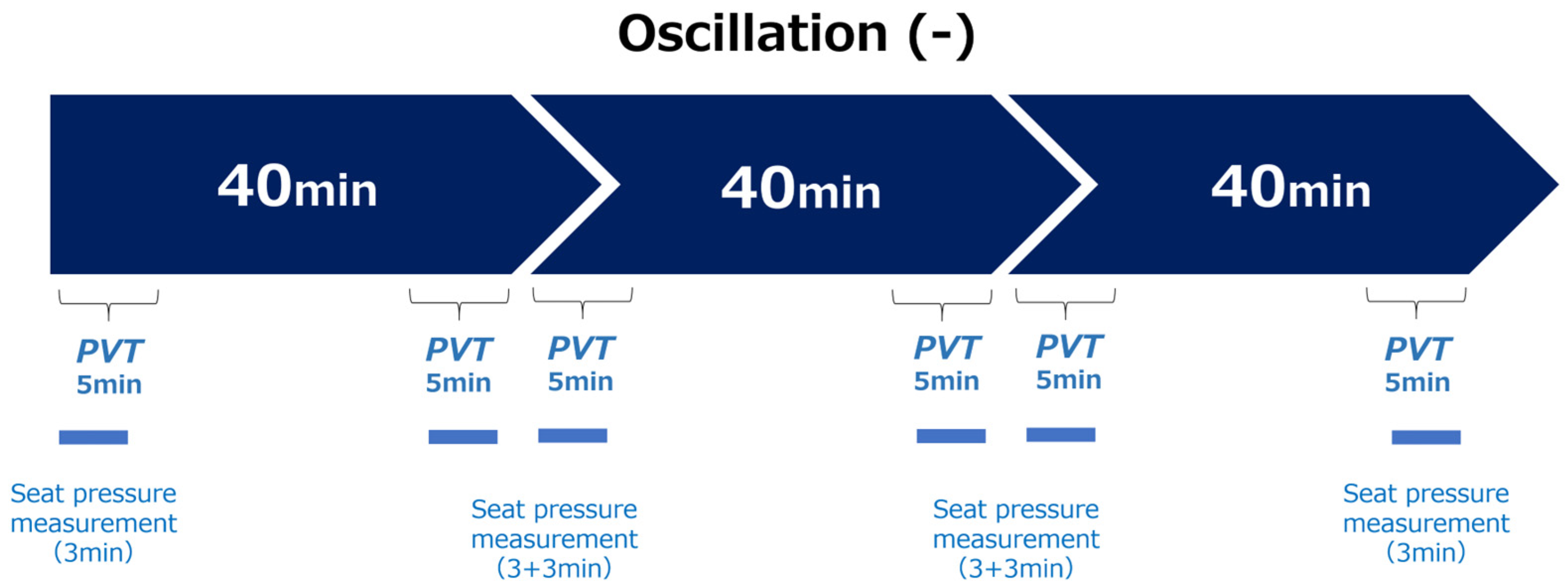
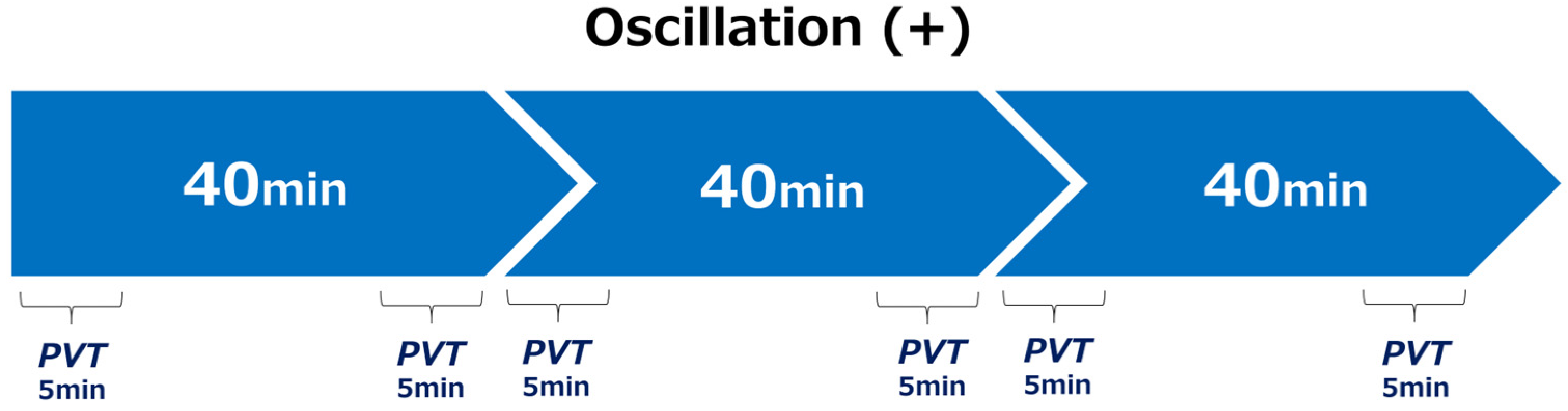
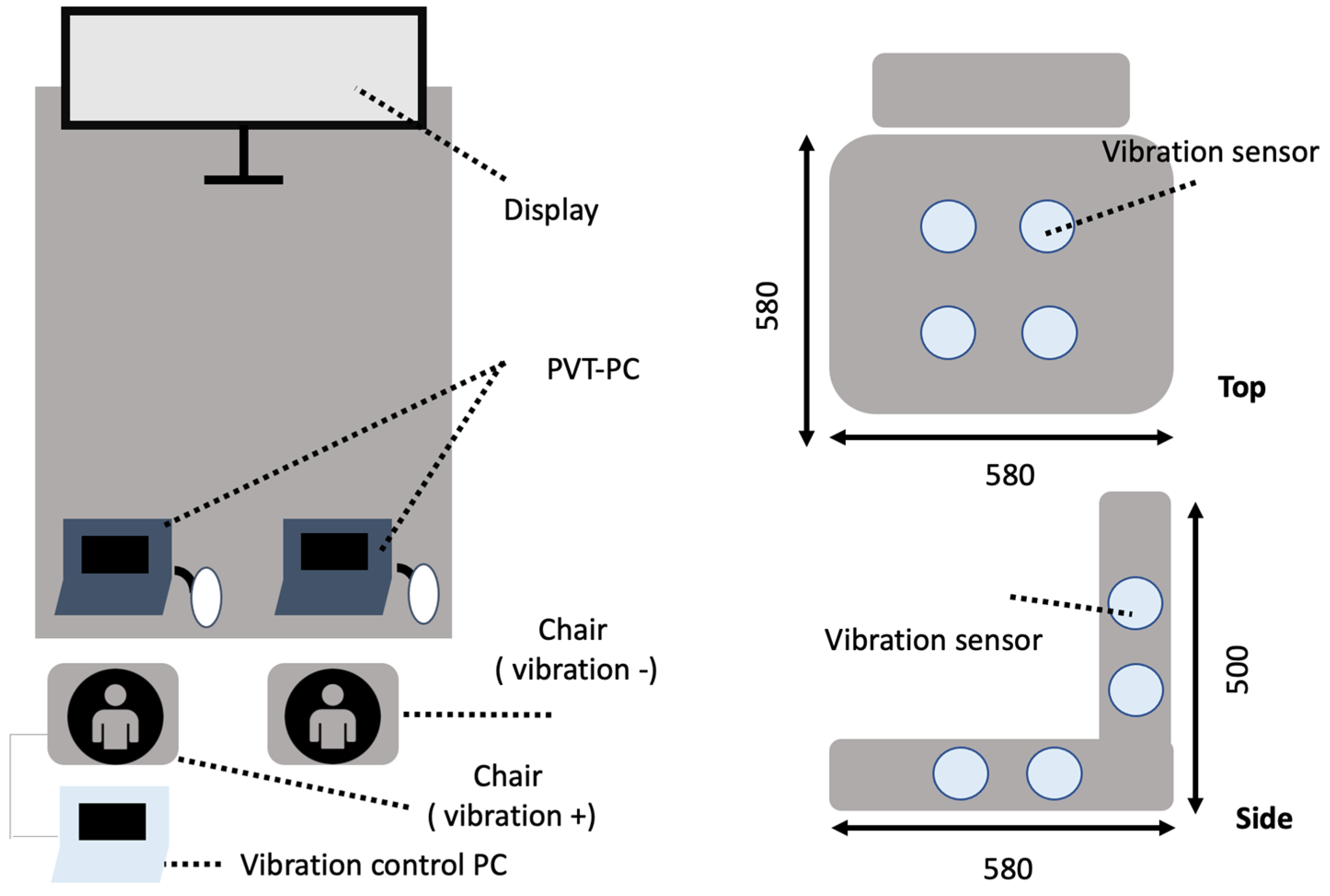
2.2. Experimental Protocol
- (1)
- Visual Stimulus
- (2)
- Vibration Conditions
- Vibration (Oscillation+): A rhythmic vibration at 2 Hz was applied to the seat to simulate the natural rhythm of spontaneous breathing (~4 s per cycle). The 2 Hz frequency was specifically chosen because it provides rhythmic stimulation without inducing discomfort, aligning with the natural breathing cycle and the resonant frequency of the seated human body. Four actuators located from the buttocks to the back were sequentially activated at 0.5-s intervals. (The low-frequency range used in this experiment (approximately 1–20 Hz) aligns with the definitions established in ISO 2631-1 and previous studies on the perceptual and physiological effects of whole-body vibration. In particular, the 2 Hz frequency applied in this study was selected because it closely matches the natural resonant frequency of the seated human body. This frequency has been associated with modulation of arousal and postural control in prior research, making it a relevant parameter for investigating the physiological effects of rhythmic seat vibration.)
- No Vibration (Oscillation−): Participants sat in the same chair without any vibration and watched the same video in a static condition.
- (3)
- Experimental Design
- (4)
- Measured Variables
- (5)
- Postural stability evaluation
2.3. Statistical Analysis
3. Results
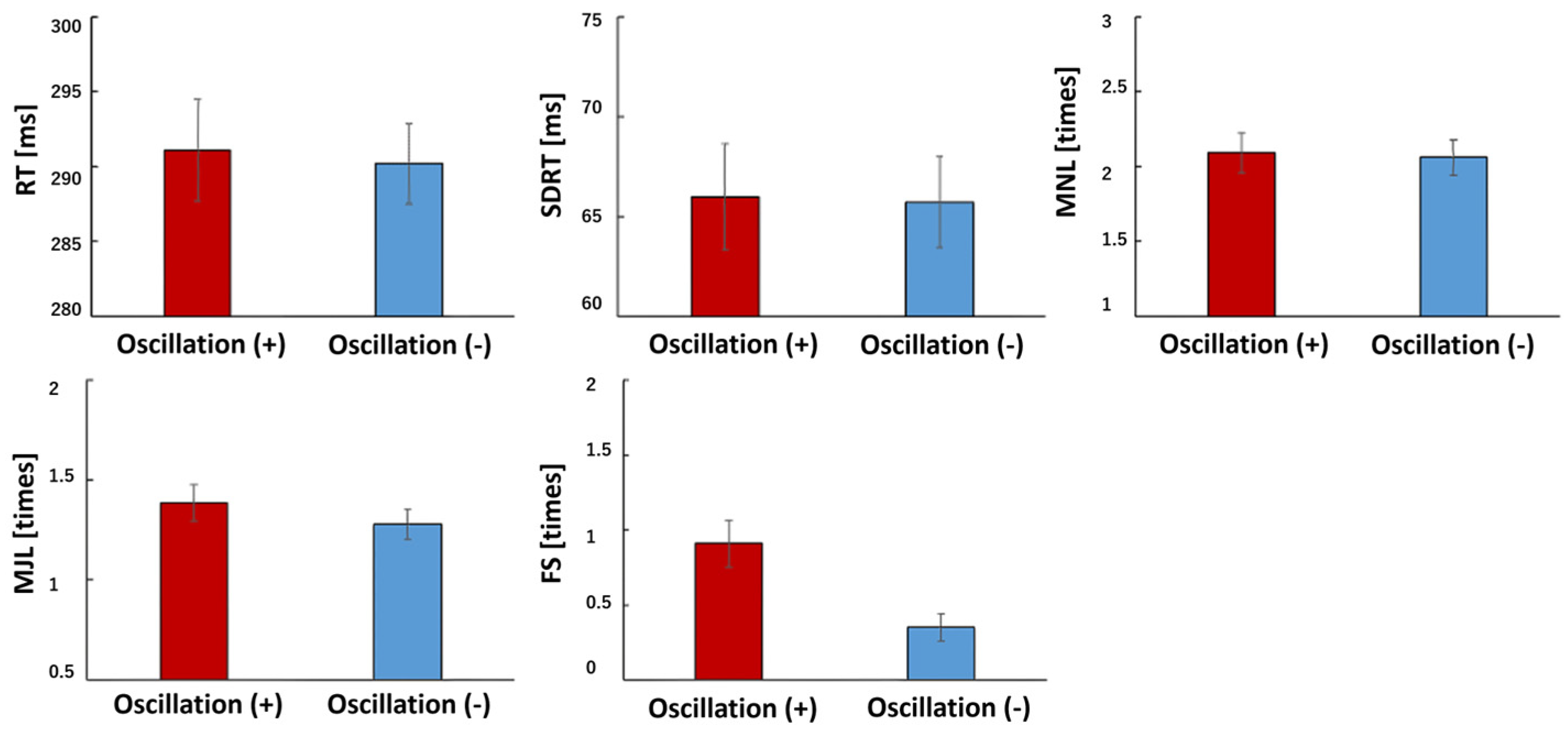
3.1. PVT
3.2. Seat Pressure Distribution
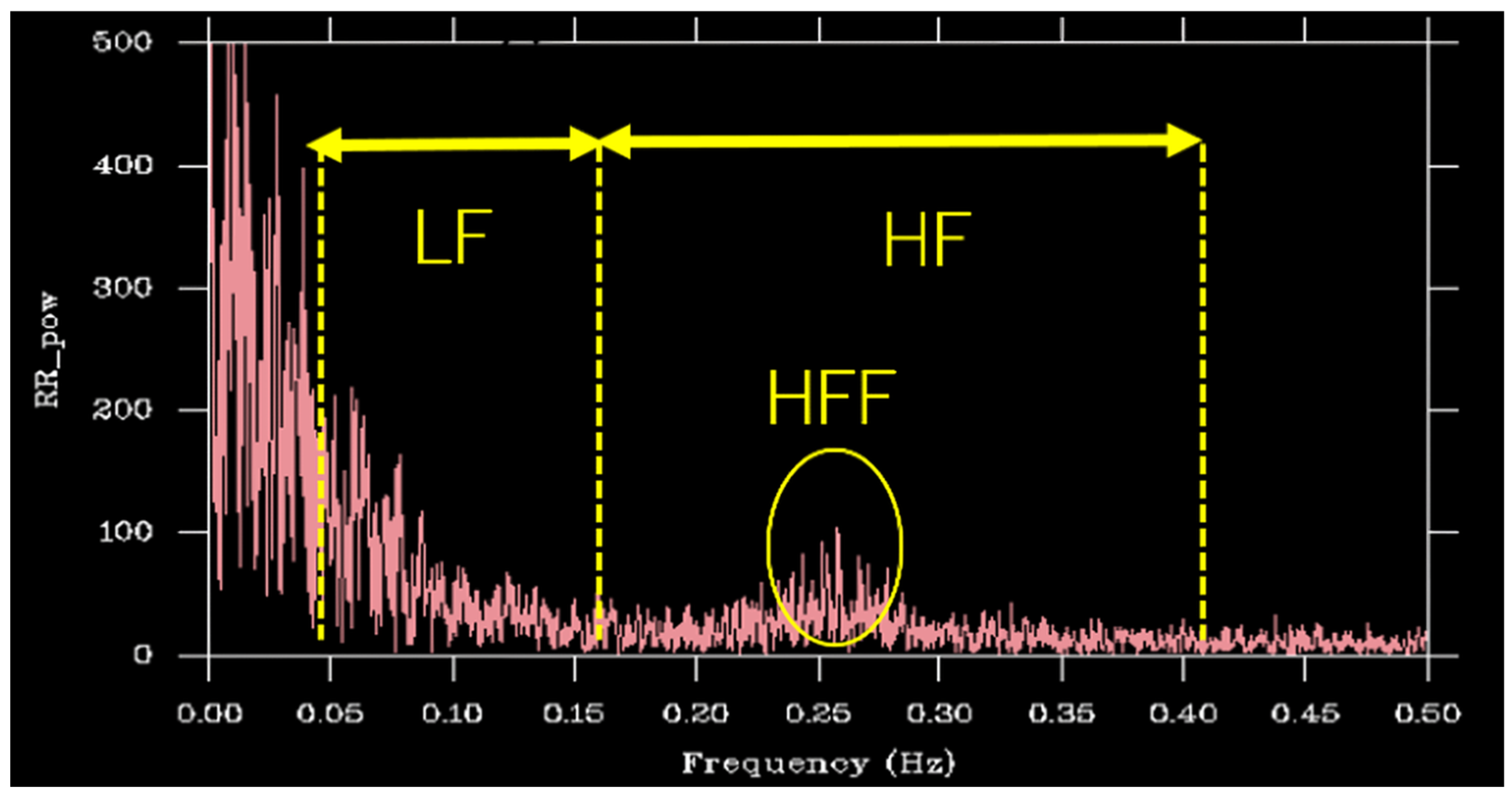
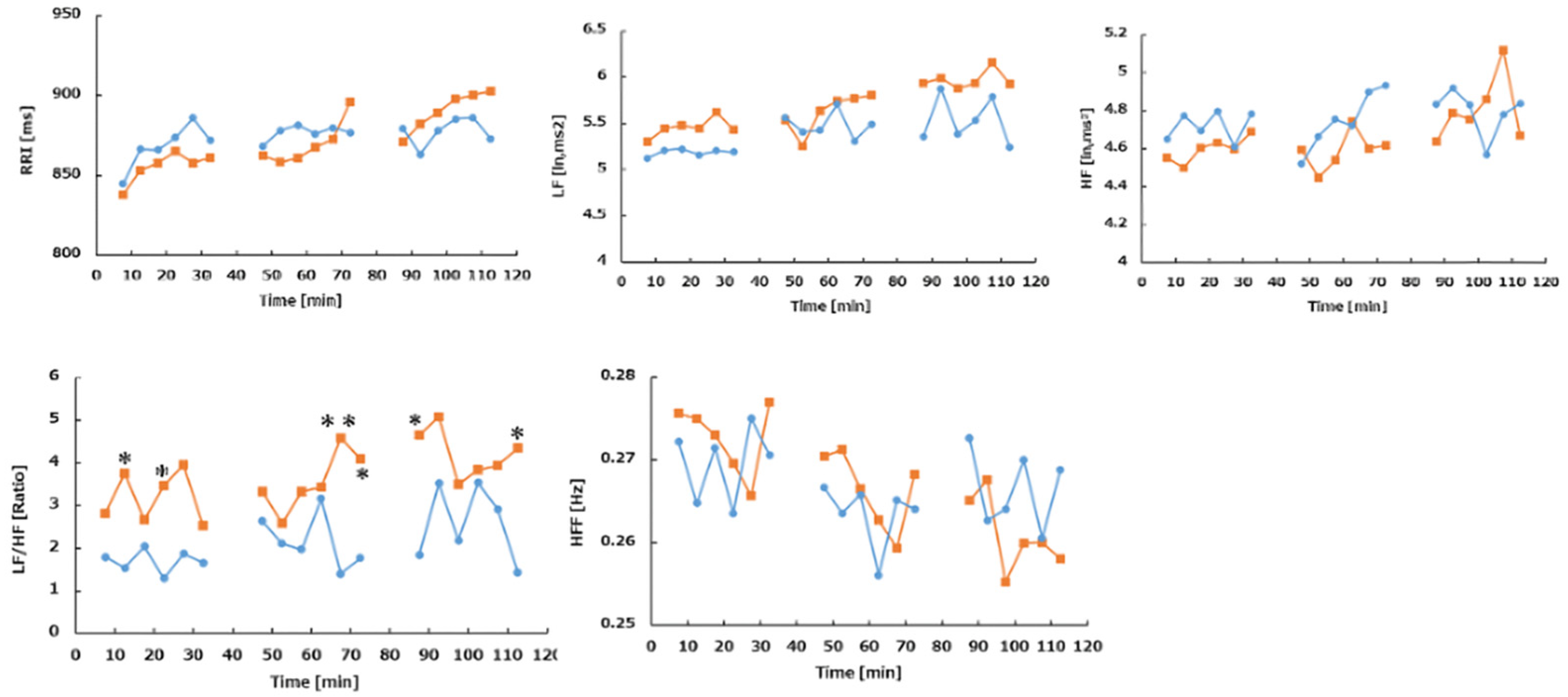
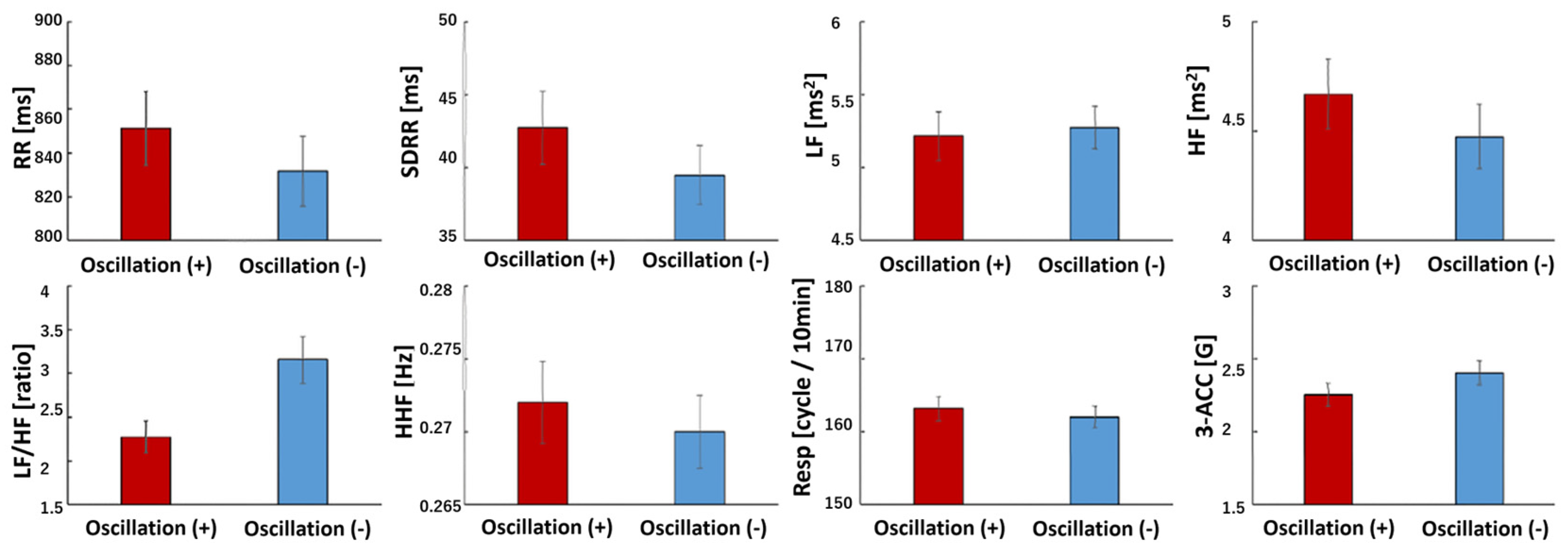
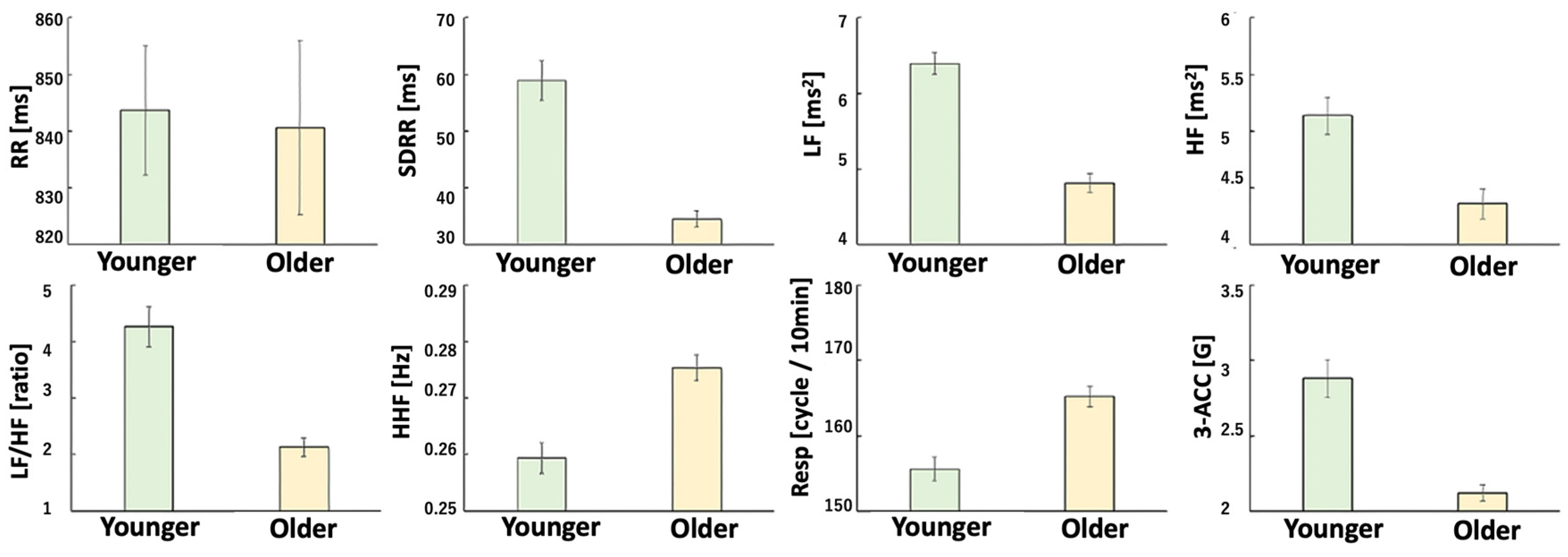
3.3. HRV Indices
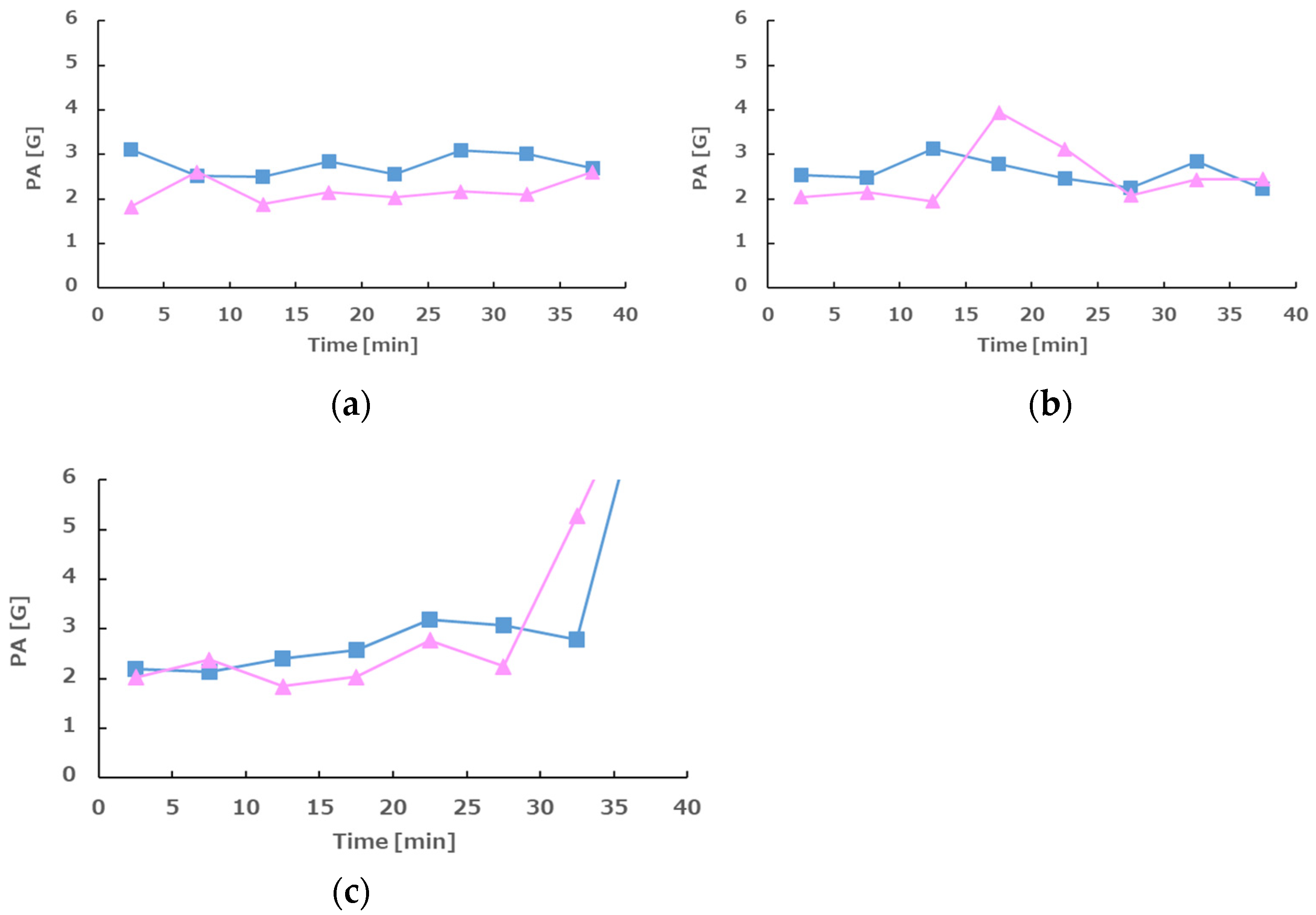
3.4. Body Acceleration (Physical Activity, PA)
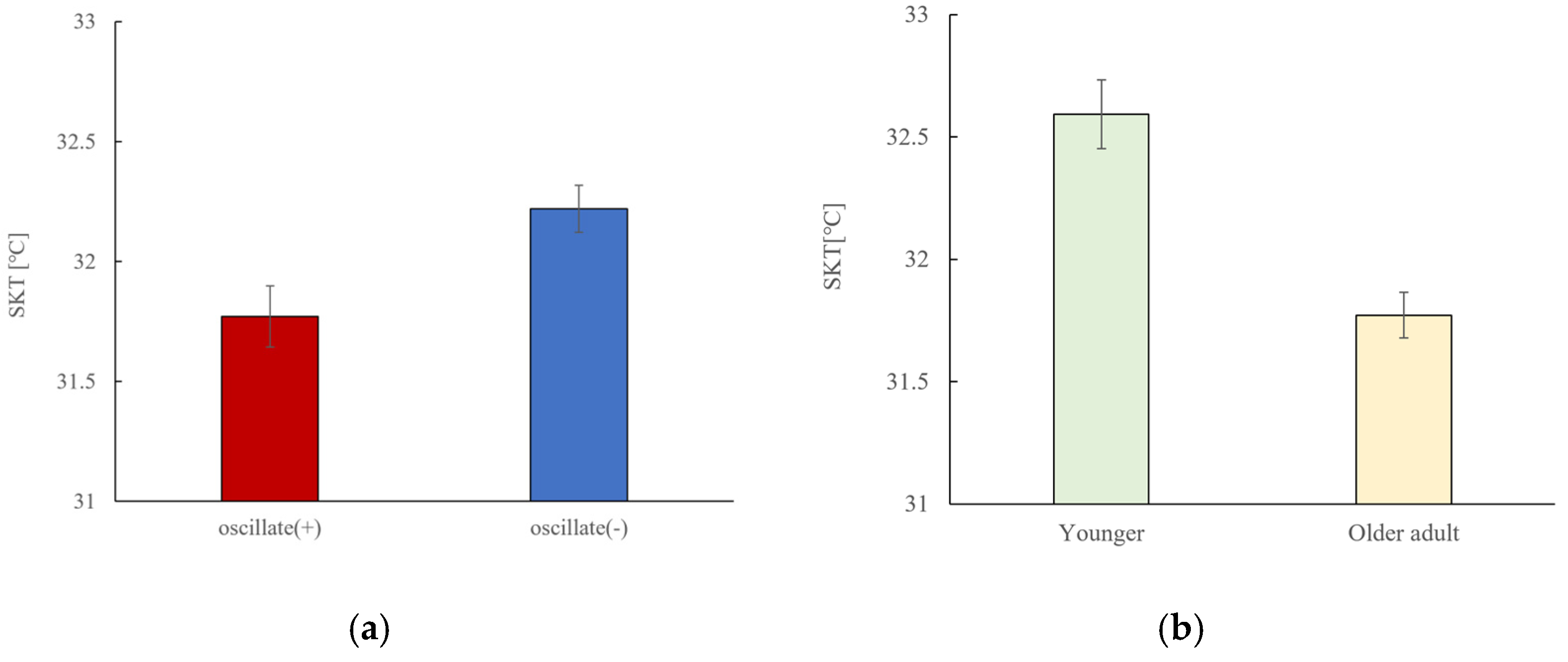
3.5. Skin Temperature
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harrison, D.D.; Harrison, S.O.; Croft, A.C.; Harrison, D.E.; Troyanovich, S.J. Sitting biomechanics, part II: Optimal car driver’s seat and optimal driver’s spinal model. J. Manip. Physiol. Ther. 2000, 23, 37–47. [Google Scholar] [CrossRef]
- Mansfield, N.; Sammonds, G.; Nguyen, L. Driver discomfort in vehicle seats—Effect of changing road conditions and seat foam composition. Appl. Ergon. 2015, 50, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Dennerlein, J.T.; Chang, C.C.; Chang, W.R.; Christiani, D.C. Seat inclination, use of lumbar support and low-back pain of taxi drivers. Scand. J. Work Environ. Health 2005, 31, 258–265. [Google Scholar] [CrossRef] [PubMed]
- de Vries, S.C.; van Erp, J.B.; Kiefer, R.J. Direction coding using a tactile chair. Appl. Ergon. 2009, 40, 477–484. [Google Scholar] [CrossRef]
- Burkhard, G.; Berger, T.; Enders, E.; Schramm, D. An extended model of the ISO-2631 standard to objectify the ride comfort in autonomous driving. Work 2021, 68, S37–S45. [Google Scholar] [CrossRef]
- Bellem, H.; Klüver, M.; Schrauf, M.; Schöner, H.P.; Hecht, H.; Krems, J.F. Can We Study Autonomous Driving Comfort in Moving-Base Driving Simulators? A Validation Study. Hum. Factors 2017, 59, 442–456. [Google Scholar] [CrossRef]
- Caballero-Bruno, I.; Wohllebe, T.; Töpfer, D.; Hernández-Castellano, P.M. The Effect of Seating Recline on Sleep Quality, Comfort and Pressure Distribution in Moving Autonomous Vehicles. Appl. Ergon. 2022, 105, 103844. [Google Scholar] [CrossRef]
- Mansfield, N.J.; Walia, K.; Singh, A. Driver Seat Comfort for Level 3–4 Autonomous Vehicles. Work 2021, 68, S111–S118. [Google Scholar] [CrossRef]
- Chourpiliadis, C.; Bhardwaj, A. Physiology, Respiratory Rate. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK537306/ (accessed on 12 July 2025).
- Russo, M.A.; Santarelli, D.M.; O’Rourke, D. The physiological effects of slow breathing in the healthy human. Breathe 2017, 13, 298–309. [Google Scholar] [CrossRef]
- Pleil, J.D.; Geer Wallace, M.A.; Davis, M.D.; Matty, C.M. The physics of human breathing: Flow, timing, volume, and pressure parameters for normal, on-demand, and ventilator respiration. J. Breath Res. 2021, 15. [Google Scholar] [CrossRef] [PubMed]
- Lantoine, P.; Lecocq, M.; Bougard, C.; Dousset, E.; Marqueste, T.; Bourdin, C.; Allègre, J.M.; Bauvineau, L.; Mesure, S. Car seat impact on driver’s sitting behavior and perceived discomfort during prolonged real driving on varied road types. PLoS ONE 2021, 16, e0259934. [Google Scholar] [CrossRef]
- El Falou, W.; Duchêne, J.; Grabisch, M.; Hewson, D.; Langeron, Y.; Lino, F. Evaluation of driver discomfort during long-duration car driving. Appl. Ergon. 2003, 34, 249–255. [Google Scholar] [CrossRef]
- Lecocq, M.; Lantoine, P.; Bougard, C.; Allègre, J.M.; Bauvineau, L.; González, D.; Bourdin, C.; Marqueste, T.; Dousset, E. Perceived Discomfort and Neuromuscular Fatigue during Long-Duration Real Driving with Different Car Seats. PLoS ONE 2022, 17, e0278131. [Google Scholar] [CrossRef]
- Lecocq, M.; Lantoine, P.; Bougard, C.; Allègre, J.M.; Bauvineau, L.; Bourdin, C.; Marqueste, T.; Dousset, E. Neuromuscular Fatigue Profiles Depend on Seat Feature during Long Duration Driving on a Static Simulator. Appl. Ergon. 2020, 87, 103118. [Google Scholar] [CrossRef]
- De Carvalho, D.; Randhawa, K.; Verville, L.; Hogg-Johnson, S.; Howarth, S.J.; Liang, C.; Mior, S.; Côté, P. The Vehicle Seating Intervention Trial: Cross-Over Randomized Controlled Trial to Evaluate the Impact of 2 Car Seat Configurations on Spinal Posture. J. Appl. Biomech. 2024, 40, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Crowther, Z.; Makhsous, M. Reducing whole-body vibration of vehicle drivers with a new sitting concept. In Proceedings of the 26th Annual International Conference of the IEEE EMBS, San Francisco, CA, USA, 1–5 September 2004; pp. 5111–5114. [Google Scholar] [CrossRef]
- Zhang, N.; Fard, M.; Bhuiyan, M.H.U.; Verhagen, D.; Azari, M.F.; Robinson, S.R. The effects of physical vibration on heart rate variability as a measure of drowsiness. Ergonomics 2018, 61, 1259–1272. [Google Scholar] [CrossRef] [PubMed]
- Vrazic, S. Robust driver heartbeat estimation: A q-Hurst exponent based automatic sensor change with interactive multi-model EKF. In Proceedings of the 37th Annual International Conference of the IEEE EMBS, Milan, Italy, 25–29 August 2015; pp. 2762–2766. [Google Scholar] [CrossRef]
- Moridani, M.K.; Mahabadi, Z.; Javadi, N. Heart Rate Variability Features for Different Stress Classification. Bratisl. Lek. Listy 2020, 121, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Servant, D.; Logier, R.; Mouster, Y.; Goudemand, M. Heart Rate Variability. Appl. Psychiatry Enceph. 2009, 35, 423–428. (In French) [Google Scholar] [CrossRef]
- Rudics, E.; Buzás, A.; Pálfi, A.; Szabó, Z.; Nagy, Á.; Hompoth, E.A.; Dombi, J.; Bilicki, V.; Szendi, I.; Dér, A. Quantifying Stress and Relaxation: A New Measure of Heart Rate Variability as a Reliable Biomarker. Biomedicines 2025, 13, 81. [Google Scholar] [CrossRef]
- Giorgi, F.; Tedeschi, R. Breathe Better, Live Better: The Science of Slow Breathing and Heart Rate Variability. Acta Neurol. Belg. 2025, 1–9. [Google Scholar] [CrossRef]
- Hernando, A.; Lazaro, J.; Gil, E.; Arza, A.; Garzon, J.M.; Lopez-Anton, R.; de la Camara, C.; Laguna, P.; Aguilo, J.; Bailon, R. Inclusion of Respiratory Frequency Information in Heart Rate Variability Analysis for Stress Assessment. IEEE J. Biomed. Health Inform. 2016, 20, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, H.; Al Khalili, Y. Physiology, Vibratory Sense. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Oliveri, D.J.; Lynn, K.; Hong, C.Z. Increased skin temperature after vibratory stimulation. Am. J. Phys. Med. Rehabil. 1989, 68, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Verrillo, R.T.; Bolanowski, S.J. Effects of temperature on the subjective magnitude of vibration. Somatosens. Mot. Res. 2003, 20, 133–137. [Google Scholar] [CrossRef]
- Forouharmajd, F.; Yadegari, M.; Ahmadvand, M.; Forouharmajd, F.; Pourabdian, S. Hand-arm Vibration Effects on Performance, Tactile Acuity, and Temperature of Hand. J. Med. Signals Sens. 2017, 7, 252–260. [Google Scholar]
- Jalilian, H.; Zamanian, Z.; Gorjizadeh, O.; Riaei, S.; Monazzam, M.R.; Abdoli-Eramaki, M. Autonomic Nervous System Responses to Whole-Body Vibration and Mental Workload: A Pilot Study. Int. J. Occup. Environ. Med. 2019, 10, 174–184. [Google Scholar] [CrossRef]
- Liu, K.C.; Wang, J.S.; Hsu, C.Y.; Liu, C.H.; Chen, C.P.; Huang, S.C. Low-Frequency Vibration Facilitates Post-Exercise Cardiovascular Autonomic Recovery. J. Sports Sci. Med. 2021, 20, 431–437. [Google Scholar] [CrossRef]
- Kim, D.; Kim, N.; Lee, Y.; Kim, S.; Kwon, J. Sound stimulation using the individual’s heart rate to improve the stability and homeostasis of the autonomic nervous system. Physiol. Rep. 2023, 11, e15816. [Google Scholar] [CrossRef]
- Makhsous, M.; Hendrix, R.; Crowther, Z.; Nam, E.; Lin, F. Reducing whole-body vibration and musculoskeletal injury with a new car seat design. Ergonomics 2005, 48, 1183–1199. [Google Scholar] [CrossRef]
- Naddeo, A.; Di Brigida, L.; Fontana, C.; Montese, J.; Quartuccia, M.; Nasti, M.; Pisani, M.M.; Turco, V.; De Stefano, M.; Fiorillo, I.; et al. A Body-Shaped Lumbar-Sacral Support for Improving Car-Seat Comfort. Work 2021, 68, S129–S138. [Google Scholar] [CrossRef]
- Jürgens, H.W. Seat Pressure Distribution. Coll. Antropol. 1997, 21, 359–366. [Google Scholar] [PubMed]
- Kim, E.; Fard, M.; Kato, K. Characterisation of the Human-Seat Coupling in Response to Vibration. Ergonomics 2017, 60, 1085–1100. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, N.J.; Griffin, M.J. Difference thresholds for automobile seat vibration. Appl. Ergon. 2000, 31, 255–261. [Google Scholar] [CrossRef]
- Petermeijer, S.M.; Cieler, S.; de Winter, J.C.F. Comparing spatially static and dynamic vibrotactile take-over requests in the driver seat. Accid. Anal. Prev. 2017, 99, 218–227. [Google Scholar] [CrossRef]
- Lewis, C.A.; Johnson, P.W. Whole-body vibration exposure in metropolitan bus drivers. Occup. Med. 2012, 62, 519–524. [Google Scholar] [CrossRef]
- Funakoshi, M.; Taoda, K.; Tsujimura, H.; Nishiyama, K. Measurement of whole-body vibration in taxi drivers. J. Occup. Health 2004, 46, 119–124. [Google Scholar] [CrossRef]
- Rohlmann, A.; Hinz, B.; Blüthner, R.; Graichen, F.; Bergmann, G. Loads on a spinal implant measured in vivo during whole-body vibration. Eur. Spine J. 2010, 19, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- de Groot, S.; de Winter, J.C.; López García, J.M.; Mulder, M.; Wieringa, P.A. The effect of concurrent bandwidth feedback on learning the lane-keeping task in a driving simulator. Hum. Factors 2011, 53, 50–62. [Google Scholar] [CrossRef] [PubMed]


| Measure | Timing | Frequency |
|---|---|---|
| PVT | At the beginning and end of each phase | 6 times total |
| Seat Pressure Mapping | At the beginning and end of each phase | 6 times total |
| Skin Temperature | Continuously throughout each 120-min session | 1-min averages |
| RRI/Body Accel. | Continuously throughout each 120-min session | Full-session recording |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuda, E.; Yoshida, Y.; Sato, K.; Sakamoto, H.; Takahashi, M. Effects of Seat Vibration on Biometric Signals and Postural Stability in a Simulated Autonomous Driving Environment. Sensors 2025, 25, 6039. https://doi.org/10.3390/s25196039
Yuda E, Yoshida Y, Sato K, Sakamoto H, Takahashi M. Effects of Seat Vibration on Biometric Signals and Postural Stability in a Simulated Autonomous Driving Environment. Sensors. 2025; 25(19):6039. https://doi.org/10.3390/s25196039
Chicago/Turabian StyleYuda, Emi, Yutaka Yoshida, Kunio Sato, Hideki Sakamoto, and Makoto Takahashi. 2025. "Effects of Seat Vibration on Biometric Signals and Postural Stability in a Simulated Autonomous Driving Environment" Sensors 25, no. 19: 6039. https://doi.org/10.3390/s25196039
APA StyleYuda, E., Yoshida, Y., Sato, K., Sakamoto, H., & Takahashi, M. (2025). Effects of Seat Vibration on Biometric Signals and Postural Stability in a Simulated Autonomous Driving Environment. Sensors, 25(19), 6039. https://doi.org/10.3390/s25196039









