Rapid Detection of Pesticide Residues in Leaf Vegetables by SERS Technology
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Instruments
2.2. Experimental Samples and Reagents
2.3. Experimental Methods
2.3.1. Gold Nanoparticle Preparation
2.3.2. Sample Preparation
2.3.3. Raman Spectroscopy Data Collection
2.3.4. Computational Methodology
2.3.5. Data Processing
3. Results and Discussion
3.1. Au Nanoparticle Characterization Analysis
3.2. Theoretical Calculations, Solid Powders, and SERS Spectra of Pesticides
3.3. Detection of Pesticide Residues and Development of Calibration Curve
3.4. Accuracy Validation of the Model
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GB 2763-2014; National Food Safety Standard-Maximum Residue Limits for Pesticides in Food. PRC National Standard the State Standard of the People’s Republic of China: Beijing, China, 2014.
- NY/T 761-2008; Pesticide Multiresidue Screen Methods for Determination of Organophosphoruspesticides, Organochlorine Pesticides, Pyrethroid Pesticides Andcarbamate Pesticedes in Vegetables and Fruits. PRC National Standard the State Standard of the People’s Republic of China: Beijing, China, 2008.
- Elmastas, A.; Umaz, A.; Pirinc, V.; Aydin, F. Quantitative determination and removal of pesticide residues in fresh vegetables and fruit products by LC–MS/MS and GC–MS/MS. Environ. Monit. Assess. 2023, 195, 277. [Google Scholar] [CrossRef]
- Ilić, M.; Pastor, K.; Romanić, R.; Vujić, Đ.; Ačanski, M. A New Challenge in Food Authenticity: Application of a Novel Mathematical Model for Rapid Quantification of Vegetable Oil Blends by Gas Chromatography—Mass Spectrometry (GC-MS). Anal. Lett. 2022, 55, 2752–2763. [Google Scholar] [CrossRef]
- Tankiewicz, M.; Berg, A. Improvement of the QuEChERS method coupled with GC–MS/MS for the determination of pesticide residues in fresh fruit and vegetables. Microchem. J. 2022, 181, 107794. [Google Scholar] [CrossRef]
- Romero-González, R. Detection of Residual Pesticides in Foods. Foods 2021, 10, 1113. [Google Scholar] [CrossRef]
- Kuižová, A.; Přikryl, M.; Procházka, M.; Kočišová, E. Drop coating deposition Raman (DCDR) spectroscopy of contaminants. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 262, 120109. [Google Scholar] [CrossRef]
- Valášková, M.; Kuižová, A.; Kočišová, E.; Profant, V. Insights into thiram fungicide: A comparative study of solution and solid phases through Raman, DCDR, and SERS measurements and DFT simulations. J. Raman Spectrosc. 2023, 54, 950–965. [Google Scholar] [CrossRef]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman-Spectra of Pyridine Adsorbed at a Silver Electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar]
- Li, C.; Wang, H.; Sun, J.; Li, P.; Dong, J.; Huang, J.; Dong, H.; Geng, L.; Yu, Z.; Zhang, P.; et al. Novel electrochemiluminescence platform utilizing AuNPs@Uio-66-NH2 bridged luminescent substrates and aptamers for the detection of pesticide residues in Chinese herbal medicines. Talanta 2025, 281, 126924. [Google Scholar] [CrossRef] [PubMed]
- Chu, B.; Lin, C.; Nie, P.; Xia, Z. Research Status in the Use of Surface-Enhanced Raman Scattering (SERS) to Detect Pesticide Residues in Foods and Plant-Derived Chinese Herbal Medicines. Int. J. Anal. Chem. 2024, 2024, 5531430. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Dai, H.; Li, G.; Zhang, Z. Rapid determination of pesticide residues in fruit and vegetable using Au@AgNPs decorated 2D Ni-MOF nanosheets as efficient surface-enhanced Raman scattering substrate. Sens. Actuators B Chem. 2022, 369, 132360. [Google Scholar] [CrossRef]
- Liu, F.; Li, Z.; Wei, H.; Xu, P.; Kang, G.; Zhu, S.; Wang, T.; He, R.; Chen, C.; Lu, Y. Coordinatively unsaturated cobalt single-atom nanozymes for visual pesticides detection by smartphone-based platform. Nano Res. 2024, 17, 2298–2307. [Google Scholar] [CrossRef]
- Kottadiyil, D.; Mehta, T.; Thasale, R.; Sivaperumal, P. Determination and dietary risk assessment of 52 pesticide residues in vegetable and fruit samples by GC-MS/MS and UHPLC-QTOF/MS from Gujarat, India. J. Food Compos. Anal. 2023, 115, 104957. [Google Scholar] [CrossRef]
- Lv, M.; Pu, H.; Sun, D. A tailored dual core-shell magnetic SERS substrate with precise shell-thickness control for trace organophosphorus pesticides residues detection. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 316, 124336. [Google Scholar] [CrossRef]
- Wu, J.; Xi, J.; Chen, H.; Li, S.; Zhang, L.; Li, P.; Wu, W. Flexible 2D nanocellulose-based SERS substrate for pesticide residue detection. Carbohydr. Polym. 2022, 277, 118890. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Pu, H.; Sun, D. A durian-shaped multilayer core-shell SERS substrate for flow magnetic detection of pesticide residues on foods. Food Chem. 2024, 433, 137389. [Google Scholar] [CrossRef]
- Wang, T.; Liu, M.; Huang, S.; Yuan, H.; Zhao, J. Detection of Ofloxacin and Norfloxacin in Duck Meat Using Surface-Enhanced Raman Spectroscopy (SERS) Coupled with Multivariate Analysis. Anal. Lett. 2023, 56, 692–702. [Google Scholar] [CrossRef]
- Adhikari, S.; Joshi, R.; Joshi, R.; Kim, M.; Jang, Y.; Tufa, L.T.; Gicha, B.B.; Lee, J.; Lee, D.; Cho, B. Rapid and ultrasensitive detection of thiram and carbaryl pesticide residues in fruit juices using SERS coupled with the chemometrics technique. Food Chem. 2024, 457, 140486. [Google Scholar] [CrossRef]
- Ungurean, A.; Leopold, N.; David, L.; Chiş, V. Vibrational spectroscopic and DFT study of trimethoprim. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 102, 52–58. [Google Scholar] [CrossRef]
- Barbosa, E.F.; Pereira, P.C.A.; Silva, F.C.D.; Mira, Á.B.D.; Dantas Filho, J.V.; Schons, S.D.V. Pesticide residues detected in Colossoma macropomum by the modified QuEChERS and GC-MS/MS methods. Acta Scientiarum. Technol. 2022, 45, e63831. [Google Scholar] [CrossRef]
- Xiong, S.; Wang, D.; Qiu, H.; He, T.; Wang, C.; Wang, Y.; Dai, C.; Liu, W. Toward Flexible Metal Semiconductor Sandwich Surface-Enhanced Raman scattering (SERS) sensors Pesticide Duplex Detection. Mater. Des. 2023, 229, 111865. [Google Scholar] [CrossRef]
- Fan, Y.; Lai, K.; Rasco, B.A.; Huang, Y. Analyses of phosmet residues in apples with surface-enhanced Raman spectroscopy. Food Control 2014, 37, 153–157. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, B.; Wan, C.; Hao, Y.; Ouyang, A.; Lan, Y. Determination of dimethoate and phosmet pesticides with surface-enhanced Raman spectroscopy. Spectrosc. Lett. 2016, 49, 198–203. [Google Scholar] [CrossRef]
- He, L.; Chen, T.; Labuza, T.P. Recovery and quantitative detection of thiabendazole on apples using a surface swab capture method followed by surface-enhanced Raman spectroscopy. Food Chem. 2014, 148, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Barcelo, S.J.; Li, Z. SERS-based pesticide detection by using nanofinger sensors. Nanotechnology 2015, 26, 15502–15507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; He, M.; Qin, C.; Wu, Z.; Cao, M.; Ni, D.; Yu, Z.; Liang, P. A highly effective SERS platform formed by the fabrication of Ag@ZIF-8@Au nanoparticles for rapid detection of acetamiprid in environment. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 308, 123754. [Google Scholar] [CrossRef] [PubMed]
- Wijaya, W.; Pang, S.; Labuza, T.P.; He, L. Rapid Detection of Acetamiprid in Foods using Surface-Enhanced Raman Spectroscopy (SERS). J. Food Sci. 2014, 79, T743–T747. [Google Scholar] [CrossRef]


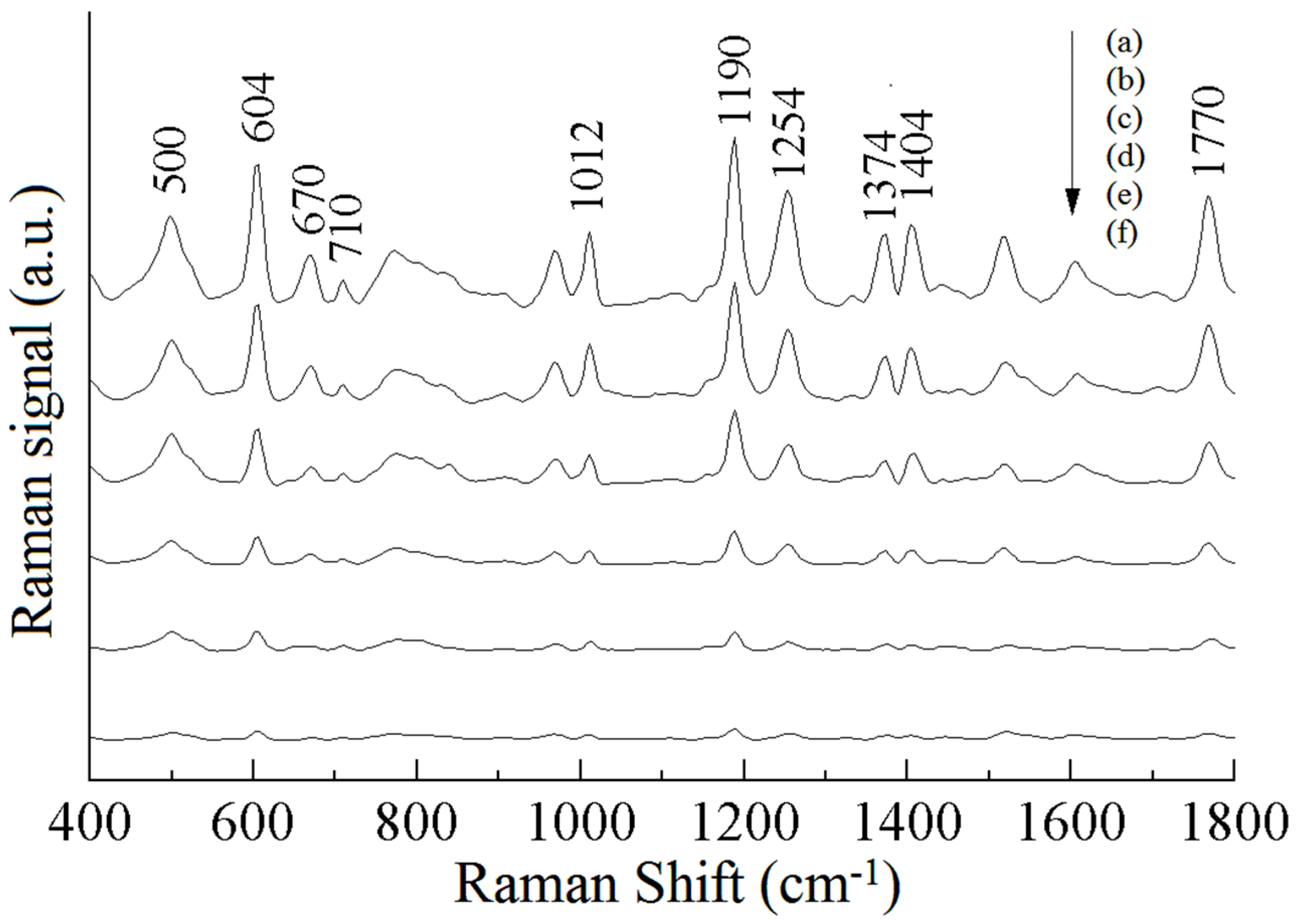
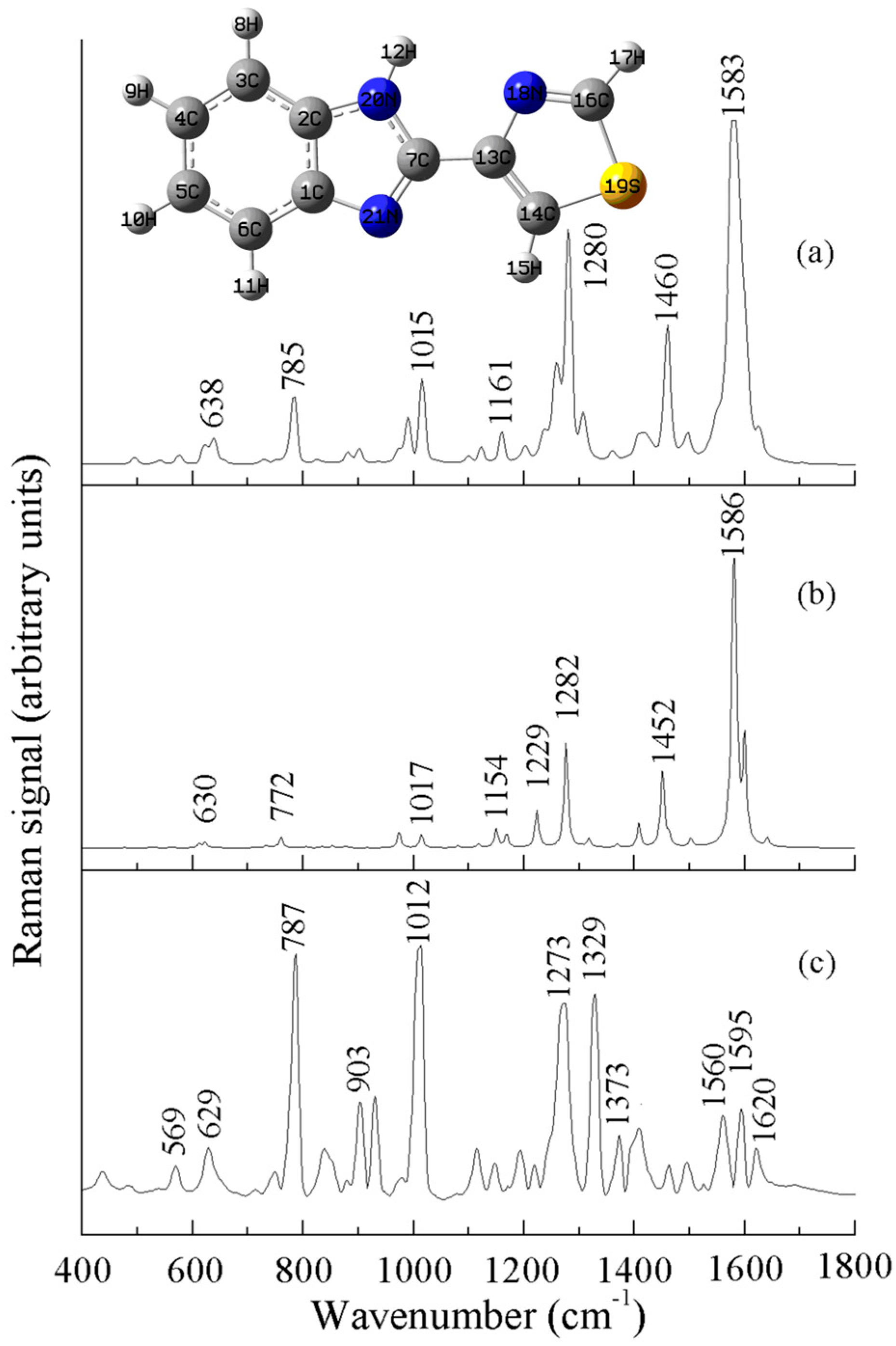
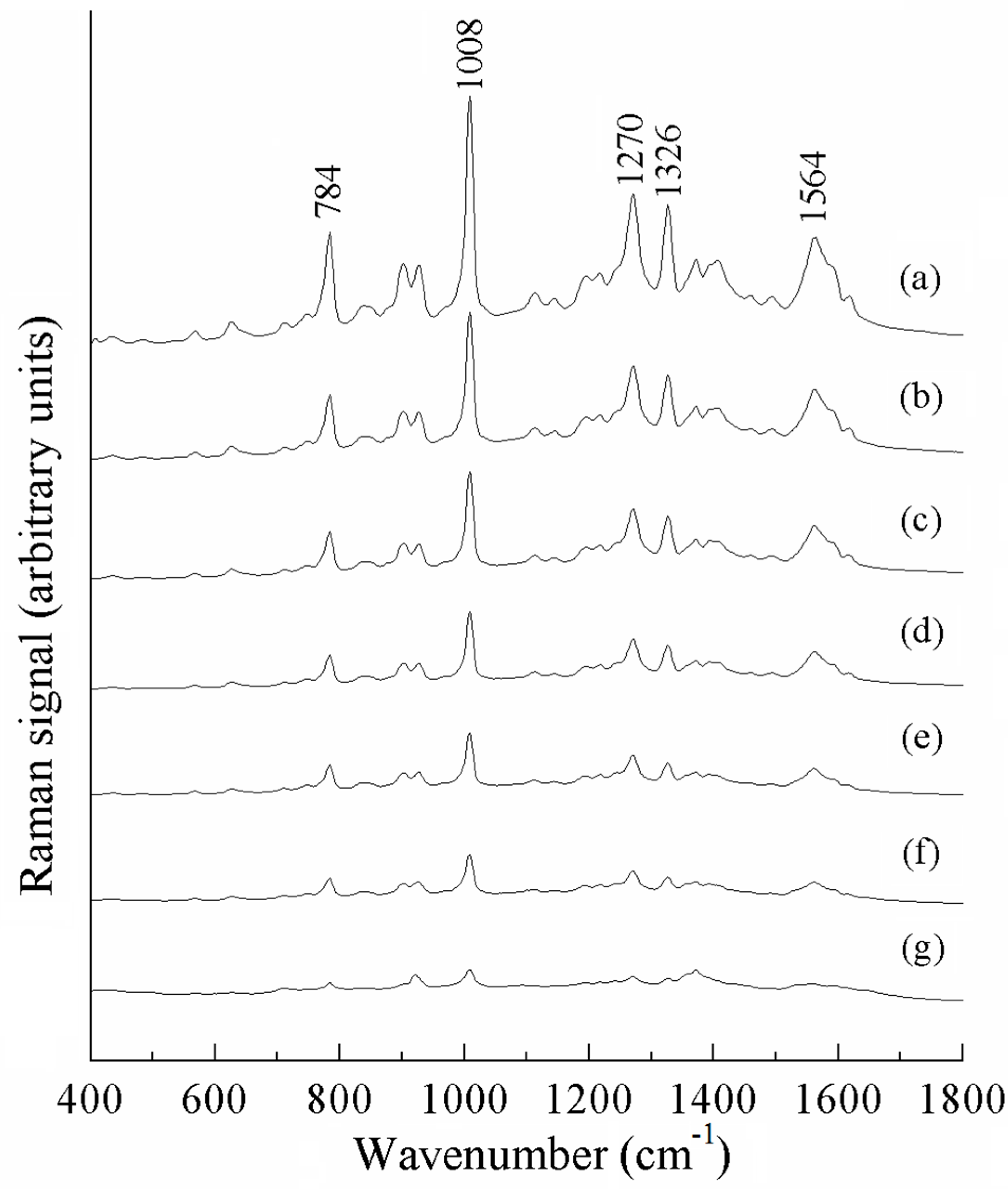
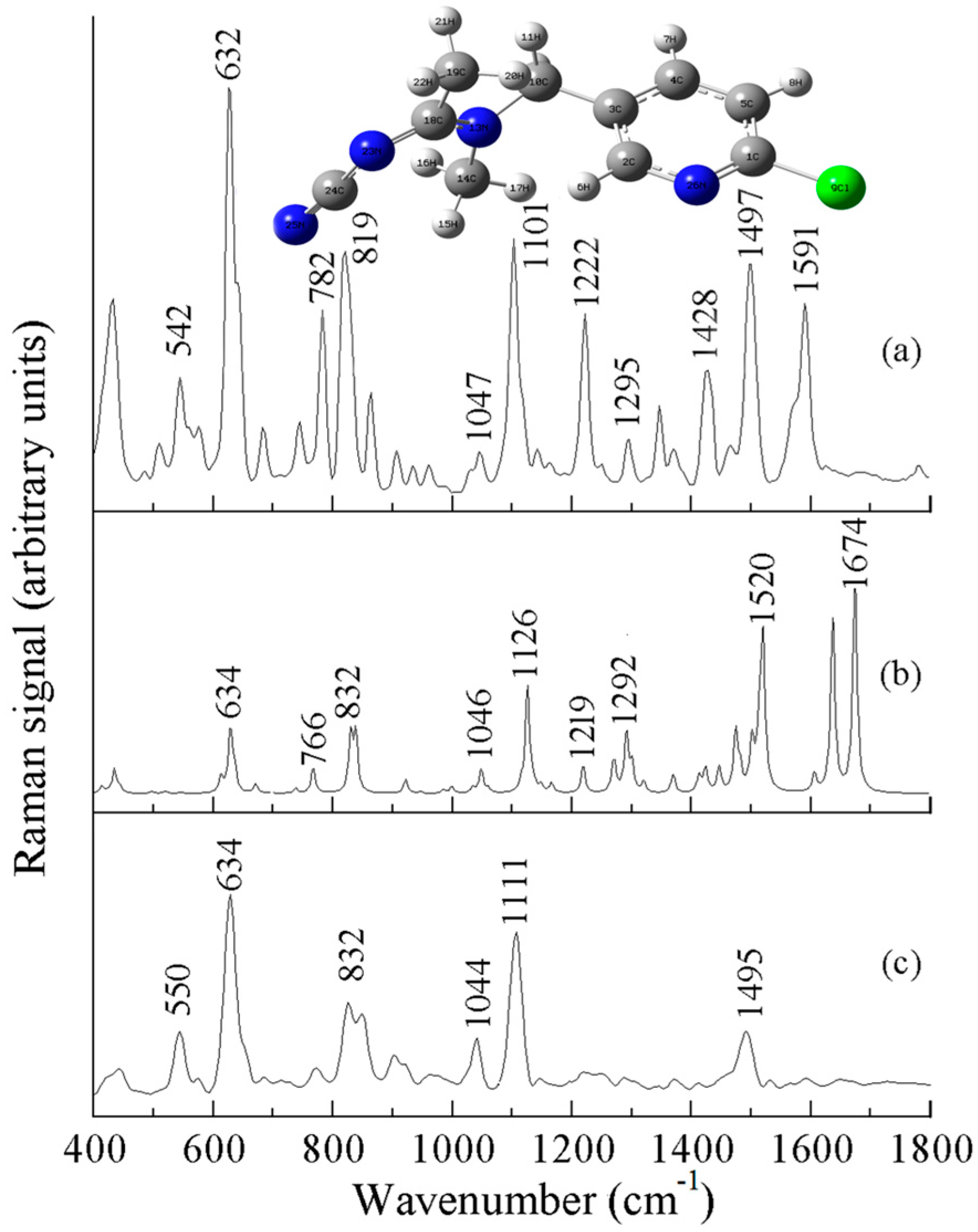
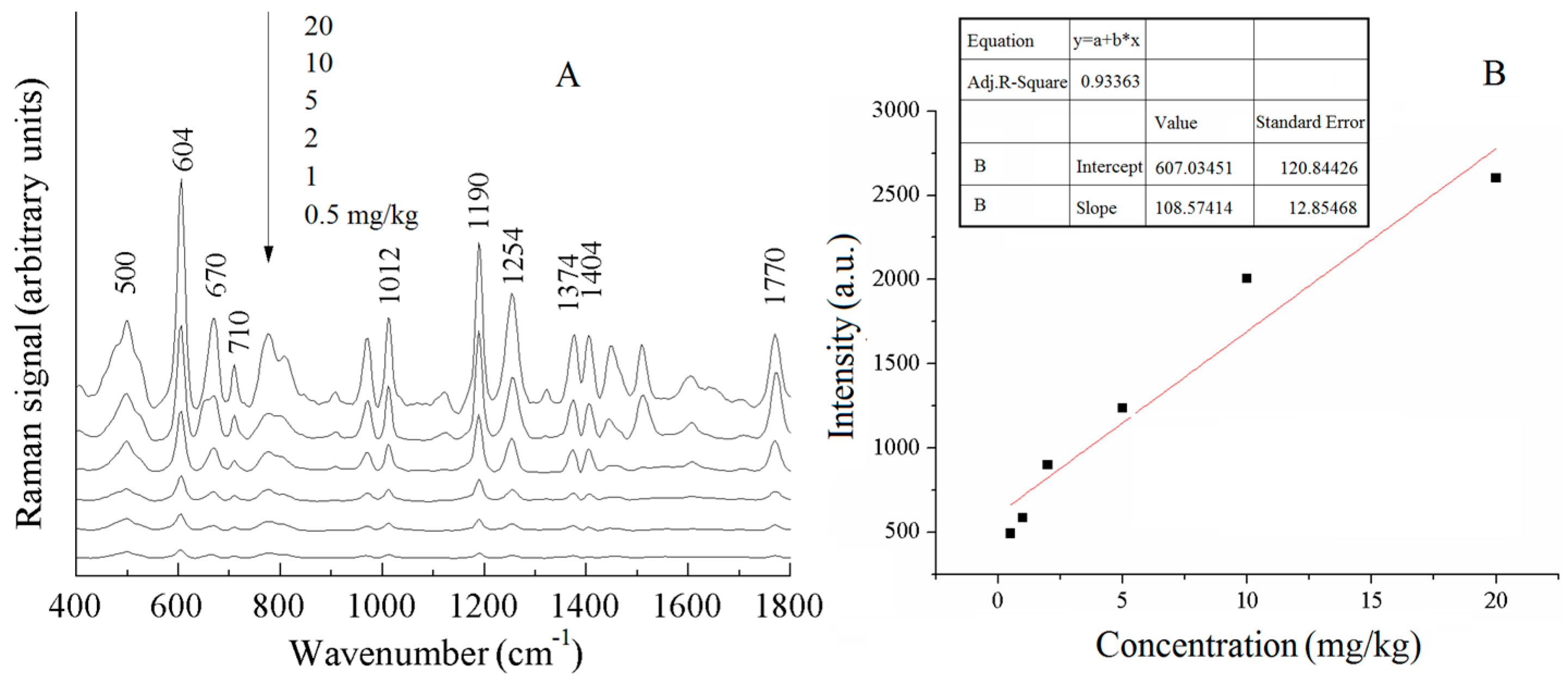

| a DFT-Calculated | Solid | b SERS | c Assignment |
|---|---|---|---|
| 500 | 510 | 500 (m) | ρ (CH2) or ρ (PO2) |
| 610 | 603 | 604 (s) | ν (C− S), ρ (C=O) |
| 634 | 650 | − | τ (P=S) |
| 676 | 670 | 670 (w) | τ (P=S) |
| − | 710 | 710 (w) | benzene ring breathing |
| 972 | 977 | − | benzene ring breathing |
| 1019 | 1016 | 1012 (m) | νas (C− O) |
| 1192 | 1188 | 1190 (s) | ρ (P-O-CH3) |
| 1286 | 1272 | 1254 (m) | ν (C− N) |
| 1408 | 1408 | 1404 (m) | ρ (C− H) |
| 1616 | 1611 | − | ν (C=O) |
| 1772 | 1772 | 1770 (m) | ν (C=O) |
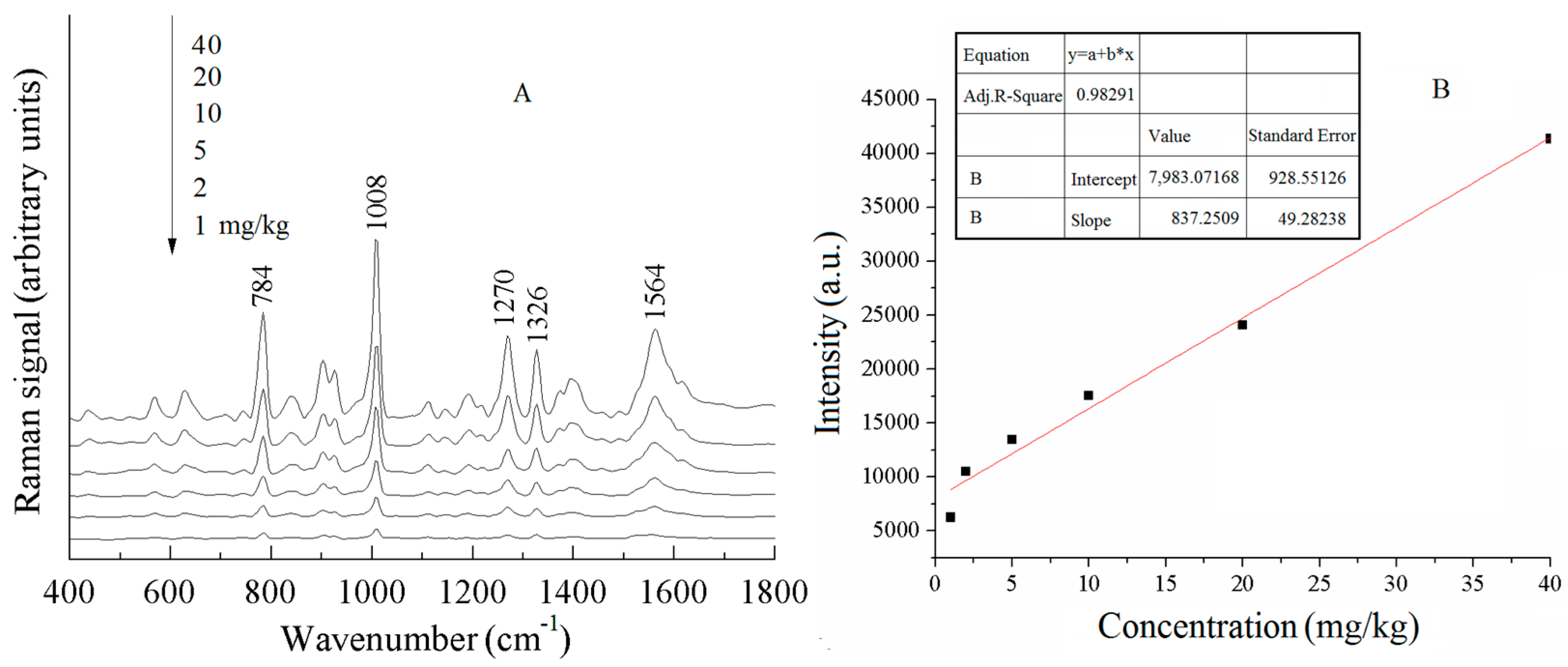
| a DFT-Calculated | Solid | b SERS | c Assignment |
|---|---|---|---|
| - | - | 569 (w) | ρ (C-C-C) |
| 630 | 638 | 629 (m) | ρ (C-S-C) |
| 772 | 785 | 784 (s) | νas (C-S-C) |
| - | - | 903 (m) | ν (benzimidazole) |
| 974 | 990 | 980 (w) | ν (C-S) |
| 1017 | 1015 | 1008 (s) | ν (C=C), ρ (C-H) |
| 1154 | 1161 | 1147 (w) | ρ (C-H) of benzimidazole |
| 1282 | 1280 | 1270 (s) | ν (benzimidazole) |
| 1326 | 1307 | 1329 (s) | ν (C=C) |
| 1452 | 1460 | 1462 (w) | νas (C-N-C) |
| 1586 | 1583 | 1560 (m) | ν (benzimidazole) |
| 1606 | 1624 | 1595 (m) | ν (C=C), ν (C-C) |
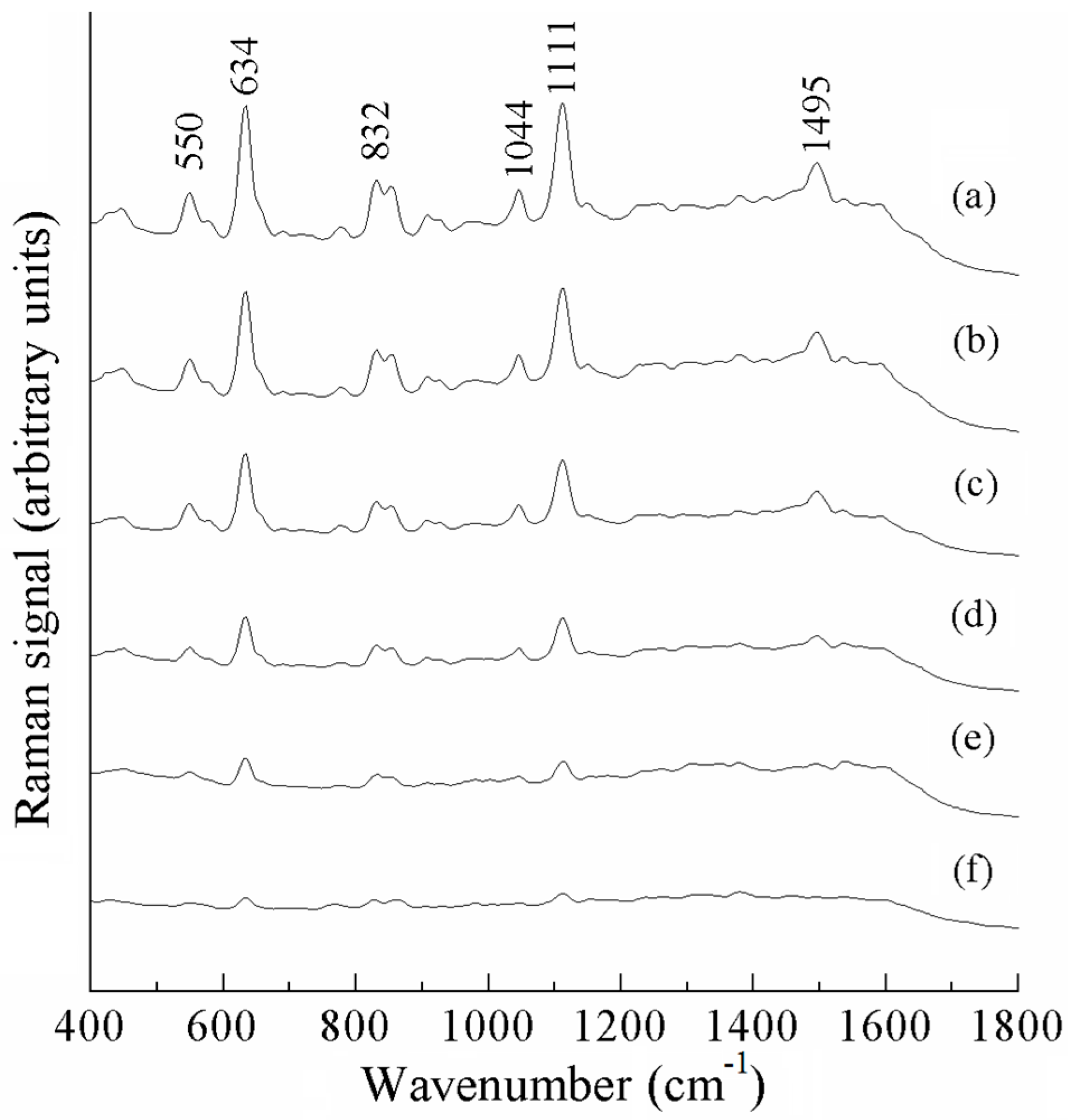
| a DFT-Calculated | Solid | b SERS | c Assignment |
|---|---|---|---|
| - | 542 | 550 (m) | ν (Cl) |
| 634 | 632 | 634 (s) | ν (C-Cl) |
| 766 | 782 | - | ν (C-Cl) |
| 832 | 819 | 832 (m) | ring breathing vibration |
| 1046 | 1047 | 1044 (w) | ν (ring) |
| 1126 | 1101 | 1111 (s) | ν (N-C=N), ring breathing |
| 1219 | 1222 | - | ν (ring) |
| 1292 | 1295 | - | ρ (CH2) |
| 1426 | 1428 | - | ν (ring) |
| 1520 | 1497 | 1495 (m) | ν (benzene ring) |
| 1674 | - | ν (C=C) |
| Calibration Band | Pesticides | Calibration Curves | R2 | LOD | LOQ |
|---|---|---|---|---|---|
| 604 cm−1 | phosmet | y = 607 + 108x | 0.93363 | 0.5 mg/kg | 0.76 mg/kg |
| 784 cm−1 | thiabendazole | y = 7983.1 + 837.2x | 0.98291 | 1 mg/kg | 1.17 mg/kg |
| 1111 cm−1 | acetamiprid | y = 1366 + 332.7x | 0.95332 | 1 mg/kg | 1.14 mg/kg |
| Pesticides | Sample | Added Value (mg/kg) | Predicted Value (mg/kg) | Standard Deviation (%) | Recovery (%) |
|---|---|---|---|---|---|
| phosmet | 1 | 3 | 3.21 | 4.78 | 107.00 |
| 2 | 6 | 6.57 | 6.41 | 109.50 | |
| 3 | 9 | 10.16 | 8.56 | 112.89 | |
| 4 | 12 | 11.36 | 3.87 | 94.67 | |
| 5 | 15 | 16.04 | 4.74 | 106.93 | |
| thiabendazole | 1 | 3 | 2.63 | 9.29 | 87.67 |
| 2 | 6 | 6.47 | 5.33 | 107.83 | |
| 3 | 9 | 9.22 | 1.71 | 102.44 | |
| 4 | 15 | 14.64 | 1.72 | 97.60 | |
| 5 | 30 | 31.93 | 4.41 | 106.43 | |
| acetamiprid | 1 | 3 | 2.72 | 6.92 | 90.67 |
| 2 | 6 | 5.67 | 4.00 | 94.50 | |
| 3 | 12 | 13.65 | 9.10 | 113.75 | |
| 4 | 18 | 19.62 | 6.09 | 109.00 | |
| 5 | 25 | 26.85 | 5.05 | 107.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, F.; Huang, S.; Chen, Q.; Tong, N.; Wu, Y. Rapid Detection of Pesticide Residues in Leaf Vegetables by SERS Technology. Sensors 2025, 25, 4912. https://doi.org/10.3390/s25164912
Peng F, Huang S, Chen Q, Tong N, Wu Y. Rapid Detection of Pesticide Residues in Leaf Vegetables by SERS Technology. Sensors. 2025; 25(16):4912. https://doi.org/10.3390/s25164912
Chicago/Turabian StylePeng, Fang, Shuanggen Huang, Qi Chen, Ni Tong, and Yan Wu. 2025. "Rapid Detection of Pesticide Residues in Leaf Vegetables by SERS Technology" Sensors 25, no. 16: 4912. https://doi.org/10.3390/s25164912
APA StylePeng, F., Huang, S., Chen, Q., Tong, N., & Wu, Y. (2025). Rapid Detection of Pesticide Residues in Leaf Vegetables by SERS Technology. Sensors, 25(16), 4912. https://doi.org/10.3390/s25164912





