A Comparative Study Between ECG- and PPG-Based Heart Rate Sensors for Heart Rate Variability Measurements: Influence of Body Position, Duration, Sex, and Age
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Apparatus
- Polar H10 Chest Strap
- Polar OH1 PPG Sensor
2.3. Study Design and Procedures
2.4. Data Analysis
3. Results
3.1. Participant Characteristics
| Overall Participants (n = 31) | ||
|---|---|---|
| Sex (number) | Female: 18—Male: 13 | |
| Age (years) | 43 ± 12 [21–66] | |
| Height (m) | 1.71 ± 0.09 [1.50–1.89] | |
| Body mass (kg) | 72 ± 16 [46–115] | |
| ≤40 years old (n = 14) | >40 years old (n = 17) | |
| Sex (number) | Female: 8—Male: 6 | Female: 10—Male: 7 |
| Age (years) | 33 ± 5 [21–40] | 52 ± 7 [41–66] |
| Height (m) | 1.73 ± 0.10 [1.59–1.89] | 1.69 ± 0.09 [1.50–1.85] |
| Body mass (kg) | 77 ± 19 [50–115] | 67 ± 13 [46–89] |
| Females (n = 18) | Males (n = 13) | |
| Age (years) | 44 ± 12 [23–66] | 43 ± 12 [21–63] |
| Height (m) | 1.65 ± 0.06 [1.50–1.75] | 1.78 ± 0.07 [1.64–1.89] |
| Body mass (kg) | 67 ± 18 [46–115] | 78 ± 12 [62–100] |
3.2. Impact of Sensor Type on HRV Parameters
3.3. Impact of Body Position on HRV Parameters
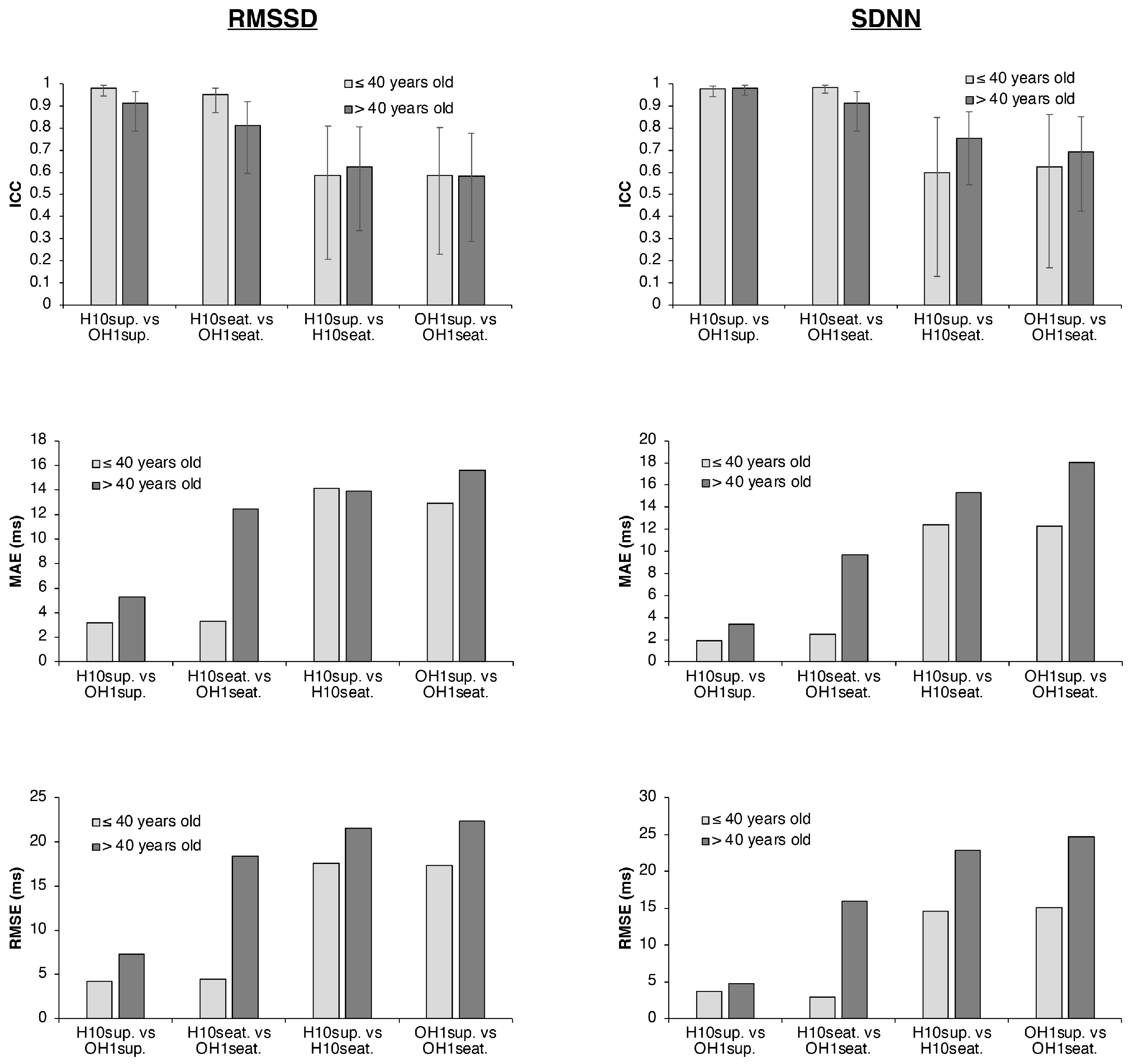
3.4. Influence of Age on HRV Parameters
3.5. Influence of Sex on HRV Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANS | Autonomic nervous system |
| ECG | Electrocardiography |
| HR | Heart rate |
| HRV | Heart rate variability |
| LoA | Limits of agreement |
| PAT | Pulse arrival time |
| PPG | Photoplethysmography |
| PPI | Peak-to-peak interval |
| PTT | Pulse transit time |
| PRV | Pulse rate variability |
| RMSSD | Root mean square of successive differences |
| SDNN | Standard deviation of normal-to-normal intervals |
References
- Rehman, R.Z.U.; Chatterjee, M.; Manyakov, N.V.; Daans, M.; Jackson, A.; O’Brisky, A.; Telesky, T.; Smets, S.; Berghmans, P.-J.; Yang, D.; et al. Assessment of Physiological Signals from Photoplethysmography Sensors Compared to an Electrocardiogram Sensor: A Validation Study in Daily Life. Sensors 2024, 24, 6826. [Google Scholar] [CrossRef]
- Gil, E.; Orini, M.; Bailón, R.; Vergara, J.M.; Mainardi, L.; Laguna, P. Photoplethysmography pulse rate variability as a surrogate measurement of heart rate variability during non-stationary conditions. Physiol. Meas. 2010, 31, 1271. [Google Scholar] [CrossRef]
- Plews, D.J.; Laursen, P.B.; Le Meur, Y.; Hausswirth, C.; Kilding, A.E.; Buchheit, M. Monitoring training with heart-rate variability: How much compliance is needed for valid assessment? Int. J. Sports Physiol. Perform. 2014, 9, 783–790. [Google Scholar] [CrossRef]
- Plews, D.J.; Laursen, P.B.; Stanley, J.; Kilding, A.E.; Buchheit, M. Training adaptation and heart rate variability in elite endurance athletes: Opening the door to effective monitoring. Sports Med. 2017, 47, 861–880. [Google Scholar] [CrossRef]
- Plews, D.J.; Scott, B.; Altini, M.; Wood, M.; Kilding, A.E.; Laursen, P.B. Comparison of heart-rate-variability recording with smartphone photoplethysmography, Polar H7 chest strap, and electrocardiography. Int. J. Sports Physiol. Perform. 2017, 12, 1324–1328. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, F.; Ginsberg, J.P. An overview of heart rate variability metrics and norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Armañac-Julián, P.; Kontaxis, S.; Lázaro, J.; Rapalis, A.; Brazaitis, M.; Marozas, V.; Laguna, P.; Bailón, R.; Gil, E. Vascular reactivity characterized by PPG-derived pulse wave velocity. Biomed. Signal Process. Control 2025, 105, 107641. [Google Scholar] [CrossRef]
- Tikhonova, I.V.; Grinevich, A.A.; Tankanag, A.V. Analysis of phase interactions between heart rate variability, respiration and peripheral microhemodynamics oscillations of upper and lower extremities in human. Biomed. Signal Process. Control 2022, 71, 103091. [Google Scholar] [CrossRef]
- Yuda, E.; Shibata, M.; Ogata, Y.; Ueda, N.; Yambe, T.; Yoshizawa, M.; Hayano, J. Pulse rate variability: A new biomarker, not a surrogate for heart rate variability. J. Physiol. Anthropol. 2020, 39, 21. [Google Scholar] [CrossRef] [PubMed]
- Constant, I.; Laude, D.; Murat, I.; Elghozi, J.L. Pulse rate variability is not a surrogate for heart rate variability. Clin. Sci. 1999, 97, 391–397. [Google Scholar] [CrossRef]
- Lu, G.; Yang, F.; Taylor, J.A.; Stein, J.F. A comparison of photoplethysmography and ECG recording to analyze heart rate variability in healthy subjects. J. Med. Eng. Technol. 2009, 33, 634–641. [Google Scholar] [CrossRef]
- Obata, Y.; Ong, Q.J.; Magruder, J.T.; Grichkevitch, H.; Berkowitz, D.E.; Nyhan, D.; Steppan, J.; Barodka, V. Noninvasive assessment of the effect of position and exercise on pulse arrival to peripheral vascular beds in healthy volunteers. Front. Physiol. 2017, 8, 47. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Task Force of the European Society of Cardiology. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Munoz, M.L.; Van Roon, A.; Riese, H.; Thio, C.; Oostenbroek, E.; Westrik, I.; de Geus, E.J.C.; Gansevoort, R.; Lefrandt, J.; Nolte, I.M.; et al. Validity of (ultra-) short recordings for heart rate variability measurements. PLoS ONE 2015, 10, e0138921. [Google Scholar] [CrossRef]
- Buchheit, M. Monitoring training status with HRV measures: Do all roads lead to Rome? Front. Physiol. 2014, 5, 73. [Google Scholar] [CrossRef] [PubMed]
- Bent, B.; Goldstein, B.A.; Kibbe, W.A.; Dunn, J.P. Investigating sources of inaccuracy in wearable optical heart rate sensors. NPJ Digit. Med. 2020, 3, 18. [Google Scholar] [CrossRef]
- Nunan, D.; Sandercock, G.R.H.; Brodie, D.A. A quantitative systematic review of normal values for short-term heart rate variability in healthy adults. Pacing Clin. Electrophysiol. 2010, 33, 1407–1417. [Google Scholar] [CrossRef]
- Almeida-Santos, M.A.; Barreto-Filho, J.A.; Oliveira, J.L.M.; Reis, F.P.; da Cunha Oliveira, C.C.; Sousa, A.C.S. Aging, heart rate variability and patterns of autonomic regulation of the heart. Arch. Gerontol. Geriatr. 2016, 63, 1–8. [Google Scholar] [CrossRef]
- Bonnemeier, H.; Wiegand, U.K.; Brandes, A.; Kluge, N.; Katus, H.A.; Richardt, G.; Potratz, J. Circadian profile of cardiac autonomic nervous modulation in healthy subjects: Differing effects of aging and gender on heart rate variability. J. Cardiovasc. Electrophysiol. 2003, 14, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Koenig, J.; Thayer, J.F. Sex differences in healthy human heart rate variability: A meta-analysis. Neurosci. Biobehav. Rev. 2016, 64, 288–310. [Google Scholar] [CrossRef]
- Sista, A.; Ittermann, T.; Gross, S.; Markus, M.R.; Stone, K.; Stoner, L.; Friedrich, N.; Dörr, M.; Bahls, M. Sex and resting heart rate influence the relation between arterial stiffness and cardiac structure and function–insights from the general population. J. Hum. Hypertens. 2025, 39, 254–261. [Google Scholar] [CrossRef]
- Koerber, D.; Khan, S.; Shamsheri, T.; Kirubarajan, A.; Mehta, S. Accuracy of heart rate measurement with wrist-worn wearable devices in various skin tones: A systematic review. J. Racial Ethn. Health Disparities 2023, 10, 2676–2684. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human participants. JAMA 2025, 333, 71–74. [Google Scholar] [CrossRef]
- Schaffarczyk, M.; Rogers, B.; Reer, R.; Gronwald, T. Validity of the polar H10 sensor for heart rate variability analysis during resting state and incremental exercise in recreational men and women. Sensors 2022, 22, 6536. [Google Scholar] [CrossRef] [PubMed]
- Hermand, E.; Cassirame, J.; Ennequin, G.; Hue, O. Validation of a photoplethysmographic heart rate monitor: Polar OH1. Int. J. Sports Med. 2019, 40, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, M. Arrhythmia classification in long-term data using relative RR intervals. In 2017 Computing in Cardiology (CinC); IEEE: New York, NY, USA, 2017; pp. 1–4. [Google Scholar] [CrossRef]
- Vollmer, M. HRVTool—An open-source MATLAB toolbox for analyzing heart rate variability. In 2019 Computing in Cardiology (CinC); IEEE: New York, NY, USA, 2019; pp. 1–4. [Google Scholar] [CrossRef]
- Natarajan, A.; Pantelopoulos, A.; Emir-Farinas, H.; Natarajan, P. Heart rate variability with photoplethysmography in 8 million individuals: A cross-sectional study. Lancet Digit. Health 2020, 2, e650–e657. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Ketelhut, S.; Wiklund, P.; Kostensalo, J.; Kolunsarka, I.; Hägglund, H.; Ahtiainen, J.P. Regular postexercise sauna bathing does not improve heart rate variability: A multi-arm randomized controlled trial. Physiol. Rep. 2025, 13, e70449. [Google Scholar] [CrossRef]
- Yuda, E.; Yamamoto, K.; Yoshida, Y.; Hayano, J. Differences in pulse rate variability with measurement site. J. Physiol. Anthropol. 2020, 39, 4. [Google Scholar] [CrossRef]
- Mejía-Mejía, E.; Kyriacou, P.A. Photoplethysmography-Based Pulse Rate Variability and Haemodynamic Changes in the Absence of Heart Rate Variability: An In-Vitro Study. Appl. Sci. 2022, 12, 7238. [Google Scholar] [CrossRef]
- Chen, X.; Chen, T.; Luo, F.; Li, J. Comparison of valley-to-valley and peak-to-peak intervals from photoplethysmographic signals to obtain heart rate variability in the sitting position. In Proceedings of the 2013 6th International Conference on Biomedical Engineering and Informatics, Hangzhou, China, 16–18 December 2013; IEEE: New York, NY, USA, 2013; pp. 214–218. [Google Scholar] [CrossRef]
- Voss, A.; Schroeder, R.; Heitmann, A.; Peters, A.; Perz, S. Short-term heart rate variability—Influence of gender and age in healthy subjects. PLoS ONE 2022, 10, e0118308. [Google Scholar] [CrossRef] [PubMed]


| Variables | Conditions (1 vs. 2) | Mean ± SD (1 vs. 2) | ICC (95% CI) | MAE | RMSE | Mean Diff. | Lower LoA | Upper LoA |
|---|---|---|---|---|---|---|---|---|
| RMSSD 5 min (ms) | H10sup. vs. OH1sup. | 37 ± 21 vs. 41 ± 20 | 0.955 (0.911–0.978) | 4.32 | 6.09 | −3.21 | −13.51 | 7.09 |
| H10seat. vs. OH1seat. | 36 ± 25 vs. 44 ± 23 | 0.834 (0.699–0.912) | 8.33 | 13.92 | −8.05 | −30.69 | 14.59 | |
| H10sup. vs. H10seat. | 37 ± 21 vs. 36 ± 25 | 0.608 (0.338–0.786) | 13.99 | 19.85 | 1.22 | −38.25 | 40.69 | |
| OH1sup. vs. OH1seat. | 41 ± 20 vs. 44 ± 23 | 0.560 (0.270–0.756) | 14.38 | 20.23 | −3.61 | −43.28 | 36.05 | |
| RMSSD 2 min (ms) | H10sup. vs. OH1sup. | 36 ± 18 vs. 39 ± 21 | 0.869 (0.757–0.931) | 5.64 | 9.98 | −2.91 | −21.93 | 16.12 |
| H10seat. vs. OH1seat. | 34 ± 22 vs. 41 ± 20 | 0.868 (0.756–0.931) | 6.89 | 10.72 | −6.14 | −23.65 | 11.37 | |
| H10sup. vs. H10seat. | 36 ± 18 vs. 34 ± 22 | 0.482 (0.169–0.707) | 13.12 | 19.90 | 1.35 | −38.19 | 40.88 | |
| OH1sup. vs. OH1seat. | 39 ± 21 vs. 41 ± 20 | 0.468 (0.145–0.701) | 13.46 | 20.95 | −1.89 | −43.47 | 39.69 | |
| SDNN 5 min (ms) | H10sup. vs. OH1sup. | 51 ± 22 vs. 54 ± 22 | 0.980 (0.959–0.990) | 2.72 | 4.32 | −2.11 | −9.62 | 5.40 |
| H10seat. vs. OH1seat. | 54 ± 31 vs. 60 ± 29 | 0.921 (0.848–0.960) | 6.43 | 11.96 | −6.02 | −26.60 | 14.57 | |
| H10sup. vs. H10seat. | 51 ± 22 vs. 54 ± 31 | 0.728 (0.545–0.845) | 14.00 | 19.54 | −2.02 | −40.75 | 36.72 | |
| OH1sup. vs. OH1seat. | 54 ± 22 vs. 60 ± 29 | 0.674 (0.454–0.817) | 15.44 | 20.90 | −5.92 | −45.86 | 34.02 | |
| SDNN 2 min (ms) | H10sup. vs. OH1sup. | 45 ± 21 vs. 48 ± 22 | 0.929 (0.861–0.964) | 4.89 | 7.96 | −2.63 | −17.60 | 12.34 |
| H10seat. vs. OH1seat. | 51 ± 31 vs. 56 ± 29 | 0.916 (0.833–0.957) | 6.77 | 12.28 | −5.23 | −27.36 | 16.90 | |
| H10sup. vs. H10seat. | 45 ± 21 vs. 51 ± 31 | 0.621 (0.392–0.778) | 13.79 | 22.56 | −5.32 | −48.99 | 38.35 | |
| OH1sup. vs. OH1seat. | 48 ± 22 vs. 56 ± 29 | 0.507 (0.223–0.712) | 17.04 | 25.92 | −7.92 | −57.09 | 41.25 |
| Variables | Conditions (1 vs. 2) | Mean ± SD (1 vs. 2) | ICC (95% CI) | MAE | RMSE | Mean Diff. | Lower LoA | Upper LoA |
|---|---|---|---|---|---|---|---|---|
| RMSSD ≤40 years old (ms) | H10sup. vs. OH1sup. | 42 ± 21 vs. 44 ± 23 | 0.981 (0.944–0.994) | 3.17 | 4.22 | −1.94 | −9.89 | 6.01 |
| H10seat. vs. OH1seat. | 35 ± 16 vs. 38 ± 15 | 0.951 (0.870–0.982) | 3.31 | 4.47 | −3.31 | −9.91 | 3.29 | |
| H10sup. vs. H10seat. | 42 ± 21 vs. 35 ± 16 | 0.584 (0.207–0.810) | 14.11 | 17.60 | 7.28 | −22.39 | 36.95 | |
| OH1sup. vs. OH1seat. | 44 ± 23 vs. 38 ± 15 | 0.585 (0.228–0.804) | 12.93 | 17.35 | 5.91 | −25.16 | 36.99 | |
| RMSSD >40 years old (ms) | H10sup. vs. OH1sup. | 31 ± 18 vs. 36 ± 17 | 0.912 (0.787–0.965) | 5.27 | 7.27 | −4.47 | −16.06 | 7.12 |
| H10seat. vs. OH1seat. | 36 ± 31 vs. 49 ± 28 | 0.812 (0.594–0.919) | 12.45 | 18.36 | −12.08 | −40.01 | 15.85 | |
| H10sup. vs. H10seat. | 31 ± 18 vs. 36 ± 31 | 0.623 (0.335–0.805) | 13.89 | 21.52 | −5.11 | −47.34 | 37.13 | |
| OH1sup. vs. OH1seat. | 36 ± 17 vs. 49 ± 28 | 0.581 (0.286–0.775) | 15.57 | 22.33 | −12.72 | −49.80 | 24.37 | |
| SDNN ≤40 years old (ms) | H10sup. vs. OH1sup. | 55 ± 17 vs. 56 ± 19 | 0.977 (0.941–0.991) | 1.89 | 3.68 | −1.08 | −8.55 | 6.39 |
| H10seat. vs. OH1seat. | 53 ± 17 vs. 55 ± 18 | 0.984 (0.957–0.994) | 2.46 | 2.96 | −2.46 | −6.22 | 1.30 | |
| H10sup. vs. H10seat. | 55 ± 17 vs. 53 ± 17 | 0.598 (0.129–0.849) | 12.43 | 14.57 | 1.97 | −28.46 | 32.39 | |
| OH1sup. vs. OH1seat. | 56 ± 19 vs. 55 ± 18 | 0.624 (0.167–0.860) | 12.26 | 15.08 | 0.58 | −31.17 | 32.33 | |
| SDNN >40 years old (ms) | H10sup. vs. OH1sup. | 48 ± 25 vs. 51 ± 25 | 0.980 (0.949–0.992) | 3.40 | 4.79 | −3.01 | −10.53 | 4.52 |
| H10seat. vs. OH1seat. | 53 ± 40 vs. 62 ± 37 | 0.912 (0.787–0.965) | 9.70 | 15.92 | −9.07 | −35.50 | 17.36 | |
| H10sup. vs. H10seat. | 48 ± 25 vs. 53 ± 40 | 0.754 (0.542–0.875) | 15.31 | 22.84 | −5.50 | −50.30 | 39.30 | |
| OH1sup. vs. OH1seat. | 51 ± 25 vs. 62 ± 37 | 0.693 (0.423–0.850) | 18.05 | 24.69 | −11.57 | −55.63 | 32.49 |
| Variables | Conditions (1 vs. 2) | Mean ± SD (1 vs. 2) | ICC (95% CI) | MAE | RMSE | Mean Diff. | Lower LoA | Upper LoA |
|---|---|---|---|---|---|---|---|---|
| RMSSD Female (ms) | H10sup. vs. OH1sup. | 29 ± 13 vs. 33 ± 11 | 0.851 (0.678–0.934) | 4.66 | 6.41 | −3.76 | −14.24 | 6.72 |
| H10seat. vs. OH1seat. | 29 ± 12 vs. 37 ± 10 | 0.521 (0.196–0.743) | 8.30 | 11.93 | −8.27 | −25.62 | 9.07 | |
| H10sup. vs. H10seat. | 29 ± 13 vs. 29 ± 12 | 0.450 (−0.002–0.750) | 9.17 | 12.49 | 0.59 | −24.57 | 25.75 | |
| OH1sup. vs. OH1seat. | 33 ± 11 vs. 37 ± 10 | 0.251 (−0.198–0.613) | 9.49 | 12.90 | −3.92 | −28.70 | 20.86 | |
| RMSSD Male (ms) | H10sup. vs. OH1sup. | 48 ± 25 vs. 51 ± 26 | 0.974 (0.921–0.991) | 3.86 | 5.60 | −2.46 | −12.73 | 7.82 |
| H10seat. vs. OH1seat. | 46 ± 34 vs. 54 ± 32 | 0.870 (0.646–0.956) | 8.36 | 16.28 | −7.74 | −36.96 | 21.49 | |
| H10sup. vs. H10seat. | 48 ± 25 vs. 46 ± 34 | 0.555 (0.076–0.826) | 20.66 | 26.89 | 2.09 | −52.61 | 56.79 | |
| OH1sup. vs. OH1seat. | 51 ± 26 vs. 54 ± 32 | 0.524 (0.010–0.819) | 21.15 | 27.31 | −3.19 | −58.53 | 52.15 | |
| SDNN Female (ms) | H10sup. vs. OH1sup. | 41 ± 11 vs. 43 ± 11 | 0.939 (0.851–0.976) | 2.49 | 3.86 | −2.13 | −8.62 | 4.35 |
| H10seat. vs. OH1seat. | 42 ± 12 vs. 50 ± 13 | 0.513 (0.145–0.757) | 7.19 | 13.09 | −7.07 | −29.28 | 15.14 | |
| H10sup. vs. H10seat. | 41 ± 11 vs. 42 ± 12 | 0.317 (−0.157–0.672) | 10.22 | 13.28 | −1.33 | −27.98 | 25.31 | |
| OH1sup. vs. OH1seat. | 43 ± 11 vs. 50 ± 13 | −0.031 (−0.424–0.373) | 14.61 | 18.28 | −6.28 | −40.90 | 28.35 | |
| SDNN Male (ms) | H10sup. vs OH1sup. | 66 ± 25 vs. 68 ± 26 | 0.980 (0.937–0.993) | 3.03 | 4.89 | −2.09 | −11.11 | 6.93 |
| H10seat. vs. OH1seat. | 69 ± 42 vs. 73 ± 39 | 0.967 (0.902–0.989) | 5.37 | 10.19 | −4.55 | −23.15 | 14.04 | |
| H10sup. vs. H10seat. | 66 ± 25 vs. 69 ± 42 | 0.702 (0.419–0.860) | 19.26 | 25.82 | −2.96 | −55.29 | 49.36 | |
| OH1sup. vs. OH1seat. | 68 ± 26 vs. 73 ± 39 | 0.720 (0.409–0.881) | 16.58 | 24.07 | −5.43 | −53.26 | 42.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coste, A.; Millour, G.; Hausswirth, C. A Comparative Study Between ECG- and PPG-Based Heart Rate Sensors for Heart Rate Variability Measurements: Influence of Body Position, Duration, Sex, and Age. Sensors 2025, 25, 5745. https://doi.org/10.3390/s25185745
Coste A, Millour G, Hausswirth C. A Comparative Study Between ECG- and PPG-Based Heart Rate Sensors for Heart Rate Variability Measurements: Influence of Body Position, Duration, Sex, and Age. Sensors. 2025; 25(18):5745. https://doi.org/10.3390/s25185745
Chicago/Turabian StyleCoste, Alexandre, Geoffrey Millour, and Christophe Hausswirth. 2025. "A Comparative Study Between ECG- and PPG-Based Heart Rate Sensors for Heart Rate Variability Measurements: Influence of Body Position, Duration, Sex, and Age" Sensors 25, no. 18: 5745. https://doi.org/10.3390/s25185745
APA StyleCoste, A., Millour, G., & Hausswirth, C. (2025). A Comparative Study Between ECG- and PPG-Based Heart Rate Sensors for Heart Rate Variability Measurements: Influence of Body Position, Duration, Sex, and Age. Sensors, 25(18), 5745. https://doi.org/10.3390/s25185745








