Combined Use of Electroencephalography and Transcranial Electrical Stimulation: A Systematic Review
Abstract
1. Introduction
- (RQ-I): Is tES guided by EEG data?
- (RQ-II): Is EEG also used to guide tES in real time?
- (RQ-III): Are treatment outcomes assessed through EEG analysis?
- (RQ-IV): Do electroencephalographic outcomes of specific tES protocols generalize across individuals?
2. Materials and Methods
2.1. Search Strategy
2.2. Exclusion Criteria
- Focusing exclusively on placebo stimulation;
- Being limited to experimental clinical protocol presentation;
- Not reporting EEG analysis results;
- Not including specific EEG-tES interaction analysis;
- Using exclusively animals or phantom models for tES treatment analysis;
- Lacking information on electrode localization or not reporting, at least, the anode position;
- Publications exclusively analyzing or commenting on experimental research (e.g., reviews, commentaries, or editorials).
2.3. Quality Assessment Strategy
3. Results
3.1. Results for Research Question I (RQ-I)
3.2. Results for Research Question II (RQ-II)
| Article | Clinical Target | Waveform | A Position | C Position | N° of Electrodes | QATQS (Sample Size) | EEG Features for tES Setup | Data Analysis Method |
|---|---|---|---|---|---|---|---|---|
| Fregni et al. (2006) [19] | Epilepsy (seizure frequency reduction) | tDCS | Silent area | EF | 2 | Moderate (19) | EF | S |
| De Ridder et al. (2012) [22] | cCronic tinnitus (tinnitus perception reduction) | tDCS | (a) Right DLPFC; (b) based on functional connectivity | (a) Left DLPFC, (b) based on functional connectivity | 2 | Weak (675) | Theta and gamma functional connectivity | S |
| San-Juan et al. (2017) [20] | Epilepsy (interictal epileptiform discharge reduction) | tDCS | Silent area | EF | 2 | Weak (28) | EF | S |
| Marinho Andrade et al. (2023) [21] | AD (cognitive decline containment) | tDCS | (a) F5, CP5, and F4; (b) F3, P4, and P5 | Contralateral; supraorbital | 4 | Strong (70) | PSD in all bands | ML |
| Mokhtarinejad et al. (2024) [23] | Healthy (time perception precision and accuracy modulation) | tACS | Oz | Cz | 2 | Moderate (24) | IAF and PAP | S |
| Article | Clinical Target | Waveform | A Position | C Position | N° of Electrodes | QATQS (Sample Size) | Adaptivity Rule |
|---|---|---|---|---|---|---|---|
| Lustenberger et al. (2016) [28] | Healthy (motor memory consolidation) | tACS | F3, F4 | Cz | 3 | Weak (17) | Stimulation triggered when ≥5 consecutive peaks of signal filtered in (11–16) Hz > mean amplitude of baseline signal filtered in (11–16) Hz × a subject-specific constant |
| Ketz et al. (2018) [24] | Healthy (declarative memory consolidation) | tACS | F10 | Upper contralateral arm | 2 | Strong (21) | Stimulation is triggered when RP (0.5–1.2 Hz) > 20% of TP. tACS frequency = peak frequency in (0.5–1.2) Hz. tACS peak aligned with the peak of the fronto-central averaged EEG signal |
| Jones et al. (2018) [25] | Healthy (declarative memory consolidation) | tACS | F3/F4 | F3/F4 | 2 | Weak (21) | Stimulation is triggered when RP (0.5–1.2 Hz) > 20% of TP. tACS frequency = peak frequency in (0.5–1.2) Hz. tACS peak aligned with the peak of the fronto-central averaged EEG signal |
| Zarubin et al. (2020) [32] | Healthy (without clinical objective) | tACS | Oz | Cz | 2 | Medium (20) | tACS frequency = IAF at POz. tACS peak aligned with the negative or positive peak of alpha-band-filtered signal |
| Pilly et al. (2020) [27] | Healthy (metamemory sensitivity improvement) | tACS | O10, TP8, P6, PO8, FT8, F6, C6, FC4, CP4, C2, P2, AF8, F2, FPz, FCz, AFz, F1, AF7, Iz, POz, P1, CPz, C1, CP3, FC3, C5, F5, FT7, PO7, P5, TP7, O9 | O10, TP8, P6, PO8, FT8, F6, C6, FC4, CP4, C2, P2, AF8, F2, FPz, FCz, AFz, F1, AF7, Iz, POz, P1, CPz, C1, CP3, FC3, C5, F5, FT7, PO7, P5, TP7, O9 | 32 | Weak (30) | Stimulation is triggered when RP (0.5–1.2 Hz) > 30% of TP. tACS frequency = peak frequency in (0.5–1.2) Hz. tACS peak aligned with the peak of the fronto-central averaged EEG signal |
| Stecher et al. (2021) [30] | Healthy (visual detection performance improvement) | tACS | Parieto-occipital cortex (Cz/Oz) | Parieto-occipital cortex (Cz/Oz) | 2 | Medium (60) | tACS frequency = IAF at Pz |
| Hubbard et al. (2021) [26] | Healthy (metamemory sensitivity improvement) | tACS | Frontal cortex | Frontal cortex | 2 | Strong (18) | Stimulation is triggered when RP (0.5–1.2 Hz) > 30% of TP. tACS frequency = peak frequency in (0.5–1.2) Hz. tACS peak aligned with the peak of the fronto-central averaged EEG signal |
| Caravati et al. (2024) [33] | Healthy (sustained attention enhancement) | tDCS | (a) F3; (b) P3 | (a) Fp2; (b) P4 | 2 | Weak (10) | Stimulation amplitude or stimulation site (frontal vs. parietal) is modulated depending on whether appropriate thresholds are reached by two features: a subject-specific metric and PSD in the theta band |
| Haslacher et al. (2024) [29] | Healthy (working memory enhancement) | tACS | Oz | Cz | 2 | Weak (50) | tACS peaks are phase-locked to alpha-band-filtered signal with a maintained constant phase lag |
| Schwippel et al. (2024) [31] | MDD (depressive symptom reduction) | tACS | F3, F4 | Cz | 3 | Weak (10) | Stimulation triggered when 10 s IAF ±2 Hz power > 60 s baseline threshold. |
3.3. Results for Research Question III (RQ-III)
3.4. Results Relevant to Both Research Questions I (RQ-I) and III (RQ-III)
| Article | Clinical Target | Waveform | A Position | C Position | N° of Electrodes | QATQS (Sample Size) | tES-Modulated EEG Features | Data Analysis Method |
|---|---|---|---|---|---|---|---|---|
| Palm et al. (2009) [34] | MDD (depressive symptom reduction) | tDCS | Left DLPFC (F3) | Right supraorbital area | 2 | Weak (1) | Absolute and relative power in delta, theta, and alpha bands | S |
| Zaehle et al. (2011) [35] | Healthy (working memory performance improvement) | tDCS | Left DLPFC (F3) | Ipsilateral left mastoid | 2 | Weak (16) | ERP and ERSP | S |
| Zaehle et al. (2011) [36] | Healthy (auditory discrimination improvement) | tDCS | (a) T7 or Cp5; (b) contralateral supraorbital | (a) contralateral supraorbital; (b) T7 or Cp5 | 2 | Weak (14) | ERP | S |
| Kasashima et al. (2012) [37] | Stroke (motor function recovery) | tDCS | M1 of affected hemisphere | Opposite supraorbital region | 2 | Weak (6) | ERD | S |
| Kongthong et al. (2013) [38] | Healthy (semantic processing efficiency improvement, faces) | tDCS | right temporal area (T6) | Left DLPFC (F3) | 2 | Weak (14) | LPC and ERP | S |
| Rütsche et al. (2013) [39] | Healthy (arithmetic performance enhancement) | tDCS | left PPC | n.a. | 2 | Weak (26) | ERS/ERD | S |
| Lazarev et al. (2013) [40] | Healthy (without clinical objective) | HD-tDCS | C3 | 5cm to C3 | 5 | Weak (15) | Amplitude spectra | S |
| Mangia et al. (2014) [41] | Healthy (visuospatial attention enhancement) | tDCS | Right PPC | Ipsilateral deltoid muscle | 2 | Weak (10) | PSD in theta, alpha, beta, and gamma bands | S |
| Romero Lauro et al. (2014) [42] | Healthy (visuospatial attention enhancement) | tDCS | Right PPC | Left supraorbital area | 2 | Weak (14) | GMFP and LMFP | S |
| Roy et al. (2014) [43] | Healthy (attention and processing efficiency improvement) | HD-tDCS | Between the C3 and CP3 | Left sensorimotor cortex | 5 | Weak (8) | ERS; ERD | S |
| Crivelli et al. (2014) [44] | Healthy (executive functions improvement) | tDCS | Right DLPFC | Cephalic area | 2 | Weak (22) | ERP | n.a. |
| von Mengden (2014) [45] | Healthy (working memory enhancement) | tACS | F3 and F4 | Mastoid | 2 | Weak (1) | PSD in theta, alpha, and beta bands | S |
| Powell et al. (2014) [46] | Affective disorder (mood symptom reduction) | tDCS | left DLPFC (F3) | F8 | 2 | Weak (18) | Relative power in alpha and theta bands; ERP | S |
| Dominguez et al. (2014) [47] | Stroke (language production improvement) | tDCS | Left frontal area | Right contralateral area | 2 | Weak (1) | Absolute power and coherence in delta, theta, alpha, and beta bands | S |
| Miller et al. (2015) [48] | Healthy (working memory accuracy enhancement) | tDCS | AFz | Under the chin | 2 | Weak (8) | frontal–midline theta amplitude | S |
| D’Atri et al. (2015) [49] | Healthy (episodic memory facilitation) | osc-tDCS | (a) Fz; (b) right deltoid muscle | (a) right deltoid muscle; (b) Fz | 2 | Weak (20) | EEG oscillatory components | S |
| Jindal et al. (2015) [50] | Stroke (motor function recovery) | tDCS | Left DLPFC (F3) | Cz | 2 | Weak (5) | MEP and log-transformed mean power | S |
| Sood et al. (2015) [51] | Stroke (ischemia- related impairment containment) | tDCS | DLPFC (F3 and F4) | Cz | 3 | Weak (5) | Log-transformed mean power in the range of (0.5–11.25 Hz) | S |
| Amatachaya et al. (2015) [52] | ASD (Attention regulation support) | tDCS | Left DLPFC (F3) | Right shoulder | 2 | Weak (24) | Peak alpha frequency | S |
| Cosmo et al. (2015b) [53] | ADHD (inhibitory control and attention improvement) | tDCS | left DLPFC (F3) | right DLPFC (F4) | 2 | Strong (60) | Functional cortical network | S |
| Del Felice et al. (2015) [54] | Epilepsy (seizure burden containment; declarative memory consolidation enhancement) | so-tDCS | Frontal–temporal (F7-T3 or F8-T8) | Ipsilateral mastoid | 2 | Weak (12) | Spindle frequency and cortical sources | S |
| Hoy et al. (2015) [55] | Schizophrenia (working memory and cognitive control improvement) | tDCS | Frontal cortex (F3) | Right supraorbital | 2 | Weak (16) | Gamma ERS and correlation | S |
| Dutta et al. (2015) [56] | Stroke (motor recovery) | tDCS | Motor cortex (Cz) | Left supraorbital notch | 2 | Moderate (4) | Log-transformed mean power in the range of (0.5–11.25 Hz) | S |
| Kasashima-Shindo et al. (2015) [37] | Stroke (motor recovery) | tDCS | Primary sensorimotor cortex | contralateral supraorbital area | 2 | Weak (18) | ERD | S |
| Wu et al. (2015) [57] | Stroke (language naming improvement) | tDCS | Left posterior perisylvian | Unaffected shoulder | 2 | Weak (12) | ApEn | S |
| Jindal et al. (2015) [58] | Stroke (motor function recovery) | tDCS | Motor cortex (Cz) | Frontal cortex (F3 or F4) | 2 | Weak (29) | Log-transformed mean power in the range of 0.5–11.25 Hz; Relative power in all bands | S |
| Ang et al. (2015) [59] | Stroke (motor imagery BCI performance and motor recovery) | tDCS | M1 of the ipsilesional hemisphere | Contralesional M1 | 2 | Moderate (19) | ERD | n.a. |
| Ulam et al. (2015) [60] | TBI (attention/ working memory improvement) | tDCS | Left DLPFC (F3) | Right supraorbital area (Fp2) | 2 | Strong (26) | Relative power in delta, theta, alpha, and beta bands | S |
| Impey et al. (2015) [61] | Healthy (auditory discrimination improvement) | tDCS | Left auditory cortex (between C5 and T7) | Contralateral forehead | 2 | Strong (12) | ERP (MMN) | S |
| Sood et al. (2016) [62] | Healthy (motor performance/ processing support) | tDCS | C3 | FC1, FC5, CP5, CP1 | 5 | Weak (5) | Log-transformed mean power in the range of 0.5–11.25 Hz | S |
| Cappon et al. (2016) [63] | Healthy (cognitive performance modulation, attention) | tACS | Fz (electrode area centroid) | C5 (electrode area centroid) | 2 | Weak (18) | ERS/ERD | S |
| Caldiroli et al. (2016) [64] | Healthy (semantic decision efficiency improvement) | tDCS | Right supraorbital region | Left DLPFC (F3) | 2 | Weak (30) | ERP | S |
| Marceglia et al. (2016) [65] | AD (cognitive symptoms mitigation) | tDCS | Bilateral temporal–parietal area | Tight deltoid muscle | 3 | Weak (7) | Absolute power and coherence in all bands | S |
| Liu et al. (2016) [66] | Epilepsy (depressive symptom reduction and memory consolidation enhancement) | tDCS | Left DLPFC (F3) | Right supraorbital area | 2 | Weak (37) | Relative power in alpha and theta bands | S |
| Dunn et al. (2016) [67] | Schizophrenia (auditory processing and working memory improvement) | tDCS | DLPFC (Fp1 and Fp2) | Right upper arm | 3 | Weak (36) | ERP (P300) | S |
| D’Agata et al. (2016) [68] | Stroke (cognitive function improvement) | tDCS | Perilesional M1(C3 or C4) | ContralesionalM1 | 2 | Weak (34) | ERP (P300, N200) | S |
| Ashikhmin et al. (2017) [69] | Healthy (autonomic regulation and cognitive vigilance modulation) | tDCS | Over T3 area | Over A2 lead | 2 | Weak (10) | Relative power in all bands | n.a. |
| Angulo-Sherman et al. (2017) [70] | Healthy (motor imagery classification/ accuracy support) | tDCS | (a) In front of C3; (b) between Cz and FC1 | Inion level (3 cm to the left hemisphere) | 2 | Weak (5) | Absolute power in the range of 9–30 Hz | S |
| Angulo-Sherman et al. (2017) [71] | Healthy (motor imagery classification/ accuracy support) | HD-tDCS | (a) C3; (b) Cz | (a) FC1, FC5, CP1, and CP5; (b) FC1, CP1, FC2, and CP2 | 5 | Weak (2) | ERS | S |
| Grande et al. (2017) [72] | Healthy (visual working memory enhancement, aging) | tACS | Parietal cortex (P3/P4) | Parietal cortex (P4/P3) | 2 | Weak (19) | ERP (N200) | S |
| Donaldson et al. (2017) [73] | Healthy (social cognition, face processing improvement) | tDCS | Right TPJ | right TPJ | Weak (n.a.) | ERP (N170, P300) | n.a. | |
| Berger et al. (2017) [74] | Healthy (motor learning facilitation) | tACS | Parietal cortex (P3/P4) | Parietal cortex (P4/P3) | 2 | Weak (15) | Relative power in alpha band | S |
| Cortes et al. (2017) [75] | Healthy (fatigue resistance/ perceived exertion modulation) | tDCS | Motor cortex (Cz) | Fpz | 2 | Weak (4) | Total EEG power in all bands | S |
| Romero Lauro et al. (2017) [42] | Healthy (visuospatial attention enhancement) | tDCS | Right PPC | n.a. | n.a. | Weak (14) | GMFP and LMFP on mean TEP | S |
| Ladenbauer et al. (2017) [76] | MCI (sleep- dependent memory consolidation enhancement) | so-tDCS | Prefrontal cortex (F3–F4) | Ipsilateral mastoid | 3 | moderate (16) | Absolute power in the range of 0.5–1 Hz) andfast spindles (12–15 Hz) | S |
| Impey et al. (2017) [77] | Schizophrenia (working memory and auditory processing improvement) | tDCS | Left auditory or left frontal cortex | Contralateral forehead | 2 | Weak (12) | ERP | S |
| Naros and Gharabaghi (2017) [78] | Stroke (motor self-regulation training improvement) | tACS | Ipsilesional sensorimotor cortex | Contralesionalforehead | 2 | Weak (20) | Relative power and ERD in beta band | S |
| Yuan et al. (2017) [79] | Stroke (swallowing apraxia improvement) | tDCS | M1 | Contralateralshoulder | Weak (9) | ApEn | S | |
| O’Neil-Pirozzi et al. (2017) [80] | TBI (immediate memory improvement) | tDCS | Left DLPFC | Right supraorbital | 2 | Weak (8) | Auditory ERP (P300) and absolute power in alpha and theta bands | S |
| Boudewyn et al. (2018) [81] | Healthy (proactive control enhancement) | tDCS | Left DLPFC | Right supraorbital | 2 | Weak (20) | Absolute power in gamma band | S |
| Kang et al. (2018) [82] | ASD (cognitive flexibility/ complexity support) | tDCS | DLPFC | Right supraorbital | 2 | Weak (13) | MER | S |
| Mane et al. (2018) [83] | Chronic stroke (motor recovery monitoring) | tDCS | The ipsilesional M1 | Contralesional M1 | 2 | Weak (19) | PRI, delta–alpha ratio, theta–beta ratio, theta–alpha ratio, theta–beta–alpha ratio, pdBSI, and Rbsi | S |
| Cucik et al. (2018) [84] | Healthy (alertness/state modulation) | tDCS | Left motor cortex | Contralateral eyebrow | 2 | Weak (16) | MSS and SV | S |
| Friedrich et al. (2018) [85] | Healthy (inhibitory control modulation) | tDCS | Contralateral orbit parallel to the eyebrow | Somatosensory cortex (C3) | 2 | Weak (17) | ERP | S |
| Mondini et al. (2018) [86] | Healthy (motor performance/ ERD training support) | tDCS | (a) Left motor cortex (C3); (b) right supraorbital (Fp2) | (a) Right supraorbital (Fp2); (b) left motor cortex (C3) | 2 | Weak (20) | Alpha-ERD and relative power in theta and alpha bands | S |
| Holgado et al. (2018) [87] | Healthy (exercise performance modulation) | tDCS | DLPFC | Shoulder | 2 | Weak (36) | Absolute power in all bands | S |
| Berger et al. (2018) [88] | Healthy (motor learning facilitation) | tACS | P3 | P4 | 2 | Weak (24) | Relative power in alpha band | S |
| Ferrucci et al. (2018) [89] | Dementia (cognitive symptoms mitigation) | tDCS | Fronto-temporal (F7–F8) | Right deltoid muscle | 3 | Moderate (13) | Absolute power in alpha and beta bands | S |
| Shahsavar et al. (2018) [90] | Depression (depressive symptom reduction) | tDCS | Left DLPFC (F3) | Right DLPFC (F4) | 2 | Weak (7) | ERP and average alpha energy | S |
| Meiron et al. (2018) [91] | Epilepsy (seizure frequency reduction) | HD-tDCS | PO3-P6- AF3-F6- FC4-O1 CP3-C1- FC8-C6- FCz-FC3 O4-F2-CP4 PO4-O2 AF8-C2 | C2, TP8,CP8, O3, T8 | 24 | Weak (1) | Mean number of spikers, mean peak amplitude, and mean absolute power | S |
| Rassovsky et al. (2018) [92] | Schizophrenia (face processing and attention improvement) | tDCS | DLPFC (F3) | Right supraorbital (Fp2) | 2 | Weak (38) | ERP (P300 and N170) | S |
| Hordacre et al. (2018) [93] | Stroke (motor network reorganization support) | tDCS | M1 | Contralateral orbit | 2 | Weak (10) | Connectivity in delta, theta, alpha, beta, and gamma bands | S |
| Nicolo et al. (2018) [94] | Stroke (motor function recovery) | tDCS | Ipsilesional supraorbital region | ContralesionalM1 | 2 | moderate (41) | Effective and functional connectivity | S |
| Straudi et al. (2019) [95] | MCS (arousal/ awareness support) | tDCS | M1 | M1 | n.a. | Weak (10) | Parietal site EEG upper alpha bandwidth | S |
| D’Atri et al. (2019) [96] | Healthy (oscillatory cognitive performance modulation) | tACS | Right fronto-temporal area | Left fronto-temporal area | 2 | Moderate (20) | Relative power in all bands | S |
| Dondè et al. (2019) [97] | Healthy (sustained attention enhancement) | tRNS | right-DLPFC (F4) | Left DLPFC (F3) | 2 | Strong (19) | Beta/alpha power ratio | S |
| Donaldson et al. (2019) [98] | Healthy (target detection/ attention improvement) | tDCS | rTPJ | rTPJ | n.a. | Weak (n.a.) | ERP (P300) | n.a. |
| Dowsett et al. (2019) [99] | Healthy (visual steady-state detection performance modulation) | tACS | Cz | O2 | 2 | Weak (30) | SSVEP | S |
| Bueno-Lopez et al. (2019) [100] | Healthy (sleep-related cognitive consolidation support) | so-tDCS | Prefrontal positions (F3–F4) | Ipsilateral mastoids (M1–M2) | 4 | moderate (23) | Relative power in all bands | S |
| Handiru et al. (2019) [101] | Stroke (motor recovery, bilateral coordination) | tDCS | Ipsilesional M1 | contralesionalM1 | n.a. | Weak (19) | Beta coherence | S |
| Willms et al. (2019) [102] | Healthy (attentional control modulation) | tDCS | Left DLPFC | right DLPFC | n.a. | Weak (n.a.) | Power in alpha band | S |
| Mastakouri et al. (2019) [103] | Healthy (motor performance/ learning support) | HD-tACS | M1 (C3) | Cz, F3, T7, and P3 | 5 | Moderate (19) | Absolute power in beta band | S |
| Emonson et al. (2019) [104] | MCI (cognitive function/TEP monitoring) | tDCS | DLPFC (F3) | Contralateral supraorbital (Fp2) | 2 | Weak (49) | ERP and TEP | S |
| Cespòn et al. (2019) [105] | AD (cognitive symptoms mitigation) | tDCS | left DLPFC (F3) | Right shoulder | 2 | moderate (26) | ERP and absolute power in theta, alpha, and beta bands | S |
| Alexander et al. (2019) [106] | MDD (depressive symptom reduction) | tACS | left/right DLPFC (F3/F4) | Cz | 2 | Strong (32) | Absolute power in alpha band | S |
| Meiron et al. (2019) [107] | Epilepsy (seizure frequency containment) | HD-tDCS | frontal–parietal cortex (AF8, F2, C2, PO4) | C6 | 5 | Weak (1) | Relative power in theta, alpha, and beta bands; delta-ERD | S |
| Ahn et al. (2019) [108] | Schizophrenia (cognitive control and network modulation) | tACS and tDCS | Prefrontal cortex (between F3 and Fp1) | TPJ (between T3 and P3) | 2 | moderate (22) | Alpha oscillations, PSD, and functional connectivity | S |
| Singh et al. (2019) [109] | Schizophrenia (negative symptoms/ cognitive slowing mitigation) | tPCS | Cerebellar vermis | Right shoulder | 2 | Weak (9) | Relative power in delta and theta bands | S |
| Schoellmann et al. (2019) [110] | PD (motor symptom relief) | tDCS | left sensorimotor (C3) | Right frontal area (FP2) | 2 | Moderate (21) | Relative power and coherence in all bands | S |
| Mane et al. (2019) [111] | Stroke (motor recovery tracking) | tDCS | ipsilesional M1 | ContralesionalM1 | 2 | Weak (19) | PSD and relative power in delta, theta, alpha, and beta bands; PRI; rBSI | S |
| Bao et al. (2019) [112] | Stroke (motor function recovery) | HD-tDCS | Ipsilesional M1 (C3) | Frontal–parietal cortex (F1, F5, P1, P5) | 5 | Weak (30) | coherence and PSD in alpha, beta, and gamma bands | S |
| Luna et al. (2020) [113] | Healthy (attention/ executive modulation, PPC/DLPFC) | HD-tDCS | (a) Right PPC; (b) right DLPFC | (a) Right PPC; (b) right DLPFC | 5 | Moderate (92) | Absolute and relative power in alpha band | S |
| El-Hagrassy (2020) [114] | Healthy (executive function and attention modulation) | tDCS | Left DLPFC | (a) Right shoulder; (b) right DLPFC | 2 | Weak (24) | PSD in delta, theta, alpha, beta, and gamma bands | S |
| de Melo et al. (2020) [115] | Fibromyalgia (pain symptom reduction) | tDCS | Left M1 (C3) | Right supraorbital | 2 | Strong (31) | Absolute power in the range of 0.5–30 Hz | S |
| Sergiou et al. (2020) [116] | Substance dependence (craving regulation support) | HD-tDCS | Fpz | AF3, AF4, F3, Fz and F4 | 6 | Weak (50) | LPP | S |
| Pross et al. (2020) [117] | Schizophrenia (alpha-linked cognitive improvement, exploratory) | tDCS | DLPFC | DLPFC | n.a. | Weak (40) | Alpha activity | n.a. |
| Gangemi et al. (2020) [118] | AD (cognitive symptoms mitigation) | tDCS | Left fronto-temporal lobe (F7-T3) | Right frontal lobe (Fp2) | 2 | Moderate (26) | Alpha/beta/theta rhythm | S |
| Nikolin et al. (2020) [119] | Depression (depressive symptom reduction; memory/ attention support) | tDCS | Left DLPFC (F3) | Right shoulder | 2 | Weak (20) | PSD in alpha and theta bands; ERP | S |
| Breitling et al. (2020) [120] | ADHD (response inhibition and attention improvement) | tDCS/ HD-tDCS | Right inferior frontal gyrus (F8) | Contralateralsupra-orbital | 2 (5 for HD) | Weak (15) | ERP (N-200 and P-300) | S |
| Boudewyn et al. (2020) [121] | Schizophrenia (proactive control/attention enhancement) | tDCS | Left DLPFC (F3) | Right supraorbital (Fp2) | 2 | Moderate (37) | Relative power in gamma band | S |
| Jahshan et al. (2020) [122] | Schizophrenia (visual processing efficiency improvement) | tDCS | Central occipital cortex | Right shoulder | n.a. | Weak (27) | VEP | n.a. |
| Zhang et al. (2020) [123] | TBI (cognitive function support: attention/ memory) | tDCS | Left DLPFC (F3) | neck/F4 | 2 | Weak (10) | ApEn; C-ApEn | S |
| Grasso et al. (2021) [124] | Healthy (attention/ executive function enhancement) | tDCS | Left PPC | Upper part of the right arm | 2 | Moderate (32) | ERP and TEP | S |
| Hasballah (2021) [125] | Post stroke (executive function and attention support) | tDCS | Left DLPFC (F3) | Right DLPFC (F4) | 2 | Weak (23) | Absolute and relative power, delta–theta–alpha–beta and delta–alpha ratios | S |
| Ghin et al. (2021) [126] | Healthy (visual processing enhancement, VEP) | hf-tRNS | PO3/P04 | PO4/PO3 | 2 | Weak (16) | PSD; VEP | S |
| Mostafavi et al. (2021) [127] | OUD (craving and relapse risk reduction support) | tDCS | (a) Left DLPFC (F3); (b) right DLPFC (F4) | (a) Right DLPFC (F4); (b) left DLPFC (F3) | 2 | Moderate (30) | Absolute power, amplitude, and coherence in all bands | S |
| Mai et al. (2021) [128] | Healthy (auditory encoding fidelity enhancement) | tDCS | Left/right auditory cortex (T7/T8) | Contralateral forehead | 2 | Strong (90) | EFR | S |
| Wang et al. (2021) [129] | Stroke (motor function recovery) | tDCS | (a)/(c) Ipsilesional M1 (C3 or C4); (b) lateral supraorbital | (a) Lateral supraorbita; (b)/(c) contralateralM1 | 2 | Weak (19) | PSD and relative power in delta, theta, alpha, and beta bands | S |
| Hu et al. (2021) [130] | Healthy (attention/ executive modulation) | tACS | DLPFC (F3/F4) | DLPFC (F4/F3) | 2 | Weak (44) | Absolute power in alpha band; ERP | S |
| Ghafoor et al. (2022) [131] | Healthy (attention/ executive modulation) | HD-tACS/HD-tDCS | FpZ | Left and right PFC | 5 | Weak (15) | Relative power in alpha and beta bands | S |
| Wang et al. (2022) [132] | Ischemic stroke (motor function recovery) | tDCS | (a)/(c) Ipsilesional M1; (b) lateral supraorbital | (a) Lateral supraorbital; (b)/(c) contralateralM1 | 2 | Moderate (32) | PSD and relative power in delta, theta, alpha, and beta bands | S |
| Liu et al. (2022) [133] | UWS (arousal/ awareness facilitation) | tDCS | (a) Prefrontal area; (b) left FTPC; (c) right FTPC; (d) left DLPFC | (a) Neck; (b)/(c) back of the opposite shoulder; (d) F4 | 2 | Strong (85) | c-ApEn | S |
| Kim et al. (2022) [134] | PTSD (PTSD symptom reduction: intrusive/ arousal) | tDCS | Left DLPFC (F3) | Right DLPFC (F4) | 2 | Weak (48) | PSD in delta, theta, alpha, and beta bands | S+ML |
| Westwood et al. (2022) [135] | ADHD (attention and impulsivity improvement) | tDCS | F8 | Right supra-orbital (Fp1) | 2 | Moderate (29) | PSD in alpha, theta, and beta bands | S |
| Maimon et al. (2022) [136] | DOC (consciousness detection/ support) | tDCS | Left DLPFC (F3) | Right supra-orbital (Fp2) | 2 | Weak (6) | MMN, ERP, and VC9 activity; Relative theta power | S+ML |
| Ayub et al. (2022) [137] | Healthy (motor task performance improvement) | tDCS | Cz | Cp1 | 2 | Weak (10) | ERDs | S |
| Palmisano et al. (2022) [138] | AD (cognitive symptoms mitigation) | tACS | 6 locations covering 4 lobes in both hemispheres | 6 locations covering 4 lobes in both hemispheres | n.a. | Weak (15) | Spectral power in all bands; theta, alpha, and beta activity | S |
| Cheng et al. (2022) [139] | OCD (compulsive symptom reduction) | tDCS | AF8, AF4, AFZ, and FPZ | Right supraorbital (Fp2) | 5 | Weak (51) | TEP ( N45, P60, N100, and P200) | S |
| Wang et al. (2022) [132] | Stroke (executive/ attention support post stroke) | tDCS | Left DLPFC (F3) | Right DLPFC (Fp2) | 2 | Moderate (4) | Relative power in delta, theta, alpha, and beta bands | S |
| de Souza Moura et al. (2022) [140] | Head and neck cancer (fatigue/ cognitive symptom mitigation) | tDCS | F4 | C5 | 2 | Weak (2) | PLI; PSD at 4/8/16/24 Hz | S |
| Mosayebi-Samani et al. (2023) [141] | Healthy (motor cortex excitability/skill learning support) | tDCS | (a) C3; (b) F3 | Contralateral supraorbital | 2 | Moderate (18) | TEP; TMS-evoked oscillations; MEP | S |
| Yeh et al. (2023) [142] | Schizophrenia (cognitive control network modulation) | tACS | (a) F1, F5, AF3, and FC3; (b) P1, P5, CP3, and PO3 | (a) CPz; (b) FCz | 10 | Strong (35) | LPS and connectivity | S |
| Dagnino et al. (2023) [143] | Healthy (sustained attention/ executive enhancement) | tDCS | (a) Left DLPFC (F3, AF3, and AF7); (b) frontal gyrus (FC6 and F8) | (a) Fp2 and T7; (b) Fp2, T8, and C6 | 5 | Strong (56) | Relative power in all bands | S+ML |
| Sergiou et al. (2023) [144] | Substance dependence (craving regulation support) | HD-tDCS | Fpz | vmPF (AF3, AF4, F3, Fz and F4) | 6 | Moderate (50) | Beta activity; Alpha and beta synchronicity | S |
| Kim et al. (2023) [145] | PTSD (PTSD symptom reduction) | tDCS | F3 | F4 | 2 | Weak (48) | EEG spectrogram | DL |
| Roy et al. (2023) [146] | Healthy (cognitive control/ attention enhancement) | tDCS | DLPFC | DLPFC | n.a. | Weak (72) | ERP | S |
| Liu et al. (2023) [147] | Stroke (motor function recovery) | tDCS | Ipsilesional M1 | Ipsilesional M1 | 2 | Weak (15) | PSD in all bands | S |
| Fabio et al. (2023) [148] | PD (executive function and motor symptom relief) | tDCS | Left DLPFC (F7) | Right supraorbital area | 2 | Weak (30) | PDS and absolute power in alpha and beta bands; ERP (P300 latency) | S |
| Chan et al. (2023) [149] | ASD (social cognition/ attention support) | tDCS | Right DLPFC (Fp2) | Left DLPFC (F3) | 2 | Moderate (60) | Theta E/I balance | S |
| Murphy et al. (2023) [150] | MDD (depressive symptom reduction) | tDCS/tRNS | Left DLPFC (F3) | Right supraorbital | 2 | Moderate (49) | ERS/ERD | S |
| Wang et al. (2023) [151] | Healthy (executive control/ attention trajectory modulation) | tACS | left DLPFC (F3) | Right DLPFC (F4) | 2 | Moderate (40) | Brain activity trajectories | S |
| Wang et al. (2024) [152] | DoC (consciousness restoration support) | HD-tDCS | Pz | Parietal cortex | 5 | Weak (8) | PSD and relative power in all bands; spectral, spectral exponent | S |
| Tarantino et al. (2024) [153] | DoC (consciousness restoration support) | tDCS | Left DLPFC | Right supraorbital | 2 | Weak (19) | Alpha/theta power ratio | S |
| Vimolratana et al. (2024) [154] | Stroke (motor function recovery) | tDCS | Lesioned hemisphere (C3/C4) | Contralateralsupraorbital | 2 | Moderate (34) | Absolute power in delta, theta, alpha, and beta bands | S |
| Singh et al. (2024) [155] | MDD (depressive symptom reduction) | tDCS | Left DLPFC (F3) | Left FTPC and FCPC | 5 | PSD in all bands; functional connectivity | S | |
| Couto et al. (2024) [156] | Comorbid anxiety– depression (anxiety and depressive symptom reduction) | tDCS | (a) rVLPFC (F6); (b) vmPFC and anterior cingulate cortex (AF3) | (a) contralateral (Fp1); (b) contralateral mastoid (TP1) | 2 | Weak (20) | Absolute power in all bands; functional connectivity; alpha activity | S |
| Liu et al. (2024) [157] | Stroke (motor function recovery) | tDCS | Ipsilesional M1 (C3/C4) | Contralesional site (FP1/FP2) | 2 | moderate (36) | Absolute power in alpha | S |
| Wynn et al. (2024) [158] | Healthy (working memory and attention enhancement) | tACS | AF4 and P5 | Cz | 3 | Weak (54) | Absolute power and peak frequency in theta and gamma bands | S |
| Yeh et al. (2024) [159] | Schizophrenia (default-mode connectivity normalization; cognitive symptom support) | tDCS | Left DLPFC (F3) | Fp1, Fz, C3, and F7 | 5 | Moderate (59) | delta DMN connectivity and LPS | S |
| Zhou et al. (2024) [160] | Healthy (motor performance improvement) | tDCS | motor cortex | n.a. | 2 | Weak (29) | Relative power in alpha and beta band | S |
| Zhang et al. (2024) [161] | Healthy (visual steady-state detection performance) | tDCS | Oz | Cz | 2 | Weak (13) | SSVEP | S |
| Xiao et al. (2025) [162] | Bipolar depression (depressive symptom reduction) | tDCS | Left DLPFC (F3) | Right DLPFC (F4) | 2 | Weak (20) | Absolute power in all bands; PLV | DL |
3.5. Results for Research Question IV (RQ-IV)
| Article | Clinical Target | Waveform | A Position | C Position | N° of Electrodes | QATQS (Sample Size) | EEG Features for tES Setup | tES-Modulated EEG Features | Data Analysis Method |
|---|---|---|---|---|---|---|---|---|---|
| Zaehle et al. (2010) [170] | Healthy (visual attention modulation) | tACS | PO9/PO10 | PO10/PO9 | 2 | Weak (20) | IAF | Absolute power in alpha band | S |
| Faria et al. (2012) [165] | Epilepsy (epileptic seizure reduction) | tDCS | CPF (FP1, FPz, and FP2) | CP6 or CP5 | 4 | Weak (17) | EF | Average number of EDs | S |
| Auvichayapat et al. (2013) [166] | Epilepsy (epileptic seizure reduction) | tDCS | contralateralshoulder area | EF | 2 | Weak (36) | EF | Average number of EDs | S |
| San-Juan et al. (2016) [20] | Epilepsy (epileptic seizure reduction) | tACS | frontal cortex (Fp1/Fp2) | Frontal cortex (Fp2/Fp1) | 2 | Weak (1) | EF | Spike-low, poli spiker-slow, slow rhythmic waves | n.a. |
| Stecher et al. (2017) [171] | Healthy (visual detection performance modulation) | tACS | Cz | Oz | 2 | Weak (33) | IAF | Absolute alpha power | S |
| Khayyer et al. (2018) [172] | MDD (depressive symptom reduction) | tDCS | Left/right DLPFC (F3/F4) | Cz | 2 | Weak (9) | LORETA EEG source localization | Absolute alpha power | S |
| Lin et al. (2018) [167] | Epilepsy (epileptic seizure reduction) | tDCS | controlateral shoulder | EF | 2 | Weak (9) | EF | PLI in delta, theta, alpha, and beta bands | n.a. |
| Tecchio et al. (2018) [168] | Epilepsy (epileptic seizure reduction) | tDCS | Opposite homologous | EF | 2 | Weak (6) | EF | Functional connectivity | S |
| P.-De Koninck et al. (2019) [173] | Healthy (alpha- mediated attention enhancement) | tACS | (a) PO7/PO8; (b) F3/F4 | (a) PO8/PO7; (b) F4/F3 | 2 | Weak (12) | IAF or ITF | Absolute alpha power | S |
| Del Felice et al. (2019) [174] | PD (motor and cognitive performance improvement) | tACS + tRNS | Based on power spectral difference | Ipsilateral mastoid | 2 | Moderate (15) | Relative power difference | Delta, theta, alpha, and beta power | S |
| Rocha et al. (2020) [163] | Healthy (shooting performance improvement) | tDCS | Contralateral supraorbital area | Right DLPFC (F4) | 2 | Moderate (60) | EEG activity | PSD in beta and gamma bands | S |
| Dallmer-Zerbe et al. (2020) [169] | ADHD (attention and inhibition improvement) | tACS | C3, C4, CP3, CP4, P3, and P4 | T7, T8, TP7, TP8, P7, and P8 | 12 | Weak (18) | P300 and ERSP max | ERP (P-300) | S |
| Aktürk et al. (2022) [164] | Healthy (working memory enhancement) | tACS | F3 | P3 | 2 | Weak (46) | ITF | Theta power and theta ERP connectivity | S |
| Radecke et al. (2023) [175] | Healthy (spatial attention enhancement) | tACS | Parietal cortex | Parietal cortex | 6 | Weak (22) | Alpha power lateralization | ERP | S |
| Gòral-Pòlrola et al. (2024) [176] | Burnout syndrome (burnout symptom reduction) | tDCS | F7 | n.a. | 2 | Weak (1) | Alpha rhythm | EEG spectra and ERP | S |
| Kim et al. (2024) [177] | Healthy (inhibitory control performance enhancement) | tACS | F5 or Fpz | F7, F3, and AF7 or Afz, Fz, and FCz | 4 | Weak (24) | ITF | Absolute theta power | S |
| Author | Sample Type | EEG Features | Results |
|---|---|---|---|
| Boudewyn et al. (2018) [81] | 20 healthy, 17 female, mean age 21, range (18–30 y.o.) | Absolute power in low-gamma and high-gamma frequency bands in frontal (FC1, Fz, and FC2), central (CP1, Cz, and CP2), and posterior (PO3, Pz, and PO4) regions | Increased frontal gamma power for B cues |
| Andrade et al. (2023) [21] | 70 AD, sex and age not reported | Absolute power of the delta, theta, alpha, beta, and gamma frequency bands in Fc1, Fc2, Fc5, Fc6, Fp1, Fp2, F3, F4, F7, F8, FT9, FT10, C3, C4, CP1, CP2, CP5, CP6, T7, T8, P3, P4, P7, P8, O1, and O2 | Increased absolute power in Fc1, F8, CP5, Oz, andF7 in responder patients |
| Boudewyn et al. (2020) [121] | 37 schizophrenia patients, 12 female, mean age 22.76 ± 3.65, range (18–30 y.o.) | Absolute power in gamma band in left frontal (F3, F7, FC5), mid frontal (AF4, AF3, Fz), right frontal (F4, F8, FC6), central (FC2, Cz, CP2, FC1, CP1), left posterior (P3, CP5, P7), mid posterior (O1, Oz, O2), and right posterior (P4, CP6, P8) regions | Increased absolute gamma power compared to the sham condition in all clusters, except the left posterior and mid posterior, when sham performed before active stimulation |
| Liu et al. (2016) [66] | 37 epilepsy patients, sex not reported, range (18–70 y.o.) | Averaged absolute power values in delta, theta, low alpha, high alpha, beta, and low gamma bands across fronto-central (Fp1, Fp2, F3, F4, C3, and C4), left temporal (F7, T3, T5, and A1), right temporal (F8, T4, T6, and A2), and occipital (O1 and O2) regions | No statistically significant results |
| Palm et al. (2009) [34] | 1 66-year-old female MD (major depression) patient | Averaged absolute and relative power in delta, theta, alpha, and beta bands for frontal (Fp1, Fp2, F3, FC1, F4, FC2, FC5, F7, F8, FC6, and Fz), central (T3, T4, CP5, CP6, C3, C4, and Cz), and posterior (T5, T6, P3, P4, Pz, O1, and O2) regions | Decreased absolute power in delta band in frontal area and decreased absolute power in alpha band in frontal and central areas. Decreased relative power in delta and theta bands in frontal area and in alpha band in frontal and central areas post tES treatment |
| Wang et al. (2022) [132] | 24 PSEI (post-stroke executive impairment) patients, 7 female, mean age 54.08 ± 10.53 | Averaged absolute power in delta, theta, alpha, and beta bands in left prefrontal (Fp1, AF3, F3, and F7), left central (C3), left occipital (O1), right prefrontal (Fp2, AF4, F4, and F8), right central (C4), right occipital (O2), prefrontal (Fp1, AF3, F3, F7, Fp2, AF4, F4, F8, and Fz), central (C3, C4, and Cz), and occipital (O1, O2, and Oz) regions | Higher theta-band absolute power after stimulation in the left central region than before stimulation |
| Maimon et al. (2022) [136] | 6 DOC patients, 1 female, range (24–81 y.o.) | Frontal MMN N1 peak amplitudes, frontal theta VC9 biomarker activity, and mean prefrontal theta-band power | Two patients with significant differences between standard-tone N1 amplitudes and deviant-tone N1 amplitudes before tDCS treatment, and three patients exhibited a significant MMN post tDCS treatment. Absolute frontal theta power increased in 4 patients and decreased in 1. VC9 activity significantly increased in 3 patients and decreased in 1 |
| Emonson et al. (2019) [104] | 20 younger adults, 10 female (mean age 24.50 ± 4.48); 20 older adults, 11 female (mean age 65.47 ± 5.62); 9 MCI patients, 4 female (mean age 72.11 ± 5.75) | For TEP at rest: P30/N40, P60, N100, and P200 in F1, FZ, and F2. ERP analysis for 2-back task: N100, P150, N250, and P300 in posterior and frontal regions | In the young, P30 and P60 reduced post-tES amplitude and N250 increased post-tES amplitude; in the elderly, N250 increased post-tES amplitude |
| Rassovsky et al. (2018) [92] | 38 schizophrenia patients, 32% females, mean age 42.7 ± 8.57, range (23–55 y.o.) | MMN in Fz using a passive-attention auditory duration deviant paradigm; P300 in Pz using an active attention auditory oddball paradigm. N170 in P7 and P8 during another task | No statistically significant results |
| Murphy et al. (2023) [150] | 49 MDD patients, 29 females, mean age = 28.46 ± 6.12, range (18–65 y.o.) | Event-Related Synchronization (ERS%) and Event-Related Desynchronization (ERD%) within the theta, upper alpha, and gamma frequency bands in all acquisition channels | Increase in upper-alpha ERS% in parieto-occipital regions 5 min post tES and in left frontal and lateral parieto-occipital regions 25 min post tES. tDCS > sham in both conditions |
| Hoy et al. (2015) [55] | 18 schizophrenia patients, 6 females, mean age 42.17 ± 11.04 | ERS% for correct trials only in the gamma band during the active interval and the reference interval in F3. | Significant ERS% increase in gamma band 40 min post stimulation 2 mA for tES. Significant decrease in gamma at 40 min post stimulation for sham tES. |
| O’Neil-Pirozzi et al. (2017) [80] | 4 Neurotypical patients, one male, mean age = 51.6, range (44–59 y.o.); 4 TBI, two males, mean age = 43, range (35–53 y.o.) | P300 in Cz and absolute power in theta and alpha bands from each electrode in frontal, parietal, and occipital areas | Increased P300 amplitude after anodal stimulation compared to sham only in TBI |
| Ulam et al. (2015) [60] | 26 TBI patients, 4 females, mean age = 33.52 ± 12.25 | Relative power Z scores in delta, theta, alpha, beta, and high beta bands at 6 different time points in F3 (anode) and Fp2 (cathode) | Active tDCS group had greater delta at Fp2 than the sham group for EEG#1 and EEG#2. Greater delta at F3 for the active tDCS group compared to the sham group at EEG#3. Greater total delta for the active tDCS group at EEG#2 and #3 compared to the sham group in Fp2. Significant decrease in theta between EEG#2 and EEG#3 for the active tDCS group in F3. Significant decrease in delta between EEG#1 and #6 for the active group in F3 and Fp2. Significant increase in alpha from EEG#1 to #6 for the active group in F3 and in Fp2. Significant difference at EEG#6 with greater alpha relative power in F3 and Fp2 for active vs. sham group |
3.6. Distribution of Participant Categories
3.7. Distribution of Current Waveform
3.8. Distribution of Anode and Cathode Positions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Meaning |
| Clinical Case | |
| MDD | Major Depressive Disorder |
| ADHD | Attention-Deficit Hyperactivity Disorder |
| ASD | Autism Spectrum Disorder |
| PD | Parkinson’s Disease |
| AD | Alzheimer’s Disease |
| OUD | Opioid Use Disorder |
| DOC/DoC | Disorder of Consciousness |
| TBI | Traumatic Brain Injury |
| PTSD | Post-Traumatic Stress Disorder |
| MCS | Minimally Conscious State |
| MCI | Mild Cognitive Impairment |
| OCD | Obsessive Compulsive Disorder |
| UWS | Unresponsive Wakefulness Syndrome |
| Stimulation electrode position | |
| A position | Anode position |
| C position | Cathode position |
| DLPFC | Dorsolateral Prefrontal Cortex |
| PFC | Prefrontal Cortex |
| rVLPFC | Ventrolateral Prefrontal Cortex |
| vmPFC | Ventromedial Prefrontal Cortex |
| M1 | Primary Motor Cortex |
| PPC | Posterior Parietal Cortex |
| TPJ | Temporal–Parietal Junction |
| EF | Epileptogenic Focus |
| EEG feature | |
| ERP | Event-Related Potential |
| RP | Relative Power |
| TP | Total Power |
| ERSP | Event-Related Spectral Perturbation |
| ERS/ERD | Event-Related Synchronization/Desynchronization |
| PSD | Power Spectral Density |
| GMFP/LMFP | Global/Local Mean Field Power |
| ApEn | Approximate Entropy |
| C-ApEn | Approximate Cross Entropy |
| SaEn | Sample Entropy |
| FuEn | Fuzzy Entropy |
| MSEI | Multiscale Sample Entropy Index |
| MFEI | Multiscale Fuzzy Entropy Index |
| TEP | Transcranial Evoked Potentials |
| MEP | Motor Evoked Potentials |
| SSVEP/VEP | Steady-State Visual Evoked Potential/Visual Evoked Potentials |
| MMN | Mismatch Negativity |
| EFR | Envelope Following Response |
| PLV | Phase-Locking Value |
| PAF | Peak Alpha Frequency |
| LPC | Late Positive Component |
| LP | Late Potential |
| DMN | Default Mode Network |
| MER | Maximum Entropy Ratio |
| PRI | Power-Ratio Index |
| pdBSI | Pairwise-Derived Brain Symmetry Index |
| rBSI | Revised Brain Symmetry Index |
| MSS | Mean State Shift |
| SV | State Variance |
| LPP | Late Positive Potential |
| PAP | Peak Alpha Power |
| EF | Epileptogenic Focus |
| IAF | Individual Alpha Frequency |
| ITF | Individual Theta Frequency |
| EDs | Epileptiform Discharges |
| LORETA | Low-Resolution Brain Electromagnetic Tomography |
| PLI | Phase Lag Index |
| Stimulation Type | |
| tDCS | Transcranial Direct Current Stimulation |
| tACS | Transcranial Alternating Current Stimulation |
| tRNS | Transcranial Random Noise Stimulation |
| so-tDCS | Slow Oscillatory Transcranial Direct Current Stimulation |
| HD-tDCS | High-Definition Transcranial Direct Current Stimulation |
| HD-tACS | High-Definition Transcranial Alternating Current Stimulation |
| tPCS | Transcranial Pulsed Current Stimulation |
| hf-tRNS | High-Frequency Transcranial Random Noise Stimulation |
| osc-tDCS | Oscillatory Transcranial Direct Current Stimulation |
| Data Analysis Method | |
| S | Statistical analysis |
| ML | Machine Learning |
| DL | Deep Learning |
References
- Ekhtiari, H.; Tavakoli, H.; Addolorato, G.; Baeken, C.; Bonci, A.; Campanella, S.; Castelo-Branco, L.; Challet-Bouju, G.; Clark, V.P.; Claus, E.; et al. Transcranial electrical and magnetic stimulation (tES and TMS) for addiction medicine: A consensus paper on the present state of the science and the road ahead. Neurosci. Biobehav. Rev. 2019, 104, 118–140. [Google Scholar] [CrossRef] [PubMed]
- Fertonani, A.; Miniussi, C. Transcranial electrical stimulation: What we know and do not know about mechanisms. Neuroscientist 2017, 23, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Bikson, M.; Esmaeilpour, Z.; Adair, D.; Kronberg, G.; Tyler, W.J.; Antal, A.; Datta, A.; Sabel, B.A.; Nitsche, M.A.; Loo, C.; et al. Transcranial electrical stimulation nomenclature. Brain Stimul. 2019, 12, 1349–1366. [Google Scholar] [CrossRef]
- Lefaucheur, J.P.; Antal, A.; Ayache, S.S.; Benninger, D.H.; Brunelin, J.; Cogiamanian, F.; Cotelli, M.; De Ridder, D.; Ferrucci, R.; Langguth, B.; et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin. Neurophysiol. 2017, 128, 56–92. [Google Scholar] [CrossRef]
- Antal, A.; Alekseichuk, I.; Bikson, M.; Brockmöller, J.; Brunoni, A.R.; Chen, R.; Cohen, L.; Dowthwaite, G.; Ellrich, J.; Flöel, A.; et al. Low intensity transcranial electric stimulation: Safety, ethical, legal regulatory and application guidelines. Clin. Neurophysiol. 2017, 128, 1774–1809. [Google Scholar] [CrossRef]
- Miranda, P.; Cox, C.D.; Alexander, M.; Danev, S.; Lakey, J.R. Overview of current diagnostic, prognostic, and therapeutic use of EEG and EEG-based markers of cognition, mental, and brain health. Integr. Mol. Med. 2019, 6, 1–9. [Google Scholar] [CrossRef]
- Choi, J.; Kwon, M.; Jun, S.C. A systematic review of closed-loop feedback techniques in sleep studies—Related issues and future directions. Sensors 2020, 20, 2770. [Google Scholar] [CrossRef]
- Ruffini, G.; Modolo, J.; Sanchez-Todo, R.; Salvador, R.; Santarnecchi, E. Clinical drivers for personalization of transcranial current stimulation (tES 3.0). In Non Invasive Brain Stimulation in Psychiatry and Clinical Neurosciences; Springer: Cham, Switzerland, 2020; pp. 353–370. [Google Scholar]
- Beumer, S.; Boon, P.; Klooster, D.C.; van Ee, R.; Carrette, E.; Paulides, M.M.; Mestrom, R.M. Personalized tdcs for focal epilepsy—A narrative review: A data-driven workflow based on imaging and eeg data. Brain Sci. 2022, 12, 610. [Google Scholar] [CrossRef]
- Simula, S.; Daoud, M.; Ruffini, G.; Biagi, M.C.; Benar, C.G.; Benquet, P.; Wendling, F.; Bartolomei, F. Transcranial current stimulation in epilepsy: A systematic review of the fundamental and clinical aspects. Front. Neurosci. 2022, 16, 909421. [Google Scholar] [CrossRef]
- Yang, D.; Shin, Y.I.; Hong, K.S. Systemic review on transcranial electrical stimulation parameters and EEG/fNIRS features for brain diseases. Front. Neurosci. 2021, 15, 629323. [Google Scholar] [CrossRef] [PubMed]
- Riva, J.J.; Malik, K.M.; Burnie, S.J.; Endicott, A.R.; Busse, J.W. What is your research question? An introduction to the PICOT format for clinicians. J. Can. Chiropr. Assoc. 2012, 56, 167. [Google Scholar]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Kitchenham, B. Procedures for Performing Systematic Reviews; Keele University: Keele, UK, 2004; Volume 33, pp. 1–26. [Google Scholar]
- Mateen, F.; OH, J.; Tergas, A.; Bhayani, N.; Kamdar, B. Title-abstract versus title-only citation screening strategies for systematic reviews and meta-analyses. In Proceedings of the Cochrane Colloquium Abstracts, Madrid, Spain, 19–23 October 2011. [Google Scholar]
- National Collaborating Centre for Methods and Tools. Quality Assessment Tool for Quantitative Studies; McMaster University: Hamilton, ON, Canada, 2010; Available online: https://www.nccmt.ca/knowledge-repositories/search/14 (accessed on 20 May 2025).
- Perestelo-Pérez, L. Standards on how to develop and report systematic reviews in Psychology and Health. Int. J. Clin. Health Psychol. 2013, 13, 49–57. [Google Scholar] [CrossRef]
- Rethlefsen, M.L.; Kirtley, S.; Waffenschmidt, S.; Ayala, A.P.; Moher, D.; Page, M.J.; Koffel, J.B. PRISMA-S: An extension to the PRISMA statement for reporting literature searches in systematic reviews. Syst. Rev. 2021, 10, 39. [Google Scholar] [CrossRef]
- Fregni, F.; Thome-Souza, S.; Nitsche, M.A.; Freedman, S.D.; Valente, K.D.; Pascual-Leone, A. A controlled clinical trial of cathodal DC polarization in patients with refractory epilepsy. Epilepsia 2006, 47, 335–342. [Google Scholar] [CrossRef]
- San-Juan, D.; Sarmiento, C.I.; Hernandez-Ruiz, A.; Elizondo-Zepeda, E.; Santos-Vázquez, G.; Reyes-Acevedo, G.; Zúñiga-Gazcón, H.; Zamora-Jarquín, C.M. Transcranial alternating current stimulation: A potential risk for genetic generalized epilepsy patients (study case). Front. Neurol. 2016, 7, 213. [Google Scholar] [CrossRef]
- Andrade, S.M.; da Silva-Sauer, L.; de Carvalho, C.D.; de Araújo, E.L.M.; Lima, E.d.O.; Fernandes, F.M.L.; Moreira, K.L.d.A.F.; Camilo, M.E.; Andrade, L.M.M.d.S.; Borges, D.T.; et al. Identifying biomarkers for tDCS treatment response in Alzheimer’s disease patients: A machine learning approach using resting-state EEG classification. Front. Hum. Neurosci. 2023, 17, 1234168. [Google Scholar] [CrossRef] [PubMed]
- De Ridder, D.; Vanneste, S. EEG driven tDCS versus bifrontal tDCS for tinnitus. Front. Psychiatry 2012, 3, 84. [Google Scholar] [CrossRef] [PubMed]
- Mokhtarinejad, E.; Tavakoli, M.; Ghaderi, A.H. Exploring the correlation and causation between alpha oscillations and one-second time perception through EEG and tACS. Sci. Rep. 2024, 14, 8035. [Google Scholar] [CrossRef]
- Ketz, N.; Jones, A.P.; Bryant, N.B.; Clark, V.P.; Pilly, P.K. Closed-loop slow-wave tACS improves sleep-dependent long-term memory generalization by modulating endogenous oscillations. J. Neurosci. 2018, 38, 7314–7326. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.P.; Choe, J.; Bryant, N.B.; Robinson, C.S.; Ketz, N.A.; Skorheim, S.W.; Combs, A.; Lamphere, M.L.; Robert, B.; Gill, H.A.; et al. Dose-dependent effects of closed-loop tACS delivered during slow-wave oscillations on memory consolidation. Front. Neurosci. 2018, 12, 867. [Google Scholar] [CrossRef]
- Hubbard, R.J.; Zadeh, I.; Jones, A.P.; Robert, B.; Bryant, N.B.; Clark, V.P.; Pilly, P.K. Brain connectivity alterations during sleep by closed-loop transcranial neurostimulation predict metamemory sensitivity. Netw. Neurosci. 2021, 5, 734–756. [Google Scholar] [CrossRef] [PubMed]
- Pilly, P.K.; Skorheim, S.W.; Hubbard, R.J.; Ketz, N.A.; Roach, S.M.; Lerner, I.; Jones, A.P.; Robert, B.; Bryant, N.B.; Hartholt, A.; et al. One-shot tagging during wake and cueing during sleep with spatiotemporal patterns of transcranial electrical stimulation can boost long-term metamemory of individual episodes in humans. Front. Neurosci. 2020, 13, 1416. [Google Scholar] [CrossRef]
- Lustenberger, C.; Boyle, M.R.; Alagapan, S.; Mellin, J.M.; Vaughn, B.V.; Fröhlich, F. Feedback-controlled transcranial alternating current stimulation reveals a functional role of sleep spindles in motor memory consolidation. Curr. Biol. 2016, 26, 2127–2136. [Google Scholar] [CrossRef]
- Haslacher, D.; Cavallo, A.; Reber, P.; Kattein, A.; Thiele, M.; Nasr, K.; Hashemi, K.; Sokoliuk, R.; Thut, G.; Soekadar, S.R. Working memory enhancement using real-time phase-tuned transcranial alternating current stimulation. Brain Stimul. 2024, 17, 850–859. [Google Scholar] [CrossRef]
- Stecher, H.I.; Notbohm, A.; Kasten, F.H.; Herrmann, C.S. A comparison of closed loop vs. fixed frequency tACS on modulating brain oscillations and visual detection. Front. Hum. Neurosci. 2021, 15, 661432. [Google Scholar] [CrossRef] [PubMed]
- Schwippel, T.; Pupillo, F.; Feldman, Z.; Walker, C.; Townsend, L.; Rubinow, D.; Frohlich, F. Closed-loop transcranial alternating current stimulation for the treatment of major depressive disorder: An open-label pilot study. Am. J. Psychiatry 2024, 181, 842–845. [Google Scholar] [CrossRef] [PubMed]
- Zarubin, G.; Gundlach, C.; Nikulin, V.; Villringer, A.; Bogdan, M. Transient amplitude modulation of alpha-band oscillations by short-time intermittent closed-loop tACS. Front. Hum. Neurosci. 2020, 14, 366. [Google Scholar] [CrossRef]
- Caravati, E.; Barbeni, F.; Chiarion, G.; Raggi, M.; Mesin, L. Closed-Loop Transcranial Electrical Neurostimulation for Sustained Attention Enhancement: A Pilot Study towards Personalized Intervention Strategies. Bioengineering 2024, 11, 467. [Google Scholar] [CrossRef]
- Palm, U.; Keeser, D.; Schiller, C.; Fintescu, Z.; Reisinger, E.; Baghai, T.C.; Mulert, C.; Padberg, F. Transcranial direct current stimulation in a patient with therapy-resistant major depression. World J. Biol. Psychiatry 2009, 10, 632–635. [Google Scholar] [CrossRef] [PubMed]
- Zaehle, T.; Sandmann, P.; Thorne, J.D.; Jäncke, L.; Herrmann, C.S. Transcranial direct current stimulation of the prefrontal cortex modulates working memory performance: Combined behavioural and electrophysiological evidence. BMC Neurosci. 2011, 12, 2. [Google Scholar] [CrossRef]
- Zaehle, T.; Beretta, M.; Jäncke, L.; Herrmann, C.S.; Sandmann, P. Excitability changes induced in the human auditory cortex by transcranial direct current stimulation: Direct electrophysiological evidence. Exp. Brain Res. 2011, 215, 135–140. [Google Scholar] [CrossRef]
- Kasashima-Shindo, Y.; Fujiwara, T.; Ushiba, J.; Matsushika, Y.; Kamatani, D.; Oto, M.; Ono, T.; Nishimoto, A.; Shindo, K.; Kawakami, M.; et al. Brain-computer interface training combined with transcranial direct current stimulation in patients with chronic severe hemiparesis: Proof of concept study. J. Rehabil. Med. 2015, 47, 318–324. [Google Scholar] [CrossRef]
- Kongthong, N.; Minami, T.; Nakauchi, S. Semantic processing in subliminal face stimuli: An EEG and tDCS study. Neurosci. Lett. 2013, 544, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Rütsche, B.; Hauser, T.; Jäncke, L.; Grabner, R. P 56. Modulating arithmetic performance: A tDCS/EEG study. Clin. Neurophysiol. 2013, 124, e91. [Google Scholar] [CrossRef]
- Lazarev, V.; Tamborino, T.; Bikson, M.; Ferreira, M.; deAzevedo, L.; Caparelli-Dáquer, E. P 235. Focal EEG effects of High Definition tDCS (HD-tDCS) detected by EEG photic driving. Clin. Neurophysiol. 2013, 124, e178–e179. [Google Scholar] [CrossRef]
- Mangia, A.L.; Pirini, M.; Cappello, A. Transcranial direct current stimulation and power spectral parameters: A tDCS/EEG co-registration study. Front. Hum. Neurosci. 2014, 8, 601. [Google Scholar] [CrossRef]
- Lauro, L.J.R.; Rosanova, M.; Mattavelli, G.; Convento, S.; Pisoni, A.; Opitz, A.; Bolognini, N.; Vallar, G. TDCS increases cortical excitability: Direct evidence from TMS–EEG. Cortex 2014, 58, 99–111. [Google Scholar] [CrossRef]
- Roy, A.; Baxter, B.; He, B. High-definition transcranial direct current stimulation induces both acute and persistent changes in broadband cortical synchronization: A simultaneous tDCS–EEG study. IEEE Trans. Biomed. Eng. 2014, 61, 1967–1978. [Google Scholar] [CrossRef]
- Crivelli, D.; Canavesio, Y.; Pala, F.; Finocchiaro, R.; Cobelli, C.; Lecci, G.; Balconi, M. Neuromodulation (tDCS) effect on executive functions in healthy aging: Clinical and EEG evidences. Neuropsychol. Trends 2014, 15, 81–98. [Google Scholar]
- von Mengden, I.; Garcia, C.; Glos, M.; Schöbel, C.; Fietze, I.; Penzel, T. Influence of Slow Oscillating Transcranial Direct Current Stimulation (so-tDCS) on Sleep EEG with focus on Spindle Density and Cognitive Performance on Healthy Subjects. Clin. Neurophysiol. 2014, 125 (Suppl. 1), S122. [Google Scholar] [CrossRef]
- Powell, T.Y.; Boonstra, T.W.; Martin, D.M.; Loo, C.K.; Breakspear, M. Modulation of cortical activity by transcranial direct current stimulation in patients with affective disorder. PLoS ONE 2014, 9, e98503. [Google Scholar] [CrossRef]
- Dominguez, A.; Socas, R.; Marrero, H.; Leon, N.; LLabres, J.; Enriquez, E. Transcranial direct current stimulation improves word production in conduction aphasia: Electroencephalographic and behavioral evidences. Int. J. Clin. Health Psychol. 2014, 14, 240–245. [Google Scholar] [CrossRef][Green Version]
- Miller, J.; Berger, B.; Sauseng, P. Anodal transcranial direct current stimulation (tDCS) increases frontal–midline theta activity in the human EEG: A preliminary investigation of non-invasive stimulation. Neurosci. Lett. 2015, 588, 114–119. [Google Scholar] [CrossRef]
- D’Atri, A.; De Simoni, E.; Gorgoni, M.; Ferrara, M.; Ferlazzo, F.; Rossini, P.M.; De Gennaro, L. Frequency-dependent effects of oscillatory-tDCS on EEG oscillations: A study with better oscillation detection method (BOSC). Arch. Ital. Biol. 2015, 153, 124–134. [Google Scholar]
- Jindal, U.; Sood, M.; Chowdhury, S.R.; Das, A.; Kondziella, D.; Dutta, A. Corticospinal excitability changes to anodal tDCS elucidated with NIRS-EEG joint-imaging: An ischemic stroke study. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 3399–3402. [Google Scholar]
- Sood, M.; Jindal, U.; Chowdhury, S.R.; Das, A.; Kondziella, D.; Dutta, A. Anterior temporal artery tap to identify systemic interference using short-separation NIRS measurements: A NIRS/EEG-tDCS study. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 1239–1242. [Google Scholar]
- Amatachaya, A.; Jensen, M.P.; Patjanasoontorn, N.; Auvichayapat, N.; Suphakunpinyo, C.; Janjarasjitt, S.; Ngernyam, N.; Aree-uea, B.; Auvichayapat, P. The short-term effects of transcranial direct current stimulation on electroencephalography in children with autism: A randomized crossover controlled trial. Behav. Neurol. 2015, 2015, 928631. [Google Scholar] [CrossRef] [PubMed]
- Cosmo, C.; Ferreira, C.; Miranda, J.G.V.; Do Rosario, R.S.; Baptista, A.F.; Montoya, P.; De Sena, E.P. Spreading effect of tDCS in individuals with attention-deficit/hyperactivity disorder as shown by functional cortical networks: A randomized, double-blind, sham-controlled trial. Front. Psychiatry 2015, 6, 111. [Google Scholar] [CrossRef] [PubMed]
- Del Felice, A.; Magalini, A.; Masiero, S. Slow-oscillatory transcranial direct current stimulation modulates memory in temporal lobe epilepsy by altering sleep spindle generators: A possible rehabilitation tool. Brain Stimul. 2015, 8, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Hoy, K.E.; Bailey, N.W.; Arnold, S.L.; Fitzgerald, P.B. The effect of transcranial direct current stimulation on gamma activity and working memory in schizophrenia. Psychiatry Res. 2015, 228, 191–196. [Google Scholar] [CrossRef]
- Dutta, A.; Jacob, A.; Chowdhury, S.R.; Das, A.; Nitsche, M.A. EEG-NIRS based assessment of neurovascular coupling during anodal transcranial direct current stimulation-a stroke case series. J. Med. Syst. 2015, 39, 1–9. [Google Scholar] [CrossRef]
- Wu, D.; Wang, J.; Yuan, Y. Effects of transcranial direct current stimulation on naming and cortical excitability in stroke patients with aphasia. Neurosci. Lett. 2015, 589, 115–120. [Google Scholar] [CrossRef]
- Jindal, U.; Sood, M.; Dutta, A.; Chowdhury, S.R. Development of point of care testing device for neurovascular coupling from simultaneous recording of EEG and NIRS during anodal transcranial direct current stimulation. IEEE J. Transl. Eng. Health Med. 2015, 3, 1–12. [Google Scholar] [CrossRef]
- Ang, K.K.; Guan, C.; Phua, K.S.; Wang, C.; Zhao, L.; Teo, W.P.; Chen, C.; Ng, Y.S.; Chew, E. Facilitating effects of transcranial direct current stimulation on motor imagery brain-computer interface with robotic feedback for stroke rehabilitation. Arch. Phys. Med. Rehabil. 2015, 96, S79–S87. [Google Scholar] [CrossRef] [PubMed]
- Ulam, F.; Shelton, C.; Richards, L.; Davis, L.; Hunter, B.; Fregni, F.; Higgins, K. Cumulative effects of transcranial direct current stimulation on EEG oscillations and attention/working memory during subacute neurorehabilitation of traumatic brain injury. Clin. Neurophysiol. 2015, 126, 486–496. [Google Scholar] [CrossRef]
- Impey, D.; Knott, V. Effect of transcranial direct current stimulation (tDCS) on MMN-indexed auditory discrimination: A pilot study. J. Neural Transm. 2015, 122, 1175–1185. [Google Scholar] [CrossRef]
- Sood, M.; Besson, P.; Muthalib, M.; Jindal, U.; Perrey, S.; Dutta, A.; Hayashibe, M. NIRS-EEG joint imaging during transcranial direct current stimulation: Online parameter estimation with an autoregressive model. J. Neurosci. Methods 2016, 274, 71–80. [Google Scholar] [CrossRef]
- Cappon, D.; Goljahani, A.; Laera, G.; Bisiacchi, P. Interactions between non invasive transcranial brain stimulation (tACS) and brain oscillations: A quantitative EEG study. Int. J. Psychophysiol. 2016, 108, 92. [Google Scholar] [CrossRef]
- Caldiroli, C.L.; Balconi, M. The effect of tDCS on EEG profile during a semantic motor task divided in a correct and incorrect ways. In Proceedings of the International Symposium on Pervasive Computing Paradigms for Mental Health, Milan, Italy, 24–25 September 2015; Springer: Cham, Switzerland, 2015; pp. 269–273. [Google Scholar]
- Marceglia, S.; Mrakic-Sposta, S.; Rosa, M.; Ferrucci, R.; Mameli, F.; Vergari, M.; Arlotti, M.; Ruggiero, F.; Scarpini, E.; Galimberti, D.; et al. Transcranial direct current stimulation modulates cortical neuronal activity in Alzheimer’s disease. Front. Neurosci. 2016, 10, 134. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Bryant, A.; Jefferson, A.; Friedman, D.; Minhas, P.; Barnard, S.; Barr, W.; Thesen, T.; O’Connor, M.; Shafi, M.; et al. Exploring the efficacy of a 5-day course of transcranial direct current stimulation (TDCS) on depression and memory function in patients with well-controlled temporal lobe epilepsy. Epilepsy Behav. 2016, 55, 11–20. [Google Scholar] [CrossRef]
- Dunn, W.; Rassovsky, Y.; Wynn, J.K.; Wu, A.D.; Iacoboni, M.; Hellemann, G.; Green, M.F. Modulation of neurophysiological auditory processing measures by bilateral transcranial direct current stimulation in schizophrenia. Schizophr. Res. 2016, 174, 189–191. [Google Scholar] [CrossRef]
- D’Agata, F.; Peila, E.; Cicerale, A.; Caglio, M.M.; Caroppo, P.; Vighetti, S.; Piedimonte, A.; Minuto, A.; Campagnoli, M.; Salatino, A.; et al. Cognitive and neurophysiological effects of non-invasive brain stimulation in stroke patients after motor rehabilitation. Front. Behav. Neurosci. 2016, 10, 135. [Google Scholar] [CrossRef]
- Ashikhmin, A.; Shishelova, A.; Aliev, R. tDCS provokes sustainable changes in EEG and reorganizes autonomic modulation of heart rate. Brain Stimul. Basic Transl. Clin. Res. Neuromodulation 2017, 10, 484–486. [Google Scholar] [CrossRef]
- Angulo-Sherman, I.N.; Rodríguez-Ugarte, M.; Sciacca, N.; Iáñez, E.; Azorín, J.M. Effect of tDCS stimulation of motor cortex and cerebellum on EEG classification of motor imagery and sensorimotor band power. J. Neuroeng. Rehabil. 2017, 14, 1–16. [Google Scholar] [CrossRef][Green Version]
- Angulo-Sherman, I.N.; Rodríguez-Ugarte, M.; Iáñez, E.; Ortíz, M.; Azorín, J.M. Effect on the classification of motor imagery in EEG after applying anodal tDCS with a 4 × 1 ring montage over the motor cortex. In Proceedings of the 2017 International Conference on Rehabilitation Robotics (ICORR), London, UK, 17–20 July 2017; pp. 818–822. [Google Scholar][Green Version]
- Grande, G.; Golemme, M.; Tatti, E.; Chiesa, S.; Van Velzen, J.; Luft, C.D.B.; Cappelletti, M. P127 A combined EEG and alpha tACS study on visual working memory in healthy ageing. Clin. Neurophysiol. 2017, 128, e77–e78. [Google Scholar] [CrossRef]
- Donaldson, P.; Kirkovski, M.; Rinehart, N.; Enticott, P. Social cognition and the temporoparietal junction: A double-blind HD-tDCS EEG study. Brain Stimul. Basic Transl. Clin. Res. Neuromodulation 2017, 10, 378. [Google Scholar] [CrossRef]
- Berger, A.; Pixa, N.; Doppelmayr, M. Frequency-specific after-effects of transcranial alternating current stimulation (tACS) on motor learning: Preliminary data of a simultaneous tACS-EEG-NIRS study. Brain Stimul. Basic Transl. Clin. Res. Neuromodulation 2017, 10, 412. [Google Scholar] [CrossRef]
- Cortes, M.; Edwards, D.; Putrino, D. Anodal tDCS decreases total EEG power at rest and alters brain signaling during fatigue in high performance athletes. Brain Stimul. Basic Transl. Clin. Res. Neuromodulation 2017, 10, e14. [Google Scholar] [CrossRef]
- Ladenbauer, J.; Ladenbauer, J.; Külzow, N.; de Boor, R.; Avramova, E.; Grittner, U.; Flöel, A. Promoting sleep oscillations and their functional coupling by transcranial stimulation enhances memory consolidation in mild cognitive impairment. J. Neurosci. 2017, 37, 7111–7124. [Google Scholar] [CrossRef] [PubMed]
- Impey, D.; Baddeley, A.; Nelson, R.; Labelle, A.; Knott, V. Effects of transcranial direct current stimulation on the auditory mismatch negativity response and working memory performance in schizophrenia: A pilot study. J. Neural Transm. 2017, 124, 1489–1501. [Google Scholar] [CrossRef]
- Naros, G.; Gharabaghi, A. Physiological and behavioral effects of β-tACS on brain self-regulation in chronic stroke. Brain Stimul. 2017, 10, 251–259. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, J.; Wu, D.; Huang, X.; Song, W. Effect of transcranial direct current stimulation on swallowing apraxia and cortical excitability in stroke patients. Top. Stroke Rehabil. 2017, 24, 503–509. [Google Scholar] [CrossRef] [PubMed]
- O’Neil-Pirozzi, T.M.; Doruk, D.; Thomson, J.M.; Fregni, F. Immediate memory and electrophysiologic effects of prefrontal cortex transcranial direct current stimulation on neurotypical individuals and individuals with chronic traumatic brain injury: A pilot study. Int. J. Neurosci. 2017, 127, 592–600. [Google Scholar] [CrossRef]
- Boudewyn, M.; Roberts, B.M.; Mizrak, E.; Ranganath, C.; Carter, C.S. Prefrontal transcranial direct current stimulation (tDCS) enhances behavioral and EEG markers of proactive control. Cogn. Neurosci. 2019, 10, 57–65. [Google Scholar] [CrossRef]
- Kang, J.; Cai, E.; Han, J.; Tong, Z.; Li, X.; Sokhadze, E.M.; Casanova, M.F.; Ouyang, G.; Li, X. Transcranial direct current stimulation (tDCS) can modulate EEG complexity of children with autism spectrum disorder. Front. Neurosci. 2018, 12, 201. [Google Scholar] [CrossRef]
- Mane, R.; Chew, E.; Phua, K.S.; Ang, K.K.; Vinod, A.P.; Guan, C. Quantitative EEG as biomarkers for the monitoring of post-stroke motor recovery in BCI and tDCS rehabilitation. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 3610–3613. [Google Scholar]
- Cukic, M.; Stokic, M.; Radenkovic, S.; Ljubisavljevic, M.; Pokrajac, D.D. The shift in brain-state induced by tDCS: An EEG study. arXiv 2018, arXiv:1812.01342. [Google Scholar]
- Friedrich, J.; Beste, C. Paradoxical, causal effects of sensory gain modulation on motor inhibitory control—A tDCS, EEG-source localization study. Sci. Rep. 2018, 8, 17486. [Google Scholar] [CrossRef]
- Mondini, V.; Mangia, A.L.; Cappello, A. Single-session tDCS over the dominant hemisphere affects contralateral spectral EEG power, but does not enhance neurofeedback-guided event-related desynchronization of the non-dominant hemisphere’s sensorimotor rhythm. PLoS ONE 2018, 13, e0193004. [Google Scholar] [CrossRef] [PubMed]
- Holgado, D.; Zandonai, T.; Hopker, J.; Zabala, M.; Ciria, L.; Sanabria, D. Null effects of tDCS over the Left Prefrontal Cortex on Self-paced Exercise and EEG. J. Sci. Cycl. 2018, 7, 4. [Google Scholar]
- Berger, A.; Pixa, N.H.; Steinberg, F.; Doppelmayr, M. Brain oscillatory and hemodynamic activity in a bimanual coordination task following transcranial alternating current stimulation (tACS): A combined EEG-fNIRS study. Front. Behav. Neurosci. 2018, 12, 67. [Google Scholar] [CrossRef]
- Ferrucci, R.; Mrakic-Sposta, S.; Gardini, S.; Ruggiero, F.; Vergari, M.; Mameli, F.; Arighi, A.; Spallazzi, M.; Barocco, F.; Michelini, G.; et al. Behavioral and neurophysiological effects of transcranial direct current stimulation (tDCS) in fronto-temporal dementia. Front. Behav. Neurosci. 2018, 12, 235. [Google Scholar] [CrossRef]
- Shahsavar, Y.; Ghoshuni, M.; Talaei, A. Quantifying clinical improvements in patients with depression under the treatment of transcranial direct current stimulation using event related potentials. Australas. Phys. Eng. Sci. Med. 2018, 41, 973–983. [Google Scholar] [CrossRef]
- Meiron, O.; Gale, R.; Namestnic, J.; Bennet-Back, O.; David, J.; Gebodh, N.; Adair, D.; Esmaeilpour, Z.; Bikson, M. High-Definition transcranial direct current stimulation in early onset epileptic encephalopathy: A case study. Brain Inj. 2018, 32, 135–143. [Google Scholar] [CrossRef]
- Rassovsky, Y.; Dunn, W.; Wynn, J.K.; Wu, A.D.; Iacoboni, M.; Hellemann, G.; Green, M.F. Single transcranial direct current stimulation in schizophrenia: Randomized, cross-over study of neurocognition, social cognition, ERPs, and side effects. PLoS ONE 2018, 13, e0197023. [Google Scholar] [CrossRef]
- Hordacre, B.; Moezzi, B.; Ridding, M.C. Neuroplasticity and network connectivity of the motor cortex following stroke: A transcranial direct current stimulation study. Hum. Brain Mapp. 2018, 39, 3326–3339. [Google Scholar] [CrossRef]
- Nicolo, P.; Magnin, C.; Pedrazzini, E.; Plomp, G.; Mottaz, A.; Schnider, A.; Guggisberg, A.G. Comparison of neuroplastic responses to cathodal transcranial direct current stimulation and continuous theta burst stimulation in subacute stroke. Arch. Phys. Med. Rehabil. 2018, 99, 862–872. [Google Scholar] [CrossRef]
- Straudi, S.; Bonsangue, V.; Mele, S.; Craighero, L.; Montis, A.; Fregni, F.; Lavezzi, S.; Basaglia, N. Bilateral M1 anodal transcranial direct current stimulation in post traumatic chronic minimally conscious state: A pilot EEG-tDCS study. Brain Inj. 2019, 33, 490–495. [Google Scholar] [CrossRef]
- D’Atri, A.; Scarpelli, S.; Gorgoni, M.; Alfonsi, V.; Annarumma, L.; Giannini, A.M.; Ferrara, M.; Ferlazzo, F.; Rossini, P.M.; De Gennaro, L. Bilateral theta transcranial alternating current stimulation (tACS) modulates EEG activity: When tACS works awake it also works asleep. Nat. Sci. Sleep 2019, 11, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Dondé, C.; Brevet-Aeby, C.; Poulet, E.; Mondino, M.; Brunelin, J. Potential impact of bifrontal transcranial random noise stimulation (tRNS) on the semantic Stroop effect and its resting-state EEG correlates. Neurophysiol. Clin. 2019, 49, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, P.H.; Kirkovski, M.; Rinehart, N.J.; Enticott, P.G. A double-blind HD-tDCS/EEG study examining right temporoparietal junction involvement in facial emotion processing. Soc. Neurosci. 2019, 14, 681–696. [Google Scholar] [CrossRef] [PubMed]
- Dowsett, J.; Herrmann, C.S.; Dieterich, M.; Taylor, P.C. Shift in lateralization during illusory self-motion: EEG responses to visual flicker at 10 Hz and frequency-specific modulation by tACS. Eur. J. Neurosci. 2020, 51, 1657–1675. [Google Scholar] [CrossRef]
- Bueno-Lopez, A.; Eggert, T.; Dorn, H.; Danker-Hopfe, H. Slow oscillatory transcranial direct current stimulation (so-tDCS) during slow wave sleep has no effects on declarative memory in healthy young subjects. Brain Stimul. 2019, 12, 948–958. [Google Scholar] [CrossRef] [PubMed]
- Handiru, V.S.; Guan, C.; Ang, K.K.; Chew, E. Abstract# 130: EEG Beta-band Coherence for Prognosis of Motor Recovery in Stroke Patients with tDCS-BCI Intervention. Brain Stimul. Basic Transl. Clin. Res. Neuromodulation 2019, 12, e45. [Google Scholar]
- Willms, M.; Brucar, L.; Muller, A.; Vila-Rodrigues, F.; Rosenblatt, C.; Babul, N.V. Exploring tDCS-induced changes in EEG power and network connectivity in youth concussion: Preliminary findings. Brain Stimul. Basic Transl. Clin. Res. Neuromodulation 2019, 12, 468. [Google Scholar] [CrossRef]
- Mastakouri, A.A.; Schölkopf, B.; Grosse-Wentrup, M. Beta power may meditate the effect of Gamma-TACS on motor performance. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 5902–5908. [Google Scholar]
- Emonson, M.; Fitzgerald, P.; Rogasch, N.; Hoy, K. Neurobiological effects of transcranial direct current stimulation in younger adults, older adults and mild cognitive impairment. Neuropsychologia 2019, 125, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Cespón, J.; Rodella, C.; Miniussi, C.; Pellicciari, M. Behavioural and electrophysiological modulations induced by transcranial direct current stimulation in healthy elderly and Alzheimer’s disease patients: A pilot study. Clin. Neurophysiol. 2019, 130, 2038–2052. [Google Scholar] [CrossRef]
- Alexander, M.L.; Alagapan, S.; Lugo, C.E.; Mellin, J.M.; Lustenberger, C.; Rubinow, D.R.; Fröhlich, F. Double-blind, randomized pilot clinical trial targeting alpha oscillations with transcranial alternating current stimulation (tACS) for the treatment of major depressive disorder (MDD). Transl. Psychiatry 2019, 9, 106. [Google Scholar] [CrossRef]
- Meiron, O.; Gale, R.; Namestnic, J.; Bennet-Back, O.; Gebodh, N.; Esmaeilpour, Z.; Mandzhiyev, V.; Bikson, M. Antiepileptic effects of a novel non-invasive neuromodulation treatment in a subject with early-onset epileptic encephalopathy: Case report with 20 sessions of HD-tDCS intervention. Front. Neurosci. 2019, 13, 547. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Mellin, J.M.; Alagapan, S.; Alexander, M.L.; Gilmore, J.H.; Jarskog, L.F.; Fröhlich, F. Targeting reduced neural oscillations in patients with schizophrenia by transcranial alternating current stimulation. Neuroimage 2019, 186, 126–136. [Google Scholar] [CrossRef]
- Singh, A.; Trapp, N.T.; De Corte, B.; Cao, S.; Kingyon, J.; Boes, A.D.; Parker, K.L. Cerebellar theta frequency transcranial pulsed stimulation increases frontal theta oscillations in patients with schizophrenia. Cerebellum 2019, 18, 489–499. [Google Scholar] [CrossRef]
- Schoellmann, A.; Scholten, M.; Wasserka, B.; Govindan, R.B.; Krüger, R.; Gharabaghi, A.; Plewnia, C.; Weiss, D. Anodal tDCS modulates cortical activity and synchronization in Parkinson’s disease depending on motor processing. NeuroImage Clin. 2019, 22, 101689. [Google Scholar] [CrossRef]
- Mane, R.; Chew, E.; Phua, K.S.; Ang, K.K.; Robinson, N.; Vinod, A.; Guan, C. Prognostic and monitory EEG-biomarkers for BCI upper-limb stroke rehabilitation. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 1654–1664. [Google Scholar] [CrossRef]
- Bao, S.C.; Wong, W.W.; Leung, T.W.H.; Tong, K.Y. Cortico-muscular coherence modulated by high-definition transcranial direct current stimulation in people with chronic stroke. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 27, 304–313. [Google Scholar] [CrossRef]
- Luna, F.G.; Román-Caballero, R.; Barttfeld, P.; Lupiáñez, J.; Martín-Arévalo, E. A High-Definition tDCS and EEG study on attention and vigilance: Brain stimulation mitigates the executive but not the arousal vigilance decrement. Neuropsychologia 2020, 142, 107447. [Google Scholar] [CrossRef] [PubMed]
- El-Hagrassy, M.; Duarte, D.; Lu, J.; Uygur-Kucukseymen, E.; Münger, M.; Thibaut, A.; Lv, P.; Morales-Quezada, L.; Fregni, F. EEG modulation by different transcranial direct current stimulation (tDCS) montages: A randomized double-blind sham-control mechanistic pilot trial in healthy participants. Expert Rev. Med. Devices 2021, 18, 107–120. [Google Scholar] [CrossRef]
- de Melo, G.A.; de Oliveira, E.A.; dos Santos Andrade, S.M.M.; Fernández-Calvo, B.; Torro, N. Comparison of two tDCS protocols on pain and EEG alpha-2 oscillations in women with fibromyalgia. Sci. Rep. 2020, 10, 18955. [Google Scholar] [CrossRef] [PubMed]
- Sergiou, C.S.; Santarnecchi, E.; Romanella, S.M.; Wieser, M.J.; Franken, I.H.A.; Rassin, E.; van Dongen, J.D.M. tDCS Targeting the Ventromedial Prefrontal Cortex Reduces Reactive Aggression and Modulates Electrophysiological Responses: A HD-tDCS/EEG Randomized Controlled Trial in a Forensic Population [Pre-Registration]. OSF. 2020. Available online: https://osf.io/cjgdt/ (accessed on 20 May 2025).
- Pross, B.; Siamouli, M.; Pogarell, O.; Falkai, P.; Hasan, A.; Strube, W. S177. Frontal cortical plasticity in schizophrenia patients examined by LTP-inducing anodal TDCS and repetitive EEG. Schizophr. Bull. 2018, 44, S393. [Google Scholar] [CrossRef]
- Gangemi, A.; Colombo, B.; Fabio, R.A. Effects of short-and long-term neurostimulation (tDCS) on Alzheimer’s disease patients: Two randomized studies. Aging Clin. Exp. Res. 2021, 33, 383–390. [Google Scholar] [CrossRef]
- Nikolin, S.; Martin, D.; Loo, C.K.; Iacoviello, B.M.; Boonstra, T.W. Assessing neurophysiological changes associated with combined transcranial direct current stimulation and cognitive-emotional training for treatment-resistant depression. Eur. J. Neurosci. 2020, 51, 2119–2133. [Google Scholar] [CrossRef] [PubMed]
- Breitling, C.; Zaehle, T.; Dannhauer, M.; Tegelbeckers, J.; Flechtner, H.H.; Krauel, K. Comparison between conventional and HD-tDCS of the right inferior frontal gyrus in children and adolescents with ADHD. Clin. Neurophysiol. 2020, 131, 1146–1154. [Google Scholar] [CrossRef]
- Boudewyn, M.A.; Scangos, K.; Ranganath, C.; Carter, C.S. Using prefrontal transcranial direct current stimulation (tDCS) to enhance proactive cognitive control in schizophrenia. Neuropsychopharmacology 2020, 45, 1877–1883. [Google Scholar] [CrossRef]
- Jahshan, C.; Wynn, J.K.; Roach, B.J.; Mathalon, D.H.; Green, M.F. Effects of Transcranial Direct Current Stimulation on Visual Neuroplasticity in Schizophrenia. Clin. EEG Neurosci. 2020, 51, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, B.; Li, N.; Li, Y.; Hou, J.; Duan, G.; Wu, D. Transcranial direct current stimulation over prefrontal areas improves psychomotor inhibition state in patients with traumatic brain injury: A pilot study. Front. Neurosci. 2020, 14, 386. [Google Scholar] [CrossRef] [PubMed]
- Grasso, P.A.; Tonolli, E.; Bortoletto, M.; Miniussi, C. tDCS over posterior parietal cortex increases cortical excitability but decreases learning: An ERPs and TMS-EEG study. Brain Res. 2021, 1753, 147227. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, H.; Valentine, O.; Mayza, A. Analysis of quantitative EEG (QEEG) parameters on the effect of transcranial direct current stimulation (TDCS) on post-stroke patients. AIP Conf. Proc. 2021, 2344, 050001. [Google Scholar] [CrossRef]
- Ghin, F.; O’Hare, L.; Pavan, A. Electrophysiological aftereffects of high-frequency transcranial random noise stimulation (hf-tRNS): An EEG investigation. Exp. Brain Res. 2021, 239, 2399–2418. [Google Scholar] [CrossRef]
- Mostafavi, H.; Dadashi, M.; Faridi, A.; Kazemzadeh, F.; Eskandari, Z. Using bilateral tDCS to modulate EEG amplitude and coherence of men with opioid use disorder under methadone therapy: A sham-controlled clinical trial. Clin. EEG Neurosci. 2022, 53, 184–195. [Google Scholar] [CrossRef]
- Mai, G.; Howell, P. Causal relationship between the right auditory cortex and speech-evoked frequency-following response: Evidence from combined tDCS and EEG. bioRxiv 2020. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Y.; Chen, Y.; Song, P.; Yu, H.; Sun, C.; Du, J. Comparison and Affecting Factors of Three tDCS Montages in Motor Recovery of Chronic Stroke Patients: A Resting-State EEG Study [Preprint]. Research Square. 2021. Available online: https://www.researchgate.net/publication/348261758_Comparison_and_Affecting_Factors_of_Three_tDCS_Montages_in_Motor_Recovery_of_Chronic_Stroke_Patients_A_Resting-State_EEG_Study (accessed on 20 May 2025).
- Hu, P.; He, Y.; Liu, X.; Ren, Z.; Liu, S. Modulating emotion processing using transcranial alternating current stimulation (tACS)-A sham-controlled study in healthy human participants. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Virtual, 1–5 November 2021; pp. 6667–6670. [Google Scholar]
- Ghafoor, U.; Yang, D.; Hong, K.S. Neuromodulatory effects of HD-tACS/tDCS on the prefrontal cortex: A resting-state fNIRS-EEG study. IEEE J. Biomed. Health Inform. 2021, 26, 2192–2203. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, Y.; Song, P.; Yu, H.; Du, J.; Zhang, Y.; Sun, C. Varied response of EEG rhythm to different tDCS protocols and lesion hemispheres in stroke subjects with upper limb dysfunction. Neural Plast. 2022, 2022, 7790730. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, X.; Li, Y.; Duan, G.; Hou, J.; Zhao, J.; Guo, T.; Wu, D. tDCS-EEG for predicting outcome in patients with unresponsive wakefulness syndrome. Front. Neurosci. 2022, 16, 771393. [Google Scholar] [CrossRef]
- Kim, S.; Yang, C.; Dong, S.Y.; Lee, S.H. Predictions of tDCS treatment response in PTSD patients using EEG based classification. Front. Psychiatry 2022, 13, 876036. [Google Scholar] [CrossRef]
- Westwood, S.J.; Bozhilova, N.; Criaud, M.; Lam, S.L.; Lukito, S.; Wallace-Hanlon, S.; Kowalczyk, O.S.; Kostara, A.; Mathew, J.; Wexler, B.E.; et al. The effect of transcranial direct current stimulation (tDCS) combined with cognitive training on EEG spectral power in adolescent boys with ADHD: A double-blind, randomized, sham-controlled trial. IBRO Neurosci. Rep. 2022, 12, 55–64. [Google Scholar] [CrossRef]
- Maimon, N.B.; Molcho, L.; Jaul, E.; Intrator, N.; Barron, J.; Meiron, O. EEG reactivity changes captured via mobile BCI device following tDCS intervention—A pilot-study in disorders of consciousness (DOC) patients. In Proceedings of the 2022 10th International Winter Conference on Brain-Computer Interface (BCI), Gangwon-do, Republic of Korea, 21–23 February 2022; pp. 1–3. [Google Scholar]
- Ayub, M.; Ullah, K.; Khan, M.J.; Farooq, H.; Khan, A. Cognitive Improvement Estimation using EEG Imaging after tDCS Therapy. In Proceedings of the 2022 International Conference on Emerging Trends in Electrical, Control, and Telecommunication Engineering (ETECTE), Lahore, Pakistan, 2–4 December 2022; pp. 1–6. [Google Scholar]
- Palmisano, A.; Tatti, E.; Pezanko, L.; Cappon, D.; Macome, J.; Koch, G.; Smeralda, C.; Rivolta, D.; El Fakhri, G.; Pascual-Leone, A.; et al. PC016/# 697 PERTURBATION-BASED TACS-EEG BIOMARKERS OF GAMMA ACTIVITY IN ALZHEIMER’S DISEASE: E-POSTER VIEWING. Neuromodulation 2022, 25, S11–S12. [Google Scholar]
- Cheng, J.; Li, P.; Tang, Y.; Zhang, C.; Lin, L.; Gao, J.; Wang, Z. Transcranial direct current stimulation improve symptoms and modulates cortical inhibition in obsessive-compulsive disorder: A TMS-EEG study. J. Affect. Disord. 2022, 298, 558–564. [Google Scholar] [CrossRef] [PubMed]
- de Souza Moura, B.; Hu, X.S.; DosSantos, M.F.; DaSilva, A.F. Study protocol of tDCS based pain modulation in head and neck cancer patients under chemoradiation therapy condition: An fNIRS-EEG study. Front. Mol. Neurosci. 2022, 15, 859988. [Google Scholar] [CrossRef]
- Mosayebi-Samani, M.; Agboada, D.; Mutanen, T.P.; Haueisen, J.; Kuo, M.F.; Nitsche, M.A. Transferability of cathodal tDCS effects from the primary motor to the prefrontal cortex: A multimodal TMS-EEG study. Brain Stimul. 2023, 16, 515–539. [Google Scholar] [CrossRef]
- Yeh, T.C.; Huang, C.C.Y.; Chung, Y.A.; Park, S.Y.; Im, J.J.; Lin, Y.Y.; Ma, C.C.; Tzeng, N.S.; Chang, H.A. Online left-hemispheric in-phase frontoparietal theta tACS modulates theta-band EEG source-based large-scale functional network connectivity in patients with schizophrenia: A randomized, double-blind, sham-controlled clinical trial. Biomedicines 2023, 11, 630. [Google Scholar] [CrossRef]
- Dagnino, P.C.; Braboszcz, C.; Kroupi, E.; Splittgerber, M.; Brauer, H.; Dempfle, A.; Breitling-Ziegler, C.; Prehn-Kristensen, A.; Krauel, K.; Siniatchkin, M.; et al. Stratification of responses to tDCS intervention in a healthy pediatric population based on resting-state EEG profiles. Sci. Rep. 2023, 13, 8438. [Google Scholar] [CrossRef]
- Sergiou, C.S.; Tatti, E.; Romanella, S.M.; Santarnecchi, E.; Weidema, A.D.; Rassin, E.G.; Franken, I.H.; van Dongen, J.D. The effect of HD-tDCS on brain oscillations and frontal synchronicity during resting-state EEG in violent offenders with a substance dependence. Int. J. Clin. Health Psychol. 2023, 23, 100374. [Google Scholar] [CrossRef]
- Kim, S.; Yang, C.; Dong, S.Y.; Lee, S.H. Deep Convolutional Neural Network based tDCS Prognosis Classification in PTSD Patients using EEG Spectrograms. Brain Stimul. Basic Transl. Clin. Res. Neuromodulation 2023, 16, 351. [Google Scholar] [CrossRef]
- Roy, S.; Fan, Y.; Nitsche, M. ASSESSING THE ROLE OF TRANSCRANIAL DIRECT CURRENT STIMULATION (TDCS) IN RESCUING STRESS-INDUCED WORKING MEMORY (WM) DEFICITS–AN EEG-BASED STUDY. IBRO Neurosci. Rep. 2023, 15, S889. [Google Scholar] [CrossRef]
- Liu, M.; Xu, G.; Yu, H.; Wang, C.; Sun, C.; Guo, L. Effects of transcranial direct current stimulation on EEG power and brain functional network in stroke patients. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 31, 335–345. [Google Scholar] [CrossRef]
- Fabio, R.A.; Suriano, R.; Gangemi, A. Effects of Transcranial Direct Current Stimulation on Potential P300-Related Events and Alpha and Beta EEG Band Rhythms in Parkinson’s Disease. J. Integr. Neurosci. 2024, 23, 25. [Google Scholar] [CrossRef]
- Chan, M.M.; Choi, C.X.; Tsoi, T.C.; Shea, C.K.; Yiu, K.W.; Han, Y.M. Effects of multisession cathodal transcranial direct current stimulation with cognitive training on sociocognitive functioning and brain dynamics in autism: A double-blind, sham-controlled, randomized EEG study. Brain Stimul. 2023, 16, 1604–1616. [Google Scholar] [CrossRef] [PubMed]
- Murphy, O.; Hoy, K.; Wong, D.; Bailey, N.; Fitzgerald, P.; Segrave, R. Effects of transcranial direct current stimulation and transcranial random noise stimulation on working memory and task-related EEG in major depressive disorder. Brain Cogn. 2023, 173, 106105. [Google Scholar] [CrossRef]
- Wang, X.; Ouyang, J.; Kang, A.; Wang, L.; Zhang, J.; Yan, T.; Zhang, J.; Yan, Z. Gamma tACS Reshapes Low-Dimensional Trajectories of Brain Activity in Working Memory. In Proceedings of the 2023 17th International Conference on Complex Medical Engineering (CME), Suzhou, China, 3–5 November 2023; pp. 98–102. [Google Scholar]
- Wang, Y.; Liu, W.; Wang, Y.; Ouyang, G.; Guo, Y. Long-term HD-tDCS modulates dynamic changes of brain activity on patients with disorders of consciousness: A resting-state EEG study. Comput. Biol. Med. 2024, 170, 108084. [Google Scholar] [CrossRef]
- Tarantino, V.; Fontana, M.L.; Buttà, A.; Ficile, S.; Oliveri, M.; Mandalà, G.; Smirni, D. Increase in EEG Alpha-to-theta Ratio After transcranial Direct Current Stimulation (tDCS) in Patients with Disorders of Consciousness: A Pilot Study. NeuroRehabilitation 2024, 55, 440–447. [Google Scholar] [CrossRef]
- Vimolratana, O.; Aneksan, B.; Siripornpanich, V.; Hiengkaew, V.; Prathum, T.; Jeungprasopsuk, W.; Khaokhiew, T.; Vachalathiti, R.; Klomjai, W. Effects of anodal tDCS on resting state eeg power and motor function in acute stroke: A randomized controlled trial. J. Neuroeng. Rehabil. 2024, 21, 6. [Google Scholar] [CrossRef]
- Singh, V.; Verma, R.; Shriyam, S.; Gandhi, T.K. Evaluating tDCS Intervention Effectiveness via Functional Connectivity Network on Resting-State EEG Data in Major Depressive Disorder. arXiv 2024, arXiv:2411.06359. [Google Scholar]
- Couto, T.A.; Gao, F.; Lak, D.C.; Yuan, Z. Combined EEG-tDCS approach in resting state to reduce comorbid anxiety and depressive symptoms in affective disorders: A sham-controlled pilot study. IBRO Neurosci. Rep. 2024, 16, 571–581. [Google Scholar] [CrossRef]
- Liu, C.L.; Su, K.H.; Horng, Y.S.; Chen, C.L.; Huang, S.H.; Wu, C.Y. Theory-driven EEG indexes for tracking motor recovery and predicting the effects of hybridizing tDCS with mirror therapy in stroke patients. IEEE Trans. Neural Syst. Rehabil. Eng. 2024, 32, 4042–4051. [Google Scholar] [CrossRef]
- Wynn, S.C.; Marshall, T.R.; Nyhus, E. Utilizing tACS to enhance memory confidence and EEG to predict individual differences in brain stimulation efficacy. Imaging Neurosci. 2025, 3, imag_a_00429. [Google Scholar] [CrossRef]
- Yeh, T.C.; Lin, Y.Y.; Tzeng, N.S.; Kao, Y.C.; Chung, Y.A.; Chang, C.C.; Fang, H.W.; Chang, H.A. Effects of online high-definition transcranial direct current stimulation over left dorsolateral prefrontal cortex on predominant negative symptoms and EEG functional connectivity in patients with schizophrenia: A randomized, double-blind, controlled trial. Psychiatry Clin. Neurosci. 2025, 79, 2–11. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhai, H.; Wei, H. Acute Effects of Transcranial Direct Current Stimulation Combined with High-Load Resistance Exercises on Repetitive Vertical Jump Performance and EEG Characteristics in Healthy Men. Life 2024, 14, 1106. [Google Scholar] [CrossRef]
- Zhang, S.; Cui, H.; Li, Y.; Chen, X.; Gao, X.; Guan, C. Improving SSVEP-BCI performance through repetitive anodal tDCS-based neuromodulation: Insights from fractal EEG and brain functional connectivity. IEEE Trans. Neural Syst. Rehabil. Eng. 2024, 32, 1647–1656. [Google Scholar] [CrossRef]
- Xiao, W.; Moncy, J.C.; Ghazi-Noori, A.R.; Woodham, R.D.; Rezaei, H.; Bramon, E.; Ritter, P.; Bauer, M.; Young, A.H.; Fu, C.H. Enhanced network synchronization connectivity following transcranial direct current stimulation (tDCS) in bipolar depression: Effects on EEG oscillations and deep learning-based predictors of clinical remission. J. Affect. Disord. 2025, 369, 576–587. [Google Scholar] [CrossRef]
- Rocha, K.; Marinho, V.; Magalhães, F.; Carvalho, V.; Fernandes, T.; Ayres, M.; Crespo, E.; Velasques, B.; Ribeiro, P.; Cagy, M.; et al. Unskilled shooters improve both accuracy and grouping shot having as reference skilled shooters cortical area: An EEG and tDCS study. Physiol. Behav. 2020, 224, 113036. [Google Scholar] [CrossRef]
- Aktürk, T.; de Graaf, T.A.; Güntekin, B.; Hanoğlu, L.; Sack, A.T. Enhancing memory capacity by experimentally slowing theta frequency oscillations using combined EEG-tACS. Sci. Rep. 2022, 12, 14199. [Google Scholar] [CrossRef]
- Faria, P.; Fregni, F.; Sebastião, F.; Dias, A.I.; Leal, A. Feasibility of focal transcranial DC polarization with simultaneous EEG recording: Preliminary assessment in healthy subjects and human epilepsy. Epilepsy Behav. 2012, 25, 417–425. [Google Scholar] [CrossRef]
- Auvichayapat, N.; Rotenberg, A.; Gersner, R.; Ngodklang, S.; Tiamkao, S.; Tassaneeyakul, W.; Auvichayapat, P. Transcranial direct current stimulation for treatment of refractory childhood focal epilepsy. Brain Stimul. 2013, 6, 696–700. [Google Scholar] [CrossRef]
- Lin, L.C.; Ouyang, C.S.; Chiang, C.T.; Yang, R.C.; Wu, R.C.; Wu, H.C. Cumulative effect of transcranial direct current stimulation in patients with partial refractory epilepsy and its association with phase lag index-A preliminary study. Epilepsy Behav. 2018, 84, 142–147. [Google Scholar] [CrossRef]
- Tecchio, F.; Cottone, C.; Porcaro, C.; Cancelli, A.; Di Lazzaro, V.; Assenza, G. Brain functional connectivity changes after transcranial direct current stimulation in epileptic patients. Front. Neural Circuits 2018, 12, 44. [Google Scholar] [CrossRef]
- Dallmer-Zerbe, I.; Popp, F.; Lam, A.P.; Philipsen, A.; Herrmann, C.S. Transcranial alternating current stimulation (tACS) as a tool to modulate P300 amplitude in attention deficit hyperactivity disorder (ADHD): Preliminary findings. Brain Topogr. 2020, 33, 191–207. [Google Scholar] [CrossRef] [PubMed]
- Zaehle, T.; Rach, S.; Herrmann, C.S. Transcranial alternating current stimulation enhances individual alpha activity in human EEG. PLoS ONE 2010, 5, e13766. [Google Scholar] [CrossRef]
- Stecher, H.I.; Pollok, T.M.; Strüber, D.; Sobotka, F.; Herrmann, C.S. Ten minutes of α-tACS and ambient illumination independently modulate EEG α-power. Front. Hum. Neurosci. 2017, 11, 257. [Google Scholar] [CrossRef] [PubMed]
- Khayyer, Z.; Ngaosuvan, L.; Sikström, S.; Ghaderi, A.H. Transcranial direct current stimulation based on quantitative electroencephalogram combining positive psychotherapy for major depression. J. Integr. Neurosci. 2018, 17, 141–155. [Google Scholar] [CrossRef]
- Beatrice, P.D.K.; Guay, S.; Proulx-Bégin, L.; Masse, I.; Lina, J.M.; Carrier, J.; De Beaumont, L. Abstract# 129: Characterization of tACS parameters to optimize the increase of EEG alpha power. Brain Stimul. Basic Transl. Clin. Res. Neuromodulation 2019, 12, e44–e45. [Google Scholar]
- Del Felice, A.; Castiglia, L.; Formaggio, E.; Cattelan, M.; Scarpa, B.; Manganotti, P.; Tenconi, E.; Masiero, S. Personalized transcranial alternating current stimulation (tACS) and physical therapy to treat motor and cognitive symptoms in Parkinson’s disease: A randomized cross-over trial. NeuroImage Clin. 2019, 22, 101768. [Google Scholar] [CrossRef]
- Radecke, J.O.; Fiene, M.; Misselhorn, J.; Herrmann, C.S.; Engel, A.K.; Wolters, C.H.; Schneider, T.R. Personalized alpha-tACS targeting left posterior parietal cortex modulates visuo-spatial attention and posterior evoked EEG activity. Brain Stimul. 2023, 16, 1047–1061. [Google Scholar] [CrossRef]
- Góral-Półrola, J.; Kochańska, E.; Cielebąk, K.; Pąchalska, M. A NEW, NEUROMARKER-BASED, FORM OF COMBINED NEUROFEED-BACK EEG/TDCS TRAINING IN THE REDUCTION OF OCCUPATIONAL BURNOUT SYNDROME IN AN ANAESTHETIC NURSE WORKING WITH COVID-19 PATIENTS. Acta Neuropsychol. 2024, 22, 301–330. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, J.H.; Yun, S.; Yang, J.; Park, J.C.; Kwon, J.; Seo, J.; Min, B.K. Enhanced inhibitory control after out-of-phase theta tACS between the lDLPFC and dACC. In Proceedings of the 2024 12th International Winter Conference on Brain-Computer Interface (BCI), Gangwon, Republic of Korea, 26–28 February 2024; pp. 1–4. [Google Scholar] [CrossRef]
- Sarkis, R.A.; Kaur, N.; Camprodon, J.A. Transcranial direct current stimulation (tDCS): Modulation of executive function in health and disease. Curr. Behav. Neurosci. Rep. 2014, 1, 74–85. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000, 527, 633. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Nitsche, M.S.; Klein, C.C.; Tergau, F.; Rothwell, J.C.; Paulus, W. Level of action of cathodal DC polarisation induced inhibition of the human motor cortex. Clin. Neurophysiol. 2003, 114, 600–604. [Google Scholar] [CrossRef]
- White, L.K.; Makhoul, W.; Teferi, M.; Sheline, Y.I.; Balderston, N.L. The role of dlPFC laterality in the expression and regulation of anxiety. Neuropharmacology 2023, 224, 109355. [Google Scholar] [CrossRef]
- Pearson, H.J. Present and Accounted For: Sensory Stimulation and Parietal Neuroplasticity. J. EMDR Pract. Res. 2009, 3, 39–49. [Google Scholar] [CrossRef]
- Malkani, R.G.; Zee, P.C. Brain stimulation for improving sleep and memory. Sleep Med. Clin. 2020, 15, 101–115. [Google Scholar] [CrossRef]
- Noetscher, G.M.; Yanamadala, J.; Makarov, S.N.; Pascual-Leone, A. Comparison of cephalic and extracephalic montages for transcranial direct current stimulation—A numerical study. IEEE Trans. Biomed. Eng. 2014, 61, 2488–2498. [Google Scholar] [CrossRef] [PubMed]
- Göldi, M.; van Poppel, E.A.M.; Rasch, B.; Schreiner, T. Increased neuronal signatures of targeted memory reactivation during slow-wave up states. Sci. Rep. 2019, 9, 2715. [Google Scholar] [CrossRef]
- Boutin, A.; Doyon, J. A sleep spindle framework for motor memory consolidation. Philos. Trans. R. Soc. B 2020, 375, 20190232. [Google Scholar] [CrossRef]
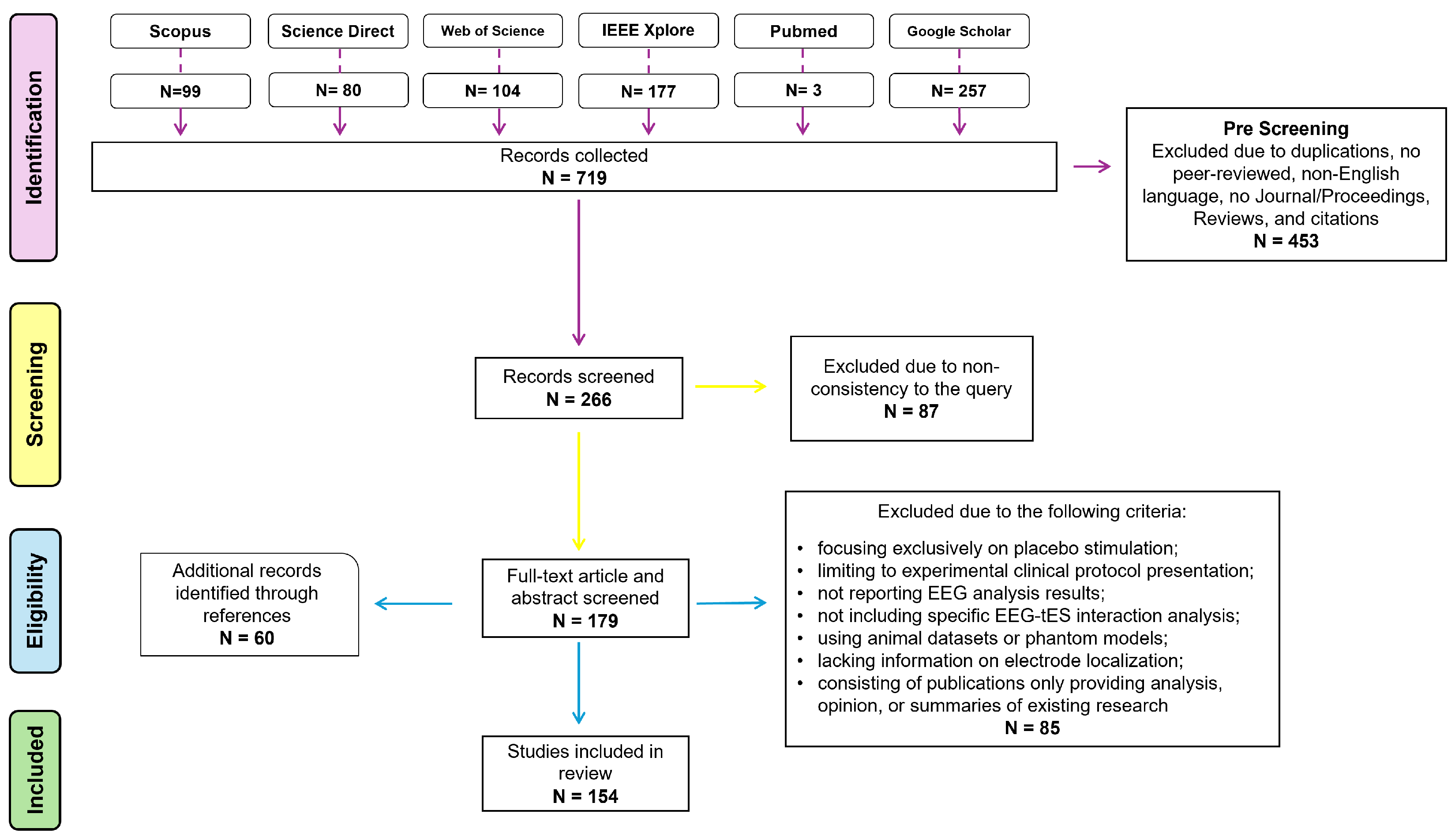
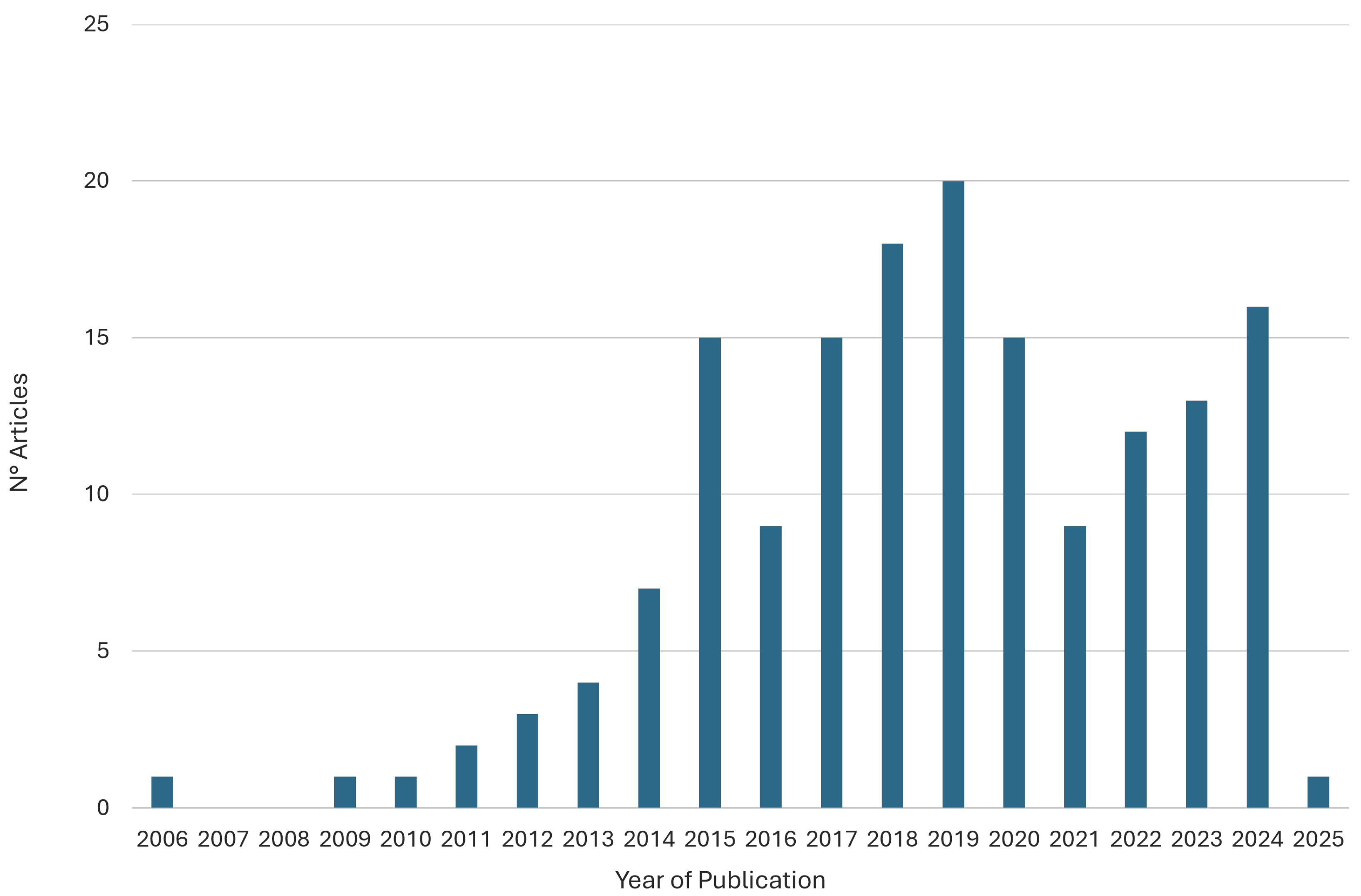

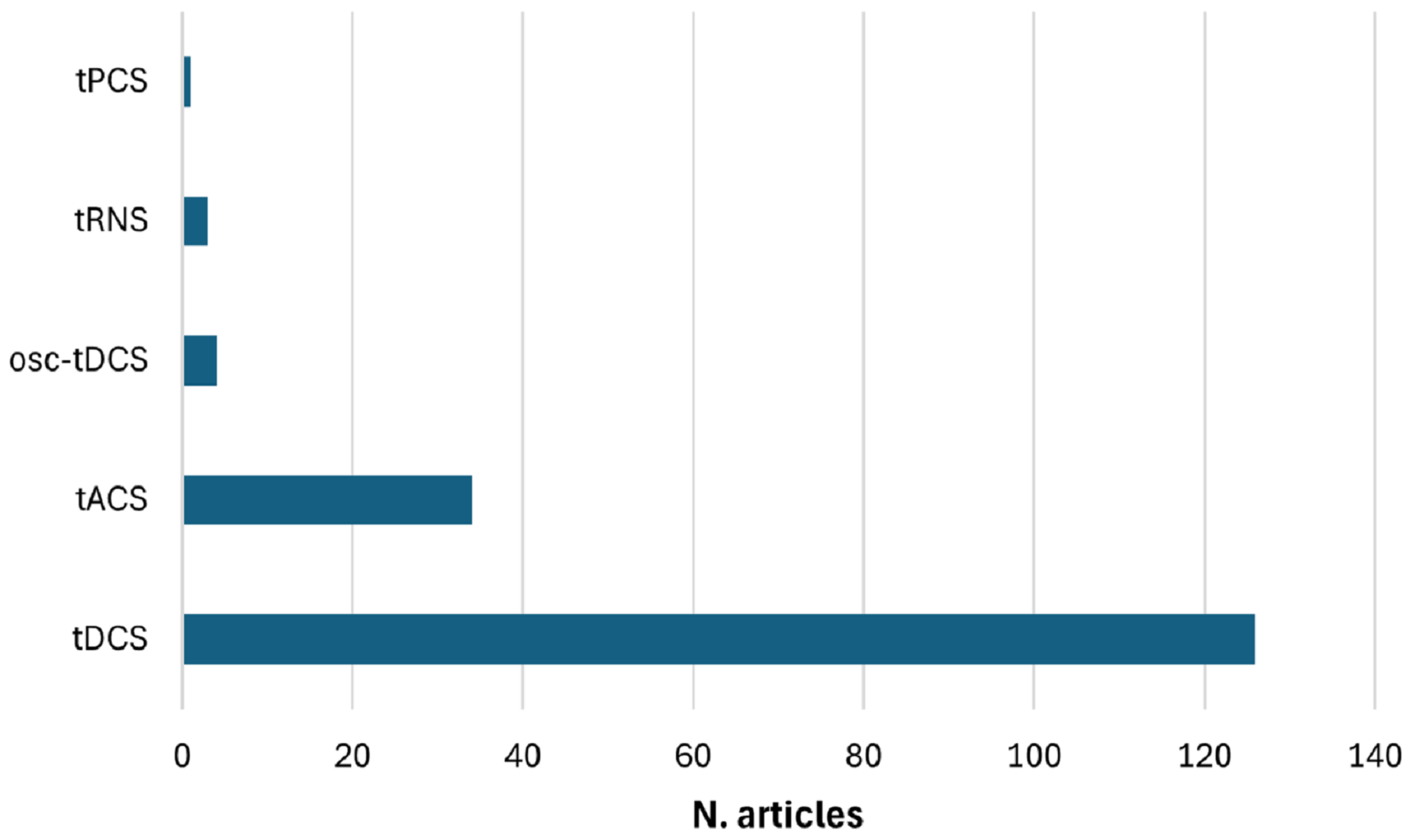
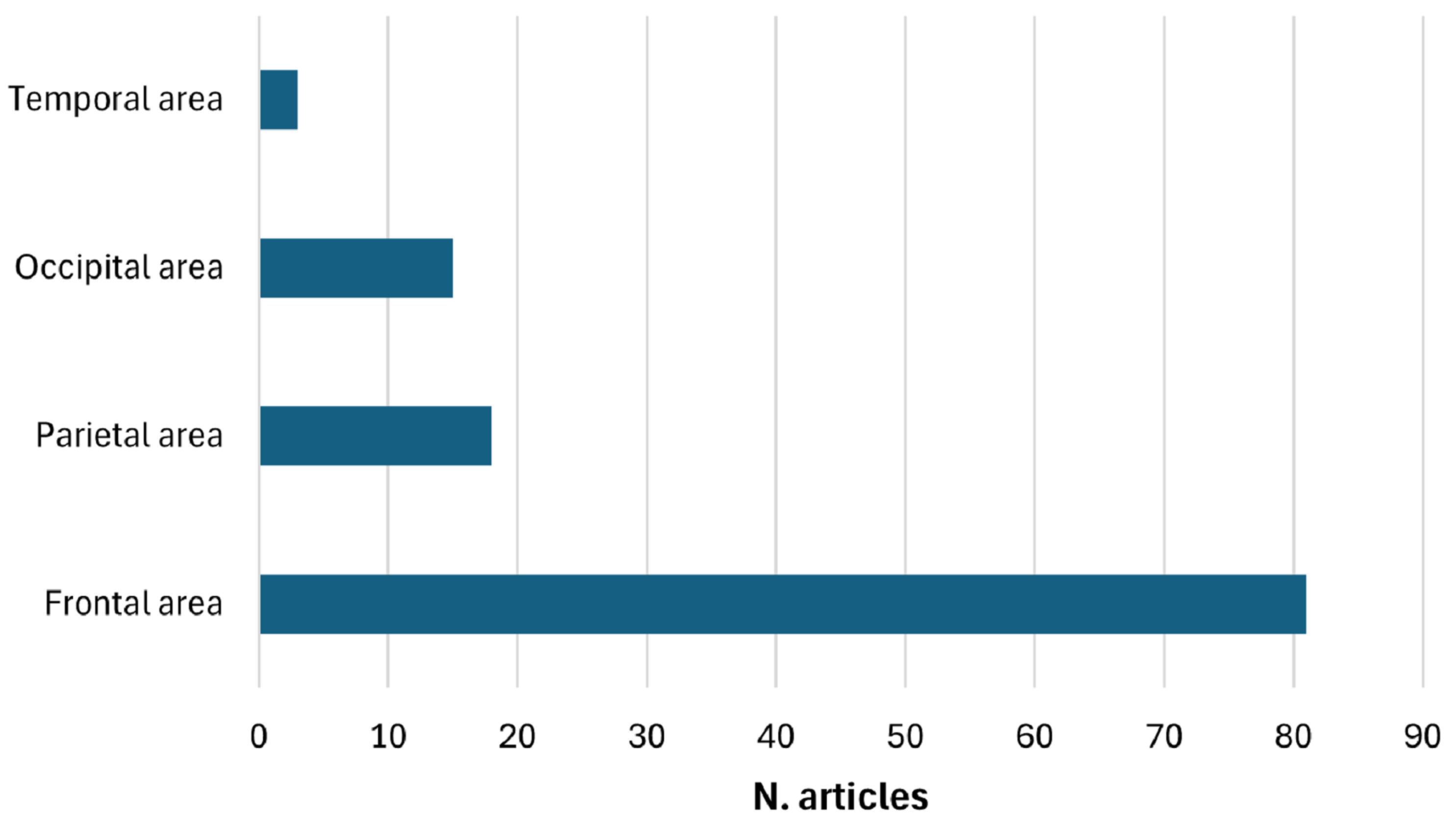

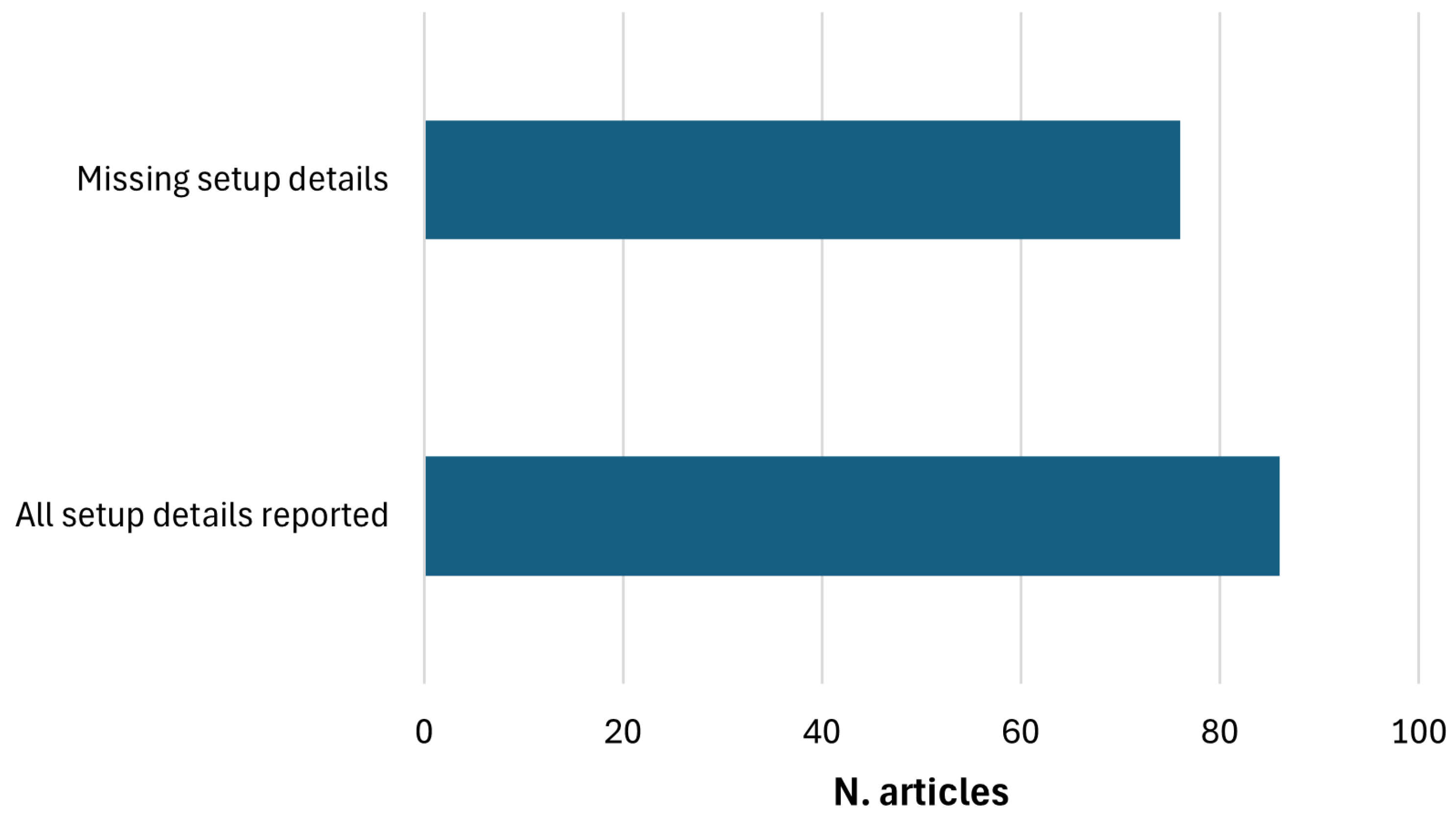
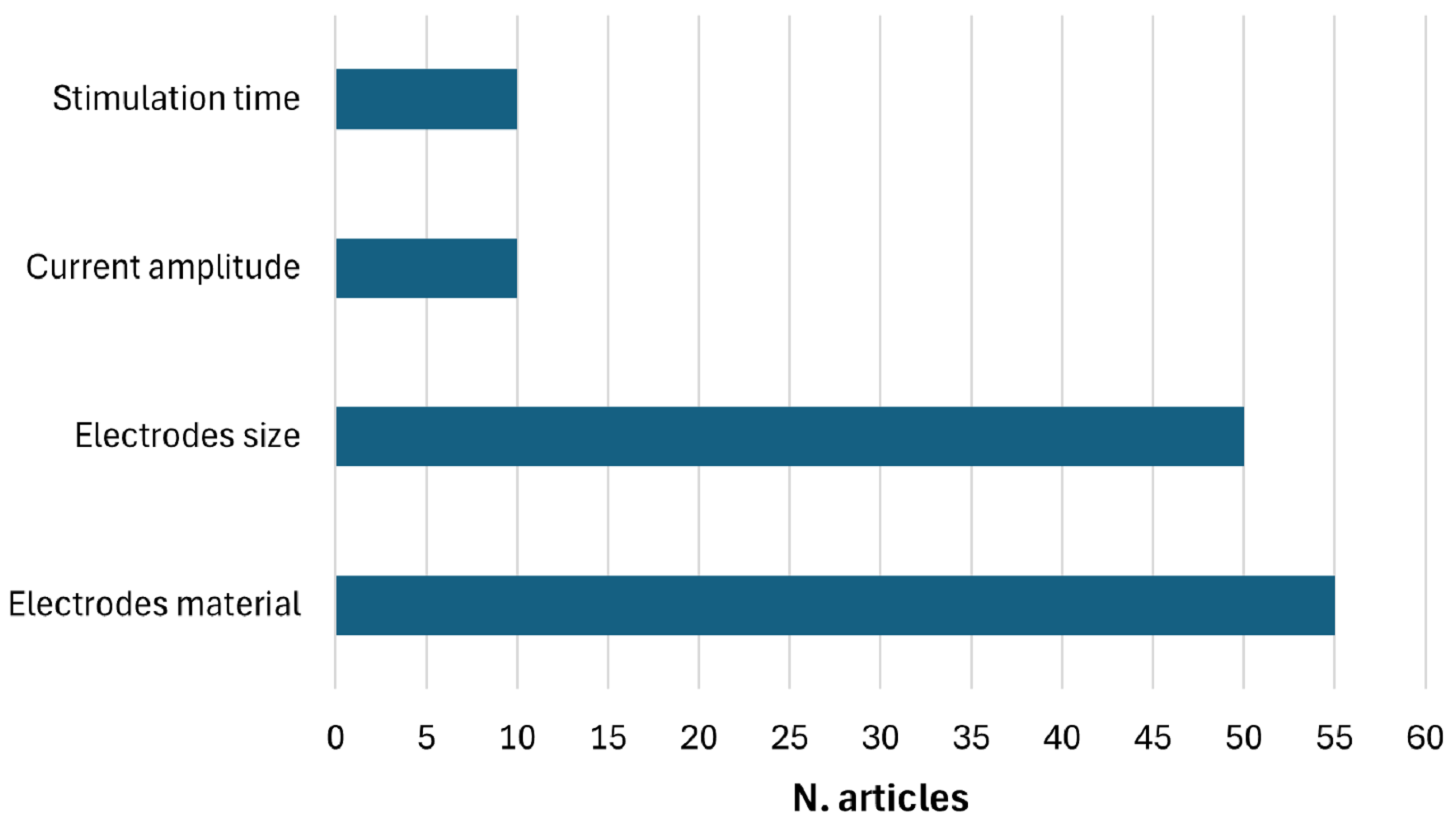
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arpaia, P.; Calce, A.D.; Di Marino, L.; Lorenzon, L.; Maffei, L.; Moccaldi, N.; Ramos, P.M. Combined Use of Electroencephalography and Transcranial Electrical Stimulation: A Systematic Review. Sensors 2025, 25, 5773. https://doi.org/10.3390/s25185773
Arpaia P, Calce AD, Di Marino L, Lorenzon L, Maffei L, Moccaldi N, Ramos PM. Combined Use of Electroencephalography and Transcranial Electrical Stimulation: A Systematic Review. Sensors. 2025; 25(18):5773. https://doi.org/10.3390/s25185773
Chicago/Turabian StyleArpaia, Pasquale, Anna Della Calce, Lucrezia Di Marino, Luciana Lorenzon, Luigi Maffei, Nicola Moccaldi, and Pedro M. Ramos. 2025. "Combined Use of Electroencephalography and Transcranial Electrical Stimulation: A Systematic Review" Sensors 25, no. 18: 5773. https://doi.org/10.3390/s25185773
APA StyleArpaia, P., Calce, A. D., Di Marino, L., Lorenzon, L., Maffei, L., Moccaldi, N., & Ramos, P. M. (2025). Combined Use of Electroencephalography and Transcranial Electrical Stimulation: A Systematic Review. Sensors, 25(18), 5773. https://doi.org/10.3390/s25185773









