Self-Sensing with Hollow Cylindrical Transducers for Histotripsy-Enhanced Aspiration Mechanical Thrombectomy Applications
Abstract
Highlights
- Miniature hollow cylindrical histotripsy transducers can detect intra-lumen cavitation without requiring external sensors.
- Voltage signals acquired across the transducer during and immediately after transmission provide insight into both the time and frequency domains.
- These self-sensing transducers offer a built-in method for cavitation monitoring, enabling feedback in therapeutic ultrasound applications such as histotripsy-mediated thrombectomy.
Abstract
1. Introduction
2. Materials and Methods
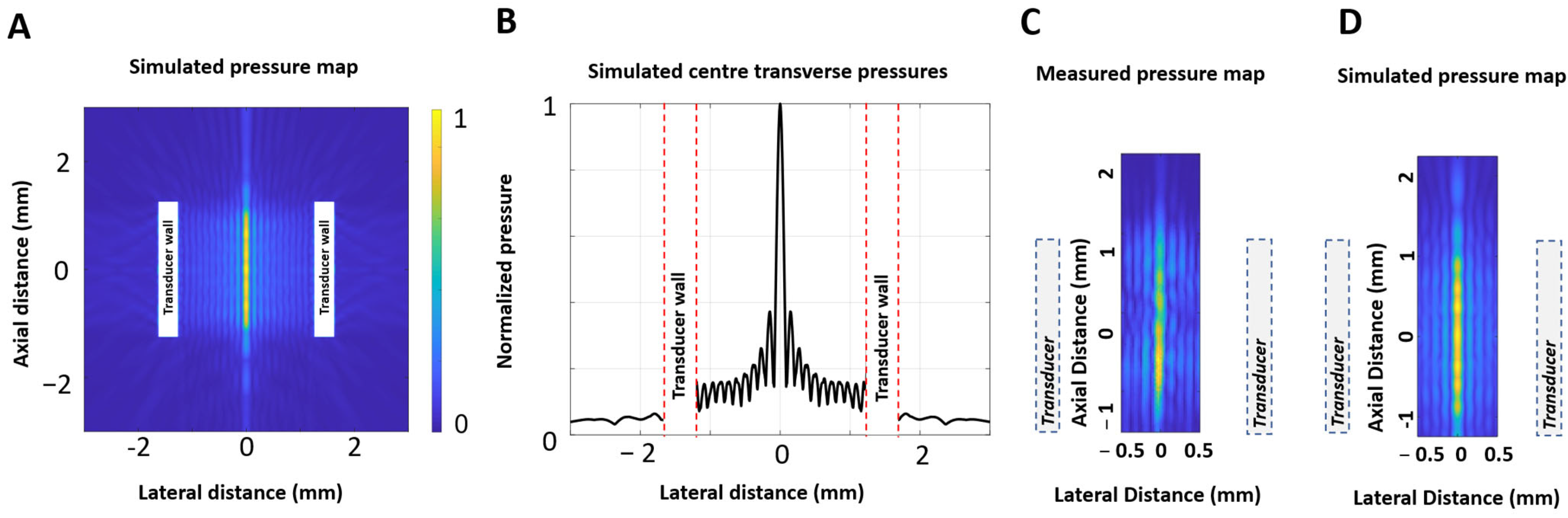
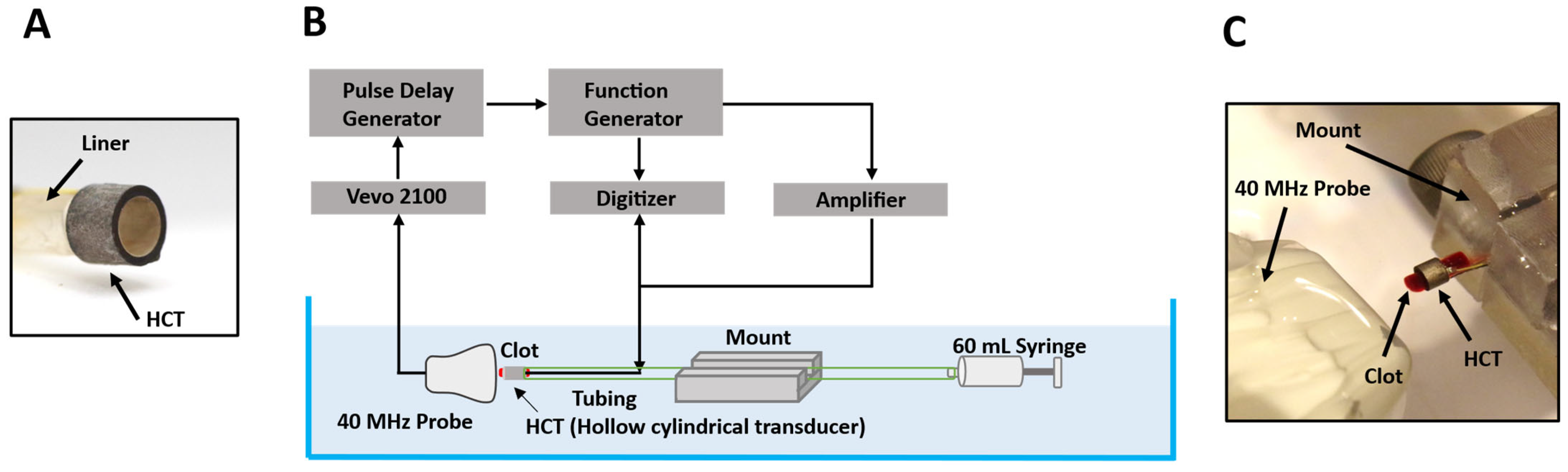
2.1. Transducer Configuration
2.2. Cavitation Observation with High-Speed Ultrasound Imaging
2.2.1. Experimental Configuration
2.2.2. Transmit Frequency Selections
2.2.3. Experimental Protocol
2.3. Cavitation Threshold
2.4. Self-Sensing Signals Analysis
2.4.1. Time Domain Analysis
2.4.2. Spectral Analysis
2.5. Blood Clot Preparation
3. Results
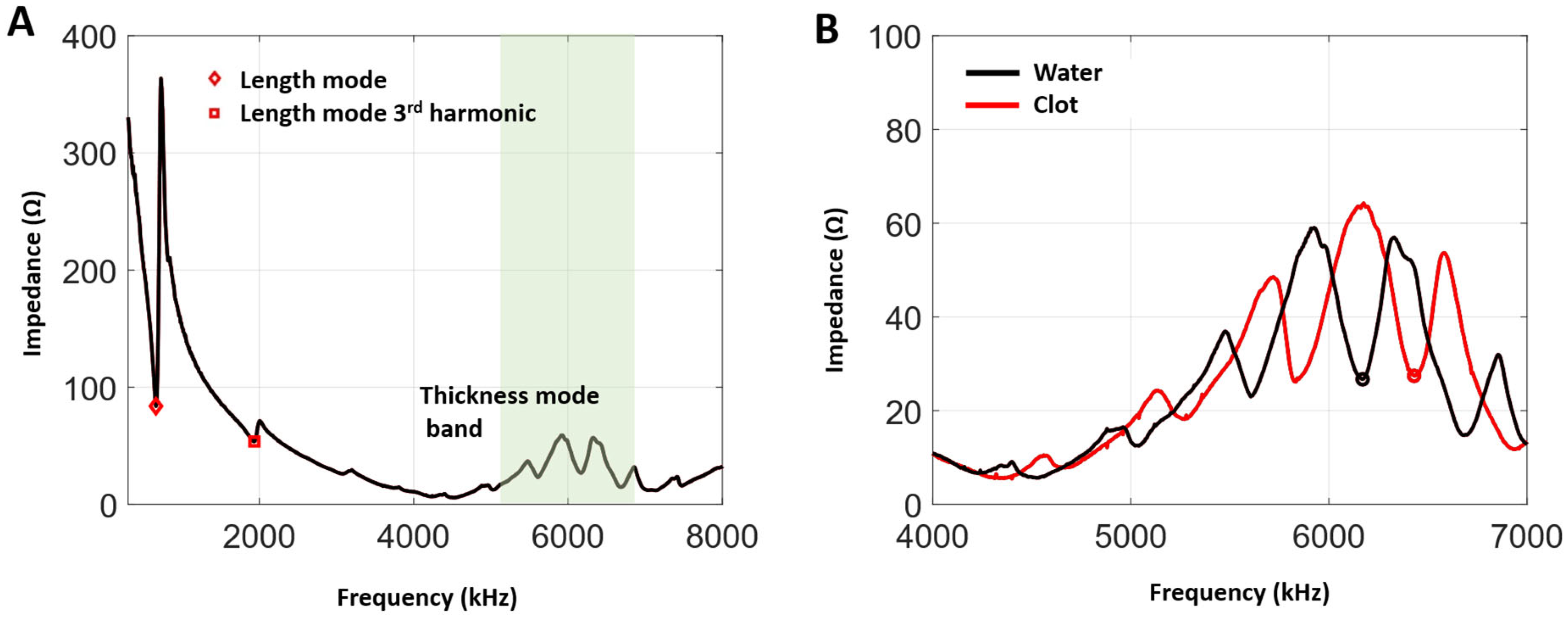
3.1. Impedance and Frequency Selection
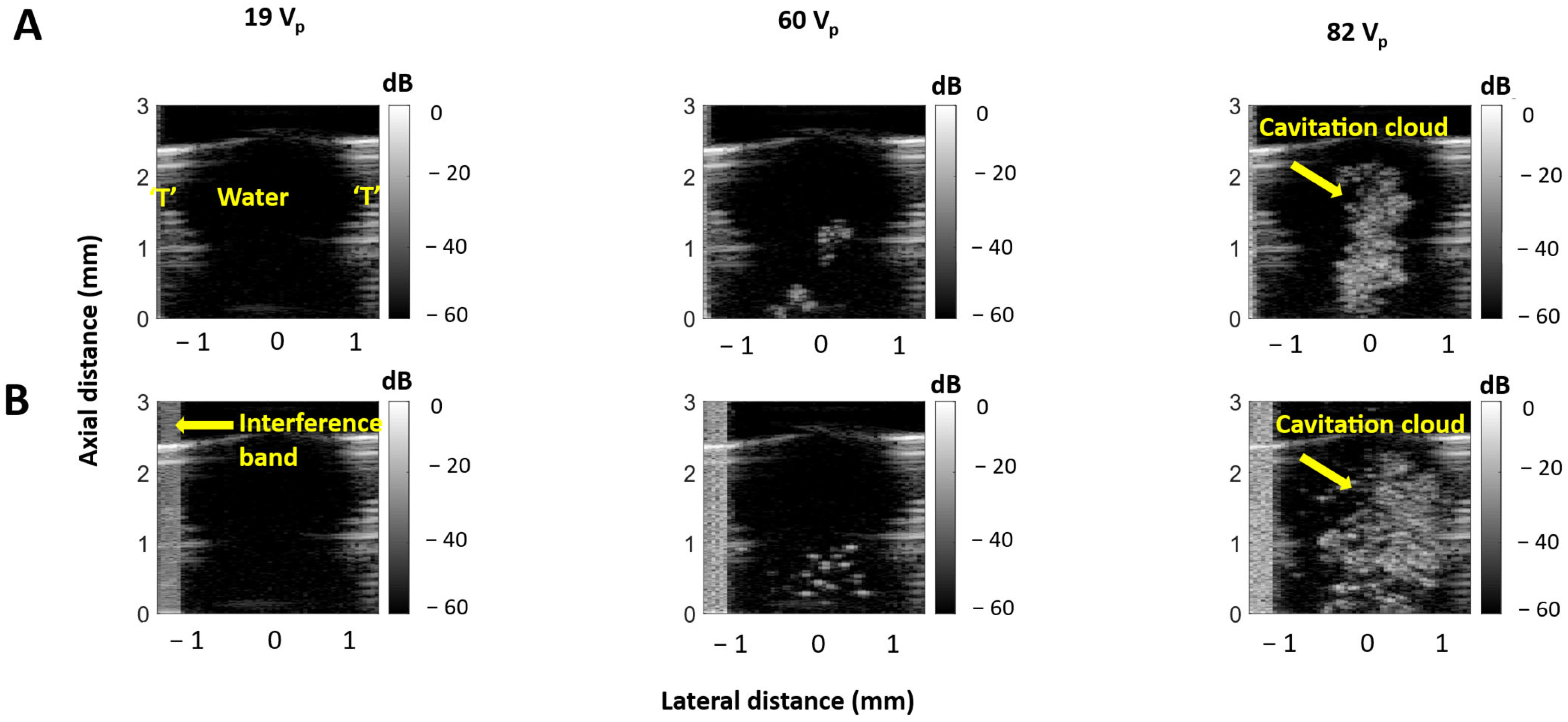
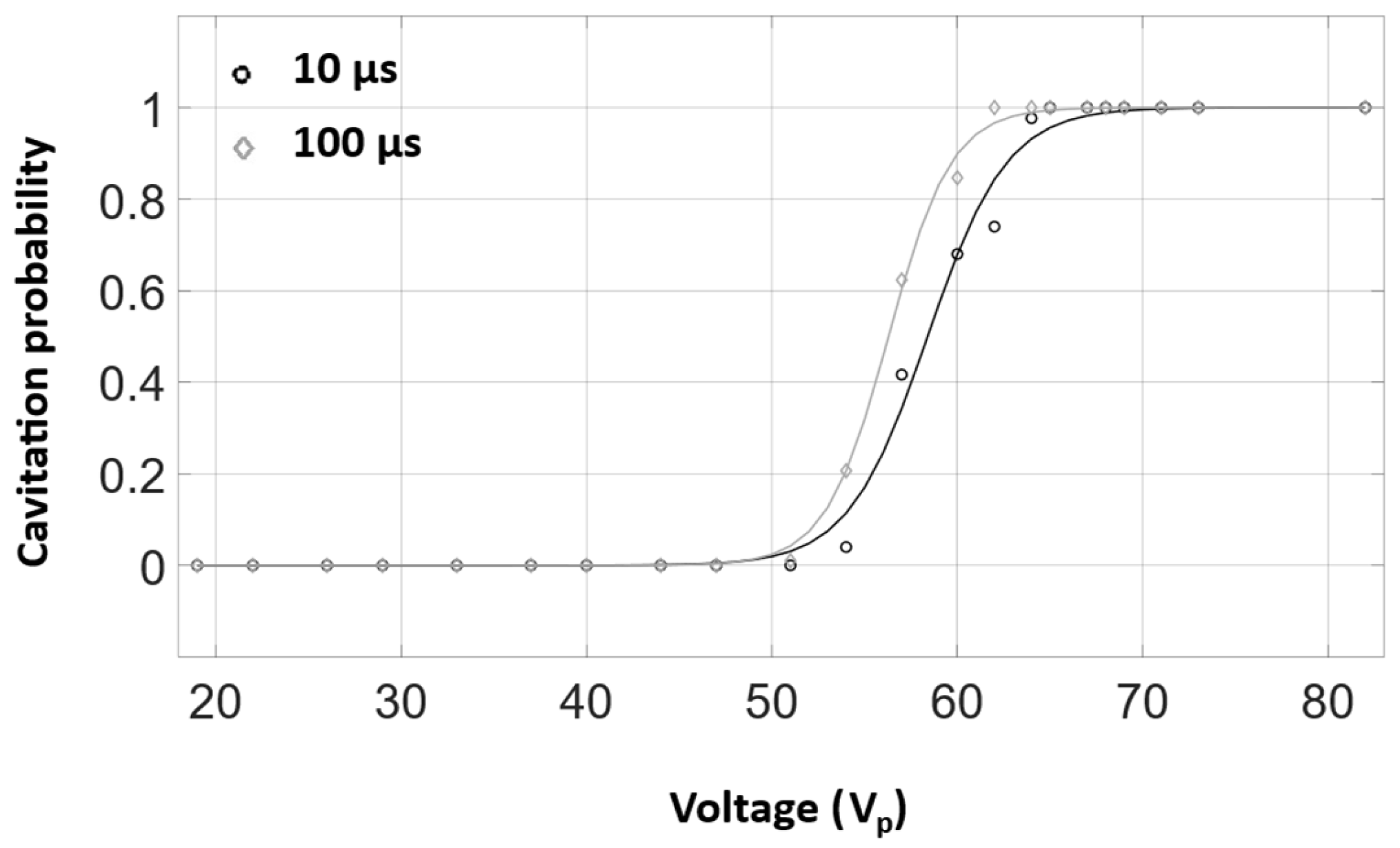
3.2. Cavitation Imaging and Cavitation Probability with Water-Filled Lumen
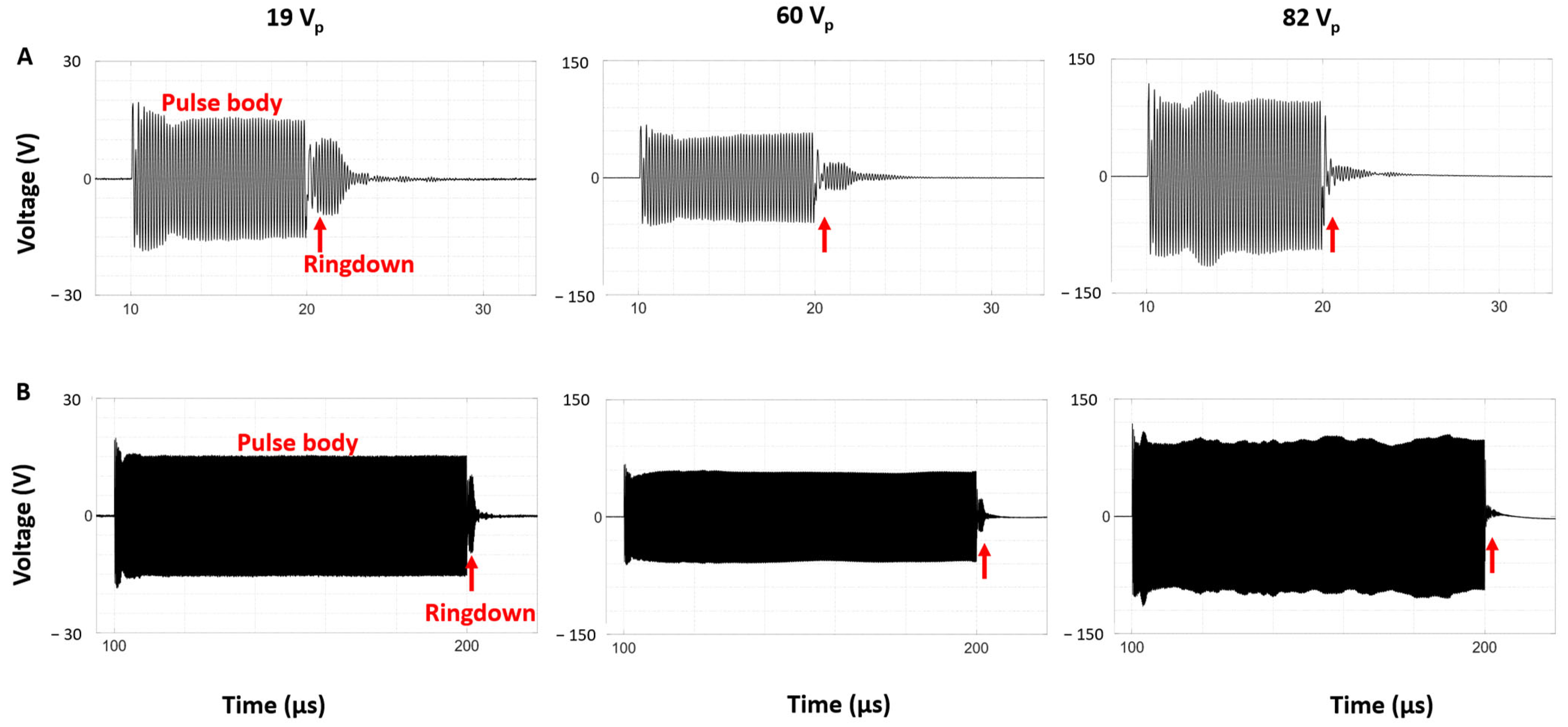
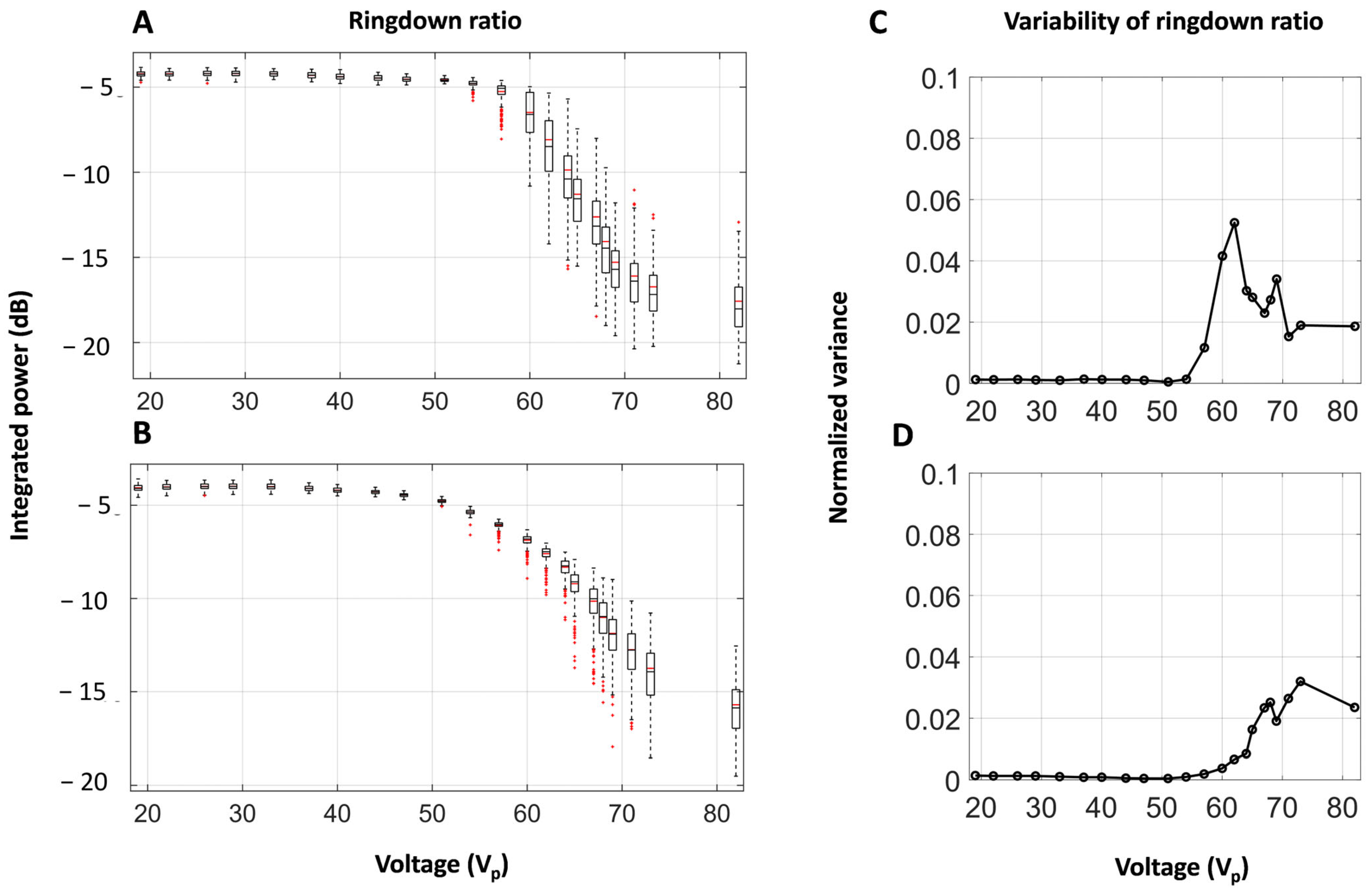
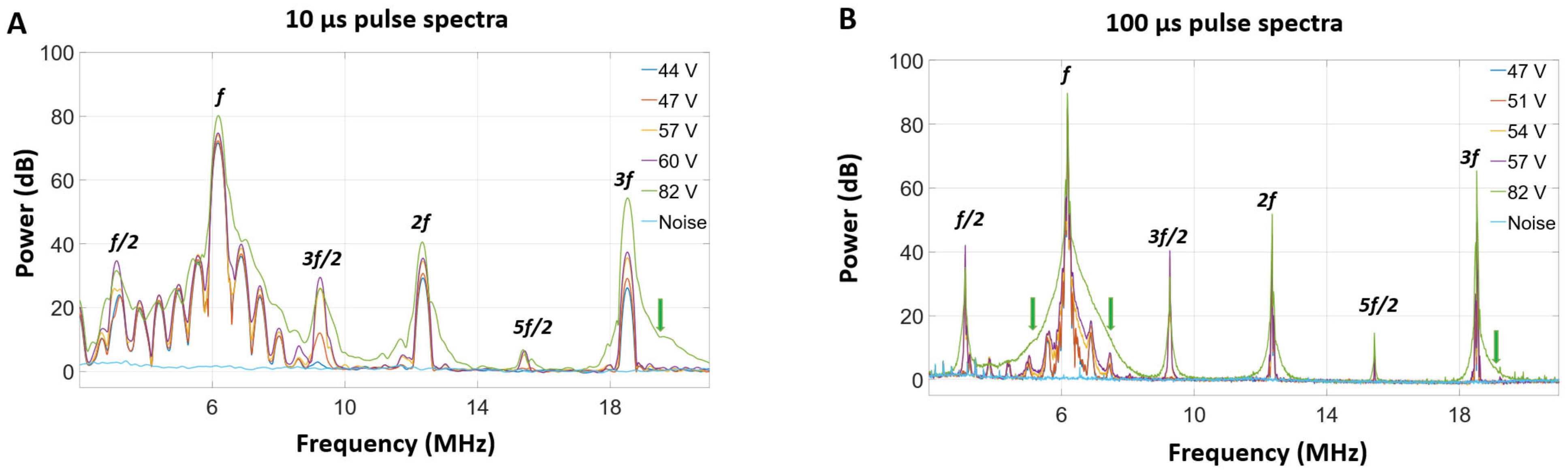
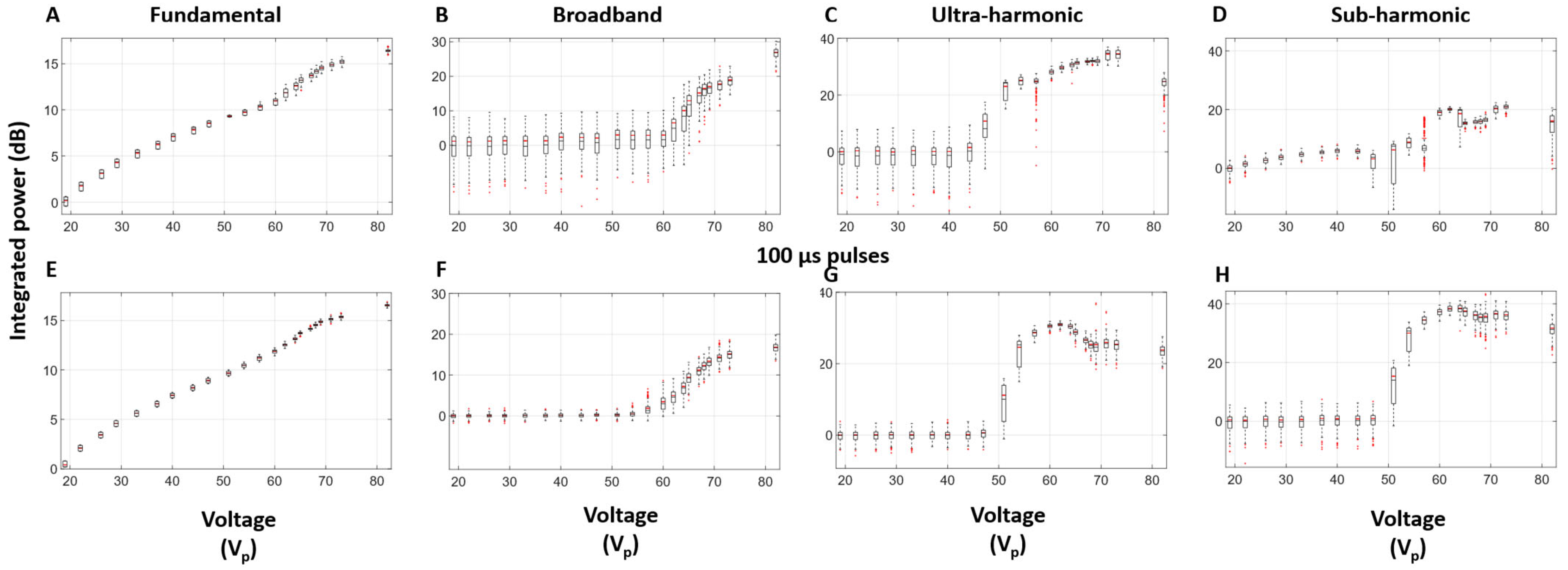
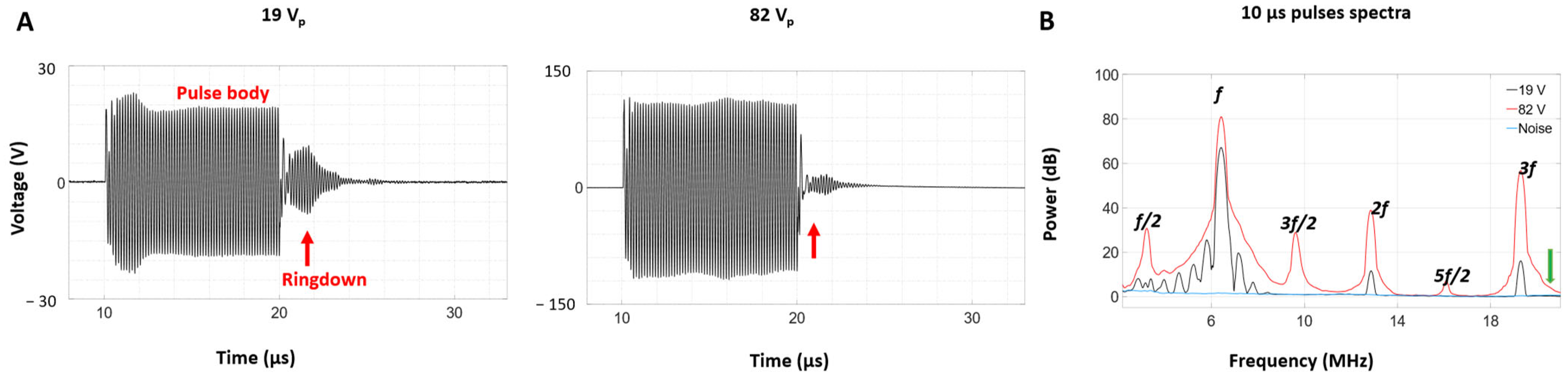
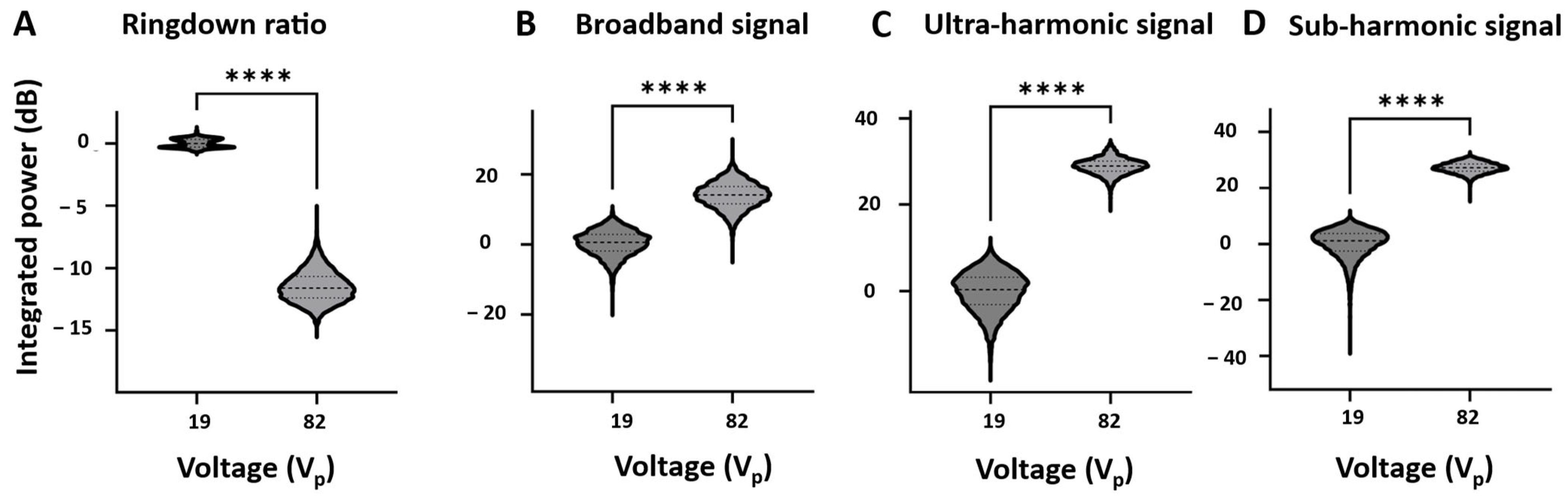
3.3. Self-Sensing Signals in Water-Filled Lumens
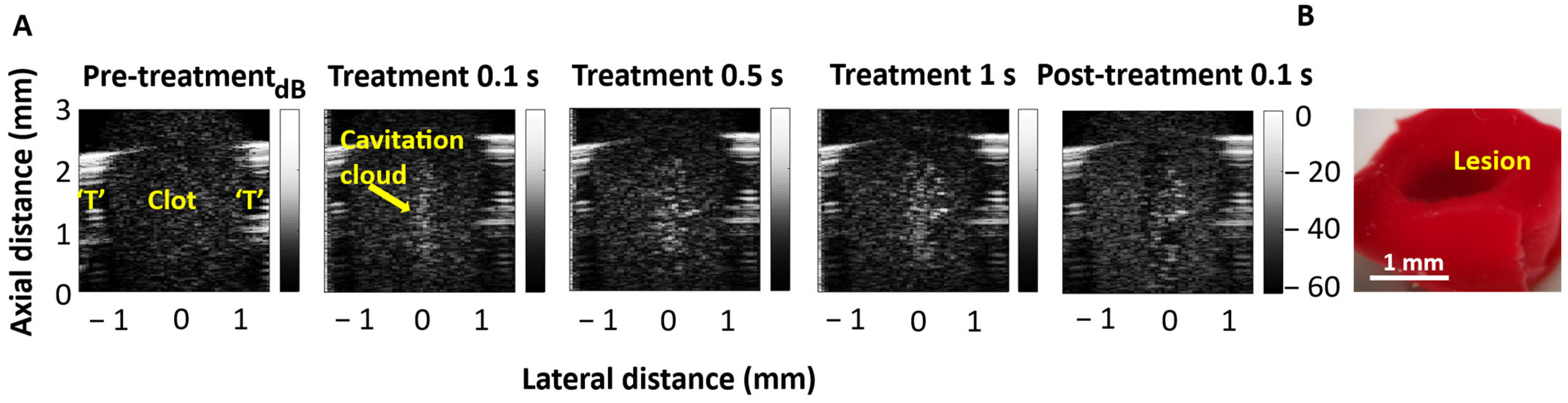
3.4. Self-Sensing Signals During Clot Treatments
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johansson, S.; Rosengren, A.; Young, K.; Jennings, E. Mortality and Morbidity Trends after the First Year in Survivors of Acute Myocardial Infarction: A Systematic Review. BMC Cardiovasc. Disord. 2017, 17, 53. [Google Scholar] [CrossRef]
- Ye, Q.; Zhang, J.; Ma, L. Predictors of All-Cause 1-Year Mortality in Myocardial Infarction Patients. Medicine 2020, 99, e21288. [Google Scholar] [CrossRef]
- Ko, D.T.; Ahmed, T.; Austin, P.C.; Cantor, W.J.; Dorian, P.; Goldfarb, M.; Gong, Y.; Graham, M.M.; Gu, J.; Hawkins, N.M.; et al. Development of Acute Myocardial Infarction Mortality and Readmission Models for Public Reporting on Hospital Performance in Canada. CJC Open 2021, 3, 1051–1059. [Google Scholar] [CrossRef]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. Heart Disease and Stroke Statistics—2023 Update: A Report From the American Heart Association. Circulation 2023, 147, e93–e621. [Google Scholar] [CrossRef]
- Tapson, V.F. Acute Pulmonary Embolism. N. Engl. J. Med. 2008, 358, 1037–1052. [Google Scholar] [CrossRef]
- Sailer, A.; Revzin, M.V.; Pollak, J.; Ayyagari, R.; Mojibian, H.R.; Nezami, N.; Pellerito, J.S.; Marino, A.G. Deep Vein Thrombosis: Update on Mechanical Thrombectomy and Intravascular US. Radiographics 2022, 42, E184–E185. [Google Scholar] [CrossRef]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Menon, B.K.; Zwam, W.H.v.; Dippel, D.W.J.; Mitchell, P.J.; Demchuk, A.M.; Dávalos, A.; Majoie, C.B.L.M.; van der Lugt, A.; de Miquel, M.A.; et al. Endovascular Thrombectomy after Large-Vessel Ischaemic Stroke: A Meta-Analysis of Individual Patient Data from Five Randomised Trials. Lancet 2016, 387, 1723–1731. [Google Scholar] [CrossRef]
- Qiu, K.; Zhao, L.-B.; Xu, X.-Q.; Wang, Y.; Liu, J.; Liu, S.; Shi, H.-B.; Zu, Q.-Q. Acute Embolic Stroke with Large-Vessel Occlusion: Does Contact Aspiration Thrombectomy Show Superiority? Clin. Radiol. 2022, 77, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, N.; Ghovvati, M.; Komuro, Y.; Guo, L.; Khatibi, K.; Mejia, L.L.P.; Saber, H.; Annabi, N.; Tateshima, S. A New Aspiration Device Equipped with a Hydro-Separator for Acute Ischemic Stroke Due to Challenging Soft and Stiff Clots. Interv. Neuroradiol. 2021, 28, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Amireh, A.O.; Kuybu, O.; Adeeb, N.; Kelley, R.E.; Javalkar, V.; Cuellar, H.; Sharma, P. Utilization of the Large-Bore Penumbra JET 7 Reperfusion Catheter in Thrombectomy for Acute Ischemic Stroke: A Single-Center Experience. Interv. Neuroradiol. 2020, 27, 99–106. [Google Scholar] [CrossRef]
- Sedhom, R.; Abdelmaseeh, P.; Haroun, M.; Megaly, M.; Narayanan, M.A.; Syed, M.; Ambrosia, A.M.; Kalra, S.; George, J.C.; Jaber, W.A. Complications of Penumbra Indigo Aspiration Device in Pulmonary Embolism: Insights From MAUDE Database. Cardiovasc. Revasc. Med. 2022, 39, 97–100. [Google Scholar] [CrossRef]
- Avgerinos, E.D.; Ali, A.A.; Toma, C.; Wu, B.; Saadeddin, Z.; McDaniel, B.; Al-Khoury, G.; Chaer, R.A. Catheter-Directed Thrombolysis versus Suction Thrombectomy in the Management of Acute Pulmonary Embolism. J. Vasc. Surg. Venous Lymphat. Disord. 2019, 7, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Sista, A.K.; Horowitz, J.M.; Tapson, V.F.; Rosenberg, M.; Elder, M.D.; Schiro, B.J.; Dohad, S.; Amoroso, N.E.; Dexter, D.J.; Loh, C.T.; et al. Indigo Aspiration System for Treatment of Pulmonary Embolism Results of the EXTRACT-PE Trial. JACC Cardiovasc. Interv. 2021, 14, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Loffroy, R.; Falvo, N.; Guillen, K.; Galland, C.; Baudot, X.; Demaistre, E.; Fréchier, L.; Ledan, F.; Midulla, M.; Chevallier, O. Single-Session Percutaneous Mechanical Thrombectomy Using the Aspirex®S Device Plus Stenting for Acute Iliofemoral Deep Vein Thrombosis: Safety, Efficacy, and Mid-Term Outcomes. Diagnostics 2020, 10, 544. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.J.; Roy, T.L. Catheter-Directed Interventions for the Treatment of Lower Extremity Deep Vein Thrombosis. Life 2022, 12, 1984. [Google Scholar] [CrossRef]
- Yeo, L.L.L.; Bhogal, P.; Gopinathan, A.; Cunli, Y.; Tan, B.; Andersson, T. Why Does Mechanical Thrombectomy in Large Vessel Occlusion Sometimes Fail? Clin. Neuroradiol. 2019, 29, 401–414. [Google Scholar] [CrossRef]
- Kaesmacher, J.; Maegerlein, C.; Kaesmacher, M.; Zimmer, C.; Poppert, H.; Friedrich, B.; Boeckh-Behrens, T.; Kleine, J.F. Thrombus Migration in the Middle Cerebral Artery: Incidence, Imaging Signs, and Impact on Success of Endovascular Thrombectomy. J. Am. Heart Assoc. 2017, 6, e005149. [Google Scholar] [CrossRef]
- Nogueira, R.G.; Thornton, J.; Connolly, K.; Mullins, L.; Fitzgerald, S. Investigation of Current and Super-Bore 088″ Treatment Strategies of Soft and Stiff Clots at Internal Carotid Artery and Middle Cerebral Artery Occlusion Sites in an In Vitro Thrombectomy Model. Stroke Vasc. Interv. Neurol. 2021, 2, e000240. [Google Scholar] [CrossRef]
- Yuki, I.; Kan, I.; Vinters, H.V.; Kim, R.H.; Golshan, A.; Vinuela, F.A.; Sayre, J.W.; Murayama, Y.; Vinuela, F. The Impact of Thromboemboli Histology on the Performance of a Mechanical Thrombectomy Device. Am. J. Neuroradiol. 2012, 33, 643–648. [Google Scholar] [CrossRef]
- Xu, Z.; Ludomirsky, A.; Eun, L.Y.; Hall, T.L.; Tran, B.C.; Fowlkes, B.; Cain, C.A. Controlled Ultrasound Tissue Erosion. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2004, 51, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Jove, J.; Serres, X.; Vlaisavljevich, E.; Cannata, J.; Duryea, A.; Miller, R.; Merino, X.; Velat, M.; Kam, Y.; Bolduan, R.; et al. First-in-Man Histotripsy of Hepatic Tumors: The THERESA Trial, a Feasibility Study. Int. J. Hyperth. 2022, 39, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Khokhlova, V.A.; Fowlkes, J.B.; Roberts, W.W.; Schade, G.R.; Xu, Z.; Khokhlova, T.D.; Hall, T.L.; Maxwell, A.D.; Wang, Y.-N.; Cain, C.A. Histotripsy Methods in Mechanical Disintegration of Tissue: Towards Clinical Applications. Int. J. Hyperth. 2015, 31, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Hall, T.L.; Vlaisavljevich, E.; Lee, F.T., Jr. Histotripsy: The First Noninvasive, Non-Ionizing, Non-Thermal Ablation Technique Based on Ultrasound. Int. J. Hyperth. 2021, 38, 561–575. [Google Scholar] [CrossRef]
- Mallay, M.G.; Woodacre, J.K.; Landry, T.G.; Campbell, N.A.; Brown, J.A.; Mallay, M. A Dual-Frequency Lens-Focused Endoscopic Histotripsy Transducer. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 2906–2916. [Google Scholar] [CrossRef]
- Landry, T.G.; Gannon, J.; Vlaisavljevich, E.; Mallay, M.G.; Woodacre, J.K.; Croul, S.; Fawcett, J.P.; Brown, J.A. Endoscopic Coregistered Ultrasound Imaging and Precision Histotripsy: Initial In Vivo Evaluation. BME Front. 2022, 2022, 9794321. [Google Scholar] [CrossRef]
- Dadgar, M.M.; Hynynen, K. High-Power Phased-Array Transducer Module for the Construction of a System for the Treatment of Deep Vein Thrombosis. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2020, 67, 2710–2716. [Google Scholar] [CrossRef]
- Gong, L.; Wright, A.R.; Hynynen, K.; Goertz, D.E. Inducing Cavitation Within Hollow Cylindrical Radially Polarized Transducers for Intravascular Applications. Ultrasonics 2024, 138, 107223. [Google Scholar] [CrossRef]
- Gong, L.; Wright, A.R.; Hynynen, K.; Goertz, D.E. Histotripsy of Blood Clots Within a Hollow Cylindrical Transducer for Aspiration Thrombectomy Applications. Ultrasound Med. Biol. 2025. submitted. [Google Scholar]
- Jones, R.M.; Deng, L.; Leung, K.; McMahon, D.; O’Reilly, M.A.; Hynynen, K. Three-Dimensional Transcranial Microbubble Imaging for Guiding Volumetric Ultrasound-Mediated Blood-Brain Barrier Opening. Theranostics 2018, 8, 2909–2926. [Google Scholar] [CrossRef]
- Coussios, C.C.; Roy, R.A. Applications of Acoustics and Cavitation to Noninvasive Therapy and Drug Delivery. Annu. Rev. Fluid Mech. 2008, 40, 395–420. [Google Scholar] [CrossRef]
- Coussios, C.C.; Farny, C.H.; Haar, G.T.; Roy, R.A. Role of Acoustic Cavitation in the Delivery and Monitoring of Cancer Treatment by High-Intensity Focused Ultrasound (HIFU). Int. J. Hyperth. 2007, 23, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Haworth, K.J.; Salgaonkar, V.A.; Corregan, N.M.; Holland, C.K.; Mast, T.D. Using Passive Cavitation Images to Classify High-Intensity Focused Ultrasound Lesions. Ultrasound Med. Biol. 2015, 41, 2420–2434. [Google Scholar] [CrossRef]
- Sukovich, J.R.; Hall, T.L.; Komaiha, M.; Haskell, S.; Lu, N.; Stocker, G.; Xu, Z. Acoustic Cavitation Localization during Histotripsy Using Transmit-Receive Capable Arrays. J. Acoust. Soc. Am. 2023, 153, A316. [Google Scholar] [CrossRef]
- Sukovich, J.R.; Macoskey, J.J.; Lundt, J.E.; Gerhardson, T.I.; Hall, T.L.; Xu, Z. Real-Time Transcranial Histotripsy Treatment Localization and Mapping Using Acoustic Cavitation Emission Feedback. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2020, 67, 1178–1191. [Google Scholar] [CrossRef]
- McLaughlan, J.; Rivens, I.; Leighton, T.; Haar, G.T. A Study of Bubble Activity Generated in Ex Vivo Tissue by High Intensity Focused Ultrasound. Ultrasound Med. Biol. 2010, 36, 1327–1344. [Google Scholar] [CrossRef] [PubMed]
- Canney, M.S.; Khokhlova, T.D.; Khokhlova, V.A.; Bailey, M.R.; Hwang, J.H.; Crum, L.A.; Hynynen, K.; Souquet, J. Tissue Erosion Using Shock Wave Heating and Millisecond Boiling in HIFU Fields. AIP Conf. Proc. 2010, 1215, 36–39. [Google Scholar] [CrossRef]
- Saalbach, K.-A.; Twiefel, J.; Wallaschek, J. Self-Sensing Cavitation Detection in Ultrasound-Induced Acoustic Cavitation. Ultrasonics 2019, 94, 401–410. [Google Scholar] [CrossRef]
- Haller, J.; Wilkens, V.; Shaw, A. Determination of Acoustic Cavitation Probabilities and Thresholds Using a Single Focusing Transducer to Induce and Detect Acoustic Cavitation Events: I. Method and Terminology. Ultrasound Med. Biol. 2018, 44, 377–396. [Google Scholar] [CrossRef]
- Samah, D.; Tierce, P.; Rouchon, J.-F. Influence of Cavitation on Ultrasonic Piezoelectric Transducers Impedance: Modelling and Experimentation. In Proceedings of the IEEE International Workshop of Electronics, Control, Measurement, Signals and Their Application to Mechatronics, Toulouse, France, 24–26 June 2013; Volume 1, pp. 1–6. [Google Scholar] [CrossRef]
- Bornmann, P.; Hemsel, T.; Sextro, W.; Memoli, G.; Hodnett, M.; Zeqiri, B. Self-Sensing Ultrasound Transducer for Cavitation Detection. In Proceedings of the 2014 IEEE International Ultrasonics Symposium, Chicago, IL, USA, 3–6 September 2014; pp. 663–666. [Google Scholar] [CrossRef]
- Lauterborn, W.; Ohl, C.-D. Cavitation Bubble Dynamics. Ultrason. Sonochem. 1997, 4, 65–75. [Google Scholar] [CrossRef]
- Lauterborn, W.; Cramer, E. Subharmonic Route to Chaos Observed in Acoustics. Phys. Rev. Lett. 1981, 47, 1445–1448. [Google Scholar] [CrossRef]
- Zhang, X.; Owens, G.E.; Cain, C.A.; Gurm, H.S.; Macoskey, J.; Xu, Z. Histotripsy Thrombolysis on Retracted Clots. Ultrasound Med. Biol. 2016, 42, 1903–1918. [Google Scholar] [CrossRef] [PubMed]
- Hendley, S.A.; Bollen, V.; Anthony, G.J.; Paul, J.D.; Bader, K.B. In Vitro Assessment of Stiffness-Dependent Histotripsy Bubble Cloud Activity in Gel Phantoms and Blood Clots. Phys. Med. Biol. 2019, 64, 145019. [Google Scholar] [CrossRef]
- Maxwell, A.D.; Haworth, K.J.; Holland, C.K.; Hendley, S.A.; Kreider, W.; Bader, K.B. Design and Characterization of an Ultrasound Transducer for Combined Histotripsy-Thrombolytic Therapy. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2022, 69, 156–165. [Google Scholar] [CrossRef]
- Sutton, J.T.; Ivancevich, N.M.; Perrin, S.R.; Vela, D.C.; Holland, C.K. Clot Retraction Affects the Extent of Ultrasound-Enhanced Thrombolysis in an Ex Vivo Porcine Thrombosis Model. Ultrasound Med. Biol. 2013, 39, 813–824. [Google Scholar] [CrossRef]
- Hendley, S.A.; Bhargava, A.; Holland, C.K.; Wool, G.D.; Ahmed, O.; Paul, J.D.; Bader, K.B. (More than) Doubling down: Effective Fibrinolysis at a Reduced Rt-PA Dose for Catheter-Directed Thrombolysis Combined with Histotripsy. PLoS ONE 2022, 17, e0261567. [Google Scholar] [CrossRef]
- Canney, M.S.; Khokhlova, V.A.; Bessonova, O.V.; Bailey, M.R.; Crum, L.A. Shock-Induced Heating and Millisecond Boiling in Gels and Tissue Due to High Intensity Focused Ultrasound. Ultrasound Med. Biol. 2010, 36, 250–267. [Google Scholar] [CrossRef]
- Shung, K.K.; Fei, D.Y.; Ballard, J.O. Further Studies on Ultrasonic Properties of Blood Clots. J. Clin. Ultrasound 1986, 14, 269–275. [Google Scholar] [CrossRef]
- Owens, C. Ultrasound-Enhanced Thrombolysis: EKOS EndoWave Infusion Catheter System. Semin. Intervent. Radiol. 2008, 25, 37–41. [Google Scholar] [CrossRef]
- Dumantepe, M.; Tarhan, I.A.; Ozler, A. Treatment of Chronic Deep Vein Thrombosis Using Ultrasound Accelerated Catheter-Directed Thrombolysis. Eur. J. Vasc. Endovasc. Surg. 2013, 46, 366–371. [Google Scholar] [CrossRef]
- Piazza, G.; Hohlfelder, B.; Jaff, M.R.; Ouriel, K.; Engelhardt, T.C.; Sterling, K.M.; Jones, N.J.; Gurley, J.C.; Bhatheja, R.; Kennedy, R.J.; et al. A Prospective, Single-Arm, Multicenter Trial of Ultrasound-Facilitated, Catheter-Directed, Low-Dose Fibrinolysis for Acute Massive and Submassive Pulmonary Embolism The SEATTLE II Study. JACC Cardiovasc. Interv. 2015, 8, 1382–1392. [Google Scholar] [CrossRef]
- Kennedy, S.R.; Lafond, M.; Haworth, K.J.; Escudero, D.S.; Ionascu, D.; Frierson, B.; Huang, S.; Klegerman, M.E.; Peng, T.; McPherson, D.D.; et al. Initiating and Imaging Cavitation from Infused Echo Contrast Agents through the EkoSonic Catheter. Sci. Rep. 2023, 13, 6191. [Google Scholar] [CrossRef] [PubMed]
- Lafond, M.; Salido, N.G.; Haworth, K.J.; Hannah, A.S.; Macke, G.P.; Genstler, C.; Holland, C.K. Cavitation Emissions Nucleated by Definity Infused through an EkoSonic Catheter in a Flow Phantom. Ultrasound Med. Biol. 2021, 47, 693–709. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lindsey, B.D.; Chang, W.-Y.; Dai, X.; Stavas, J.M.; Dayton, P.A.; Jiang, X. Intravascular Forward-Looking Ultrasound Transducers for Microbubble-Mediated Sonothrombolysis. Sci. Rep. 2017, 7, 3454. [Google Scholar] [CrossRef] [PubMed]
- Goel, L.; Wu, H.; Zhang, B.; Kim, J.; Dayton, P.A.; Xu, Z.; Jiang, X. Nanodroplet-Mediated Catheter-Directed Sonothrombolysis of Retracted Blood Clots. Microsyst. Nanoeng. 2021, 7, 3. [Google Scholar] [CrossRef]
- Goel, L.; Wu, H.; Zhang, B.; Kim, J.; Dayton, P.A.; Xu, Z.; Jiang, X. Safety Evaluation of a Forward-Viewing Intravascular Transducer for Sonothrombolysis: An in Vitro and Ex Vivo Study. Ultrasound Med. Biol. 2021, 47, 3231–3239. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, H.; Kim, J.; Dayton, P.; Xu, Z.; Jiang, X. Integration of Forward-Viewing and Side-Viewing Ultrasound Transducers in an Intravascular Sonothrombolysis Catheter. In Proceedings of the 2022 IEEE International Ultrasonics Symposium (IUS), Venice, Italy, 10–13 October 2022; pp. 1–4. [Google Scholar] [CrossRef]
- Wu, H.; Kim, J.; Zhang, B.; Owens, G.; Stocker, G.; Chen, M.; Kreager, B.C.; Cornett, A.; Bautista, K.; Kaovasia, T.; et al. Rotational Intravascular Multidirectional Ultrasound Catheter for Sonothrombolysis of Retracted Clots: An in Vitro and in Vivo Study. Engineering 2024, 42, 235–243. [Google Scholar] [CrossRef]
- Vu, P.T.; Rojas, S.S.; Ott, C.C.; Lindsey, B.D. A 9-Fr Endovascular Therapy Transducer With an Acoustic Metamaterial Lens for Rapid Stroke Thrombectomy. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2024, 71, 1627–1640. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, L.; Wright, A.R.; Hynynen, K.; Goertz, D.E. Self-Sensing with Hollow Cylindrical Transducers for Histotripsy-Enhanced Aspiration Mechanical Thrombectomy Applications. Sensors 2025, 25, 5417. https://doi.org/10.3390/s25175417
Gong L, Wright AR, Hynynen K, Goertz DE. Self-Sensing with Hollow Cylindrical Transducers for Histotripsy-Enhanced Aspiration Mechanical Thrombectomy Applications. Sensors. 2025; 25(17):5417. https://doi.org/10.3390/s25175417
Chicago/Turabian StyleGong, Li, Alex R. Wright, Kullervo Hynynen, and David E. Goertz. 2025. "Self-Sensing with Hollow Cylindrical Transducers for Histotripsy-Enhanced Aspiration Mechanical Thrombectomy Applications" Sensors 25, no. 17: 5417. https://doi.org/10.3390/s25175417
APA StyleGong, L., Wright, A. R., Hynynen, K., & Goertz, D. E. (2025). Self-Sensing with Hollow Cylindrical Transducers for Histotripsy-Enhanced Aspiration Mechanical Thrombectomy Applications. Sensors, 25(17), 5417. https://doi.org/10.3390/s25175417






