Temporal Sculpting of Laser Pulses for Functional Engineering of Al2O3/AgO Films: From Structural Control to Enhanced Gas Sensing Performance
Abstract
1. Introduction
2. Experimental Procedure
2.1. Preparation of the /AgO Solid Target
2.2. Preparation of the /AgO Thin Films
2.3. Gas Sensor
3. Results and Discussion
3.1. X-Ray Diffraction (XRD)
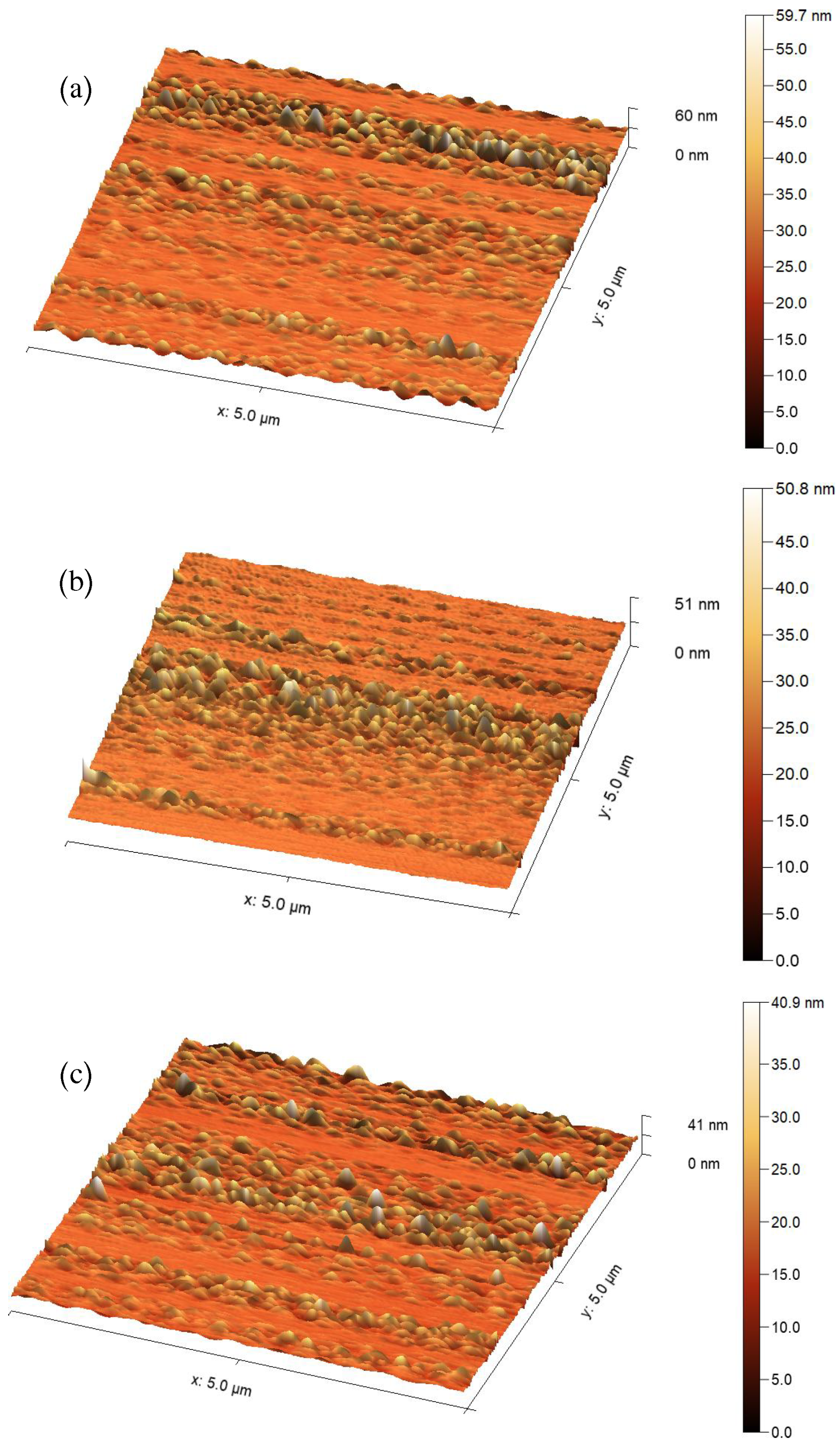
3.2. Atomic Force Microscope (AFM)
3.3. Field Emission Scanning Electron Microscopes (FE-SEM)
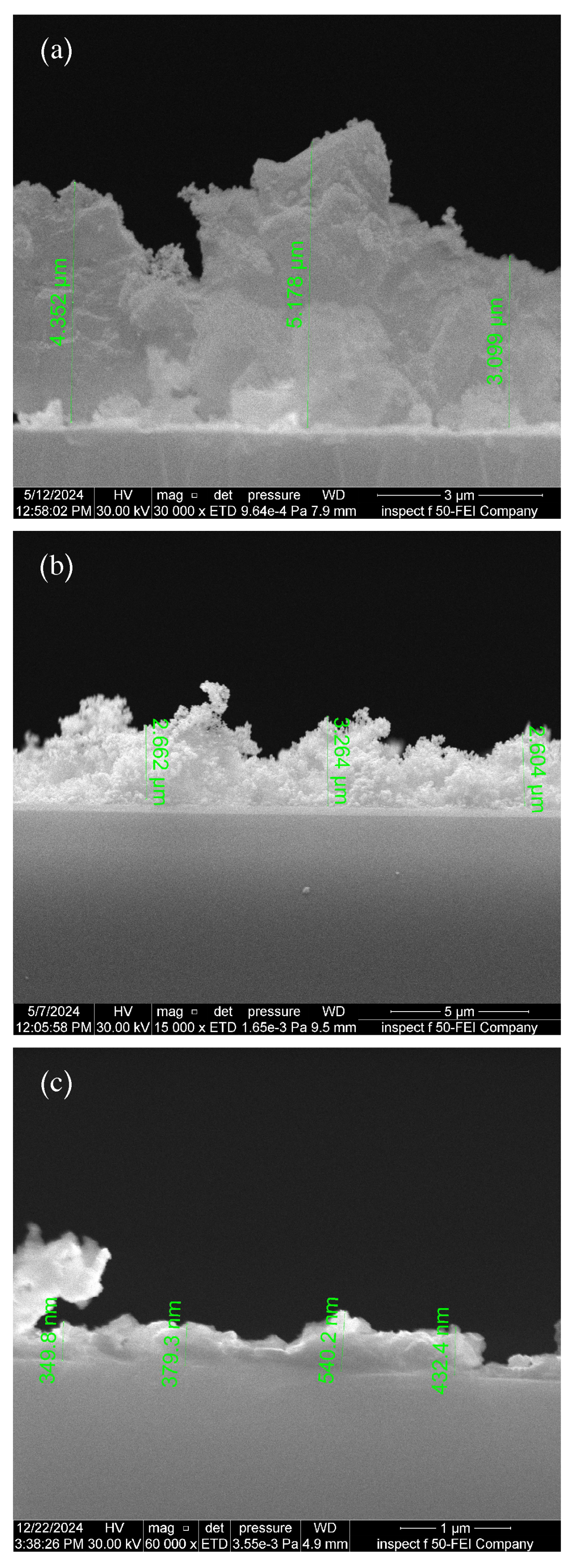
3.4. Cross-Section FE-SEM Images
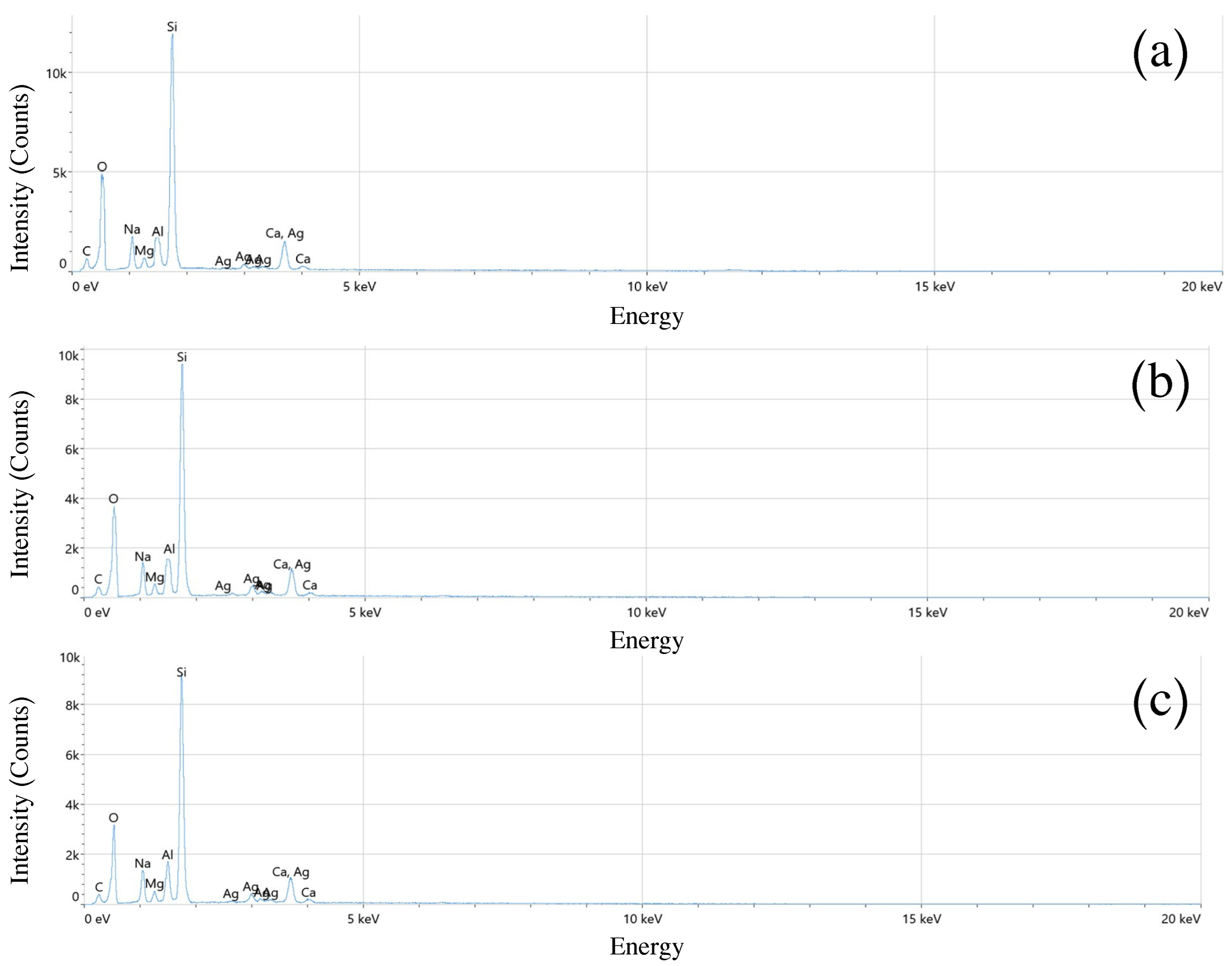
3.5. Energy Dispersive Spectroscopy (EDS)
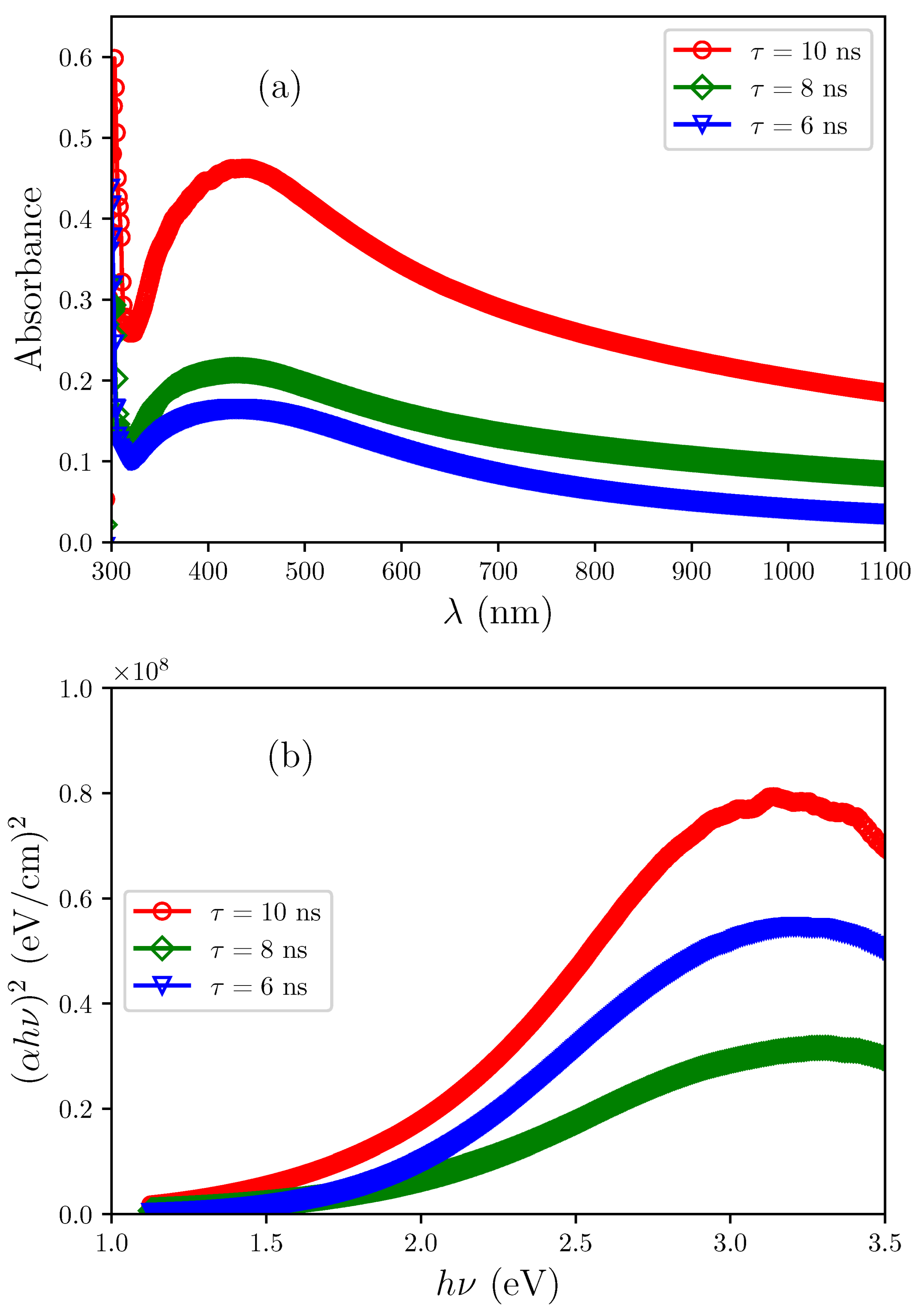
3.6. Optical Properties
3.7. Hall Effect
3.8. Current-Voltage Characteristics
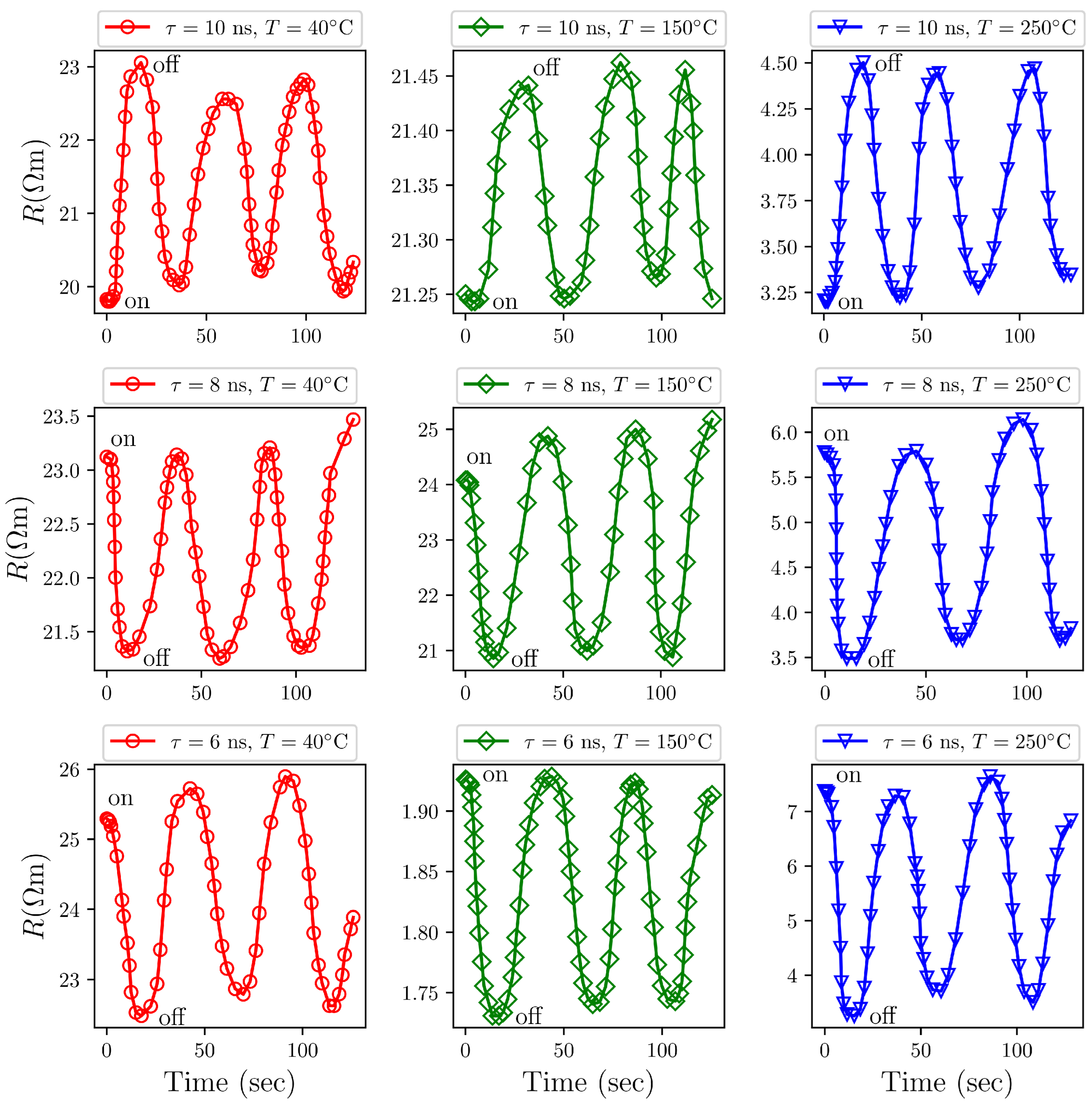
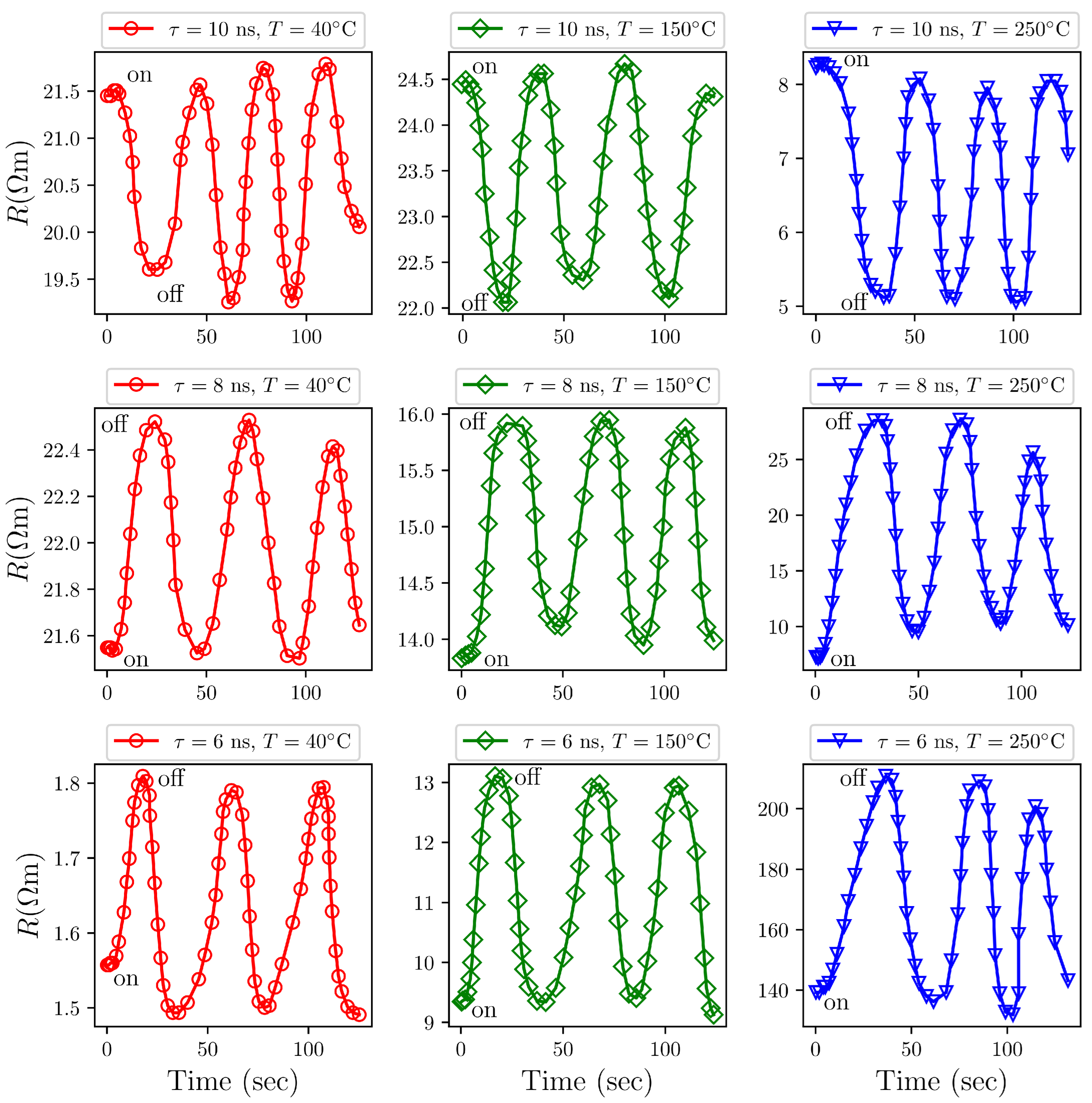
3.9. Sensing Characteristics of /AgO Towards Concentration of and Gases
3.9.1. Sensing Mechanism
3.9.2. Response and Recovery Times
3.9.3. Effect of Sensor Operation Temperature
3.9.4. Effect of Decreasing Pulse Duration on Film Sensitivity
Exposure to Gas
Exposure to H2S Gas
4. Benchmark Comparison of PLD-Grown /AgO Films Versus Contemporary Thin-Film Platforms for and S Gas Sensing
5. Potential Application Areas of the Developed Sensors
5.1. Industrial Safety and Leak Detection ()
5.2. Process and Emissions Monitoring ( Near Source)
5.3. Laboratory/Process Cabinets and Closed Lines
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Kanan, S.M.; El-Kadri, O.M.; Abu-Yousef, I.A.; Kanan, M.C. Semiconducting metal oxide based sensors for selective gas pollutant detection. Sensors 2009, 9, 8158–8196. [Google Scholar] [CrossRef] [PubMed]
- Sekimoto, S.; Nakagawa, H.; Okazaki, S.; Fukuda, K.; Asakura, S.; Shigemori, T.; Takahashi, S. A fiber-optic evanescent-wave hydrogen gas sensor using palladium-supported tungsten oxide. Sens. Actuators B Chem. 2000, 66, 142–145. [Google Scholar] [CrossRef]
- Morazzoni, F.; Scotti, R.; Origoni, L.; D’Arienzo, M.; Jimenez, I.; Cornet, A.; Morante, J. Mechanism of NH3 interaction with transition metal-added nanosized WO3 for gas sensing: In situ electron paramagnetic resonance study. Catal. Today 2007, 126, 169–176. [Google Scholar] [CrossRef]
- Nazemi, H.; Joseph, A.; Park, J.; Emadi, A. Advanced micro-and nano-gas sensor technology: A review. Sensors 2019, 19, 1285. [Google Scholar] [CrossRef]
- Wang, J.; Wang, R. Development of Gas Sensors and Their Applications in Health Safety, Medical Detection, and Diagnosis. Chemosensors 2025, 14, 190. [Google Scholar] [CrossRef]
- Mahapatra, C. Recent advances in medical gas sensing with artificial intelligence–enabled technology. Med. Gas Res. 2025, 15, 318–326. [Google Scholar] [CrossRef]
- Ko, J.K.; Park, I.H.; Hong, K.; Kwon, K.C. Recent advances in chemoresistive gas sensors using two-dimensional materials. Nanomaterials 2024, 14, 1397. [Google Scholar] [CrossRef]
- Wang, P.; Xu, S.; Shi, X.; Zhu, J.; Xiong, H.; Wen, H. Recent Advances in Resistive Gas Sensors: Fundamentals, Material and Device Design, and Intelligent Applications. Chemosensors 2025, 13, 224. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B. Synthesis and deposition of sensor materials. Chem. Sens. Fundam. Sens. Mater. Gen. Approaches 2010, 1, 215–304. [Google Scholar]
- Ma, C.; Chen, C. Advanced Nano Deposition Methods; Wiley: Hoboken, NJ, USA, 2016; Chapter 1; pp. 1–31. [Google Scholar]
- Bellucci, A.; De Bonis, A.; Curcio, M.; Santagata, A.; Pace, M.L.; Bolli, E.; Mastellone, M.; Polini, R.; Salerno, R.; Valentini, V.; et al. WO3-Based Thin Films Grown by Pulsed Laser Deposition as Gas Sensors for NO2 Detection. Sensors 2024, 24, 7366. [Google Scholar] [CrossRef]
- Cieniek, L.; Kopia, A.; Kowalski, K.; Moskalewicz, T. High-Quality Perovskite Thin Films for NO2 Detection: Optimizing Pulsed Laser Deposition of Pure and Sr-Doped LaMO3 (M = Co, Fe). Materials 2025, 18, 1175. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Guo, J.; Sun, J.; Tang, P.; Chen, A.; Luo, R.; Li, D. Enhancement of NO2-sensing performance at room temperature by graphene-modified polythiophene. Ind. Eng. Chem. Res. 2016, 55, 5788–5794. [Google Scholar] [CrossRef]
- Fan, C.; Sun, F.; Wang, X.; Majidi, M.; Huang, Z.; Kumar, P.; Liu, B. Enhanced H2S gas sensing properties by the optimization of p-Cuo/n-ZnO composite nanofibers. J. Mater. Sci. 2020, 55, 7702–7714. [Google Scholar] [CrossRef]
- Jiang, J.; Chan, A.; Ali, S.; Saha, A.; Haushalter, K.J.; Lam, W.L.M.; Glasheen, M.; Parker, J.; Brenner, M.; Mahon, S.B.; et al. Hydrogen sulfide—Mechanisms of toxicity and development of an antidote. Sci. Rep. 2016, 6, 20831. [Google Scholar] [CrossRef] [PubMed]
- Taha, S.S.; Idoudi, S.; Alhamdan, N.; Ibrahim, R.H.; Surkatti, R.; Amhamed, A.; Alrebei, O.F. Comprehensive Review of Health Impacts of the Exposure to Nitrogen Oxides (NOx), Carbon Dioxide (CO2), and Particulate Matter (PM). J. Hazard. Mater. Adv. 2025, 19, 100771. [Google Scholar] [CrossRef]
- Chen, H.; Chen, H.; Chen, J.; Song, M. Gas sensors based on semiconductor metal oxides fabricated by electrospinning: A review. Sensors 2024, 24, 2962. [Google Scholar] [CrossRef]
- Liu, Z.L.; Yang, Z.; Liu, B.; Chen, Y.N.; Feng, W. Construction of Hybrid ZnO/SnO2 n–n Heterojunction with Hierarchical Porous Biomorphic Nanostructure as a High-Response Sensor for Methanol Gas. Crystals 2024, 14, 1049. [Google Scholar] [CrossRef]
- Saleh, B.E.A.; Teich, M.C. Fundamentals of Photonics; Wiley: New York, NY, USA, 2007. [Google Scholar]
- Li, S.; Wang, X.; Chen, G.; Wang, Z. Interaction Energy Dependency on Pulse Width in ns NIR Laser Scanning of Silicon. Micromachines 2022, 14, 119. [Google Scholar] [CrossRef]
- Xiong, C.W.; Ho, C.Y.; Qiao, D.K. Analysis of direct optical ablation and sequent thermal ablation for the ultrashort pulsed laser photo-thermal micromachining. Coatings 2020, 10, 1151. [Google Scholar] [CrossRef]
- Lorenz, P.; Bez, E.; Himmerlich, M.; Ehrhardt, M.; Taborelli, M.; Zimmer, K. Pulse Duration Dependence of Infrared Laser-Induced Secondary Electron Yield Reduction of Copper Surfaces. J. Laser Micro/Nanoeng. 2023, 18, 121. [Google Scholar]
- Motevalli, K.; Ebadi, M.; Salehi, Z. Synthesis of Ag–AgO/Al2O3 nanocomposite via a facile two-step method for photodegradation of methylene blue. J. Mater. Sci. Mater. Electron. 2017, 28, 13024–13031. [Google Scholar] [CrossRef]
- Shepelin, N.A.; Tehrani, Z.P.; Ohannessian, N.; Schneider, C.W.; Pergolesi, D.; Lippert, T. A practical guide to pulsed laser deposition. Chem. Soc. Rev. 2023, 52, 2294–2321. [Google Scholar] [CrossRef]
- Jia, X.; Zhao, X. Ultrafast laser ablation of copper with GHz bursts: A numerical study on the effects of repetition rate, pulse number and burst fluence on ablation efficiency and heat-affected zone. Opt. Laser Technol. 2023, 158, 108803. [Google Scholar] [CrossRef]
- Sissa, A.Q.; Delgado Sancho, L.; Roudier, S.; Scalet, B.M.; Garcia Muñoz, M. Best Available Techniques (BAT) Reference Document for the Manufacture of Glass-Industrial Emissions Directive 2010/75/EU: Integrated Pollution Prevention and Control; Publications Office: Seville, Spain, 2013. [Google Scholar]
- Shimizu, T.; Zanáška, M.; Villoan, R.; Brenning, N.; Helmersson, U.; Lundin, D. Experimental verification of deposition rate increase, with maintained high ionized flux fraction, by shortening the HiPIMS pulse. Plasma Sources Sci. Technol. 2021, 30, 045006. [Google Scholar] [CrossRef]
- Skjærvø, S.H.; Wefring, E.T.; Nesdal, S.K.; Gaukås, N.H.; Olsen, G.H.; Glaum, J.; Tybell, T.; Selbach, S.M. Interstitial oxygen as a source of p-type conductivity in hexagonal manganites. Nat. Commun. 2016, 7, 13745. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Yu, Y.; Jia, S.; Zhou, C.; Cojocaru-Mirédin, O.; Wuttig, M. Strong charge carrier scattering at grain boundaries of PbTe caused by the collapse of metavalent bonding. Nat. Commun. 2023, 14, 719. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal oxide gas sensors: Sensitivity and influencing factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef]
- Al-Dujayli, S.; Ali, N. The effects of CuO doping on structural, electrical and optical properties of CdO thin films deposited by pulsed laser deposition technique. J. Ovonic Res. 2022, 18, 579–590. [Google Scholar] [CrossRef]
- Usha, N.; Sivakumar, R.; Sanjeeviraja, C.; Arivanandhan, M. Niobium pentoxide (Nb2O5) thin films: rf Power and substrate temperature induced changes in physical properties. Opt.-Int. J. Light Electron Opt. 2015, 126, 1945–1950. [Google Scholar] [CrossRef]
- Jeong, H.S.; Park, M.J.; Kwon, S.H.; Joo, H.J.; Kwon, H.I. Highly sensitive and selective room-temperature NO2 gas-sensing characteristics of SnOX-based p-type thin-film transistor. Sens. Actuators B Chem. 2019, 288, 625–633. [Google Scholar] [CrossRef]
- Kneer, J.; Wöllenstein, J.; Palzer, S. Manipulating the gas–surface interaction between copper(II) oxide and mono-nitrogen oxides using temperature. Sens. Actuators B Chem. 2016, 229, 57–62. [Google Scholar] [CrossRef]
- Filipovic, L.; Selberherr, S.; Mutinati, G.C.; Brunet, E.; Steinhauer, S.; Köck, A.; Teva, J.; Kraft, J.; Siegert, J.; Schrank, F.; et al. A method for simulating spray pyrolysis deposition in the level set framework. Eng. Lett. 2013, 21, 224–240. [Google Scholar]
- Rzaij, J. Characterization of CuO thin films for gas sensing applications. Iraqi J. Phys. 2019, 14, 1–12. [Google Scholar] [CrossRef]
- Vaishampayan, M.V.; Deshmukh, R.G.; Walke, P.; Mulla, I. Fe-doped SnO2 nanomaterial: A low temperature hydrogen sulfide gas sensor. Mater. Chem. Phys. 2008, 109, 230–234. [Google Scholar] [CrossRef]
- Liu, Y.L.; Wang, H.; Yang, Y.; Liu, Z.M.; Yang, H.F.; Shen, G.L.; Yu, R.Q. Hydrogen sulfide sensing properties of NiFe2O4 nanopowder doped with noble metals. Sens. Actuators B Chem. 2004, 102, 148–154. [Google Scholar] [CrossRef]
- Hennemann, J.; Kohl, C.D.; Smarsly, B.M.; Sauerwald, T.; Teissier, J.M.; Russ, S.; Wagner, T. CuO thin films for the detection of H2S doses: Investigation and application. Phys. Status Solidi A 2015, 212, 1281–1288. [Google Scholar] [CrossRef]
- Gattinoni, C.; Ewen, J.P.; Dini, D. Adsorption of Surfactants on α-Fe2O3(0001): A Density Functional Theory Study. J. Phys. Chem. C 2018, 122, 20817–20826. [Google Scholar] [CrossRef]
- Sood, T.; Thundiyil, R.; Anusha; Chattopadhyay, S.; Poornesh, P. Optimized nanostructured In2O3 gas sensors: Harnessing annealing-induced defects and oxygen vacancies for ultra-sensitive and selective H2S detection at trace levels. RSC Adv. 2025, 15, 16555–16569. [Google Scholar] [CrossRef]
- Ambi, R.; Mane, A.; Tasgaonkar, R.; Mane, R. Highly porous NiO microstructure for NO2 detection. Phys. B Condens. Matter 2024, 674, 415567. [Google Scholar] [CrossRef]
- Ganbavle, V.; Mohite, S.; Agawane, G.; Kim, J.; Rajpure, K. Nitrogen dioxide sensing properties of sprayed tungsten oxide thin film sensor: Effect of film thickness. J. Colloid Interface Sci. 2015, 451, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Nie, L.; Zhou, Q.; Song, Z.D.; Pan, G. Chemiresistive H2S gas sensors based on composites of ZnO nanocrystals and foam-like GaN fabricated by photoelectrochemical etching and a sol-gel method. Sens. Actuators B Chem. 2023, 393, 134148. [Google Scholar] [CrossRef]
- Mousavi, S.S.; Goudarzi, G.; Sabzalipour, S.; Rouzbahani, M.M.; Hassan, E.M. Evaluating CO, NO2, and SO2 Emissions From Stacks of Turbines and Gas Furnaces of Oil and Gas Processing Complex Using AERMOD. Arch. Hyg. Sci. 2022, 11, 113. [Google Scholar] [CrossRef]
- Poeta, E.; Núñez-Carmona, E.; Sberveglieri, V. A Review: Applications of MOX Sensors from Air Quality Monitoring to Biomedical Diagnosis and Agro-Food Quality Control. J. Sens. Actuator Netw. 2025, 14, 50. [Google Scholar] [CrossRef]
- Broas, M.; Kanninen, O.; Vuorinen, V.; Tilli, M.; Paulasto-Krockel, M. Chemically stable atomic-layer-deposited Al2O3 films for processability. ACS Omega 2017, 2, 3390–3398. [Google Scholar] [CrossRef] [PubMed]
- Dallaev, R.; Sobola, D.; Tofel, P.; Skvarenina, L.; Sedlak, P. Aluminum Nitride Nanofilms by Atomic Layer Deposition Using Alternative Precursors Hydrazinium Chloride and Triisobutylaluminum. Coatings 2020, 10, 954. [Google Scholar] [CrossRef]



| (nm) | D (nm) | FWHM (Degree) | (Degree) | Pulse Duration (ns) |
|---|---|---|---|---|
| 9.703 | 13.300 | 0.702 | 37.953 | 10 |
| 3.909 | 2.438 | 44.157 | ||
| 6.735 | 1.423 | 45.730 | ||
| 6.388 | 1.632 | 64.265 | ||
| 11.699 | 0.904 | 66.773 | ||
| 16.186 | 0.698 | 77.289 | ||
| 4.093 | 4.018 | 2.330 | 38.778 | 8 |
| 3.879 | 2.464 | 44.982 | ||
| 2.234 | 4.303 | 46.566 | ||
| 4.561 | 2.299 | 65.288 | ||
| 5.772 | 1.840 | 67.554 | ||
| 2.706 | 1.305 | 6.457 | 38.734 | 6 |
| 5.211 | 1.650 | 44.850 | ||
| 1.603 | 5.398 | 46.643 |
| Pulse Duration (ns) | Average Grain Size (nm) | RMS Roughness (nm) | Mean Roughness (nm) |
|---|---|---|---|
| 10 | 27.19 | 3.87 | 2.54 |
| 8 | 23.95 | 3.13 | 2.04 |
| 9 | 17.87 | 2.91 | 1.95 |
| Pulse Duration (ns) | Elements | Atomic % | Weight % |
|---|---|---|---|
| 10 | C | 13.9 | 10.1 |
| O | 51.9 | 29.6 | |
| Na | 6.3 | 7.3 | |
| Mg | 1.5 | 1.8 | |
| Al | 3.8 | 5.2 | |
| Si | 18.8 | 35.5 | |
| Ca | 2.2 | 6.4 | |
| Ag | 1.6 | 4.1 | |
| 8 | C | 13.0 | 9.9 |
| O | 53.7 | 30.6 | |
| Na | 5.6 | 6.6 | |
| Mg | 1.4 | 1.6 | |
| Al | 3.2 | 4.8 | |
| Si | 19.7 | 36.4 | |
| Ca | 2.1 | 6.2 | |
| Ag | 1.3 | 3.9 | |
| 6 | C | 11.2 | 8.5 |
| O | 55.2 | 31.5 | |
| Na | 5.6 | 6.6 | |
| Mg | 1.4 | 1.7 | |
| Al | 2.7 | 4.2 | |
| Si | 20.3 | 37.4 | |
| Ca | 2.1 | 6.3 | |
| Ag | 1.1 | 3.8 |
| Type (p/n) | (/cm)−1 | (/Vs) | () | (/C) | Pulse Duration (ns) |
|---|---|---|---|---|---|
| n | 10 | ||||
| p | 8 | ||||
| p | 6 |
| Rec. Time (s) | Res. Time (s) | S (%) | Rec. Time (s) | Res. Time (s) | (%) | Temp. (°C) | Pulse Duration (ns) |
|---|---|---|---|---|---|---|---|
| 20.7 | 15.57 | 8.41 | 31.23 | 10.53 | 16.1 | 40 | 10 |
| 14.13 | 14.4 | 9.46 | 20.61 | 21.6 | 0.9 | 150 | |
| 17.1 | 19.62 | 37.03 | 17.64 | 14.04 | 37.5 | 250 | |
| 20.7 | 17.82 | 4.65 | 22.95 | 7.65 | 7.63 | 40 | 8 |
| 19.35 | 18.81 | 14.38 | 23.85 | 11.25 | 12.55 | 150 | |
| 16.02 | 24.84 | 74.20 | 26.28 | 9.27 | 41.7 | 250 | |
| 14.22 | 12.69 | 15.38 | 21.78 | 14.13 | 10.35 | 40 | 6 |
| 17.73 | 13.95 | 38.29 | 23.22 | 13.86 | 8.9 | 150 | |
| 20.88 | 31.68 | 50.2 | 20.43 | 11.61 | 55.56 | 250 |
| Material | Gas (Concentration) | T (°C) | Sensitivity | Response Time (s)/Recovery Time (s) | References |
|---|---|---|---|---|---|
| /AgO | S (200 ppm) | 250 | / | This work | |
| S (50 ppm) | 200 | 100/109 | [41] | ||
| NiO | S (200 ppm) | 400 | 108/47 | [41] | |
| /rGO | S (100 ppm) | 150 | 8/38 | [41] | |
| /AgO | (150 ppm) | 250 | / | This work | |
| NiO | (100 ppm) | 200 | 13/146 | [42] | |
| (100 ppm) | 200 | 12/412 | [42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doohee, D.Y.; Azarian, A.; Mozaffari, M.R. Temporal Sculpting of Laser Pulses for Functional Engineering of Al2O3/AgO Films: From Structural Control to Enhanced Gas Sensing Performance. Sensors 2025, 25, 5836. https://doi.org/10.3390/s25185836
Doohee DY, Azarian A, Mozaffari MR. Temporal Sculpting of Laser Pulses for Functional Engineering of Al2O3/AgO Films: From Structural Control to Enhanced Gas Sensing Performance. Sensors. 2025; 25(18):5836. https://doi.org/10.3390/s25185836
Chicago/Turabian StyleDoohee, Doaa Yaseen, Abbas Azarian, and Mohammad Reza Mozaffari. 2025. "Temporal Sculpting of Laser Pulses for Functional Engineering of Al2O3/AgO Films: From Structural Control to Enhanced Gas Sensing Performance" Sensors 25, no. 18: 5836. https://doi.org/10.3390/s25185836
APA StyleDoohee, D. Y., Azarian, A., & Mozaffari, M. R. (2025). Temporal Sculpting of Laser Pulses for Functional Engineering of Al2O3/AgO Films: From Structural Control to Enhanced Gas Sensing Performance. Sensors, 25(18), 5836. https://doi.org/10.3390/s25185836





