A New Methodological Approach Integrating Motion Capture and Pressure-Sensitive Gait Data to Assess Functional Mobility in Parkinson’s Disease: A Two-Phase Study
Abstract
Highlights
- We report the development of a novel measure of functional mobility (FMA-P) that provides detailed insight into symptom-related movement impairments in Parkinson’s disease.
- By integrating motion capture and pressure-sensitive gait mat technology, the FMA-P evaluates multiple aspects of mobility—including balance, posture, gait, sit-to-stand transitions, turning, and reaching—that are often overlooked by standard assessments focused primarily on task duration.
- Specific movement tasks, particularly those involving yaw rotation, are sensitive indicators of changes in Parkinson’s disease symptom severity.
- Rehabilitation programs should prioritize these tasks, as targeting them may optimize functional gains and better monitor disease progression.
Abstract
1. Introduction
2. Materials and Methods
- Study 1 was a cross-sectional, mixed-methods study primarily focused on the development of the FMA-P, alongside its preliminary evaluation.
- Study 2 applied the FMA-P in a repeated-measures design to evaluate its sensitivity to change following an intervention program.
2.1. Participants and Ethical Considerations
2.2. Materials
2.2.1. Motion Capture System
2.2.2. Walkway Gait Analysis System
2.3. Study 1: Development and Pilot Testing of the Functional Mobility Assessment for Parkinson’s (FMA-P)
2.3.1. Development of the FMA-P Study Protocol
- Sit-to-stand and Stand-to-sit
- Turning
- Functional reach
2.3.2. Additional Tasks
- Standing upright
- Locomotion
- Freezing
2.3.3. FMA-P Performance Score
2.3.4. Timed up and Go (TUG)
2.3.5. The WHO−5 Measure of Wellbeing
2.3.6. Study Design and Procedure
2.3.7. Data Processing and Functional Mobility Analysis
- Sit-to-stand and Stand-to-Sit
- Turning
- Functional reach
- Standing upright
- Locomotion

- Freezing
2.3.8. Performance Score
2.3.9. Statistical Analysis: Analysis of Covariance for Group and Protocol Differences
- The number of parameters considered for the Bonferroni corrections was defined by parameter type, such as spatiotemporal (e.g., duration), kinetic (e.g., COP), and upper/lower kinematics (e.g., trunk inclination, stride velocity). In the spatiotemporal category, only one parameter was compared across groups and protocols, and no Bonferroni correction was applied to this parameter.
- The Shapiro–Wilk test was applied to assess the normality of our data. Sphericity was assessed using Mauchly’s test. Data with sphericity assumption violation was corrected using the Greenhouse-Geiser method. To control observed and unobserved differences in our sample, age, sex, and body height as fixed effects were included in all analyses. Foot length was added as a covariate for biomechanical parameters such as TO angle, HS angle, and foot height comparisons.
2.4. Study 2: Application of the FMA-P to Measure Functional Mobility Outcomes of a Music and Movement-Based Intervention
2.4.1. Study Design
2.4.2. Statistical Analysis: RM-ANOVA of Intervention Phases
3. Results
3.1. Results of Study 1
3.1.1. Complementary Clinical Assessments
3.1.2. Clinical Measures
3.1.3. Biomechanical Analysis
- Sit-to-stand
- Turning
- Stand-to-sit
- Functional reach
3.1.4. Additional Tasks
- Standing upright
- Locomotion
- Freezing
3.1.5. Performance Score
3.2. Results of Study 2
3.2.1. Clinical Analysis and Performance Score
3.2.2. Biomechanical Analysis
- Sit-to-stand
- Turning
- Stand-to-sit
- Functional Reach
4. Discussion of Study 1
5. Discussion of Study 2
6. Implications, Strengths, and Limitations
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANCOVA | Analysis of covariance |
| AP | Antero-posterior |
| ASA | Arm asymmetry factor |
| COM | Center of mass |
| COP | Center of pressure |
| DBS | Deep Brain Stimulation |
| FLE | Freeze-like events |
| FMA-P | Functional Mobility Assessment in Parkinson’s |
| FOG | Freeze of gait |
| HS | Heel strike |
| MDS-UPDRS | Movement Disorders Society Unified Parkinson’s Disease Rating Scale |
| ML | Medio-lateral |
| MRI | Magnetic Resonance Imaging |
| PADLS | Parkinson’s disease Activities of Daily Living Scale |
| PD | Parkinson’s disease |
| PDQ-39 | Parkinson’s Disease Questionnaire |
| PLM | Postural-Locomotion-Manual Test |
| PwP | People with Parkinson’s |
| RMS | Root-mean-square |
| SEM | Standard Error of the Mean |
| TO | Toe-off |
| TUG | Timed Up and Go |
| WHO-5 | 5-item WHO Well-Being Index |
Appendix A
| Parameter | Group | TUG Mean (SD) | FMA-P Mean (SD) | Protocol Effect (p) | Group Effect (p) | Interaction (p) |
|---|---|---|---|---|---|---|
| Peak Trunk AP Inclination (deg) a | Control | 123.5 (2.7) | 124.7 (3.8) | 0.498 | 0.006 ** | 0.365 |
| PwP | 120.3 (3.2) | 119.7 (4.9) | ||||
| Peak Trunk ML Inclination (deg) a | Control | 4.8 (2.4) | 4.8 (1.7) | 0.159 | 0.105 | 0.151 |
| PwP | 5.1 (2.1) | 6.5 (2.5) | ||||
| Max. trunk velocity (m/s) a | Control | 1.5 (0.3) | 2.6 (2.4) | 0.089 | 0.597 | 0.246 |
| PwP | 1.2 (0.2) | 1.4 (1.1) | ||||
| RMS of trunk acceleration (m/s2) a | Control | 7.4 (1.8) | 17.0 (23.2) | 0.101 | 0.565 | 0.254 |
| PwP | 5.2 (1.4) | 6.9 (7.2) | ||||
| Mean trunk jerk (m/s3) a | Control | −4.0 (1.6) | −4.2 (1.5) | 0.270 | 0.003 ** | 0.687 |
| PwP | −2.0 (1.0) | −2.4 (1.5) | ||||
| Mean COP displacement in AP (mm) b | Control | −0.9 (1.7) | −1.6 (7.2) | 0.040 | <0.001 | 0.121 |
| PwP | 0.9 (5.2) | −2.7 (7.0) | ||||
| Mean COP displacement in ML (mm) b | Control | 1.0 (0.3) | 0.8 (13.3) | 0.572 | 1.000 | 0.938 |
| PwP | 2.1 (1.2) | −3.6 (6.5) |
| Parameter | Group | TUG Mean (SD) | FMA-P Mean (SD) | Protocol Effect (p) | Group Effect (p) | Interaction (p) |
|---|---|---|---|---|---|---|
| Task duration (s) | Control | 1.5 (0.3) | 1.2 (0.2) | 0.064 | 0.026 | 0.951 |
| PwP | 1.9 (0.5) | 1.6 (0.5) | ||||
| Mean pelvis jerk (m/s3) a | Control | −1.1 (0.7) | −1.5 (0.9) | 0.080 | 0.175 | 0.965 |
| PwP | −0.5 (0.4) | −0.9 (1.7) | ||||
| Absolute head yaw rotation (deg) a | Control | 202.3 (72.8) | 149.9 (14.7) | <0.001 | 0.052 | 0.245 |
| PwP | 200.4 (74.0) | 138.5 (11.9) | ||||
| Absolute trunk yaw rotation (deg)a | Control | 200.1 (74.1) | 161.8 (13.0) | 0.372 | 0.461 | 0.625 |
| PwP | 197.4 (75.6) | 146.8 (11.2) | ||||
| Absolute pelvis yaw rotation (deg) a | Control | 216.4 (66.8) | 160.2 (38.0) | <0.001 | 0.403 | 0.756 |
| PwP | 205.9 (71.7) | 155.1 (12.8) | ||||
| Head relative to trunk yaw rotation (deg) a | Control | 2.2 (4.7) | −11.9 (13.0) | <0.001 | 0.521 | 0.848 |
| PwP | 3.0 (5.4) | −8.4 (8.1) | ||||
| Trunk relative to pelvis yaw rotation (deg) a | Control | −16.3 (16.8) | 1.5 (37.7) | 0.257 | 0.780 | 0.156 |
| PwP | −8.4 (9.4) | −8.2 (11.3) | ||||
| Head relative to pelvis yaw rotation (deg) a | Control | −14.2 (14.4) | −10.3 (33.0) | 0.353 | 0.978 | 0.155 |
| PwP | −5.5 (7.7) | −16.6 (9.2) | ||||
| Head onset (%) a | Control | 3.5 (5.8) | 6.5 (1.2) | <0.001 | 0.218 | 0.904 |
| PwP | 3.1 (6.5) | 4.7 (0.9) | ||||
| Trunk onset (%) a | Control | 8.3 (8.1) | 9.3 (1.7) | 0.327 | 0.115 | 0.815 |
| PwP | 5.3 (8.3) | 6.9 (1.4) | ||||
| Pelvis onset (%) a | Control | 6.5 (6.3) | 2.9 (3.9) | 0.100 | 0.517 | 0.520 |
| PwP | 8.5 (7.4) | 5.8 (7.0) | ||||
| Max. trunk AP inclination (deg) a | Control | 3.4 (1.3) | 3.4 (1.6) | 0.691 | 0.213 | 0.738 |
| PwP | 2.3 (0.4) | 2.3 (1.0) | ||||
| Max. trunk ML inclination (deg) a | Control | 9.3 (2.7) | 10.3 (3.5) | 0.183 | 0.045 | 0.873 |
| PwP | 7.5 (3.7) | 8.9 (3.2) | ||||
| Mean toe-off angle (deg) b | Control | 17.3 (4.0) | 14.3 (4.7) | 0.168 | 0.509 | 0.167 |
| PwP | 12.2 (2.6) | 12.0 (3.0) | ||||
| Mean heel-strike angle (deg) b | Control | 6.9 (3.1) | 6.1 (3.5) | 0.527 | 0.007 | 0.209 |
| PwP | 5.8 (2.2) | 3.2 (2.4) | ||||
| Mean COP displacement in AP (mm) b | Control | 1.4 (2.4) | 2.1 (8.6) | 0.089 | 1.000 | 0.348 |
| PwP | 0.8 (2.4) | 1.3 (7.1) | ||||
| Mean COP displacement in ML (mm) b | Control | −4.1 (6.8) | 44.0 (24.4) | 0.752 | 1.000 | 0.072 |
| PwP | −1.7 (5.6) | 52.0 (23.2) |
| Parameter | Group | TUG Mean (SD) | FMA-P Mean (SD) | Protocol Effect (p) | Group Effect (p) | Interaction (p) |
|---|---|---|---|---|---|---|
| Peak Trunk AP Inclination (deg) a | Control | 109.8 (5.0) | 100.7 (26.3) | 0.629 | 0.105 | 0.114 |
| PwP | 105.7 (5.9) | 110.3 (11.7) | ||||
| Peak Trunk ML Inclination (deg) a | Control | 5.6 (4.4) | 6.0 (4.7) | 0.916 | 0.171 | 0.700 |
| PwP | 7.6 (4.7) | 6.6 (5.9) | ||||
| Max. trunk velocity (m/s) a | Control | 2.1 (2.5) | 2.0 (2.4) | 0.775 | 0.177 | 0.540 |
| PwP | 1.0 (0.2) | 1.5 (1.4) | ||||
| RMS of trunk acceleration (m/s2) a | Control | 8.5 (12.1) | 8.6 (13.0) | 0.580 | 0.187 | 0.580 |
| PwP | 2.9 (0.8) | 6.2 (9.1) | ||||
| Mean trunk jerk (m/s3) a | Control | 5.0 (2.4) | 5.0 (3.8) | 0.925 | 0.024 | 0.913 |
| PwP | 2.9 (1.5) | 2.8 (1.9) | ||||
| Max. pelvis flexion (deg) a | Control | 76.4 (16.4) | 78.4 (23.8) | 0.863 | 0.922 | 0.684 |
| PwP | 76.1 (23.3) | 75.7 (19.9) | ||||
| Max. knee flexion (deg) a | Control | 100.5 (5.5) | 115.4 (16.3) | 0.160 | 0.322 | 0.806 |
| PwP | 101.8 (5.3) | 109.8 (15.8) | ||||
| Mean COP displacement in AP (mm) b | Control | −2.4 (2.2) | 6.7 (13.8) | 0.046 | 1.000 | 0.156 |
| PwP | 1.5 (0.6) | 2.3 (12.6) | ||||
| Mean COP displacement in ML (mm) b | Control | −3.5 (6.7) | −33.5 (47.7) | 0.313 | 1.000 | 0.552 |
| PwP | −7.3 (2.1) | −48.0 (28.2) |
Appendix B
| Group | F | p | ηp2 | ||||
|---|---|---|---|---|---|---|---|
| PwP | Control | ||||||
| Parameter | M | 95% CI | M | 95% CI | |||
| Mean toe-off angle (deg) a | 22.4 | [21.2; 23.5] | 26.9 | [25.7; 28.1] | 24.3 | <0.001 *** | 0.15 |
| Mean heel-strike angle (deg) a | 9.1 | [8.3; 9.9] | 11.5 | [10.7; 12.3] | 15.6 | <0.001 *** | 0.1 |
| Mean walking velocity (m/s) a | 1.3 | [1.3; 1.4] | 1.5 | [1.5; 1.6] | 12.93 | <0.001 *** | 0.09 |
| Stride length (m) a | 1.28 | [1.2; 1.4] | 1.5 | [1.4; 1.6] | 9.80 | <0.001 *** | 0.67 |
| Stride width (m) a | 0.1 | [0.1; 0.2] | 0.1 | [0.1; 0.2] | 0.51 | 0.730 | 0.09 |
| Stride time (s) a | 0.91 | [0.8; 0.9] | 1.0 | [0.9; 1.1] | 3.83 | 0.019 | 0.49 |
| Stride velocity (m/s) a | 1.3 | [1.1; 1.4] | 1.7 | [1.5; 1.8] | 10.39 | <0.001 *** | 0.69 |
| Stance time (s) a | 0.7 | [0.6; 0.7] | 0.6 | [0.5; 0.6] | 4.62 | 0.009 | 0.49 |
| Swing time (s) a | 0.4 | [0.3; 0.4] | 0.3 | [0.3; 0.4] | 2.77 | 0.057 | 0.37 |
| Single support time (s) a | 0.4 | [0.3; 0.4] | 0.3 | [0.3; 0.4] | 1.40 | 0.270 | 0.23 |
| Double support time (s) a | 0.3 | [0.3; 0.4] | 0.2 | [0.2; 0.3] | 5.54 | 0.004 | 0.54 |
| Left foot angle (deg) a | −6.0 | [−8.3; −3.7] | −5.7 | [−8.0; −3.4] | 6.20 | 0.792 | 0.57 |
| Right foot angle (deg) a | 7.9 | [4.3; 9.3] | 6.8 | [4.3; 9.3] | 2.62 | 0.505 | 0.36 |
| Stride length variability (%) a | 10.9 | [8.1; 13.6] | 9.7 | [7.0; 12.5] | 3.19 | 0.036 | 0.40 |
| Stride time variability (%) a | 6.2 | [2.7; 9.6] | 4.4 | [1.0; 7.9] | 0.28 | 0.886 | 0.06 |
| Stride velocity variability (%) a | 12.3 | [9.5; 15.0] | 9.5 | [6.7; 12.3] | 2.29 | 0.097 | 0.33 |
| Stance time variability (%) a | 4.1 | [2.6; 5.6] | 5.4 | [3.9; 6.9] | 1.17 | 0.355 | 0.19 |
| Double support time variability (%) a | 9.0 | [6.7; 11.3] | 11.0 | [8.7; 13.3] | 0.51 | 0.728 | 0.09 |
| Walking asymmetry (pressure units) a | 13.1 | [5.3; 14.5] | 9.9 | [5.3; 14.4] | 1.30 | 0.411 | 0.22 |
| Mean shoulder-elbow angle. Vel. (deg/s) b | 5.2 | [3.8; 6.6] | 7.1 | [5.7; 8.5] | 2.78 | 0.098 | 0.02 |
| Mean shoulder-wrist ang. Vel. (deg/s) b | 6.4 | [4.9; 8] | 7.8 | [6.3; 9.4] | 1.29 | 0.259 | 0.01 |
| Mean elbow-wrist ang. Vel. (deg/s) b | 11.4 | [7.1; 15.6] | 12.8 | [8.6; 17.1] | 0.19 | 0.663 | 0.001 |
| Arm asymmetry factor (ASA) (%) b | 11.2 | [8.5; 13.8] | 12.9 | [10.2; 15.6] | 0.69 | 0.408 | 0.01 |
Appendix C
| Group | ||||||
|---|---|---|---|---|---|---|
| PwP | Control | |||||
| Task | M | SD | n | M | SD | n |
| Sit to stand (postural stability) | ||||||
| Score | 1.5 | 0.8 | 12 | 0.76 | 0.7 | 12 |
| Single foot offset | 1 | 0 | ||||
| Double foot offset | 7 | 4 | ||||
| Hand’s on chair | 4 | 3 | ||||
| Hand’s on knee/thigh | 4 | 2 | ||||
| Arm swing assist | 4 | 4 | ||||
| More than one attempt to stand up | 1 | 0 | ||||
| Walking forward | ||||||
| Score | 1.1 | 0.5 | 12 | 0.1 | 0.3 | 12 |
| Symmetrical arm swing with good swing amplitude | 1 | 9 | ||||
| Symmetrical arm swing with less amplitude | 3 | 1 | ||||
| Asymmetrical arm swing | 1 | 2 | ||||
| Elbow arm swing (both) | 1 | 0 | ||||
| Elbow arm swing (one side) | 2 | 0 | ||||
| Turning | ||||||
| Score | 1.5 | 0.9 | 12 | 0.1 | 0.3 | 12 |
| Functional reach (postural stability) | ||||||
| Score | 0.9 | 0.7 | 12 | 0.3 | 0.3 | 12 |
| Lunge to reach | 0 | 3 | ||||
| Stop and stoop | 3 | 0 | ||||
| Stop and knee bend | 7 | 9 | ||||
| Hand on knee support for bending | 2 | 0 | ||||
| Extra steps for reaching and/or placing | 3 | 1 | ||||
| More than one attempt to reach or/and place | 5 | 3 | ||||
| Stand-to-sit | ||||||
| Score | 1.1 | 1.0 | 12 | 0.1 | 0.3 | 12 |
| Hands on chair | 7 | 2 | ||||
| Obvious trunk bending | 1 | 1 | ||||
| Slump | 1 | 0 | ||||
Appendix D
| Parameter | Comparison | Mean Difference | 95% CI | t | p |
|---|---|---|---|---|---|
| MDS-UPDRS III | Baseline-Pre | −1.7 | [−11.7; 8.4] | 0.74 | 1.0 |
| Baseline-Post | −4.9 | [−15.0; 5.2] | 0.33 | 0.983 | |
| Pre-Post | −3.3 | [−13.3; 6.8] | 0.52 | 1.0 | |
| TUG time (s) | Baseline-Pre | 0.2 | [−4.1; 4.0] | −0.02 | 1.0 |
| Baseline-Post | −0.1 | [−4.3; 3.8] | −0.13 | 1.0 | |
| Pre-Post | −2.6 | [−3.9; 4.3] | 0.11 | 1.0 | |
| FMA-P time (s) | Baseline-Pre | −0.3 | [−5.1; 4.0] | −0.02 | 1.0 |
| Baseline-Post | −0.8 | [−5.1; 4.0] | −0.13 | 1.0 | |
| Pre-Post | −0.5 | [−4.8; 4.3] | 0.11 | 1.0 | |
| Performance Score | Baseline-Pre | −23.7 | [−5.3; 3.7] | −0.48 | 1.0 |
| Baseline-Post | 0.8 | [−5.6; 3.5] | −0.36 | 1.0 | |
| Pre-Post | −24.5 | [−4.8; 4.3] | −0.12 | 1.0 |
Appendix E
| Task | Parameter | Comparison | Mean Difference | 95% CI | t | p |
|---|---|---|---|---|---|---|
| Sit-to-stand | Peak Trunk AP Inclination (deg) | Baseline-Pre | −0.5 | [−2.6; 1.5] | −0.74 | 1.0 |
| Baseline-Post | −1.1 | [−3.9; 1.7] | −1.13 | 0.853 | ||
| Pre-Post | −0.6 | [−2.4; 2.3] | 0.88 | 1.0 | ||
| Peak Trunk ML Inclination (deg) | Baseline-Pre | 0.3 | [−1.4; 2] | 0.44 | 1.0 | |
| Baseline-Post | 1.0 | [−1.1; 3.2] | 1.34 | 0.622 | ||
| Pre-Post | 0.8 | [−0.6; 2.1] | 1.58 | 0.429 | ||
| Max. trunk velocity (m/s) | Baseline-Pre | −0.1 | [−0.2; 0.1] | −1.23 | 0.733 | |
| Baseline-Post | −0.1 | [−0.2; 0.1] | −1.22 | 0.744 | ||
| Pre-Post | 0 | [−0.1; 0.1] | −0.17 | 1.0 | ||
| RMS of trunk acceleration (m/s2) | Baseline-Pre | −0.2 | [−0.8; 0.4] | −0.91 | 1 | |
| Baseline-Post | −0.6 | [−1.2; 0] | −2.7 | 0.066 | ||
| Pre-Post | −0.4 | [−0.2; 0.9] | −1.98 | 0.228 | ||
| Mean trunk jerk (m/s3) | Baseline-Pre | 0.5 | [−0.1; 1.2] | 2.3 | 0.132 | |
| Baseline-Post | 0.6 | [−0.3; 1.5] | 1.99 | 0.223 | ||
| Pre-Post | −0.1 | [−0.7; 0.5] | −0.5 | 1.0 | ||
| Max. pelvis flexion (deg) | Baseline-Pre | 4.9 | [−18.9; 28.7] | 0.58 | 1 | |
| Baseline-Post | 5 | [−11.9; 21.9] | 0.83 | 1 | ||
| Pre-Post | 0.1 | [−15.3; 15.5] | 0.01 | 1 | ||
| Max. knee flexion (deg) | Baseline-Pre | −8 | [−37.3; 21.3] | −0.77 | 1 | |
| Baseline-Post | −17.3 | [−40.3; 5.8] | −2.11 | 0.174 | ||
| Pre-Post | 9.3 | [27.6; −9] | 1.43 | 0.539 |
| Task | Parameter | Comparison | Mean Difference | 95% CI | t | p |
|---|---|---|---|---|---|---|
| Turning | Mean pelvis jerk (m/s3) | Baseline-Pre | 189.2 | [−387.4; 765.8] | 0.96 | 1.0 |
| Baseline-Post | 556.3 | [−3.8; 1116.3] | 2.91 | 0.05 * | ||
| Pre-Post | 367.0 | [−192.1; 926.2] | 1.92 | 0.258 | ||
| Absolute head yaw rotation (deg) | Baseline-Pre | 0.6 | [−5.4; 6.5] | 0.27 | 1.0 | |
| Baseline-Post | 25.5 | [10.4; 40.6] | 4.75 | 0.002 ** | ||
| Pre-Post | 24.9 | [10.6; 39.2] | 4.92 | 0.001 ** | ||
| Absolute trunk yaw rotation (deg) | Baseline-Pre | 2.1 | [−5.9; 10.1] | 0.74 | 1.0 | |
| Baseline-Post | 27.4 | [11.9; 42.9] | 4.98 | 0.001 ** | ||
| Pre-Post | 25.3 | [10.5; 40.0] | 4.83 | 0.002 ** | ||
| Absolute pelvis yaw rotation (deg) | Baseline-Pre | −2.6 | [−16.4; 11.3] | −0.52 | 1.0 | |
| Baseline-Post | 21.0 | [6.3; 35.7] | 4.03 | 0.006 ** | ||
| Pre-Post | 23.6 | [5.2; 42.0] | 3.61 | 0.012 * | ||
| Head relative to trunk yaw rotation (deg) | Baseline-Pre | −2 | [−6.8; 2.8] | −1.19 | 0.775 | |
| Baseline-Post | −0.7 | [−7.8; 6.4] | −0.28 | 1.0 | ||
| Pre-Post | 1.3 | [−3.1; 5.8] | 0.84 | 1.0 | ||
| Trunk relative to pelvis yaw rotation (deg) | Baseline-Pre | 2.2 | [−7.6; 5.2] | −0.53 | 1.0 | |
| Baseline-Post | 2.9 | [−7.2; 9.2] | 0.36 | 1.0 | ||
| Pre-Post | −3.7 | [−8.5; 12.9] | 0.6 | 1.0 | ||
| Head relative to pelvis yaw rotation (deg) | Baseline-Pre | 1.0 | [−8.4; 6.3] | −0.41 | 1.0 | |
| Baseline-Post | 6.1 | [−0.7; 12.8] | 2.57 | 0.083 | ||
| Pre-Post | −7.1 | [2; 3] | 2.01 | 0.217 | ||
| Head onset (%) | Baseline-Pre | 0.2 | [−0.1; −0.02] | 1.8 | 0.240 | |
| Baseline-Post | 0.3 | [−0.6; −0.06] | 2.3 | 0.088 | ||
| Pre-Post | 0.03 | [−0.6; −0.1] | 0.2 | 1.0 | ||
| Trunk onset (%) a | Baseline-Pre | 0.3 | [−0.1; −0.03] | 1.8 | 0.240 | |
| Baseline-Post | 0.3 | [−0.6; −0.06] | 2.3 | 0.088 | ||
| Pre-Post | 0.03 | [−0.6; −0.1] | 0.2 | 1.0 | ||
| Pelvis onset (%) a | Baseline-Pre | 0.3 | [−2; 2.6] | 0.39 | 1.0 | |
| Baseline-Post | 0.7 | [−1.2; 2.7] | 1.08 | 0.903 | ||
| Pre-Post | 0.4 | [−1.9; 2.8] | 0.51 | 1.0 | ||
| Max. trunk AP inclination (deg) | Baseline-Pre | −0.1 | [−0.8; 0.5] | −0.6 | 1 | |
| Baseline-Post | 0.4 | [0; 0.9] | 2.66 | 0.071 | ||
| Pre-Post | 0.6 | [0; 1.2] | −2.77 | 0.059 | ||
| Max. trunk ML inclination (deg) | Baseline-Pre | −0.1 | [−1.9; 1.7] | −0.12 | 1.0 | |
| Baseline-Post | 2.0 | [−0.1; 4.2] | 2.68 | 0.064 | ||
| Pre-Post | 2.1 | [0.7; 3.5] | 4.24 | 0.004 ** | ||
| Mean toe-off angle (deg) | Baseline-Pre | 0.5 | [−1.2; 2.2] | 0.85 | 1.0 | |
| Baseline-Post | 0.4 | [−2.5; 3.2] | 0.37 | 1.0 | ||
| Pre-Post | −0.1 | [3.3; 3.6] | −0.1 | 1.0 | ||
| Mean heel-strike angle (deg) | Baseline-Pre | −0.6 | [−2.1; 0.8] | −1.25 | 0.719 | |
| Baseline-Post | −1.4 | [−3.4; 0.6] | −1.96 | 0.237 | ||
| Pre-Post | 0.7 | [1.3; 2.8] | 1.07 | 0.931 |
| Task | Parameter | Comparison | Mean Difference | 95% CI | t | p |
|---|---|---|---|---|---|---|
| Stand-to-sit | Task duration (s) | Baseline-Pre | 0.2 | [−0.2; 0.6] | 1.3 | 0.656 |
| Baseline-Post | 0.2 | [−0.4; 0.8] | 0.98 | 1.0 | ||
| Pre-Post | 0 | [−0.5; 0.5] | −0.08 | 1.0 | ||
| Peak Trunk AP Inclination (deg) a | Baseline-Pre | 1 | [−1.5; 3.4] | 1.11 | 0.875 | |
| Baseline-Post | 1.3 | [−1.7; 4.3] | 1.24 | 0.724 | ||
| Pre-Post | 0.4 | [−2.2; 3] | −0.39 | 1 | ||
| Peak Trunk ML Inclination (deg) | Baseline-Pre | −0.9 | [−4.1; 2.4] | −0.77 | 1.0 | |
| Baseline-Post | −0.2 | [−4.6; 4.2] | −0.16 | 1.0 | ||
| Pre-Post | 0.6 | [−2.1; 3.3] | 0.67 | 1.0 | ||
| Max. trunk velocity (m/s) | Baseline-Pre | 0 | [−0.3; 0.3] | 0.37 | 1.0 | |
| Baseline-Post | 0.2 | [−0.1; 0.4] | 1.68 | 0.362 | ||
| Pre-Post | 0.1 | [0; 0.3] | 2.16 | 0.161 | ||
| RMS of trunk acceleration (m/s2) | Baseline-Pre | 0.3 | [−0.3; 1] | 1.46 | 0.521 | |
| Baseline-Post | −0.1 | [−1.2; 1] | −0.31 | 1.0 | ||
| Pre-Post | 0.4 | [−1.2; 0.3] | 1.78 | 0.314 | ||
| Mean trunk jerk (m/s3) | Baseline-Pre | −0.2 | [−0.7; 0.4] | −1 | 1.0 | |
| Baseline-Post | −0.4 | [−1.3; 0.5] | −1.33 | 0.635 | ||
| Pre-Post | 0.2 | [−1; 0.5] | −0.85 | 1.0 | ||
| Max. pelvis flexion (deg) | Baseline-Pre | −12 | [−19.9; -4.2] | −4.32 | 0.004 ** | |
| Baseline-Post | −3.8 | [−12.4; 4.9] | −1.23 | 0.737 | ||
| Pre-Post | 8.3 | [−3.1; 19.7] | 2.05 | 0.194 | ||
| Max. knee flexion (deg) | Baseline-Pre | −11.7 | [−16.9; −6.5] | −6.39 | <0.001 *** | |
| Baseline-Post | −9.5 | [−14.1; −4.8] | −5.76 | <0.001 *** | ||
| Pre-Post | 2.2 | [−5.7; 10.2] | 0.79 | 1.0 |
| Task | Parameter | Comparison | Mean Difference | 95% CI | t | p |
|---|---|---|---|---|---|---|
| Functional reach | Task duration (s) | Baseline-Pre | 0.7 | [−0.5; 1.8] | 1.65 | 0.39 |
| Baseline-Post | 0.6 | [−0.5; 1.6] | 1.51 | 0.483 | ||
| Pre-Post | 0.1 | [−1.4; 1.2] | 0.23 | 1.0 | ||
| Reaching arm-body alignment (deg) | Baseline-Pre | −0.8 | [−4.5; 3] | −0.57 | 1.0 | |
| Baseline-Post | 2.3 | [−3.4; 8] | 1.15 | 0.826 | ||
| Pre-Post | 3 | [−3.1; 9.2] | 1.42 | 0.557 | ||
| Max. pelvis flexion (deg) | Baseline-Pre | 0 | [−10.4; 10.4] | 0 | 1 | |
| Baseline-Post | −3.8 | [−16; 8.3] | −0.89 | 1 | ||
| Pre-Post | −3.8 | [−12.7; 5.1] | −1.22 | 0.747 | ||
| Max. knee flexion (deg) | Baseline-Pre | 0.2 | [−10.7; 11] | 0.04 | 1.0 | |
| Baseline-Post | 0.9 | [−7; 8.8] | 0.32 | 1.0 | ||
| Pre-Post | −0.7 | [−5.9; 7.3] | −0.31 | 1.0 | ||
| AP foot distance (m) | Baseline-Pre | 26.3 | [−37.6; 90.3] | 1.16 | 0.81 | |
| Baseline-Post | 61.3 | [−37.4; 160] | 1.75 | 0.323 | ||
| Pre-Post | 35 | [−37.8; 107.8] | 1.35 | 0.608 | ||
| Feet aperture angle (deg) | Baseline-Pre | −6.7 | [−17.4; 4.1] | −1.75 | 0.326 | |
| Baseline-Post | −4.2 | [−13.6; 5.3] | −1.24 | 0.719 | ||
| Pre-Post | 2.5 | [−5.5; 10.5] | 0.88 | 1.0 | ||
| Max. stance width (m) | Baseline-Pre | −23.7 | [−53.1; 5.7] | −2.31 | 0.129 | |
| Baseline-Post | 0.8 | [−30.3; 31.8] | 0.07 | 1.0 | ||
| Pre-Post | 24.5 | [−2; 51] | 2.65 | 0.073 | ||
| Max. Toe-off angle (deg) | Baseline-Pre | 0.4 | [−10.2; 10.9] | 0.09 | 1.0 | |
| Baseline-Post | 2.6 | [−6.2; 11.5] | 0.84 | 1.0 | ||
| Pre-Post | 2.3 | [−6.6; 11.2] | −0.72 | 1.0 |
References
- Jankovic, J. Parkinson’s Disease: Clinical Features and Diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef]
- Obeso, J.A.; Stamelou, M.; Goetz, C.G.; Poewe, W.; Lang, A.E.; Weintraub, D.; Burn, D.; Halliday, G.M.; Bezard, E.; Przedborski, S.; et al. Past, Present, and Future of Parkinson’s Disease: A Special Essay on the 200th Anniversary of the Shaking Palsy. Mov. Disord. 2017, 32, 1264–1310. [Google Scholar] [CrossRef] [PubMed]
- Poewe, W. Non-motor Symptoms in Parkinson’s Disease. Euro J. Neurol. 2008, 15, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020, 323, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Schrag, A.; Schott, J.M. Epidemiological, Clinical, and Genetic Characteristics of Early-Onset Parkinsonism. Lancet Neurol. 2006, 5, 355–363. [Google Scholar] [CrossRef]
- Rana, A.Q.; Siddiqui, I.; Yousuf, M.S. Challenges in Diagnosis of Young Onset Parkinson’s Disease. J. Neurol. Sci. 2012, 323, 113–116. [Google Scholar] [CrossRef]
- Mirelman, A.; Bonato, P.; Camicioli, R.; Ellis, T.D.; Giladi, N.; Hamilton, J.L.; Hass, C.J.; Hausdorff, J.M.; Pelosin, E.; Almeida, Q.J. Gait Impairments in Parkinson’s Disease. Lancet Neurol. 2019, 18, 697–708. [Google Scholar] [CrossRef]
- Bae, Y.J.; Kim, J.-M.; Sohn, C.-H.; Choi, J.-H.; Choi, B.S.; Song, Y.S.; Nam, Y.; Cho, S.J.; Jeon, B.; Kim, J.H. Imaging the Substantia Nigra in Parkinson Disease and Other Parkinsonian Syndromes. Radiology 2021, 300, 260–278. [Google Scholar] [CrossRef] [PubMed]
- Chougar, L.; Arsovic, E.; Gaurav, R.; Biondetti, E.; Faucher, A.; Valabrègue, R.; Pyatigorskaya, N.; Dupont, G.; Lejeune, F.-X.; Cormier, F.; et al. Regional Selectivity of Neuromelanin Changes in the Substantia Nigra in Atypical Parkinsonism. Mov. Disord. 2022, 37, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Chen, Y.; LeWitt, P.A.; Yan, F.; Haacke, E.M. Application of Neuromelanin MR Imaging in Parkinson Disease. J. Magn. Reson. Imaging 2023, 57, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Bloem, B.R.; Marinus, J.; Almeida, Q.; Dibble, L.; Nieuwboer, A.; Post, B.; Ruzicka, E.; Goetz, C.; Stebbins, G.; Martinez-Martin, P.; et al. Measurement Instruments to Assess Posture, Gait, and Balance in Parkinson’s Disease: Critique and Recommendations: Posture, Gait, and Balance Instruments in PD. Mov. Disord. 2016, 31, 1342–1355. [Google Scholar] [CrossRef] [PubMed]
- Pelicioni, P.H.S.; Menant, J.C.; Latt, M.D.; Lord, S.R. Falls in Parkinson’s Disease Subtypes: Risk Factors, Locations and Circumstances. Int. J. Environ. Res. Public Health 2019, 16, 2216. [Google Scholar] [CrossRef]
- Saluja, A.; Goyal, V.; Dhamija, R.K. Multi-Modal Rehabilitation Therapy in Parkinson’s Disease and Related Disorders. Ann. Indian Acad. Neurol. 2023, 26, S15–S25. [Google Scholar] [CrossRef]
- Xu, G.; Ma, C.; Yang, Y. Intervention Strategies for Parkinson’s Disease: The Role of Exercise and Mitochondria. Front. Aging Neurosci. 2025, 17, 1519672. [Google Scholar] [CrossRef]
- El Hayek, M.; Lobo Jofili Lopes, J.L.M.; LeLaurin, J.H.; Gregory, M.E.; Abi Nehme, A.-M.; McCall-Junkin, P.; Au, K.L.K.; Okun, M.S.; Salloum, R.G. Type, Timing, Frequency, and Durability of Outcome of Physical Therapy for Parkinson Disease: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2023, 6, e2324860. [Google Scholar] [CrossRef]
- Opara, J.; Małecki, A.; Małecka, E.; Socha, T. Motor Assessment in Parkinson’s Disease. Ann. Agric. Environ. Med. 2017, 24, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-sponsored Revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale Presentation and Clinimetric Testing Results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef] [PubMed]
- Hoehn, M.M.; Yahr, M.D. Parkinsonism: Onset, Progression and Mortality. Neurology 2001, 57, S11–S26. [Google Scholar]
- Bouça-Machado, R.; Fernandes, A.; Ranzato, C.; Beneby, D.; Nzwalo, H.; Ferreira, J.J. Measurement Tools to Assess Activities of Daily Living in Patients with Parkinson’s Disease: A Systematic Review. Front. Neurosci. 2022, 16, 945398. [Google Scholar] [CrossRef]
- Jonasson, S.B.; Hagell, P.; Hariz, G.-M.; Iwarsson, S.; Nilsson, M.H. Psychometric Evaluation of the Parkinson’s Disease Activities of Daily Living Scale. Park. Dis. 2017, 2017, 4151738. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, C.; Fitzpatrick, R.; Peto, V.; Greenhall, R.; Hyman, N. The Parkinson’s Disease Questionnaire (PDQ-39): Development and Validation of a Parkinson’s Disease Summary Index Score. Age Ageing 1997, 26, 353–357. [Google Scholar] [CrossRef]
- Zolfaghari, S.; Thomann, A.E.; Lewandowski, N.; Trundell, D.; Lipsmeier, F.; Pagano, G.; Taylor, K.I.; Postuma, R.B. Self-Report versus Clinician Examination in Early Parkinson’s Disease. Mov. Disord. 2022, 37, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, T.; Fang, H.; Sugiyama, K.; Nozaki, T.; Kobayashi, S.; Hong, Z.; Suzuki, K.; Mori, N.; Yang, Y.; Hua, F.; et al. Human Behavioral Assessments in Current Research of Parkinson’s Disease. Neurosci. Biobehav. Rev. 2016, 68, 741–772. [Google Scholar] [CrossRef] [PubMed]
- Dean, C.E.; Russell, J.M.; Kuskowski, M.A.; Caligiuri, M.P.; Nugent, S.M. Clinical Rating Scales and Instruments: How Do They Compare in Assessing Abnormal, Involuntary Movements? J. Clin. Psychopharmacol. 2004, 24, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Kalia, L.V.; Lang, A.E. Parkinson’s Disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Crouse, J.J.; Phillips, J.R.; Jahanshahi, M.; Moustafa, A.A. Postural Instability and Falls in Parkinson’s Disease. Rev. Neurosci. 2016, 27, 549–555. [Google Scholar] [CrossRef]
- Sprint, G.; Cook, D.J.; Weeks, D.L. Toward Automating Clinical Assessments: A Survey of the Timed Up and Go. IEEE Rev. Biomed. Eng. 2015, 8, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Nocera, J.R.; Stegemöller, E.L.; Malaty, I.A.; Okun, M.S.; Marsiske, M.; Hass, C.J. Using the Timed Up & Go Test in a Clinical Setting to Predict Falling in Parkinson’s Disease. Arch. Phys. Med. Rehabil. 2013, 94, 1300–1305. [Google Scholar] [CrossRef]
- Zampieri, C.; Salarian, A.; Carlson-Kuhta, P.; Aminian, K.; Nutt, J.G.; Horak, F.B. The Instrumented Timed up and Go Test: Potential Outcome Measure for Disease Modifying Therapies in Parkinson’s Disease. J. Neurol. Neurosurg. Psychiatry 2010, 81, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Baudendistel, S.T.; Schmitt, A.C.; Roemmich, R.T.; Harrison, I.L.; Hass, C.J. Levodopa Facilitates Improvements in Gait Kinetics at the Hip, Not the Ankle, in Individuals with Parkinson’s Disease. J. Biomech. 2021, 121, 110366. [Google Scholar] [CrossRef] [PubMed]
- Del Olmo, M.F.; Cudeiro, J. Temporal Variability of Gait in Parkinson Disease: Effectsof a Rehabilitation Programme Based on Rhythmic Sound Cues. Park. Relat. Disord. 2005, 11, 25–33. [Google Scholar] [CrossRef]
- Di Biase, L.; Di Santo, A.; Caminiti, M.L.; De Liso, A.; Shah, S.A.; Ricci, L.; Di Lazzaro, V. Gait Analysis in Parkinson’s Disease: An Overview of the Most Accurate Markers for Diagnosis and Symptoms Monitoring. Sensors 2020, 20, 3529. [Google Scholar] [CrossRef]
- Duncan, R.P.; Earhart, G.M. Randomized Controlled Trial of Community-Based Dancing to Modify Disease Progression in Parkinson Disease. Neurorehabil. Neural Repair 2012, 26, 132–143. [Google Scholar] [CrossRef]
- Salarian, A.; Horak, F.B.; Zampieri, C.; Carlson-Kuhta, P.; Nutt, J.G.; Aminian, K. iTUG, a Sensitive and Reliable Measure of Mobility. IEEE Trans. Neural Syst. Rehabil. Eng. 2010, 18, 303–310. [Google Scholar] [CrossRef]
- Espay, A.J.; Giuffrida, J.P.; Chen, R.; Payne, M.; Mazzella, F.; Dunn, E.; Vaughan, J.E.; Duker, A.P.; Sahay, A.; Kim, S.J.; et al. Differential Response of Speed, Amplitude, and Rhythm to Dopaminergic Medications in Parkinson’s Disease. Mov. Disord. 2011, 26, 2504–2508. [Google Scholar] [CrossRef]
- King, L.A.; Mancini, M.; Priest, K.; Salarian, A.; Rodrigues-de-Paula, F.; Horak, F. Do Clinical Scales of Balance Reflect Turning Abnormalities in People with Parkinson’s Disease? J. Neurol. Phys. Ther. 2012, 36, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Toosizadeh, N.; Mohler, J.; Lei, H.; Parvaneh, S.; Sherman, S.; Najafi, B. Motor Performance Assessment in Parkinson’s Disease: Association between Objective In-Clinic, Objective In-Home, and Subjective/Semi-Objective Measures. PLoS ONE 2015, 10, e0124763. [Google Scholar] [CrossRef]
- Muro-de-la-Herran, A.; Garcia-Zapirain, B.; Mendez-Zorrilla, A. Gait Analysis Methods: An Overview of Wearable and Non-Wearable Systems, Highlighting Clinical Applications. Sensors 2014, 14, 3362–3394. [Google Scholar] [CrossRef]
- Picerno, P.; Iosa, M.; D’Souza, C.; Benedetti, M.G.; Paolucci, S.; Morone, G. Wearable Inertial Sensors for Human Movement Analysis: A Five-Year Update. Expert. Rev. Med. Devices 2021, 18, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Neto, P.; Pires, J.N.; Moreira, A.P. 3-D Position Estimation from Inertial Sensing: Minimizing the Error from the Process of Double Integration of Accelerations. In Proceedings of the IECON 2013—39th Annual Conference of the IEEE Industrial Electronics Society, Vienna, Austria, 10–13 November 2013; pp. 4026–4031. [Google Scholar]
- Lambrecht, S.; Nogueira, S.L.; Bortole, M.; Siqueira, A.A.G.; Terra, M.H.; Rocon, E.; Pons, J.L. Inertial Sensor Error Reduction through Calibration and Sensor Fusion. Sensors 2016, 16, 235. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, L.; Suglia, V.; Grieco, S.; Buongiorno, D.; Brunetti, A.; Carnimeo, L.; Amitrano, F.; Coccia, A.; Pagano, G.; D’Addio, G.; et al. A Deep Learning-Based Framework Oriented to Pathological Gait Recognition with Inertial Sensors. Sensors 2025, 25, 260. [Google Scholar] [CrossRef]
- Bortone, I.; Buongiorno, D.; Lelli, G.; Di Candia, A.; Cascarano, G.D.; Trotta, G.F.; Fiore, P.; Bevilacqua, V. Gait Analysis and Parkinson’s Disease: Recent Trends on Main Applications in Healthcare. In Converging Clinical and Engineering Research on Neurorehabilitation III; Springer: Cham, Switzerland, 2019; pp. 1121–1125. [Google Scholar]
- Tripathi, R.; McKay, J.L.; Esper, C.D. Movement Disorders Moment: Use of 3D Motion Capture for Kinematic Analysis in Movement Disorders—Practical Neurology. Pract. Neurol. 2023. [Google Scholar]
- Jakob, V.; Küderle, A.; Kluge, F.; Klucken, J.; Eskofier, B.M.; Winkler, J.; Winterholler, M.; Gassner, H. Validation of a Sensor-Based Gait Analysis System with a Gold-Standard Motion Capture System in Patients with Parkinson’s Disease. Sensors 2021, 21, 7680. [Google Scholar] [CrossRef]
- Buckley, C.; Alcock, L.; McArdle, R.; Rehman, R.Z.U.; Del Din, S.; Mazzà, C.; Yarnall, A.J.; Rochester, L. The Role of Movement Analysis in Diagnosing and Monitoring Neurodegenerative Conditions: Insights from Gait and Postural Control. Brain Sci. 2019, 9, 34. [Google Scholar] [CrossRef]
- Del Din, S.; Godfrey, A.; Galna, B.; Lord, S.; Rochester, L. Free-Living Gait Characteristics in Ageing and Parkinson’s Disease: Impact of Environment and Ambulatory Bout Length. J. Neuroeng. Rehabil. 2016, 13, 46. [Google Scholar] [CrossRef]
- Stephenson, D.; Badawy, R.; Mathur, S.; Tome, M.; Rochester, L. Digital Progression Biomarkers as Novel Endpoints in Clinical Trials: A Multistakeholder Perspective. J. Park. Dis. 2021, 11, S103–S109. [Google Scholar] [CrossRef]
- Lu, R.; Xu, Y.; Li, X.; Fan, Y.; Zeng, W.; Tan, Y.; Ren, K.; Chen, W.; Cao, X. Evaluation of Wearable Sensor Devices in Parkinson’s Disease: A Review of Current Status and Future Prospects. Park. Dis. 2020, 2020, 4693019. [Google Scholar] [CrossRef]
- Hartmann, C.J.; Fliegen, S.; Groiss, S.J.; Wojtecki, L.; Schnitzler, A. An Update on Best Practice of Deep Brain Stimulation in Parkinson’s Disease. Ther. Adv. Neurol. Disord. 2019, 12, 1756286419838096. [Google Scholar] [CrossRef]
- Harris, D.M.; Latella, C.; Tripodi, N.; O’Bryan, S.J. Exploring Non-Invasive Brain Stimulation Effects on Physical Outcomes in People with Parkinson’s Disease: An Umbrella Evidence Mapping Review with Meta-Analyses. Neurorehabil. Neural Repair 2025, 39, 321–340. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xu, H.; Xu, B.; Wang, Y.; Shi, Y.; Xiao, L. Automatic Localization of Key Structures for Subthalamic Nucleus–Deep Brain Stimulation Surgery via Prior-Enhanced Multi-Object Magnetic Resonance Imaging Segmentation. World Neurosurg. 2023, 178, e472–e479. [Google Scholar] [CrossRef]
- Altini, N.; Lasaracina, E.; Galeone, F.; Prunella, M.; Suglia, V.; Carnimeo, L.; Triggiani, V.; Ranieri, D.; Brunetti, G.; Bevilacqua, V. A Comparison Between Unimodal and Multimodal Segmentation Models for Deep Brain Structures from T1- and T2-Weighted MRI. Mach. Learn. Knowl. Extr. 2025, 7, 84. [Google Scholar] [CrossRef]
- Pyatigorskaya, N.; Sanz-Morère, C.B.; Gaurav, R.; Biondetti, E.; Valabregue, R.; Santin, M.; Yahia-Cherif, L.; Lehéricy, S. Iron Imaging as a Diagnostic Tool for Parkinson’s Disease: A Systematic Review and Meta-Analysis. Front. Neurol. 2020, 11, 366. [Google Scholar] [CrossRef]
- Pletcher, C.; Dabbs, K.; Barzgari, A.; Pozorski, V.; Haebig, M.; Wey, S.; Krislov, S.; Theisen, F.; Okonkwo, O.; Cary, P.; et al. Cerebral Cortical Thickness and Cognitive Decline in Parkinson’s Disease. Cereb. Cortex Commun. 2023, 4, tgac044. [Google Scholar] [CrossRef] [PubMed]
- Sisodia, D.; Singh, L.; Sisodia, S. Clustering Techniques: A Brief Survey of Different Clustering Algorithms. Int. J. Latest Trends Eng. Technol. 2012, 1, 82–87. [Google Scholar]
- Olson, M.C.; Shill, H.; Ponce, F.; Aslam, S. Deep Brain Stimulation in PD: Risk of Complications, Morbidity, and Hospitalizations: A Systematic Review. Front. Aging Neurosci. 2023, 15, 1258190. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhang, M.; Ren, W.; Zheng, X.; Chang, Y. Neuromelanin-Sensitive Magnetic Resonance Imaging: Possibilities and Promises as an Imaging Biomarker for Parkinson’s Disease. Eur. J. Neurosci. 2024, 59, 2616–2627. [Google Scholar] [CrossRef]
- Lorenzo-García, P.; Cavero-Redondo, I.; de Arenas-Arroyo, S.N.; Guzmán-Pavón, M.J.; Priego-Jiménez, S.; Álvarez-Bueno, C. Effects of Physical Exercise Interventions on Balance, Postural Stability and General Mobility in Parkinson’S Disease: A Network Meta-Analysis. J. Rehabil. Med. 2024, 56, 10329. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhou, R.; Wei, W.; Luan, R.; Li, K. Effects of Music-Based Movement Therapy on Motor Function, Balance, Gait, Mental Health, and Quality of Life for Patients with Parkinson’s Disease: A Systematic Review and Meta-Analysis. Clin. Rehabil. 2021, 35, 937–951. [Google Scholar] [CrossRef]
- Ye, X.; Li, L.; He, R.; Jia, Y.; Poon, W. Rhythmic Auditory Stimulation Promotes Gait Recovery in Parkinson’s Patients: A Systematic Review and Meta-Analysis. Front. Neurol. 2022, 13, 940419. [Google Scholar] [CrossRef]
- Wang, L.; Peng, J.-L.; Ou-Yang, J.-B.; Gan, L.; Zeng, S.; Wang, H.-Y.; Zuo, G.-C.; Qiu, L. Effects of Rhythmic Auditory Stimulation on Gait and Motor Function in Parkinson’s Disease: A Systematic Review and Meta-Analysis of Clinical Randomized Controlled Studies. Front. Neurol. 2022, 13, 818559. [Google Scholar] [CrossRef]
- Hackney, M.E.; Earhart, G.M. Effects of Dance on Gait and Balance in Parkinson’s Disease: A Comparison of Partnered and Nonpartnered Dance Movement. Neurorehabil. Neural Repair 2010, 24, 384–392. [Google Scholar] [CrossRef]
- Hadley, R.; Eastwood-Gray, O.; Kiddier, M.; Rose, D.; Ponzo, S. “Dance Like Nobody’s Watching”: Exploring the Role of Dance-Based Interventions in Perceived Well-Being and Bodily Awareness in People with Parkinson’s. Front. Psychol. 2020, 11, 531567. [Google Scholar] [CrossRef] [PubMed]
- Doshi, P.K.; Mehrotra, B.; Rai, N.; Rajani, M.R.; Budhakar, A.; Aggarwal, R.; Thomas, M.; Patole, S.; Agarbattiwala, R.V. Impact of Dance/Music and Meditation on the Progression of Parkinson’s Disease with Mild/Moderate Severity: A Single-Blinded Randomized Controlled Pilot Study. Clin. Park. Relat. Disord. 2025, 13, 100366. [Google Scholar] [CrossRef]
- Zackrisson, T.; Holmberg, B.; Johnels, B.; Thorlin, T. A New Automated Implementation of the Posturo-Locomotion-Manual (PLM) Method for Movement Analysis in Patients with Parkinson’s Disease: Automated Movement Analysis in Patients with PD. Acta Neurol. Scand. 2011, 123, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.; Ungerer, M.; Köchli, S.; Paolantonio, P.; Dinacci, D.; Foletti, A.; Molteni, D.; Greenwood, A.; Thomas, M.; Truran, L.; et al. Songlines for Parkinson’s: The Process of Co-Developing a New Music-and-Movement Group-Based Intervention to Improve Mood and Movement for Parkinson’s. Int. J. Qual. Methods 2025, 24, 16094069251335453. [Google Scholar] [CrossRef]
- Gill, D.J.; Freshman, A.; Blender, J.A.; Ravina, B. The Montreal Cognitive Assessment as a Screening Tool for Cognitive Impairment in Parkinson’s Disease. Mov. Disord. 2008, 23, 1043–1046. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.B.; Õunpuu, S.; Tyburski, D.; Gage, J.R. A Gait Analysis Data Collection and Reduction Technique. Hum. Mov. Sci. 1991, 10, 575–587. [Google Scholar] [CrossRef]
- Kadaba, M.P.; Ramakrishnan, H.K.; Wootten, M.E. Measurement of Lower Extremity Kinematics during Level Walking. J. Orthop. Res. 1990, 8, 383–392. [Google Scholar] [CrossRef]
- Johnels, B.; Ingvarsson, P.E.; Thorselius, M.; Valls, M.; Steg, G. Disability Profiles and Objective Quantitative Assessment in Parkinson’s Disease. Acta Neurol. Scand. 1989, 79, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Zackrisson, T.; Bergquist, F.; Holmberg, B.; Johnels, B.; Thorlin, T. Evaluation of the Objective Posturo-Locomotor-Manual Method in Patients with Parkinsonian Syndromes. Front. Neurol. 2013, 4, 95. [Google Scholar] [CrossRef] [PubMed]
- Dolan, R.J.; Dayan, P. Goals and Habits in the Brain. Neuron 2013, 80, 312–325. [Google Scholar] [CrossRef]
- Redgrave, P.; Rodriguez, M.; Smith, Y.; Rodriguez-Oroz, M.C.; Lehericy, S.; Bergman, H.; Agid, Y.; DeLong, M.R.; Obeso, J.A. Goal-Directed and Habitual Control in the Basal Ganglia: Implications for Parkinson’s Disease. Nat. Rev. Neurosci. 2010, 11, 760–772. [Google Scholar] [CrossRef]
- Bannard, C.; Leriche, M.; Bandmann, O.; Brown, C.H.; Ferracane, E.; Sánchez-Ferro, Á.; Obeso, J.; Redgrave, P.; Stafford, T. Reduced Habit-Driven Errors in Parkinson’s Disease. Sci. Rep. 2019, 9, 3423. [Google Scholar] [CrossRef]
- Mi, T.-M.; Zhang, W.; McKeown, M.J.; Chan, P. Impaired Formation and Expression of Goal-Directed and Habitual Control in Parkinson’s Disease. Front. Aging Neurosci. 2021, 13, 734807. [Google Scholar] [CrossRef]
- de Wit, S.; Barker, R.A.; Dickinson, A.D.; Cools, R. Habitual versus Goal-Directed Action Control in Parkinson Disease. J. Cogn. Neurosci. 2011, 23, 1218–1229. [Google Scholar] [CrossRef]
- Bond, J.M.; Morris, M. Goal-Directed Secondary Motor Tasks: Their Effects on Gait in Subjects with Parkinson Disease. Arch. Phys. Med. Rehabil. 2000, 81, 110–116. [Google Scholar] [CrossRef]
- Janssen, S.; de Ruyter van Steveninck, J.; Salim, H.S.; Cockx, H.M.; Bloem, B.R.; Heida, T.; van Wezel, R.J.A. The Effects of Augmented Reality Visual Cues on Turning in Place in Parkinson’s Disease Patients with Freezing of Gait. Front. Neurol. 2020, 11, 185. [Google Scholar] [CrossRef]
- Cowie, D.; Limousin, P.; Peters, A.; Day, B.L. Insights into the Neural Control of Locomotion from Walking through Doorways in Parkinson’s Disease. Neuropsychologia 2010, 48, 2750–2757. [Google Scholar] [CrossRef] [PubMed]
- Cowie, D.; Limousin, P.; Peters, A.; Hariz, M.; Day, B.L. Doorway-Provoked Freezing of Gait in Parkinson’s Disease. Mov. Disord. 2012, 27, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Bryant, M.S.; Kang, G.E.; Protas, E.J. Relation of Chair Rising Ability to Activities of Daily Living and Physical Activity in Parkinson’s Disease. Arch. Physiother. 2020, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Brod, M.; Mendelsohn, G.A.; Roberts, B. Patients’ Experiences of Parkinson’s Disease. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 1998, 53B, P213–P222. [Google Scholar] [CrossRef]
- Berg, K. Measuring Balance in the Elderly: Preliminary Development of an Instrument. Physiother. Can. 1989, 41, 304–311. [Google Scholar] [CrossRef]
- Podsiadlo, D.; Richardson, S. The Timed “Up & Go”: A Test of Basic Functional Mobility for Frail Elderly Persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Inkster, L.M.; Eng, J.J. Postural Control during a Sit-to-Stand Task in Individuals with Mild Parkinson’s Disease. Exp. Brain Res. 2004, 154, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Horak, F.B.; Mancini, M. Objective Biomarkers of Balance and Gait for Parkinson’s Disease Using Body-Worn Sensors. Mov. Disord. 2013, 28, 1544–1551. [Google Scholar] [CrossRef] [PubMed]
- Nikfekr, E.; Kerr, K.; Attfield, S.; Playford, E.D. Trunk Movement in Parkinson’s Disease during Rising from Seated Position. Mov. Disord. 2002, 17, 274–282. [Google Scholar] [CrossRef]
- Stack, E.; Ashburn, A. Fall Events Described by People with Parkinson’s Disease: Implications for Clinical Interviewing and the Research Agenda. Physiother. Res. Int. 1999, 4, 190–200. [Google Scholar] [CrossRef]
- Hong, M.; Perlmutter, J.S.; Earhart, G.M. A Kinematic and Electromyographic Analysis of Turning in People with Parkinson Disease. Neurorehabil. Neural Repair 2009, 23, 166–176. [Google Scholar] [CrossRef]
- Yang, W.-C.; Hsu, W.-L.; Wu, R.-M.; Lu, T.-W.; Lin, K.-H. Motion Analysis of Axial Rotation and Gait Stability during Turning in People with Parkinson’s Disease. Gait Posture 2016, 44, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.-Y.; Yang, Y.-R.; Wang, C.-J.; Wu, Y.-R.; Cheng, S.-J.; Wang, H.-C.; Wang, R.-Y. Factors Influencing Turning and Its Relationship with Falls in Individuals with Parkinson’s Disease. PLoS ONE 2014, 9, e93572. [Google Scholar] [CrossRef]
- Huxham, F.; Baker, R.; Morris, M.E.; Iansek, R. Head and Trunk Rotation during Walking Turns in Parkinson’s Disease. Mov. Disord. 2008, 23, 1391–1397. [Google Scholar] [CrossRef] [PubMed]
- Magrinelli, F.; Picelli, A.; Tocco, P.; Federico, A.; Roncari, L.; Smania, N.; Zanette, G.; Tamburin, S. Pathophysiology of Motor Dysfunction in Parkinson’s Disease as the Rationale for Drug Treatment and Rehabilitation. Park. Dis. 2016, 2016, 9832839. [Google Scholar] [CrossRef] [PubMed]
- Stack, E.L.; Ashburn, A.M.; Jupp, K.E. Strategies Used by People with Parkinson’s Disease Who Report Difficulty Turning. Park. Relat. Disord. 2006, 12, 87–92. [Google Scholar] [CrossRef]
- Alexander, G.E. Biology of Parkinson’s Disease: Pathogenesis and Pathophysiology of a Multisystem Neurodegenerative Disorder. Dialogues Clin. Neurosci. 2004, 6, 259–280. [Google Scholar] [CrossRef] [PubMed]
- Turner, T.H.; Dale, M.L. Inconsistent Movement Disorders Society–Unified Parkinson’s Disease Rating Scale Part III Ratings in the Parkinson’s Progression Marker Initiative. Mov. Disord. 2020, 35, 1488–1489. [Google Scholar] [CrossRef]
- de Waroquier-Leroy, L.; Bleuse, S.; Serafi, R.; Watelain, E.; Pardessus, V.; Tiffreau, A.-V.; Thevenon, A. The Functional Reach Test: Strategies, Performance and the Influence of Age. Ann. Phys. Rehabil. Med. 2014, 57, 452–464. [Google Scholar] [CrossRef]
- Jenkins, M.E.; Johnson, A.M.; Holmes, J.D.; Stephenson, F.F.; Spaulding, S.J. Predictive Validity of the UPDRS Postural Stability Score and the Functional Reach Test, When Compared with Ecologically Valid Reaching Tasks. Park. Relat. Disord. 2010, 16, 409–411. [Google Scholar] [CrossRef] [PubMed]
- Griner, D.; Smith, T.B. Culturally Adapted Mental Health Intervention: A Meta-Analytic Review. Psychother. Theory Res. Pract. Train. 2006, 43, 531–548. [Google Scholar] [CrossRef] [PubMed]
- Blin, O.; Ferrandez, A.M.; Serratrice, G. Quantitative Analysis of Gait in Parkinson Patients: Increased Variability of Stride Length. J. Neurol. Sci. 1990, 98, 91–97. [Google Scholar] [CrossRef]
- Shin, K.J.; Park, J.; Ha, S.; Park, K.M.; Kim, S.E.; Lee, B.I.; Lee, D.A.; Kim, H.-T.; Yoon, J.-Y. Decreased Foot Height May Be a Subclinical Shuffling Gait in Early Stage of Parkinson’s Disease: A Study of Three-Dimensional Motion Analysis. Gait Posture 2020, 76, 64–67. [Google Scholar] [CrossRef]
- Moore, S.R.; Martinez, A.; Kröll, J.; Strutzenberger, G.; Schwameder, H. Simple Foot Strike Angle Calculation from Three-Dimensional Kinematics: A Methodological Comparison. J. Sports Sci. 2022, 40, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Švehlík, M.; Zwick, E.B.; Steinwender, G.; Linhart, W.E.; Schwingenschuh, P.; Katschnig, P.; Ott, E.; Enzinger, C. Gait Analysis in Patients with Parkinson’s Disease Off Dopaminergic Therapy. Arch. Phys. Med. Rehabil. 2009, 90, 1880–1886. [Google Scholar] [CrossRef]
- Lowry, K.A.; Smiley-Oyen, A.L.; Carrel, A.J.; Kerr, J.P. Walking Stability Using Harmonic Ratios in Parkinson’s Disease. Mov. Disord. 2009, 24, 261–267. [Google Scholar] [CrossRef]
- Latt, M.D.; Menz, H.B.; Fung, V.S.; Lord, S.R. Walking Speed, Cadence and Step Length Are Selected to Optimize the Stability of Head and Pelvis Accelerations. Exp. Brain Res. 2008, 184, 201–209. [Google Scholar] [CrossRef]
- Latt, M.D.; Menz, H.B.; Fung, V.S.; Lord, S.R. Acceleration Patterns of the Head and Pelvis During Gait in Older People with Parkinson’s Disease: A Comparison of Fallers and Nonfallers. J. Gerontol. Ser. A 2009, 64A, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Sejdić, E.; Lowry, K.A.; Bellanca, J.; Redfern, M.S.; Brach, J.S. A Comprehensive Assessment of Gait Accelerometry Signals in Time, Frequency and Time-Frequency Domains. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 22, 603–612. [Google Scholar] [CrossRef]
- Mancini, M.; Bloem, B.R.; Horak, F.B.; Lewis, S.J.G.; Nieuwboer, A.; Nonnekes, J. Clinical and Methodological Challenges for Assessing Freezing of Gait: Future Perspectives. Mov. Disord. 2019, 34, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Rahimpour, S.; Gaztanaga, W.; Yadav, A.P.; Chang, S.J.; Krucoff, M.O.; Cajigas, I.; Turner, D.A.; Wang, D.D. Freezing of Gait in Parkinson’s Disease: Invasive and Noninvasive Neuromodulation. Neuromodul. Technol. Neural Interface 2021, 24, 829–842. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-S.; Gao, C.; Tan, Y.-Y.; Chen, S.-D. Prevalence of Freezing of Gait in Parkinson’s Disease: A Systematic Review and Meta-Analysis. J. Neurol. 2021, 268, 4138–4150. [Google Scholar] [CrossRef] [PubMed]
- Bech, P.; Olsen, L.R.; Kjoller, M.; Rasmussen, N.K. Measuring Well-Being Rather than the Absence of Distress Symptoms: A Comparison of the SF-36 Mental Health Subscale and the WHO-Five Well-Being Scale. Int. J. Methods Psychiatr. Res. 2003, 12, 85–91. [Google Scholar] [CrossRef]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories; American Thoracic Society. ATS Statement: Guidelines for the Six-Minute Walk Test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.C.; Sigrist, C.; Alessandri, E. Hard Work and Hopefulness: A Mixed Methods Study of Music Students’ Status and Beliefs in Relation to Health, Wellbeing, and Success as They Enter Specialized Higher Education. Front. Psychol. 2021, 12, 740775. [Google Scholar] [CrossRef] [PubMed]
- Garland, A.F.; Deyessa, N.; Desta, M.; Alem, A.; Zerihun, T.; Hall, K.G.; Goren, N.; Fish, I. Use of the WHO’s Perceived Well-Being Index (WHO-5) as an Efficient and Potentially Valid Screen for Depression in a Low Income Country. Fam. Syst. Health 2018, 36, 148–158. [Google Scholar] [CrossRef]
- Yeatts, S.D.; Martin, R.H. What Is Missing from My Missing Data Plan? Stroke 2015, 46, e130–e132. [Google Scholar] [CrossRef] [PubMed]
- Lewek, M.D.; Poole, R.; Johnson, J.; Halawa, O.; Huang, X. Arm Swing Magnitude and Asymmetry during Gait in the Early Stages of Parkinson’s Disease. Gait Posture 2010, 31, 256–260. [Google Scholar] [CrossRef]
- Zifchock, R.A.; Davis, I.; Higginson, J.; Royer, T. The Symmetry Angle: A Novel, Robust Method of Quantifying Asymmetry. Gait Posture 2008, 27, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Kellis, E.; Ellinoudis, A.; Kofotolis, N. Effect of Hip Flexion Angle on the Hamstring to Quadriceps Strength Ratio. Sports 2019, 7, 43. [Google Scholar] [CrossRef]
- Russo, M.; Amboni, M.; Pisani, N.; Volzone, A.; Calderone, D.; Barone, P.; Amato, F.; Ricciardi, C.; Romano, M. Biomechanics Parameters of Gait Analysis to Characterize Parkinson’s Disease: A Scoping Review. Sensors 2025, 25, 338. [Google Scholar] [CrossRef]
- Horak, F.B.; Dimitrova, D.; Nutt, J.G. Direction-Specific Postural Instability in Subjects with Parkinson’s Disease. Exp. Neurol. 2005, 193, 504–521. [Google Scholar] [CrossRef]
- Palmerini, L.; Mellone, S.; Avanzolini, G.; Valzania, F.; Chiari, L. Quantification of Motor Impairment in Parkinson’s Disease Using an Instrumented Timed Up and Go Test. IEEE Trans. Neural Syst. Rehabil. Eng. 2013, 21, 664–673. [Google Scholar] [CrossRef]
- Mancini, M.; Horak, F.B.; Zampieri, C.; Carlson-Kuhta, P.; Nutt, J.G.; Chiari, L. Trunk Accelerometry Reveals Postural Instability in Untreated Parkinson’s Disease. Park. Relat. Disord. 2011, 17, 557–562. [Google Scholar] [CrossRef]
- Carvalho, D.V.; Santos, R.M.S.; de Magalhães, H.C.; de Souza, M.S.; Christo, P.P.; de Almeida-Leite, C.M.; Scalzo, P.L. Can Fatigue Predict Walking Capacity of Patients with Parkinson’s Disease? Arq. Neuropsiquiatr. 2020, 78, 70–75. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.E. Movement Disorders in People with Parkinson Disease: A Model for Physical Therapy. Phys. Ther. 2000, 80, 578–597. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.; Rocchi, L.; Horak, F.B.; Chiari, L. Effects of Parkinson’s Disease and Levodopa on Functional Limits of Stability. Clin. Biomech. 2008, 23, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Akram, S.; Frank, J.S.; Jog, M. Parkinson’s Disease and Segmental Coordination during Turning: I. Standing Turns. Can. J. Neurol. Sci. 2013, 40, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Schlachetzki, J.C.M.; Barth, J.; Marxreiter, F.; Gossler, J.; Kohl, Z.; Reinfelder, S.; Gassner, H.; Aminian, K.; Eskofier, B.M.; Winkler, J.; et al. Wearable Sensors Objectively Measure Gait Parameters in Parkinson’s Disease. PLoS ONE 2017, 12, e0183989. [Google Scholar] [CrossRef] [PubMed]
- Beuter, A.; Hernández, R.; Rigal, R.; Modolo, J.; Blanchet, P.J. Postural Sway and Effect of Levodopa in Early Parkinson’s Disease. Can. J. Neurol. Sci. 2008, 35, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Schoneburg, B.; Mancini, M.; Horak, F.; Nutt, J.G. Framework for Understanding Balance Dysfunction in Parkinson’s Disease. Mov. Disord. 2013, 28, 1474–1482. [Google Scholar] [CrossRef]
- Horak, F.B.; Mancini, M.; Carlson-Kuhta, P.; Nutt, J.G.; Salarian, A. Balance and Gait Represent Independent Domains of Mobility in Parkinson Disease. Phys. Ther. 2016, 96, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- Frenklach, A.; Louie, S.; Koop, M.M.; Bronte-Stewart, H. Excessive Postural Sway and the Risk of Falls at Different Stages of Parkinson’s Disease. Mov. Disord. 2009, 24, 377–385. [Google Scholar] [CrossRef]
- Castiglia, S.F.; Tatarelli, A.; Trabassi, D.; De Icco, R.; Grillo, V.; Ranavolo, A.; Varrecchia, T.; Magnifica, F.; Di Lenola, D.; Coppola, G.; et al. Ability of a Set of Trunk Inertial Indexes of Gait to Identify Gait Instability and Recurrent Fallers in Parkinson’s Disease. Sensors 2021, 21, 3449. [Google Scholar] [CrossRef] [PubMed]
- de Azevedo, A.K.e.C.; Claudino, R.; Conceição, J.S.; Swarowsky, A.; dos Santos, M.J. Anticipatory and Compensatory Postural Adjustments in Response to External Lateral Shoulder Perturbations in Subjects with Parkinson’s Disease. PLoS ONE 2016, 11, e0155012. [Google Scholar] [CrossRef]
- Brichetto, G.; Pelosin, E.; Marchese, R.; Abbruzzese, G. Evaluation of Physical Therapy in Parkinsonian Patients with Freezing of Gait: A Pilot Study. Clin. Rehabil. 2006, 20, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Nieuwhof, F.; Bloem, B.R.; Reelick, M.F.; Aarts, E.; Maidan, I.; Mirelman, A.; Hausdorff, J.M.; Toni, I.; Helmich, R.C. Impaired Dual Tasking in Parkinson’s Disease Is Associated with Reduced Focusing of Cortico-Striatal Activity. Brain 2017, 140, 1384–1398. [Google Scholar] [CrossRef] [PubMed]
- Heinzel, S.; Roeben, B.; Ben-Shlomo, Y.; Lerche, S.; Alves, G.; Barone, P.; Behnke, S.; Berendse, H.W.; Bloem, B.R.; Burn, D.; et al. Prodromal Markers in Parkinson’s Disease: Limitations in Longitudinal Studies and Lessons Learned. Front. Aging Neurosci. 2016, 8, 147. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.; Koechli, S.; Ungerer, M.; Karageorghis, C.; Whyatt, C.; Annett, L.; Bohlhalter, S.; Vanbellingen, T.; Galati, S.; Dinacci, S. Songlines for Parkinson’s. 2023. Available online: https://osf.io/329gh/ (accessed on 26 March 2025).
- Bonanno, M.; De Pasquale, P.; Fonti, B.; Gjonaj, E.; De Salvo, S.; Quartarone, A.; Calabrò, R.S. Neural Control Meets Biomechanics in the Motor Assessment of Neurological Disorders: A Narrative Review. Front. Neural Circuits 2025, 19, 1608328. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Fernández, L.P. Biomechanics of Parkinson’s Disease with Systems Based on Expert Knowledge and Machine Learning: A Scoping Review. Computation 2024, 12, 230. [Google Scholar] [CrossRef]






| Parameter | Tasks | Description | Predictor/Expected Difference | References |
|---|---|---|---|---|
| Max trunk inclination (AP, ML) | Sit-to-Stand/Stand-to-Sit; Turning | Max. angle between trunk and vertical axis with respect to AP/ML planes. | Larger values may indicate compensatory leaning. Excessive tilt expected in PwP, especially in the ML axis during turning. | [94] |
| Peak trunk velocity (AP) | Sit-to-Stand/Stand-to-Sit | Derivative of trunk displacement. | Higher values indicate momentum-driven strategies; lower values may indicate rigid movement; lower values expected in PwP. | [94] |
| RMS trunk acceleration (AP) | Sit-to-Stand/Stand-to-Sit; Standing Upright | Measure of anticipatory postural adjustments. | Lower values in PwP indicate reduced anticipatory postural adjustments. | [94] |
| Mean/Trunk & Pelvis jerk | Sit-to-Stand/Stand-to-Sit; Turning; Standing Upright | Derivative of trunk acceleration, measures the smoothness of movement. | Higher values in PwP may indicate less fluid/postural control. During Turning, reduced jerk reflects less dynamic compensatory movement. In Standing Upright, indicator of movement smoothness. | [73,94,108] |
| Peak pelvis flexion | Sit-to-Stand/Stand-to-Sit; Functional Reach | Angle between trunk–pelvis and trunk–knees vectors. | During Sit-to-stand/Stand-to-sit greater flexion in PwP reflects reliance on pelvis flexion to stabilize COM during transition. In Functional Reach, reduced pelvis flexion may indicate compensatory while bending down. | [103,105] |
| Knee flexion | Sit-to-Stand/Stand-to-Sit; Functional Reach | Angle between knee–pelvis and knee–heel. | Greater knee flexion values in PwP suggest a compensatory strategy for stability during sit-to-stand and stand-to-sit, and similarly, greater flexion during Functional Reach reflects a compensatory strategy while bending down. | [87,104,105] |
| COP displacement/sway (AP, ML) | Sit-to-Stand/Stand-to-Sit; Turning; Functional Reach; Standing Upright | Postural stability indicator. Obtained from the PKMAS system. | Greater displacement in PwP reflects impaired balance/anticipatory postural control. | [8,73,107] |
| Absolute yaw rotation angle | Turning | Segment rotation (head, trunk, pelvis) around the vertical axis. | Reduced angles indicate in-bloc turning strategy. Higher angles suggest a craniocaudal turning strategy. | [91] |
| Relative yaw angle | Turning | Relative rotation between head–trunk, trunk–pelvis, and head–pelvis. | Indicator of flexibility and coordination between adjacent body segments. Smaller differences in PwP suggest less coordinated turning strategy. | [91,92] |
| Onset of rotation (% gait cycle) | Turning | Percentage of gait cycle for head, trunk, pelvis normalized to the first stride of turn. | Additional indicator to determine turning strategy. Lower onset values = earlier rotation; differences indicate altered coordination in PwP. | [91,92] |
| Arm alignment angle | Functional Reach | Shoulder–wrist segment alignment with respect to the sagittal plane. | Indicator of upper body control while bending down. Values close to 90° indicate a diagonal reach further from the body; PwP expected to show higher variability. | [75] |
| Stance stability: AP distance, stance width, feet aperture angle | Functional Reach | Frontal and mediolateral distance between feet. Angle between the long axes of the feet in the transverse plane. | Larger distances/angles may indicate a compensatory strategy to maintain COM stability while bending down. | [105,106] |
| Toe-off (TO) angle | Functional Reach; Locomotion | Maximum heel-ground angle (see Figure 3). | Reduced values may indicate impaired dorsiflexion of the ankle joint in PwP. | [88,104] |
| Heel-strike (HS) angle | Locomotion | Max toe-ground angle (see Figure 3). | Reduced values may indicate shuffling. | [88,104] |
| Foot height, (minimum foot clearance, mFC) | Locomotion | Shortest vertical foot–ground distance. | Reduced values suggest impaired foot clearance in PwP. | [88,104] |
| Spatiotemporal gait parameters | Locomotion | Stride length, width, velocity, time, double support, asymmetry, variability. | PwP expected to show higher asymmetry, longer double support, shorter strides, wider stance, slower velocity, and higher variability. | [29] |
| Arm swing displacement & angular velocities | Locomotion | Arm segment kinematics (shoulder–elbow, elbow–wrist, shoulder–wrist). Calculated using markers coordinated projected onto the sagittal plane, with the pelvis midpoint as the reference point. | Reduced magnitude indicating impaired upper-limb contribution to gait. Lower velocities in PwP, reflecting bradykinesia and reduced rhythmic coordination. | [34] |
| Arm Swing Asymmetry (ASA) | Locomotion | Asymmetry percentage between dominant and non-dominant arm swing (see Equation (2)). | High asymmetry percentages suggest an impaired arm coordination during gait. | [109,110] |
| Freezing-like events (FLE) | Locomotion Gait during FMA-P | Based on pelvis midpoint displacement. Detected when the velocity decreases to less than 10% of a baseline. Baseline speed was determined from TUG. FLE duration was calculated considering a minimum duration threshold of 0.5 s. | Higher frequency and longer duration in PwP, reflecting impaired gait initiation and motor blocks. | [118,119] |
| Parameter | Study 1 PwP, n = 12 | Study 1 Control, n = 12 | Study 2, n = 12 | |||
|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | |
| Age (years) | 69.5 | 6.1 | 61.0 | 7.3 | 72.2 | 7.0 |
| Gender | ||||||
| Female (%) | 58 | 33 | 67 | |||
| Male (%) | 42 | 67 | 33 | |||
| Measure | Group | |
|---|---|---|
| PwP | Controls | |
| TUG | ||
| Mean completion time (s) | 10.4 | 7.3 |
| SD | 3.6 | 1.0 |
| FMA-P | ||
| Mean completion time (s) | 13.4 | 9.7 |
| SD | 4.3 | 1.4 |
| Task | Parameter | Group | TUG M (SD) | FMA-P M (SD) | Protocol Effect (p) | Group Effect (p) | Interaction (p) |
|---|---|---|---|---|---|---|---|
| Sit-to-stand | Peak Trunk AP Inclination (deg) a | Control | 123.5 (2.7) | 124.7 (3.8) | 0.498 | 0.006 ** | 0.365 |
| PwP | 120.3 (3.2) | 119.7 (4.9) | |||||
| Mean trunk jerk (m/s3) a | Control | −4.0 (1.6) | −4.2 (1.5) | 0.270 | 0.003 ** | 0.687 | |
| PwP | −2.0 (1.0) | −2.4 (1.5) | |||||
| Turning | Task duration (s) | Control | 1.5 (0.3) | 1.2 (0.2) | 0.064 | 0.026 * | 0.951 |
| PwP | 1.9 (0.5) | 1.6 (0.5) | |||||
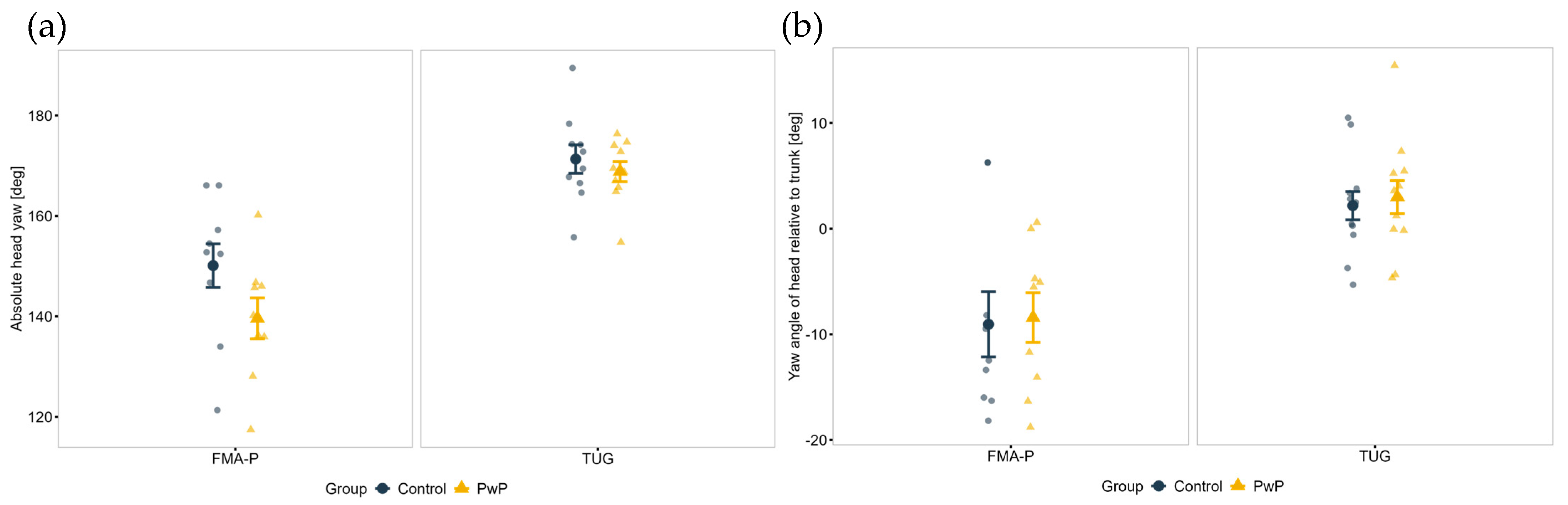
| Absolute head yaw rotation (deg) a | Control | 171.3 (9.0) | 150.1 (13.7) | <0.001 *** | 0.052 | 0.245 | |
| PwP | 168.8 (6.3) | 139.6 (12.3) | |||||
| Head relative to trunk yaw rotation (deg) a | Control | 2.2 (4.7) | −9.1 (9.2) | <0.001 *** | 0.521 | 0.848 | |
| PwP | 3.0 (5.4) | −8.4 (7.0) | |||||
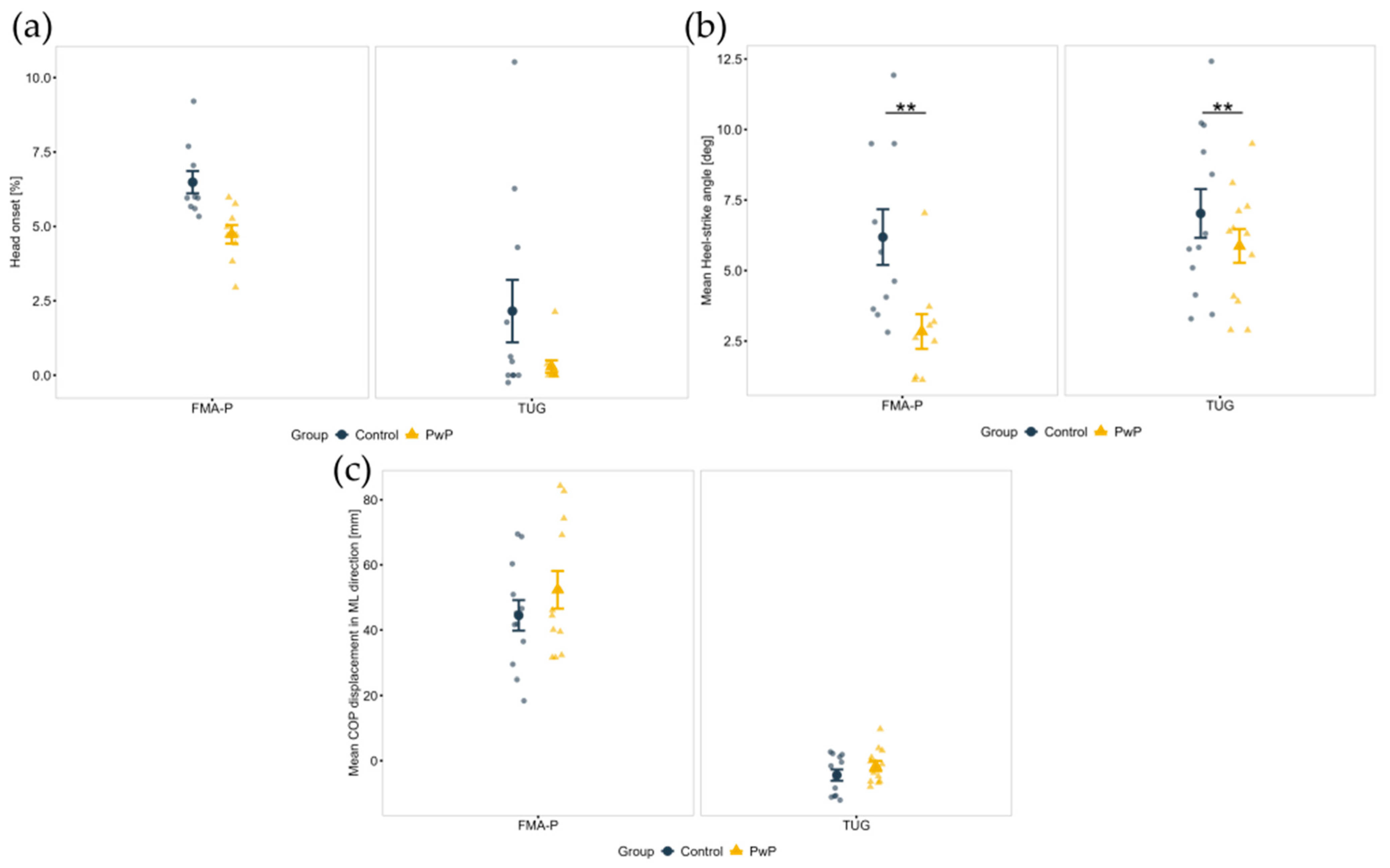
| Head onset (%) a | Control | 2.2 (3.5) | 6.5 (1.2) | <0.001 *** | 0.218 | 0.904 | |
| PwP | 0.3 (0.7) | 4.7 (0.9) | |||||
| Mean heel-strike angle (deg) b | Control | 7.0 (3.1) | 6.2 (3.1) | 0.527 | 0.007 ** | 0.209 | |
| PwP | 5.9 (2.1) | 2.8 (1.8) | |||||
| Mean COP displacement in ML (mm) b | Control | −4.1 (5.9) | 44.5 (16.2) | 0.002 ** | 0.386 | 0.098 | |
| PwP | −1.7 (5.6) | 52.3 (19.9) |
| Task | Parameter | Comparison | Mean Difference | 95% CI | t | p |
|---|---|---|---|---|---|---|
| Turning | Task duration (s) | Baseline-Pre | −0.02 | [−0.3; 0.2] | 0.22 | 1.0 |
| Baseline-Post | 0.4 | [0.1; 0.6] | 3.97 | 0.006 ** | ||
| Pre-Post | 0.4 | [0.1; 0.7] | 3.98 | 0.006 ** | ||
| Mean pelvis jerk (m/s3) | Baseline-Pre | 189.2 | [−387.4; 765.8] | 0.96 | 1.0 | |
| Baseline-Post | 556.3 | [−3.8; 1116.3] | 2.91 | 0.05 * | ||
| Pre-Post | 367.0 | [−192.1; 926.2] | 1.92 | 0.258 | ||
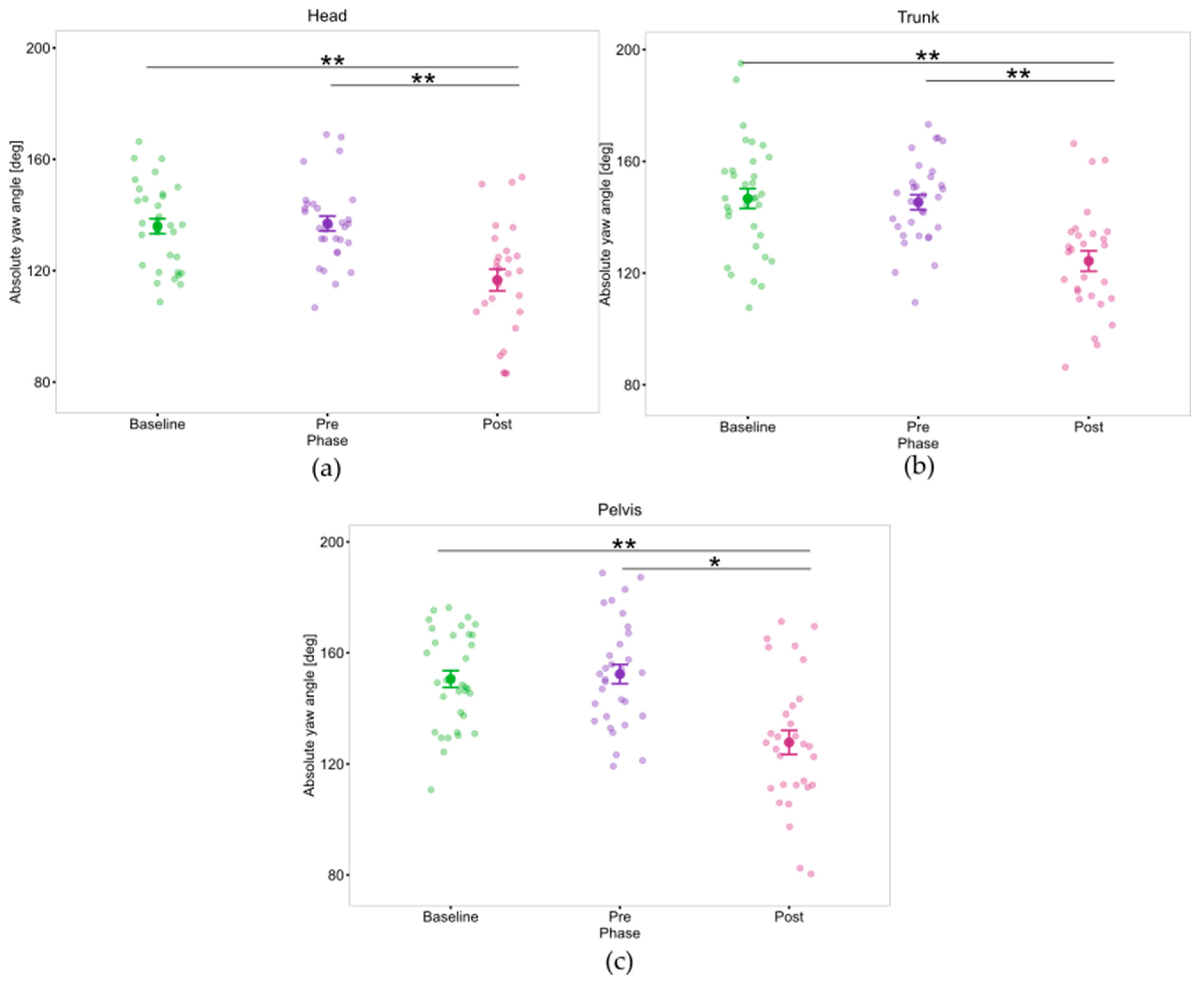
| Absolute head yaw rotation (deg) | Baseline-Pre | 0.6 | [−5.4; 6.5] | 0.27 | 1.0 | |
| Baseline-Post | 25.5 | [10.4; 40.6] | 4.75 | 0.002 ** | ||
| Pre-Post | 24.9 | [10.6; 39.2] | 4.92 | 0.001 ** | ||
| Absolute trunk yaw rotation (deg) | Baseline-Pre | 2.1 | [−5.9; 10.1] | 0.74 | 1.0 | |
| Baseline-Post | 27.4 | [11.9; 42.9] | 4.98 | 0.001 ** | ||
| Pre-Post | 25.3 | [10.5; 40.0] | 4.83 | 0.002 ** | ||
| Absolute pelvis yaw rotation (deg) | Baseline-Pre | −2.6 | [−16.4; 11.3] | −0.52 | 1.0 | |
| Baseline-Post | 21.0 | [6.3; 35.7] | 4.03 | 0.006 ** | ||
| Pre-Post | 23.6 | [5.2; 42.0] | 3.61 | 0.012 * | ||
| Max. trunk ML inclination (deg) | Baseline-Pre | −0.1 | [−1.9; 1.7] | −0.12 | 1.0 | |
| Baseline-Post | 2.0 | [−0.1; 4.2] | 2.68 | 0.064 | ||
| Pre-Post | 2.1 | [0.7; 3.5] | 4.24 | 0.004 ** | ||
| Stand-to-sit | Max. pelvis flexion (deg) | Baseline-Pre | −12 | [−19.9; −4.2] | −4.32 | 0.004 ** |
| Baseline-Post | −3.8 | [−12.4; 4.9] | −1.23 | 0.737 | ||
| Pre-Post | 8.3 | [−3.1; 19.7] | 2.05 | 0.194 | ||
| Max. knee flexion (deg) | Baseline-Pre | −11.7 | [−16.9; −6.5] | −6.39 | <0.001 *** | |
| Baseline-Post | −9.5 | [−14.1; −4.8] | −5.76 | <0.001 *** | ||
| Pre-Post | 2.2 | [−5.7; 10.2] | 0.79 | 1.0 | ||
| Functional reach | Max. stance width (m) | Baseline-Pre | −23.7 | [−53.1; 5.7] | −2.31 | 0.129 |
| Baseline-Post | 0.8 | [−30.3; 31.8] | 0.07 | 1.0 | ||
| Pre-Post | 24.5 | [−2; 51] | 2.65 | 0.073 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Köchli, S.; Casso, I.; Delevoye-Turrell, Y.N.; Schmid, S.; Rose, D.C.; Whyatt, C. A New Methodological Approach Integrating Motion Capture and Pressure-Sensitive Gait Data to Assess Functional Mobility in Parkinson’s Disease: A Two-Phase Study. Sensors 2025, 25, 5999. https://doi.org/10.3390/s25195999
Köchli S, Casso I, Delevoye-Turrell YN, Schmid S, Rose DC, Whyatt C. A New Methodological Approach Integrating Motion Capture and Pressure-Sensitive Gait Data to Assess Functional Mobility in Parkinson’s Disease: A Two-Phase Study. Sensors. 2025; 25(19):5999. https://doi.org/10.3390/s25195999
Chicago/Turabian StyleKöchli, Sabrina, Isabel Casso, Yvonne N. Delevoye-Turrell, Stefan Schmid, Dawn C. Rose, and Caroline Whyatt. 2025. "A New Methodological Approach Integrating Motion Capture and Pressure-Sensitive Gait Data to Assess Functional Mobility in Parkinson’s Disease: A Two-Phase Study" Sensors 25, no. 19: 5999. https://doi.org/10.3390/s25195999
APA StyleKöchli, S., Casso, I., Delevoye-Turrell, Y. N., Schmid, S., Rose, D. C., & Whyatt, C. (2025). A New Methodological Approach Integrating Motion Capture and Pressure-Sensitive Gait Data to Assess Functional Mobility in Parkinson’s Disease: A Two-Phase Study. Sensors, 25(19), 5999. https://doi.org/10.3390/s25195999






