Sex Differences in Cortical Hemodynamic Responses During Interactive and Passive Tasks: An fNIRS Study Using the Nefroball System
Abstract
1. Introduction
2. Materials and Methods
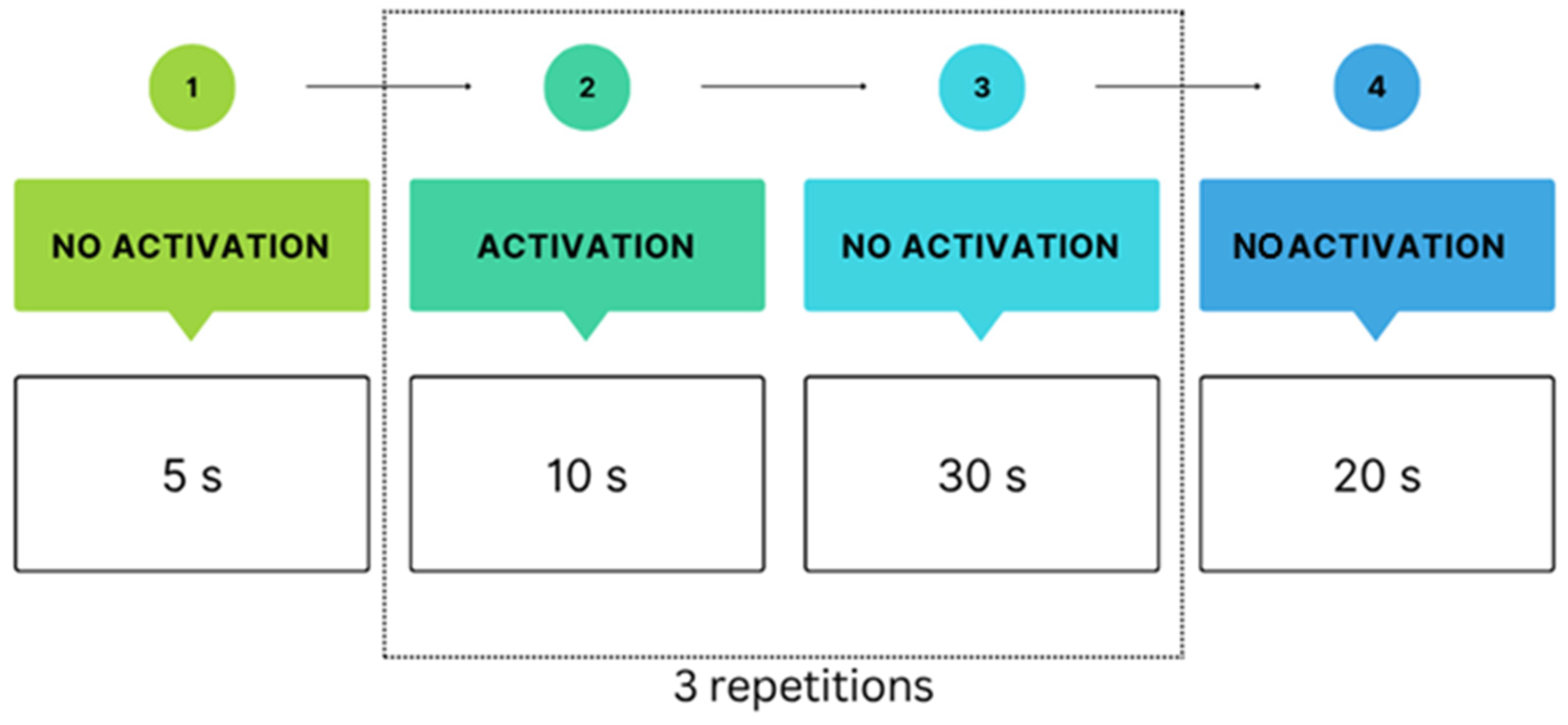
- In Protocol I (Interactive task), participants performed the same ball-squeezing movement. However, in this condition, the NefroBall system registered each movement, allowing the participant to shoot down virtual objects in the “Space Invaders” game. The goal was to shoot down as many objects as possible within a set time, requiring eye-hand coordination as ball compression directly controlled the number of projectiles fired by the plane. During the interactive task, participants used the NefroBall system by pressing the ball with a predefined force threshold set in the application. Once the required pressure was reached, a virtual aeroplane on the screen fired projectiles, thereby “shooting down” the flying objects. In this way, foot-applied pressure was directly translated into an action within the game.
- In Protocol II (Passive task), participants rhythmically squeezed the pressure controller approximately every 2 s. Simultaneously, they observed a computer screen displaying the “Space Invaders” game with an aeroplane moving, but their actions did not affect the game. The objective was to maintain consistent ball compression without reacting to on-screen changes. Protocol II was a control study.
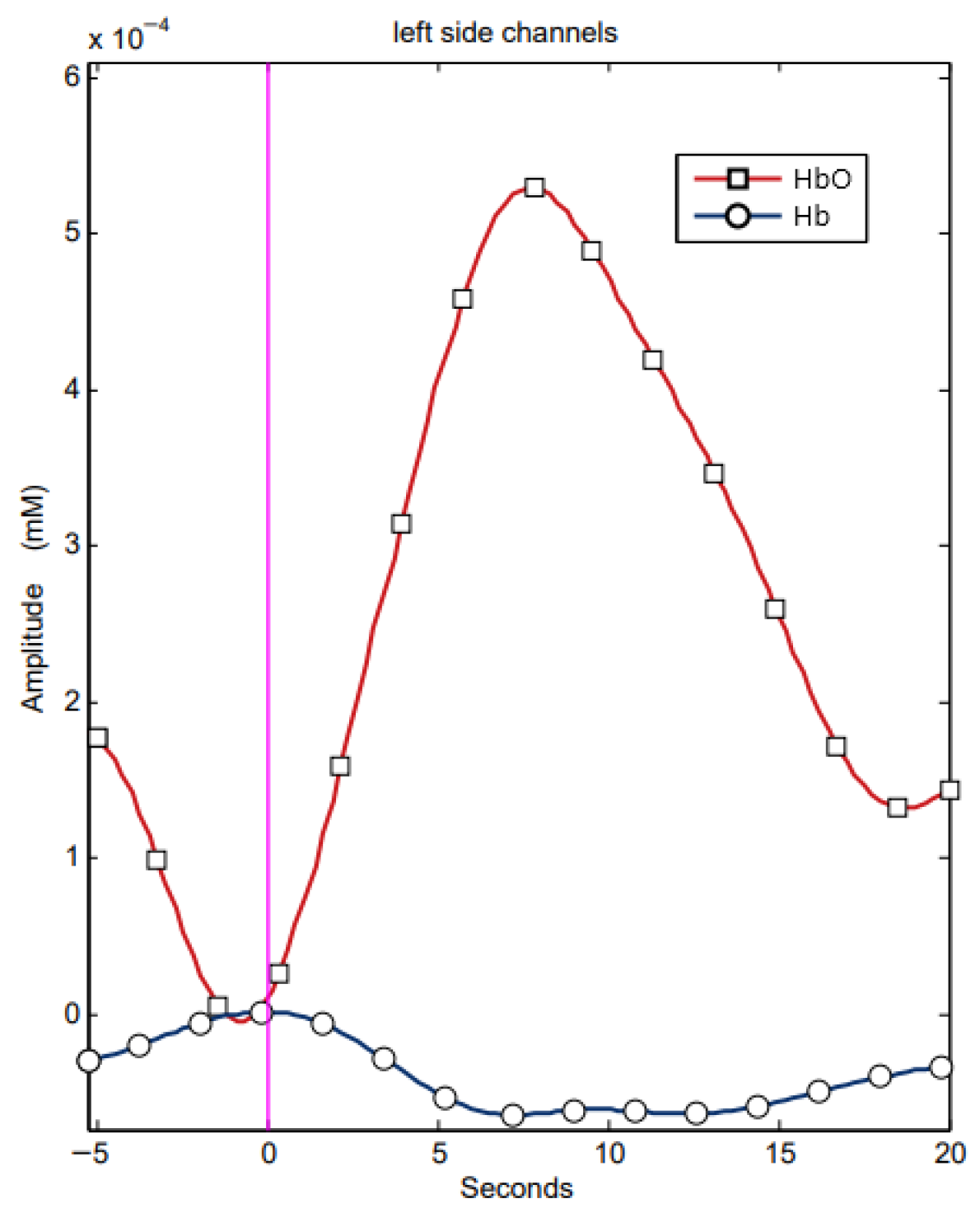
- The amplitude of the hemodynamic response, ΔHbO, which is the difference between the maximum (after the stimulus) and the minimum (before the stimulus) of the signal for HbO
- Latency time (tmax), which is the time from the onset of the stimulus (beginning of task execution) to the maximum HbO signal.
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| fNIRS | Functional near-infrared spectroscopy |
| tmax | The time from the beginning of the task to the HbO signal reaching its maximum value |
| HbO | Oxygenated reduced haemoglobin |
| Hb | Reduced haemoglobin |
| ΔHbO | The average amplitude of the increase in oxygenated oxyhaemoglobin |
| EEG | Electroencephalopathy |
References
- Gur, R.C.; Turetsky, B.I.; Matsui, M.; Yan, M.; Bilker, W.; Hughett, P.; Gur, R.E. Sex Differences in Brain Gray and White Matter in Healthy Young Adults: Correlations with Cognitive Performance. J. Neurosci. 1999, 19, 4065–4072. [Google Scholar] [CrossRef] [PubMed]
- Cahill, L. Why Sex Matters for Neuroscience. Nat. Rev. Neurosci. 2006, 7, 477–484. [Google Scholar] [CrossRef]
- Semrud-Clikeman, M.; Fine, J.G.; Bledsoe, J.; Zhu, D.C. Gender Differences in Brain Activation on a Mental Rotation Task. Int. J. Neurosci. 2012, 122, 590–597. [Google Scholar] [CrossRef]
- Scholkmann, F.; Kleiser, S.; Metz, A.J.; Zimmermann, R.; Mata Pavia, J.; Wolf, U.; Wolf, M. A Review on Continuous Wave Functional Near-Infrared Spectroscopy and Imaging Instrumentation and Methodology. NeuroImage 2014, 85, 6–27. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Quaresima, V. A Brief Review on the History of Human Functional Near-Infrared Spectroscopy (fNIRS) Development and Fields of Application. NeuroImage 2012, 63, 921–935. [Google Scholar] [CrossRef] [PubMed]
- Jezierska, K.; Sękowska-Namiotko, A.; Pala, B.; Lietz-Kijak, D.; Gronwald, H.; Podraza, W. Searching for the Mechanism of Action of Extremely Low Frequency Electromagnetic Field—The Pilot fNIRS Research. Int. J. Environ. Res. Public Health 2022, 19, 4012. [Google Scholar] [CrossRef]
- Jezierska, K.; Lietz-Kijak, D.; Gronwald, H.; Oleksy, B.; Gronwald, B.J.; Podraza, W. Taste Dysfunction after COVID-19: Analysis with Functional near-Infrared Spectroscopy. Otolaryngol. Pol. 2023, 78, 14–19. [Google Scholar] [CrossRef]
- Chen, W.-L.; Wagner, J.; Heugel, N.; Sugar, J.; Lee, Y.-W.; Conant, L.; Malloy, M.; Heffernan, J.; Quirk, B.; Zinos, A.; et al. Functional Near-Infrared Spectroscopy and Its Clinical Application in the Field of Neuroscience: Advances and Future Directions. Front. Neurosci. 2020, 14, 724. [Google Scholar] [CrossRef]
- Ehlis, A.-C.; Schneider, S.; Dresler, T.; Fallgatter, A.J. Application of Functional Near-Infrared Spectroscopy in Psychiatry. NeuroImage 2014, 85 Pt 1, 478–488. [Google Scholar] [CrossRef]
- Cutini, S.; Moro, S.B.; Bisconti, S. Functional near Infrared Optical Imaging in Cognitive Neuroscience: An Introductory Review. J. Near Infrared Spectrosc. 2012, 20, 75–92. [Google Scholar] [CrossRef]
- Shaywitz, B.A.; Shaywitz, S.E.; Pugh, K.R.; Constable, R.T.; Skudlarski, P.; Fulbright, R.K.; Bronen, R.A.; Fletcher, J.M.; Shankweiler, D.P.; Katz, L. Sex Differences in the Functional Organization of the Brain for Language. Nature 1995, 373, 607–609. [Google Scholar] [CrossRef] [PubMed]
- Ingalhalikar, M.; Smith, A.; Parker, D.; Satterthwaite, T.D.; Elliott, M.A.; Ruparel, K.; Hakonarson, H.; Gur, R.E.; Gur, R.C.; Verma, R. Sex Differences in the Structural Connectome of the Human Brain. Proc. Natl. Acad. Sci. USA 2014, 111, 823–828. [Google Scholar] [CrossRef]
- Frederikse, M.E.; Lu, A.; Aylward, E.; Barta, P.; Pearlson, G. Sex Differences in the Inferior Parietal Lobule. Cereb. Cortex 1999, 9, 896–901. [Google Scholar] [CrossRef]
- Bell, E.C.; Willson, M.C.; Wilman, A.H.; Dave, S.; Silverstone, P.H. Males and Females Differ in Brain Activation during Cognitive Tasks. NeuroImage 2006, 30, 529–538. [Google Scholar] [CrossRef]
- Cahill, L. Equal ≠ The Same: Sex Differences in the Human Brain. Cerebrum 2014, 2014, 5. [Google Scholar] [PubMed]
- Cazzell, M.; Li, L.; Lin, Z.-J.; Patel, S.J.; Liu, H. Comparison of Neural Correlates of Risk Decision Making between Genders: An Exploratory fNIRS Study of the Balloon Analogue Risk Task (BART). NeuroImage 2012, 62, 1896–1911. [Google Scholar] [CrossRef]
- Huang, X.; Bai, L.; Chen, Y.; Cui, H.; Wang, L. Gender Differences in Oxyhemoglobin (Oxy-Hb) Changes during Drawing Interactions in Romantic Couples: An fNIRS Study. Front. Behav. Neurosci. 2025, 18, 1476535. [Google Scholar] [CrossRef]
- Altınkaynak, M.; Güven, A.; Dolu, N.; İzzetoğlu, M.; Pektaş, F.; Özmen, S.; Demirci, E. Gender Effects On Prefrontal Cortex Oxygenation Levels During Auditory Oddball Task In Children. J. Intell. Syst. Appl. 2018, 1, 1–7. [Google Scholar] [CrossRef]
- Mihara, M.; Miyai, I.; Hattori, N.; Hatakenaka, M.; Yagura, H.; Kawano, T.; Okibayashi, M.; Danjo, N.; Ishikawa, A.; Inoue, Y.; et al. Neurofeedback Using Real-Time Near-Infrared Spectroscopy Enhances Motor Imagery Related Cortical Activation. PLoS ONE 2012, 7, e32234. [Google Scholar] [CrossRef] [PubMed]
- Sharmin, S.; Barmaki, R.L. A Scoping Review of Functional Near-Infrared Spectroscopy (fNIRS) Applications in Game-Based Learning Environments. arXiv 2024, arXiv:2411.02650. [Google Scholar]
- Jezierska, K.; Turoń-Skrzypińska, A.; Rotter, I.; Syroka, A.; Łukowiak, M.; Rawojć, K.; Rawojć, P.; Rył, A. Latency and Amplitude of Cortical Activation in Interactive vs. Passive Tasks: An fNIRS Study Using the NefroBall System. Sensors 2025, 25, 4135. [Google Scholar] [CrossRef] [PubMed]
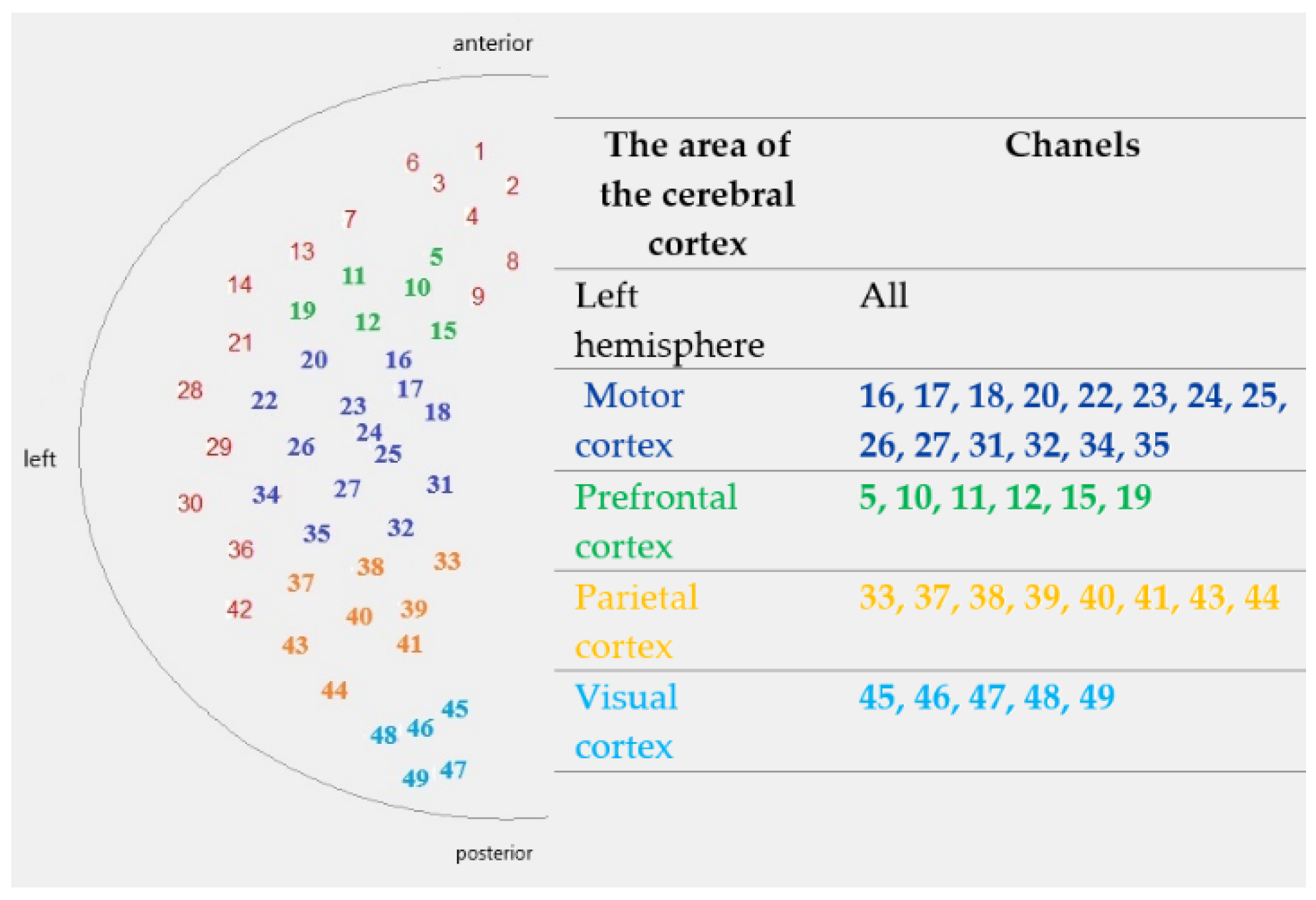

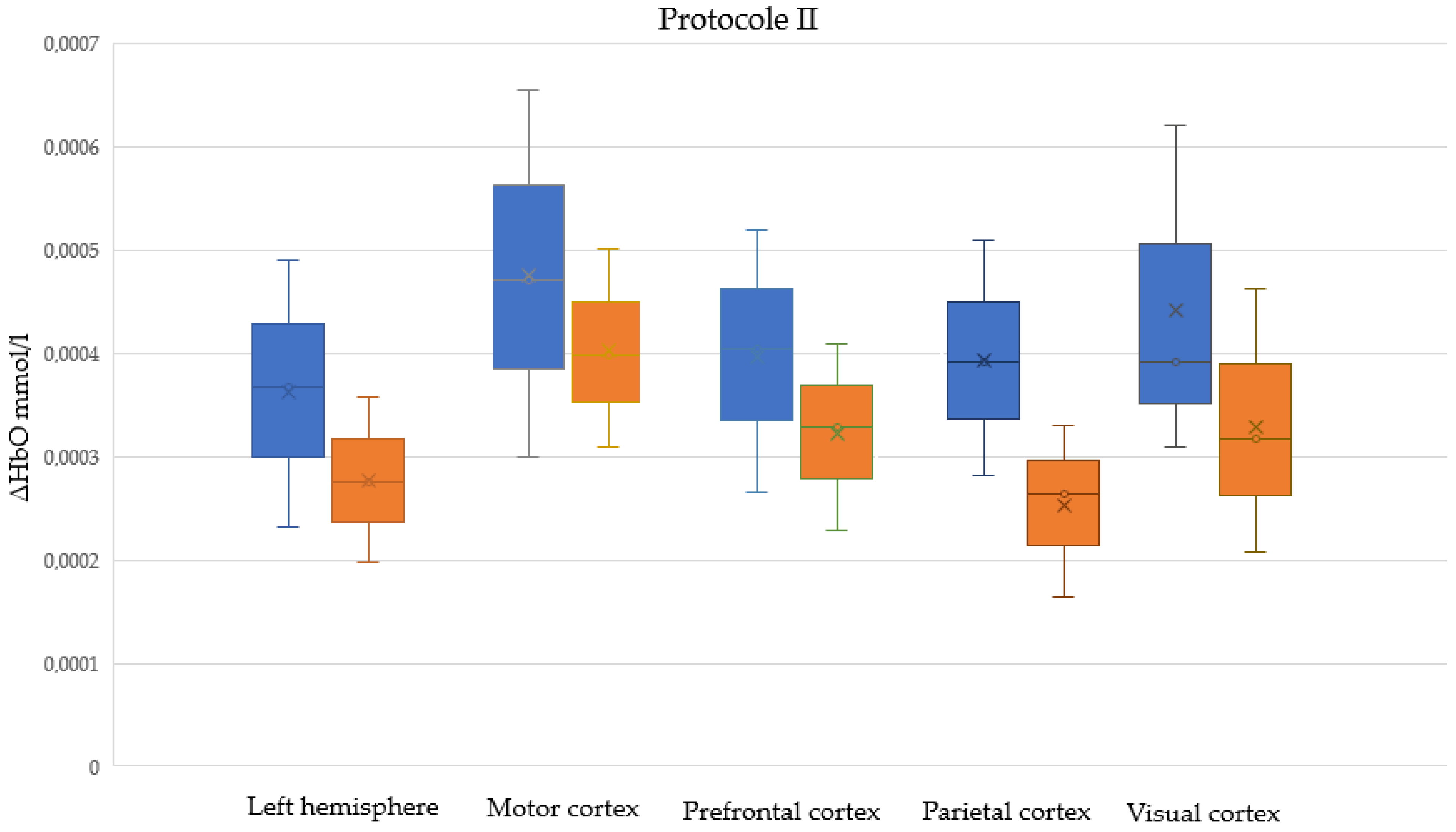
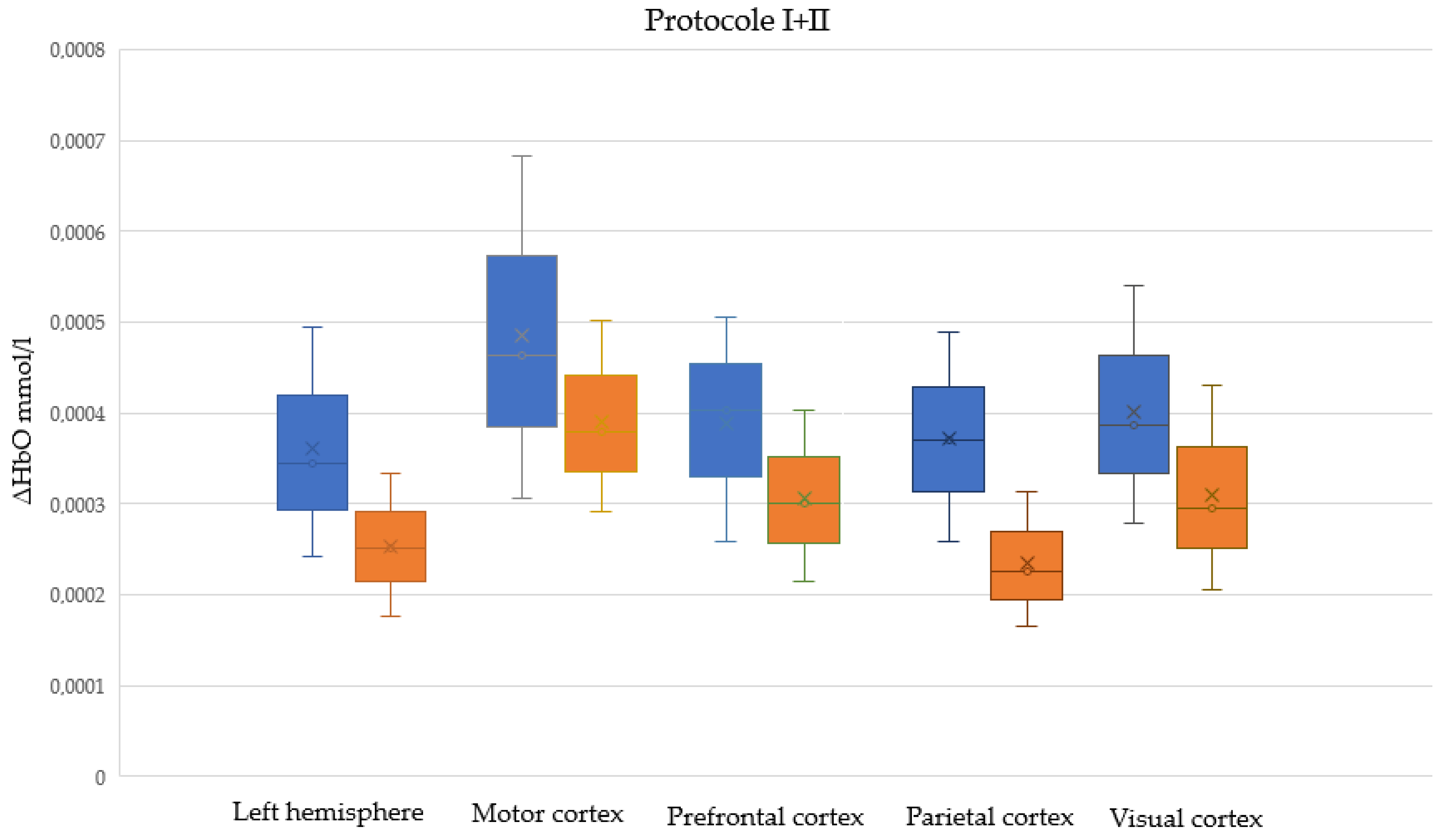
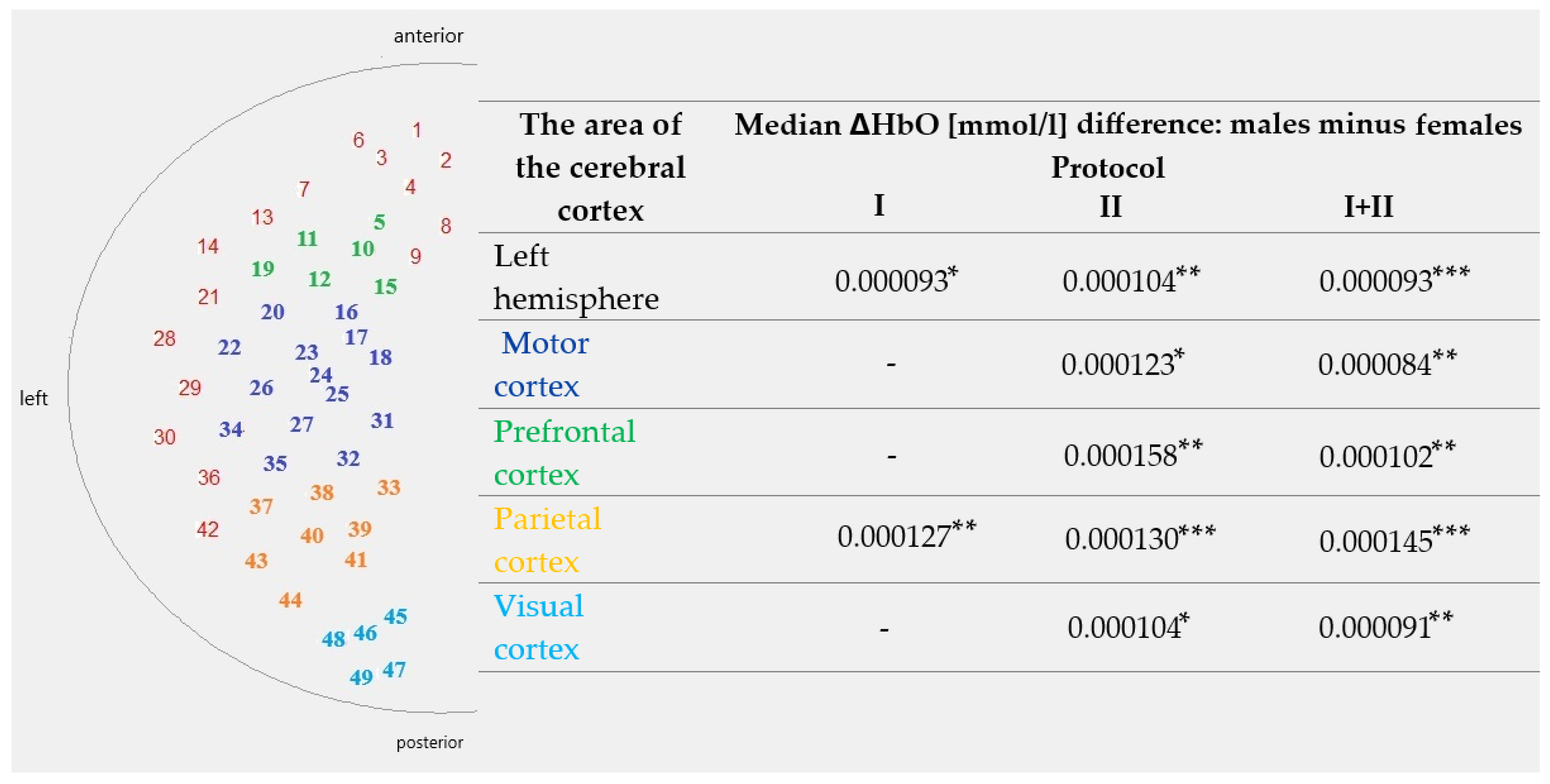
| The Area of the Cerebral Cortex | Protocol | Median ΔHbO [mmol/L] Difference: Males Minus Females | p-Value | U Test Statistic |
|---|---|---|---|---|
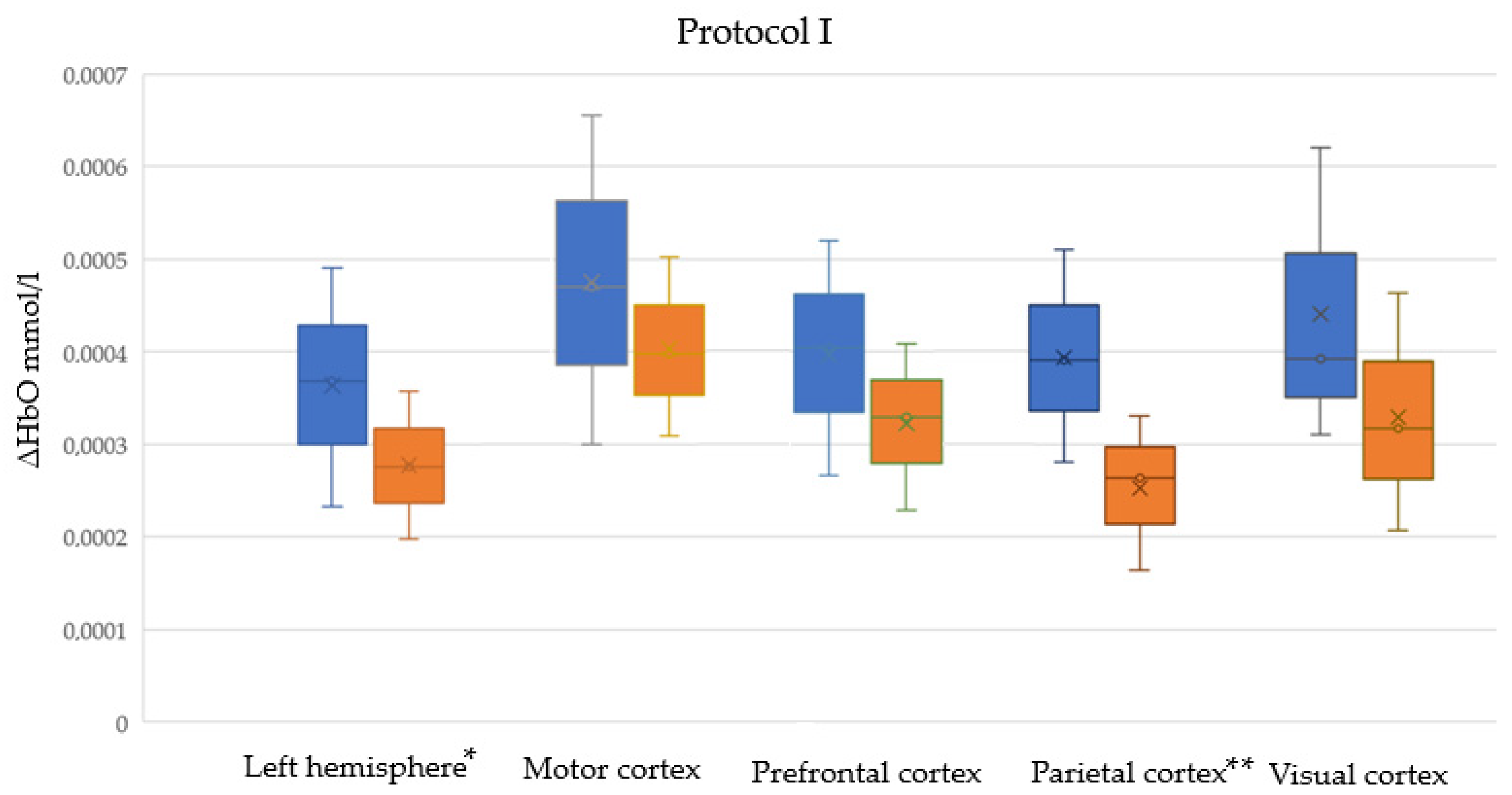
| Left hemisphere | I | 0.000093 | 0.022 | 741 |
| II | 0.000104 | 0.001 | 599 | |
| I + II | 0.000093 | <0.001 | 2695 | |
| Motor cortex | I | 0.000072 | 0.165 | 831 |
| II | 0.000123 | 0.014 | 694 | |
| I + II | 0.000084 | 0.009 | 3098 | |
| Prefrontal cortex | I | 0.000075 | 0.060 | 755 |
| II | 0.000158 | 0.004 | 631 | |
| I + II | 0.000102 | 0.001 | 2757 | |
| Parietal cortex | I | 0.000127 | 0.001 | 473 |
| II | 0.000130 | <0.001 | 456 | |
| I + II | 0.000145 | <0.001 | 1870 | |
| Visual cortex | I | 0.000076 | 0.065 | 576 |
| II | 0.000104 | 0.014 | 514 | |
| I + II | 0.000091 | 0.002 | 2186 |
| The Area of the Cerebral Cortex | Protocol | Median tmax [s] Difference: Males Minus Females | p-Value | U Test Statistic |
|---|---|---|---|---|
| Left hemisphere | I | 0.125 | 0.892 | 1019 |
| II | 0.375 | 0.516 | 953 | |
| I + II | 0.625 | 0.589 | 3951 | |
| Motor cortex | I | 0.125 | 0.732 | 964 |
| II | 0.250 | 0.579 | 937 | |
| I + II | 0.250 | 0.768 | 3926 | |
| Prefrontal cortex | I | 0.250 | 0.621 | 928 |
| II | 0.750 | 0.492 | 904 | |
| I + II | 0.500 | 0.429 | 3682 | |
| Parietal cortex | I | −0.125 | 0.377 | 742 |
| II | 2.125 | 0.391 | 759 | |
| I + II | 0.500 | 0.917 | 3357 | |
| Visual cortex | I | −1.000 | 0.029 | 541 |
| II | −0.875 | 0.244 | 646 | |
| I + II | −3.250 | 0.014 | 2355 |
| Female | |||
|---|---|---|---|
| The Area of the Cerebral Cortex | Correlation Analysis Between ΔHbO and tmax for Protocols: | R | p-Value |
| Left hemisphere | I | 0.08 | 0.555 |
| II | 0.21 | 0.103 | |
| I + II | 0.10 | 0.257 | |
| Motor cortex | I | 0.14 | 0.271 |
| II | 0.25 | 0.049 | |
| I + II | 0.17 | 0.052 | |
| Prefrontal cortex | I | 0.13 | 0.321 |
| II | 0.23 | 0.086 | |
| I + II | 0.12 | 0.196 | |
| Parietal cortex | I | 0.03 | 0.857 |
| II | 0.13 | 0.342 | |
| I + II | 0.04 | 0.700 | |
| Visual cortex | I | 0.24 | 0.087 |
| II | −0.14 | 0.340 | |
| I + II | 0.04 | 0.695 | |
| Male | |||
|---|---|---|---|
| The Area of the Cerebral Cortex | Correlation Analysis Between ΔHbO and tmax for Protocols: | R | p-Value |
| Left hemisphere | I | 0.26 | 0.139 |
| II | −0.04 | 0.830 | |
| I + II | 0.15 | 0.221 | |
| Motor cortex | I | 0.18 | 0.317 |
| II | 0.21 | 0.254 | |
| I + II | 0.23 | 0.070 | |
| Prefrontal cortex | I | 0.26 | 0.144 |
| II | 0.38 | 0.031 | |
| I + II | 0.27 | 0.030 | |
| Parietal cortex | I | 0.25 | 0.190 |
| II | 0.01 | 0.970 | |
| I + II | 0.07 | 0.615 | |
| Visual cortex | I | 0.29 | 0.121 |
| II | 0.10 | 0.614 | |
| I + II | 0.15 | 0.237 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jezierska, K.; Turoń-Skrzypińska, A.; Rotter, I.; Syroka, A.; Rył, A. Sex Differences in Cortical Hemodynamic Responses During Interactive and Passive Tasks: An fNIRS Study Using the Nefroball System. Sensors 2025, 25, 5897. https://doi.org/10.3390/s25185897
Jezierska K, Turoń-Skrzypińska A, Rotter I, Syroka A, Rył A. Sex Differences in Cortical Hemodynamic Responses During Interactive and Passive Tasks: An fNIRS Study Using the Nefroball System. Sensors. 2025; 25(18):5897. https://doi.org/10.3390/s25185897
Chicago/Turabian StyleJezierska, Karolina, Agnieszka Turoń-Skrzypińska, Iwona Rotter, Anna Syroka, and Aleksandra Rył. 2025. "Sex Differences in Cortical Hemodynamic Responses During Interactive and Passive Tasks: An fNIRS Study Using the Nefroball System" Sensors 25, no. 18: 5897. https://doi.org/10.3390/s25185897
APA StyleJezierska, K., Turoń-Skrzypińska, A., Rotter, I., Syroka, A., & Rył, A. (2025). Sex Differences in Cortical Hemodynamic Responses During Interactive and Passive Tasks: An fNIRS Study Using the Nefroball System. Sensors, 25(18), 5897. https://doi.org/10.3390/s25185897





