Dual-Responsive Starch Hydrogels via Physicochemical Crosslinking for Wearable Pressure and Ultra-Sensitive Humidity Sensing
Abstract
1. Introduction
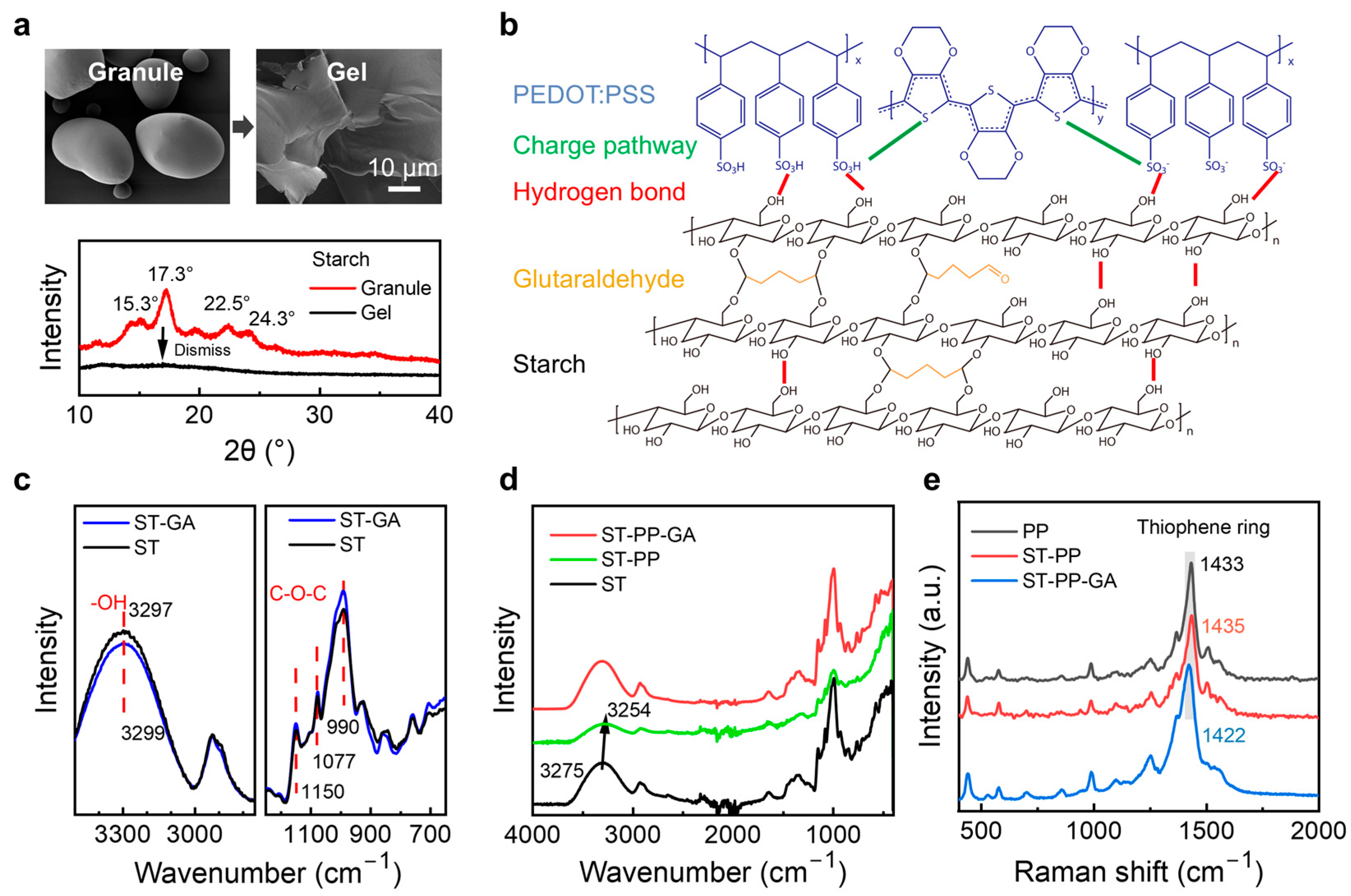
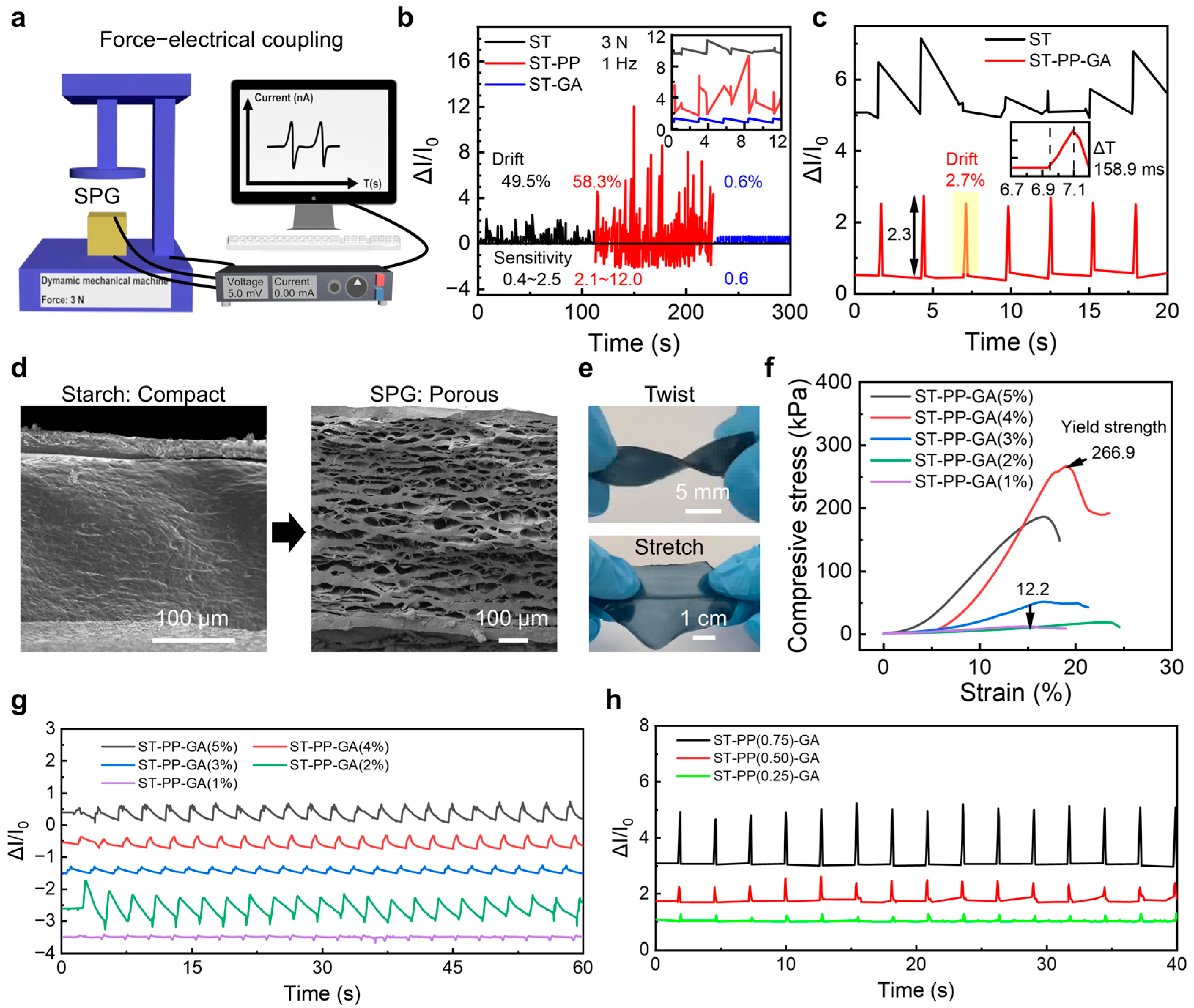
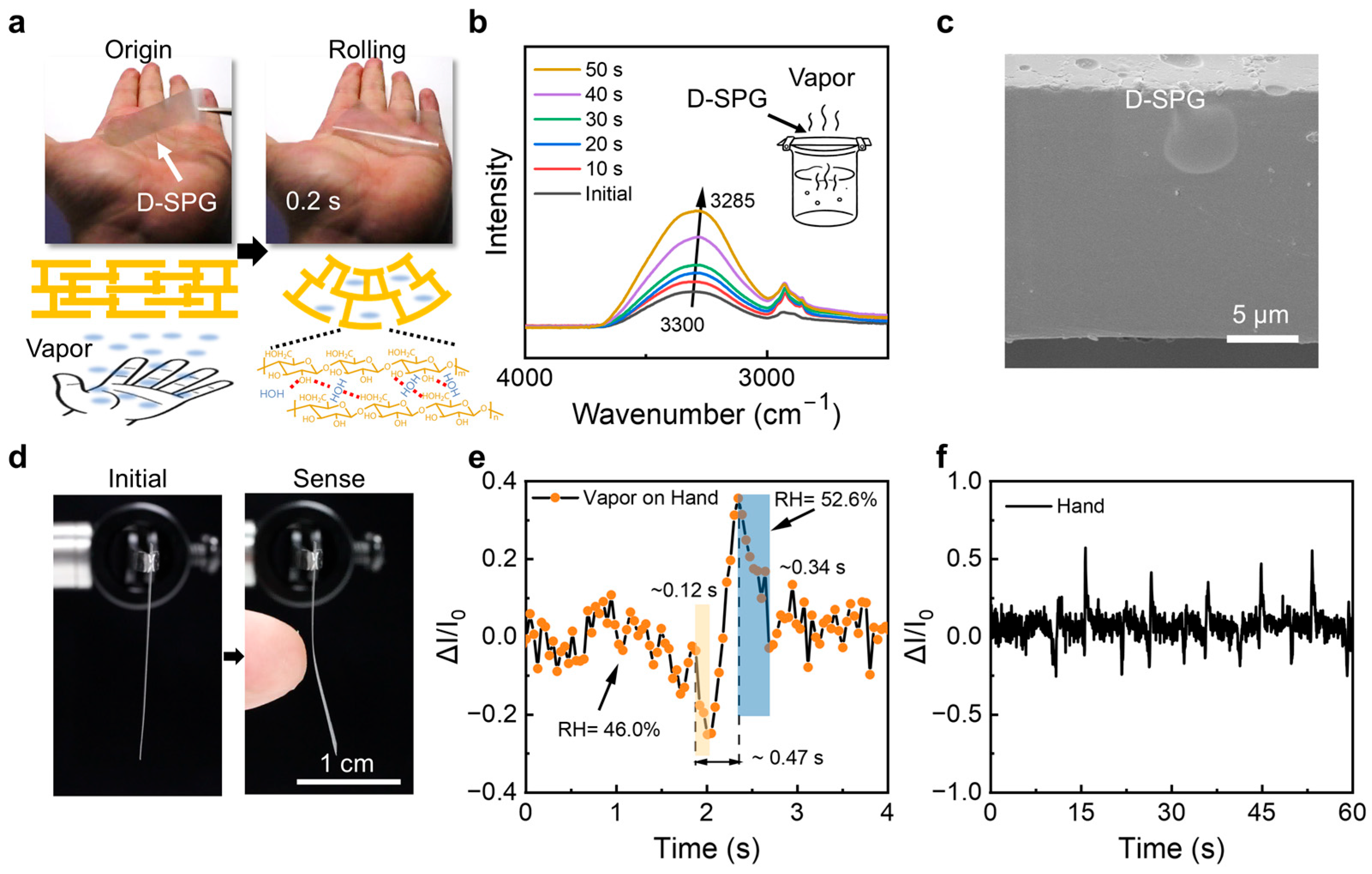
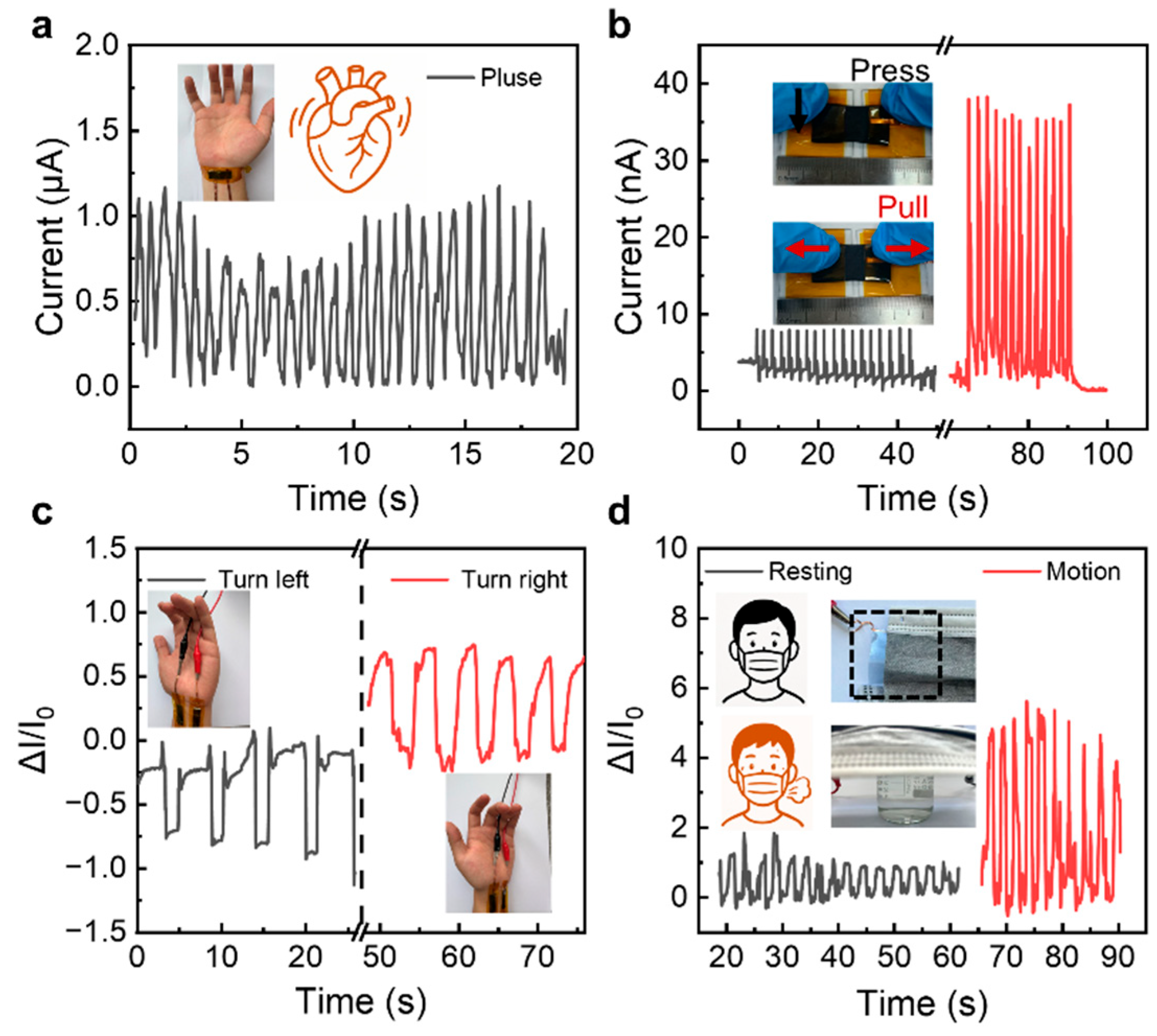
2. Results and Discussion
3. Conclusions
4. Experimental Section
4.1. Materials
4.2. Preparation of Dried Starch-PEDOT:PSS-GA (D-SPG) and Starch-PEDOT:PSS-GA (SPG) Hydrogel
4.3. Calculation Method of Signal Sensitivity
4.4. Calculation Method of Swelling/Deswelling Ratio
4.5. Characterization
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, Q.; Xu, C.; Jiang, Z.; Zhu, M.; Chen, C.; Fu, F. Smart actuators based on external stimulus response. Front. Chem. 2021, 9, 650358. [Google Scholar] [CrossRef]
- Pu, W.; Wei, F.; Yao, L.; Xie, S. A review of humidity-driven actuator: Toward high response speed and practical applications. J. Mater. Sci. 2022, 57, 12202–12235. [Google Scholar] [CrossRef]
- Le, X.; Lu, W.; Zhang, J.; Chen, T. Recent progress in biomimetic anisotropic hydrogel actuators. Adv. Sci. 2019, 6, 1801584. [Google Scholar] [CrossRef]
- Liu, X.; Gao, M.; Chen, J.; Guo, S.; Zhu, W.; Bai, L.; Zhai, W.; Du, H.; Wu, H.; Yan, C.; et al. Recent advances in stimuli-responsive shape-morphing hydrogels. Adv. Funct. Mater. 2022, 32, 2203323. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, H.; Zhao, Q.; Du, X. Chameleon-inspired structural-color actuators. Matter 2019, 1, 626–638. [Google Scholar] [CrossRef]
- Cui, H.; Zhao, Q.; Wang, Y.; Du, X. Bioinspired actuators based on stimuli-responsive polymers. Chem.-Asian J. 2019, 14, 2369–2387. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Hwang, Y.; Yang, Y.; Fan, T.; Song, J.; Suresh, S.; Cho, N.J. Actuation and locomotion driven by moisture in paper made with natural pollen. Proc. Natl. Acad. Sci. USA 2020, 117, 8711–8718. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Huang, C.; Galluzzi, M.; Ye, Q.; Xiao, R.; Yu, X.; Du, X. Editing the shape morphing of monocomponent natural polysaccharide hydrogel films. Research 2021, 2021, 9786128. [Google Scholar] [CrossRef] [PubMed]
- Dawson, C.; Vincent, J.; Rocca, A.M. How pines open up. Nature 1997, 390, 668. [Google Scholar] [CrossRef]
- Erb, R.M.; Sander, J.S.; Grisch, R.; Studart, A.R. Self-shaping composites with programmable bioinspired microstructures. Nat. Commun. 2013, 4, 1712. [Google Scholar] [CrossRef]
- Wei, Y.; Lan, C.; Luo, Y.; Li, F.; Meng, Y.; Yip, S.; Li, C.; Yin, Y.; Ho, J.C. Ultrasensitive, fast and flexible piezoresistive strain sensor based on te nanomesh. Chem. Eng. J. 2025, 511, 162024. [Google Scholar] [CrossRef]
- Zeng, S.; Zhang, J.; Zu, G.; Huang, J. Transparent, flexible, and multifunctional starch-based double-network hydrogels as high-performance wearable electronics. Carbohydr. Polym. 2021, 267, 118198. [Google Scholar] [CrossRef]
- Lei, K.; Wang, K.; Sun, Y.; Zheng, Z.; Wang, X. Rapid-fabricated and recoverable dual-network hydrogel with inherently anti-bacterial abilities for potential adhesive dressings. Adv. Funct. Mater. 2020, 31, 2008010. [Google Scholar] [CrossRef]
- Cui, C.; Wu, T.; Chen, X.; Liu, Y.; Li, Y.; Xu, Z.; Fan, C.; Liu, W. A janus hydrogel wet adhesive for internal tissue repair and anti-postoperative adhesion. Adv. Funct. Mater. 2020, 30, 2005689. [Google Scholar] [CrossRef]
- Cianchetti, M.; Laschi, C.; Menciassi, A.; Dario, P. Biomedical applications of soft robotics. Nat. Rev. Mater. 2018, 3, 143–153. [Google Scholar] [CrossRef]
- Fan, H.; Wang, J.; Gong, J.P. Barnacle cement proteins-inspired tough hydrogels with robust, long-lasting, and repeatable underwater adhesion. Adv. Funct. Mater. 2020, 31, 2009334. [Google Scholar] [CrossRef]
- Calderón Moreno, J.M.; Chelu, M.; Popa, M. Eco-friendly conductive hydrogels: Towards green wearable electronics. Gels 2025, 11, 220. [Google Scholar] [CrossRef] [PubMed]
- Landi, G.; Pagano, S.; Granata, V.; Avallone, G.; La Notte, L.; Palma, A.L.; Sdringola, P.; Puglisi, G.; Barone, C. Regeneration and long-term stability of a low-power eco-friendly temperature sensor based on a hydrogel nanocomposite. Nanomaterials 2024, 14, 283. [Google Scholar] [CrossRef]
- Veeramuthu, L.; Weng, R.-J.; Chiang, W.-H.; Pandiyan, A.; Liu, F.-J.; Liang, F.-C.; Kumar, G.R.; Hsu, H.-Y.; Chen, Y.-C.; Lin, W.-Y.; et al. Bio-inspired sustainable electrospun quantum nanostructures for high quality factor enabled face masks and self-powered intelligent theranostics. Chem. Eng. J. 2024, 502, 157752. [Google Scholar] [CrossRef]
- Zhang, L.; Naumov, P.; Du, X.; Hu, Z.; Wang, J. Vapomechanically responsive motion of microchannel-programmed actuators. Adv. Mater. 2017, 29, 1702231. [Google Scholar] [CrossRef]
- Ma, M.; Guo, L.; Anderson, D.G.; Langer, R. Bio-inspired polymer composite actuator and generator driven by water gradients. Science 2013, 339, 186–189. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindstrom, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A new family of nature-based materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, J.; Chen, Y.; Zheng, X.; Liu, H.; Li, H. Multiple-stimuli-responsive and cellulose conductive ionic hydrogel for smart wearable devices and thermal actuators. ACS Appl. Mater. Interfaces 2021, 13, 1353–1366. [Google Scholar] [CrossRef]
- Rostami, M.S.; Khodaei, M.M. Recent advances of chitosan-based nanocomposites for supercapacitor applications: Key challenges and future research directions. J. Energy Storage 2023, 72, 108344. [Google Scholar] [CrossRef]
- Xie, F. Natural polymer starch-based materials for flexible electronic sensor development: A review of recent progress. Carbohydr. Polym. 2024, 337, 122116. [Google Scholar] [CrossRef]
- Wang, W.; Xiang, C.; Liu, Q.; Li, M.; Zhong, W.; Yan, K.; Wang, D. Natural alginate fiber-based actuator driven by water or moisture for energy harvesting and smart controller applications. J. Mater. Chem. A 2018, 6, 22599–22608. [Google Scholar] [CrossRef]
- Wei, J.; Jia, S.; Guan, J.; Ma, C.; Shao, Z. Robust and highly sensitive cellulose nanofiber-based humidity actuators. ACS Appl. Mater. Interfaces 2021, 13, 54417–54427. [Google Scholar] [CrossRef]
- Yang, L.; Cui, J.; Zhang, L.; Xu, X.; Chen, X.; Sun, D. A moisture-driven actuator based on polydopamine-modified mxene/bacterial cellulose nanofiber composite film. Adv. Funct. Mater. 2021, 31, 2101378. [Google Scholar] [CrossRef]
- Wang, Y.; Song, L.; Wang, Q.; Wang, L.; Li, S.; Du, H.; Wang, C.; Wang, Y.; Xue, P.; Nie, W.C.; et al. Multifunctional acetylated distarch phosphate based conducting hydrogel with high stretchability, ultralow hysteresis and fast response for wearable strain sensors. Carbohydr. Polym. 2023, 318, 121106. [Google Scholar] [CrossRef]
- Hu, H.; Nie, M.; Galluzzi, M.; Yu, X.; Du, X. Mimosa-inspired high-sensitive and multi-responsive starch actuators. Adv. Funct. Mater. 2023, 33, 2306634. [Google Scholar] [CrossRef]
- Dodda, J.M.; Azar, M.G.; Bělský, P.; Šlouf, M.; Brož, A.; Bačáková, L.; Kadlec, J.; Remiš, T. Biocompatible hydrogels based on chitosan, cellulose/starch, pva and pedot: Pss with high flexibility and high mechanical strength. Cellulose 2022, 29, 6697–6717. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Yue, S.; He, H.; Ouyang, J. Biocompatible blends of an intrinsically conducting polymer as stretchable strain sensors for real-time monitoring of starch-based food processing. Adv. Funct. Mater. 2021, 31, 2102745. [Google Scholar] [CrossRef]
- Skansberger, T.; Kocherbitov, V. The reversible and irreversible phenomena in potato starch gelatinization. Starch—Stärke 2019, 71, 1800233. [Google Scholar] [CrossRef]
- Wan, S.; Wu, N.; Ye, Y.; Li, S.; Huang, H.; Chen, L.; Bi, H.; Sun, L. Highly stretchable starch hydrogel wearable patch for electrooculographic signal detection and human–machine interaction. Small Struct. 2021, 2, 2100105. [Google Scholar] [CrossRef]
- Lu, J.; Gu, J.; Hu, O.; Fu, Y.; Ye, D.; Zhang, X.; Zheng, Y.; Hou, L.; Liu, H.; Jiang, X. Highly tough, freezing-tolerant, healable and thermoplastic starch/poly(vinyl alcohol) organohydrogels for flexible electronic devices. J. Mater. Chem. A 2021, 9, 18406–18420. [Google Scholar] [CrossRef]
- Chen, S.; Liang, L.; Zhang, Y.; Lin, K.; Yang, M.; Zhu, L.; Yang, X.; Zang, L.; Lu, B. PEDOT:PSS-based electronic materials: Preparation, performance tuning, processing, applications, and future prospect. Prog. Polym. Sci. 2025, 166, 101990. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Zhu, J.; Wang, Z.; Hu, H.; Zhang, T. Dual-Responsive Starch Hydrogels via Physicochemical Crosslinking for Wearable Pressure and Ultra-Sensitive Humidity Sensing. Sensors 2025, 25, 5006. https://doi.org/10.3390/s25165006
Li Z, Zhu J, Wang Z, Hu H, Zhang T. Dual-Responsive Starch Hydrogels via Physicochemical Crosslinking for Wearable Pressure and Ultra-Sensitive Humidity Sensing. Sensors. 2025; 25(16):5006. https://doi.org/10.3390/s25165006
Chicago/Turabian StyleLi, Zi, Jinhui Zhu, Zixuan Wang, Hao Hu, and Tian Zhang. 2025. "Dual-Responsive Starch Hydrogels via Physicochemical Crosslinking for Wearable Pressure and Ultra-Sensitive Humidity Sensing" Sensors 25, no. 16: 5006. https://doi.org/10.3390/s25165006
APA StyleLi, Z., Zhu, J., Wang, Z., Hu, H., & Zhang, T. (2025). Dual-Responsive Starch Hydrogels via Physicochemical Crosslinking for Wearable Pressure and Ultra-Sensitive Humidity Sensing. Sensors, 25(16), 5006. https://doi.org/10.3390/s25165006




