Automated Shoulder Girdle Rigidity Assessment in Parkinson’s Disease via an Integrated Model- and Data-Driven Approach
Abstract
Highlights
- A hybrid framework integrating model-driven (damping ratio, decay rate) and data-driven (maximum detail coefficient) features via weak supervision achieved a strong correlation (r = 0.78, p < 0.001) with UPDRS rigidity scores, outperforming traditional Wartenberg pendulum test metrics like maximum velocity.
- The integrated model improved PD/HC classification accuracy by 10% over data-driven methods, with damping ratio and maximum detail coefficient identified as highly predictive biomarkers.
- Combining biomechanical and statistical features through weak supervision enables objective, interpretable shoulder rigidity assessment in Parkinson’s Disease. The results suggest that rigidity, generally considered velocity-independent, can be inferred by velocity-dependent features like the damping ratio.
- Because rigidity assessment typically requires in-person, hands-on examination, wearable sensors in the current framework enable scalable, remote monitoring that facilitates earlier diagnosis and ongoing longitudinal tracking within telemedicine settings.
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Ethical Approval
2.2. Experimental Protocol and Data Collection
Wearable Sensors
2.3. Feature Extraction
2.3.1. Model-Driven Features
2.3.2. Data-Driven Features
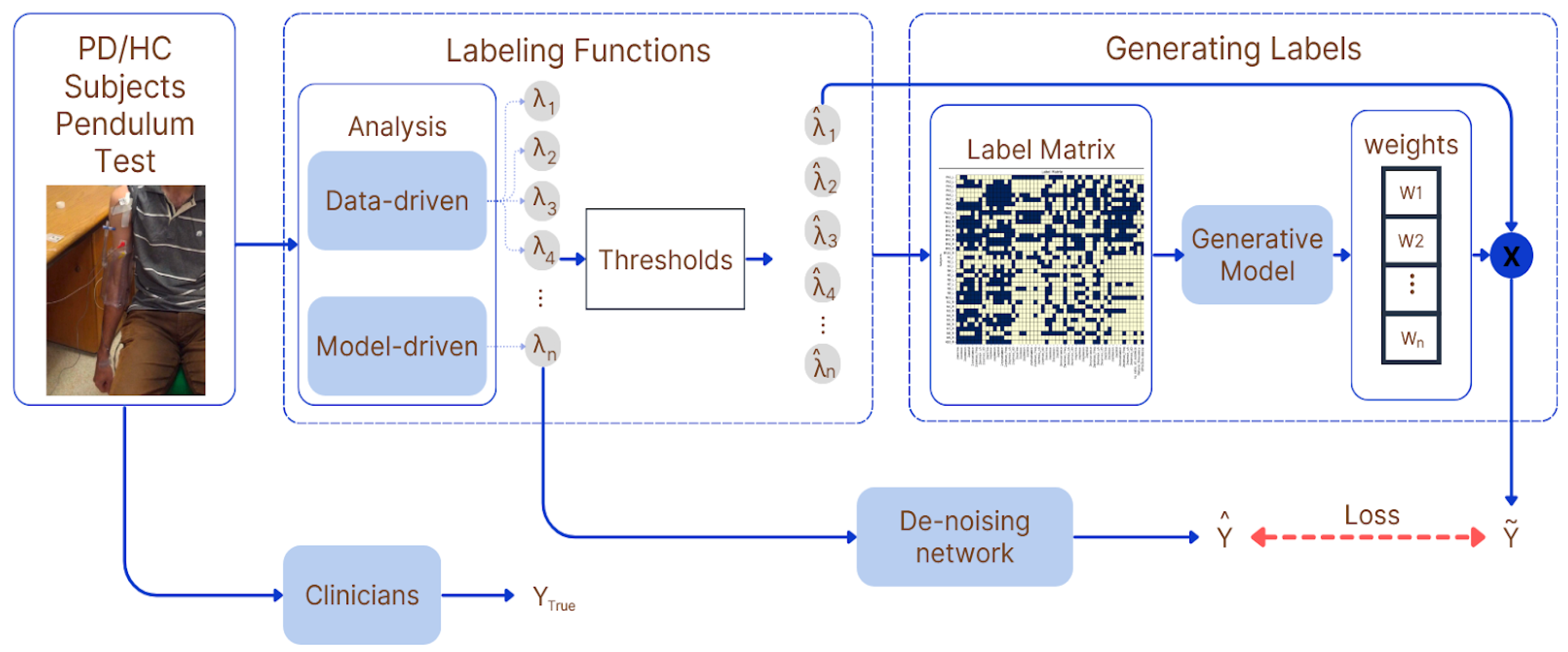
2.4. Weak Supervision and Label Generation
2.5. Cross-Validation Strategy
2.6. Feature Fusion and Classification
3. Results
3.1. PD/HC Classification
3.2. Rigidity Score Estimation
4. Discussion
4.1. Advantages of the Integrated Approach
4.2. Role of Weak Supervision in Harmonizing Features
4.3. Refining Traditional Pendulum Tests with Novel Biomarkers
4.4. Re-Evaluating Rigidity’s Biomechanical Dynamics
4.5. Limitations and Future Work
4.6. Clinical Implications and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PD | Parkinson’s Disease |
| UPDRS | Unified Parkinson’s Disease Rating Scale |
| LTI | Linear Time Invariant |
References
- Dorsey, E.R.; Sherer, T.; Okun, M.S.; Bloem, B.R. The Emerging Evidence of the Parkinson Pandemic. J. Park. Dis. 2018, 8 (Suppl. S1), S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Sánchez, M.R.; Moreno-Verdú, M.; Cano-de-la-Cuerda, R. Quantitative Measurement of Rigidity in Parkinson’s Disease: A Systematic Review. Sensors 2020, 20, 880. [Google Scholar] [CrossRef] [PubMed]
- Martino, G.; McKay, J.L.; Factor, S.A.; Ting, L.H. Neuromechanical Assessment of Activated vs. Resting Leg Rigidity Using the Pendulum Test Is Associated with a Fall History in People with Parkinson’s Disease. Front. Hum. Neurosci. 2020, 14, 602595. [Google Scholar] [CrossRef] [PubMed]
- Werning, A.; Umbarila, D.; Fite, M.; Fergus, S.; Zhang, J.; Molnar, G.F.; Johnson, L.A.; Wang, J.; Vitek, J.L.; Escobar Sanabria, D. Quantifying Viscous Damping and Stiffness in Parkinsonism Using Data-Driven Model Estimation and Admittance Control. J. Med. Devices 2022, 16, 041004. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Xu, Y.; Li, X.; Fan, Y.; Zeng, W.; Tan, Y.; Ren, K.; Chen, W.; Cao, X. Evaluation of Wearable Sensor Devices in Parkinson’s Disease: A Review of Current Status and Future Prospects. Park. Dis. 2020, 2020, 4693019. [Google Scholar] [CrossRef] [PubMed]
- Karniadakis, G.E.; Kevrekidis, I.G.; Lu, L.; Perdikaris, P.; Wang, S.; Yang, L. Physics-informed machine learning. Nat. Rev. Phys. 2021, 3, 422–440. [Google Scholar] [CrossRef]
- Ratner, A.; Bach, S.H.; Ehrenberg, H.; Fries, J.; Wu, S.; Ré, C. Snorkel: Rapid training data creation with weak supervision. Proc. VLDB Endow. 2017, 11, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Cebulla, A.; Panev, S.; Hodgins, J.; De la Torre, F. Weakly-supervised learning for Parkinson’s Disease tremor detection. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Jeju, Republic of Korea, 11–15 July 2017; IEEE: New York, NY, USA, 2017; pp. 143–147. [Google Scholar] [CrossRef]
- Yue, P.; Li, Z.; Zhou, M.; Wang, X.; Yang, P. Wearable-Sensor-Based Weakly Supervised Parkinson’s Disease Assessment with Data Augmentation. Sensors 2024, 24, 1196. [Google Scholar] [CrossRef] [PubMed]
- Djurić-Jovicić, M.D.; Jovicić, N.S.; Radovanović, S.M.; Stanković, I.D.; Popović, M.B.; Kostić, V.S. Automatic identification and classification of freezing of gait episodes in Parkinson’s disease patients. IEEE Trans. Neural Syst. Rehabil. Eng. A Publ. IEEE Eng. Med. Biol. Soc. 2014, 22, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Ashoori, A. Analysis of Motor Performance in Parkinson’s Disease Through LTI Dynamical Systems; University of British Columbia: Vancouver, BC, Canada, 2014. [Google Scholar] [CrossRef]
- Cooper, P. DeJong’s The Neurologic Examination. 2005. Sixth edition. By William W. Campbell. Published by Lippincott, Williams & Wilkins. 671 pages. C$140 approx. Can. J. Neurol. Sci. J. Can. Des Sci. Neurol. 2005, 32, 558. [Google Scholar] [CrossRef]
- Patera, M.; Zampogna, A.; Pietrosanti, L.; Asci, F.; Falletti, M.; Pinola, G.; Bianchini, E.; Di Lazzaro, G.; Rosati, V.; Grillo, P.; et al. Abnormal arm swing movements in Parkinson’s disease: Onset, progression and response to L-Dopa. J. Neuroeng. Rehabil. 2025, 22, 47. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Mahoney, J.M.; Lewis, M.M.; Du, G.; Piazza, S.J.; Cusumano, J.P. Both coordination and symmetry of arm swing are reduced in Parkinson’s disease. Gait Posture 2012, 35, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.T.; MacDougall, H.G.; Ondo, W.G. Ambulatory monitoring of freezing of gait in Parkinson’s disease. J. Neurosci. Methods 2008, 167, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Gourrame, K.; Griškevičius, J.; Haritopoulos, M.; Lukšys, D.; Jatužis, D.; Kaladytė-Lokominienė, R.; Bunevičiūtė, R.; Mickutė, G. Parkinson’s disease classification with CWNN: Using wavelet transformations and IMU data fusion for improved accuracy. Technol. Health Care Off. J. Eur. Soc. Eng. Med. 2023, 31, 2447–2455. [Google Scholar] [CrossRef] [PubMed]
- Caramia, C.; Torricelli, D.; Schmid, M.; Munoz-Gonzalez, A.; Gonzalez-Vargas, J.; Grandas, F.; Pons, J.L. IMU-Based Classification of Parkinson’s Disease from Gait: A Sensitivity Analysis on Sensor Location and Feature Selection. IEEE J. Biomed. Health Inform. 2008, 22, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Carvajal-Castaño, H.A.; Pérez-Toro, P.A.; Orozco-Arroyave, J.R. Classification of Parkinson’s Disease Patients—A Deep Learning Strategy. Electronics 2022, 11, 2684. [Google Scholar] [CrossRef]
- Rovini, E.; Maremmani, C.; Cavallo, F. How Wearable Sensors Can Support Parkinson’s Disease Diagnosis and Treatment: A Systematic Review. Front. Neurosci. 2017, 11, 555. [Google Scholar] [CrossRef] [PubMed]
- Moreau, C.; Rouaud, T.; Grabli, D.; Benatru, I.; Remy, P.; Marques, A.-R.; Drapier, S.; Mariani, L.-L.; Roze, E.; Devos, D.; et al. Overview on wearable sensors for the management of Parkinson’s disease. Npj Park. Dis. 2023, 9, 153. [Google Scholar] [CrossRef] [PubMed]


| Method | Accuracy | Precision | F1-Score |
|---|---|---|---|
| Integrated Approach (Ours) | 0.71 | 0.71 | 0.71 |
| Data-Driven Approach (Ours) | 0.66 | 0.67 | 0.64 |
| Model-Driven Approach (Ours) | 0.71 | 0.72 | 0.70 |
| Baseline (RF, Leave-One-Out) | 0.73 | 0.36 | 0.34 |
| Baseline (DT, Leave-One-Out) | 0.60 | 0.31 | 0.23 |
| Baseline (RF, 5-Fold Cross Validation) | 0.70 | 0.75 | 0.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khosrobeygi, F.; Abouhadi, Z.; Mahdizadeh, A.; Ashoori, A.; Niksirat, N.; Mirian, M.S.; McKeown, M.J. Automated Shoulder Girdle Rigidity Assessment in Parkinson’s Disease via an Integrated Model- and Data-Driven Approach. Sensors 2025, 25, 6019. https://doi.org/10.3390/s25196019
Khosrobeygi F, Abouhadi Z, Mahdizadeh A, Ashoori A, Niksirat N, Mirian MS, McKeown MJ. Automated Shoulder Girdle Rigidity Assessment in Parkinson’s Disease via an Integrated Model- and Data-Driven Approach. Sensors. 2025; 25(19):6019. https://doi.org/10.3390/s25196019
Chicago/Turabian StyleKhosrobeygi, Fatemeh, Zahra Abouhadi, Ailar Mahdizadeh, Ahmad Ashoori, Negin Niksirat, Maryam S. Mirian, and Martin J. McKeown. 2025. "Automated Shoulder Girdle Rigidity Assessment in Parkinson’s Disease via an Integrated Model- and Data-Driven Approach" Sensors 25, no. 19: 6019. https://doi.org/10.3390/s25196019
APA StyleKhosrobeygi, F., Abouhadi, Z., Mahdizadeh, A., Ashoori, A., Niksirat, N., Mirian, M. S., & McKeown, M. J. (2025). Automated Shoulder Girdle Rigidity Assessment in Parkinson’s Disease via an Integrated Model- and Data-Driven Approach. Sensors, 25(19), 6019. https://doi.org/10.3390/s25196019







