Recent Advances in Micro- and Nano-Enhanced Intravascular Biosensors for Real-Time Monitoring, Early Disease Diagnosis, and Drug Therapy Monitoring
Abstract
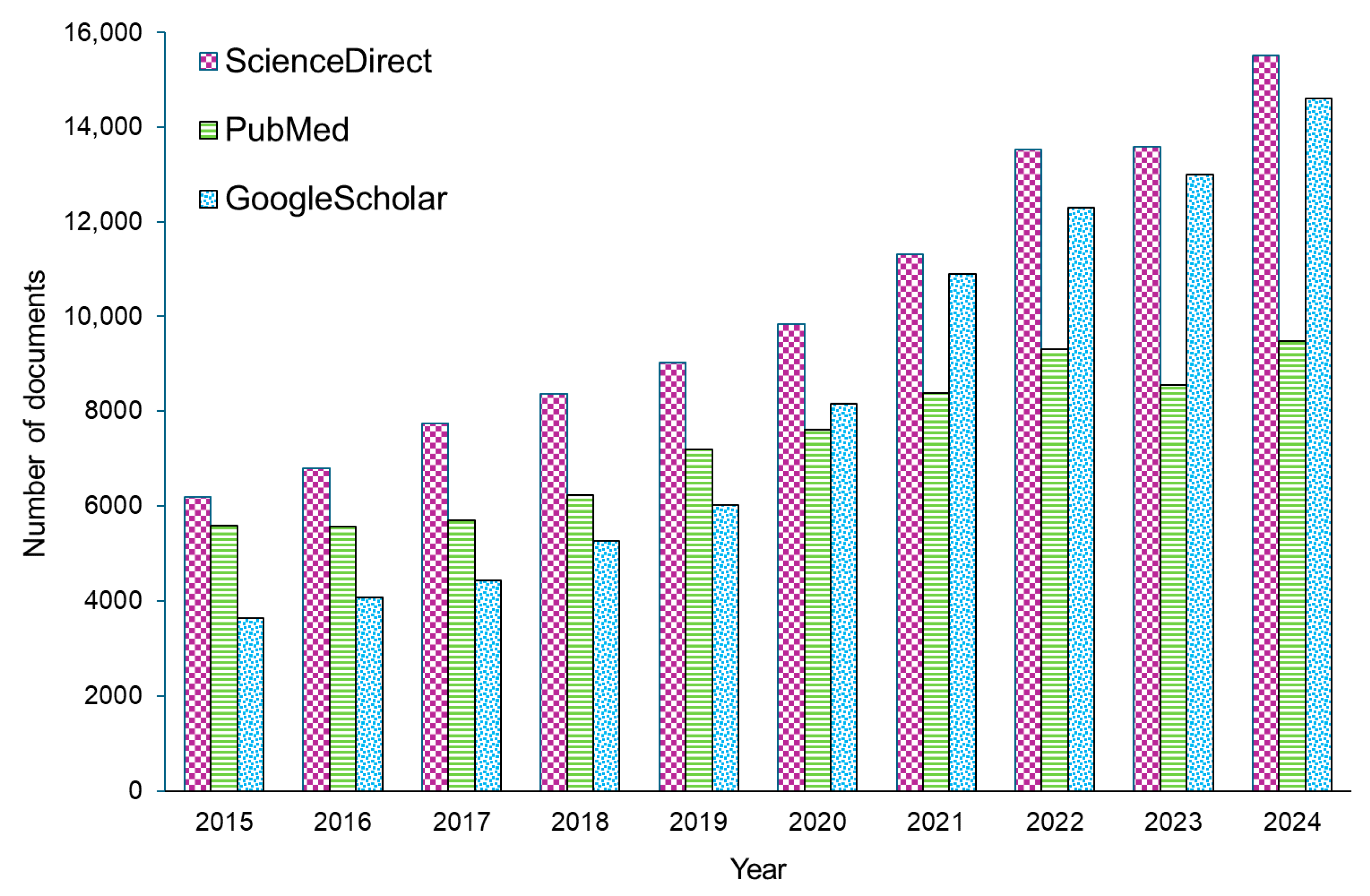
1. Introduction
2. Monitoring of Physiological Parameters
2.1. Glucose Level Monitoring
2.2. Oxygen Level Monitoring
2.3. Intravascular Lactate Biosensors
2.4. Blood Pressure and Heart Rate Monitoring
2.5. Technological Platforms for Advanced Biosensing
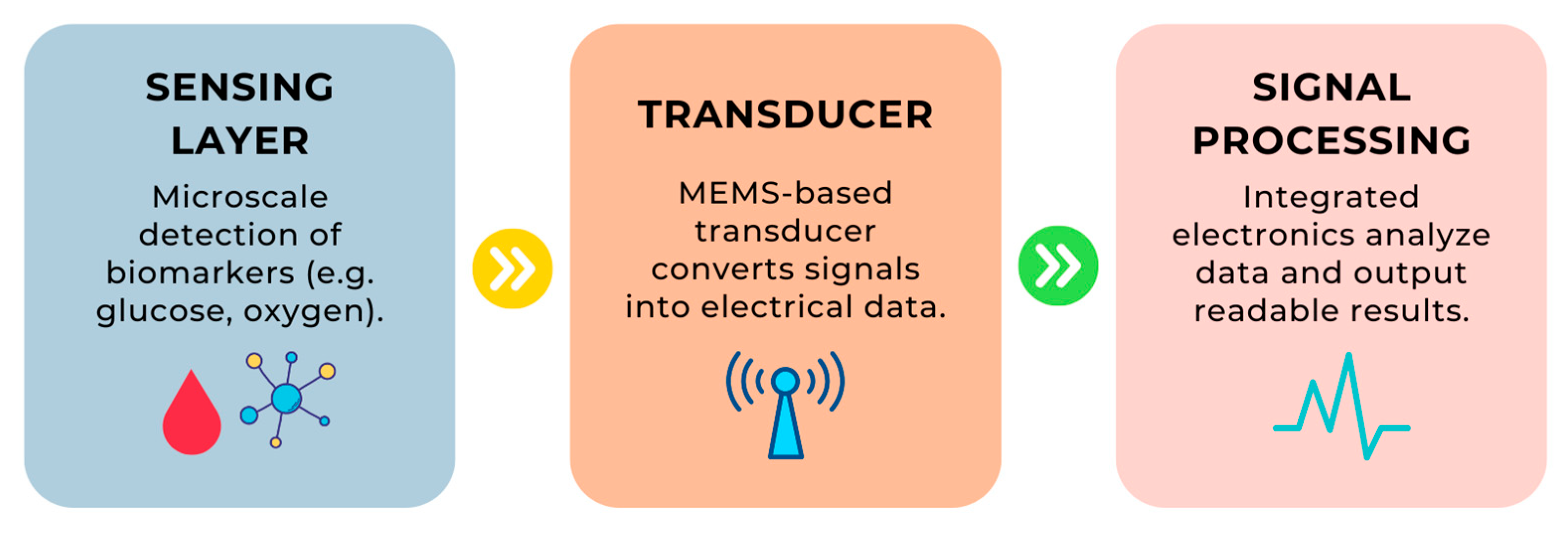
- Step 1: A biomarker (e.g., a glucose molecule) interacts with the sensing layer, which recognizes its presence.
- Step 2: Information about this interaction is converted into a signal by the MEMS transducer.
- Step 3: The signal is processed by the signal processor, which ultimately delivers the result in an understandable format.
2.6. Advantages of Intravascular Biosensors Compared to Conventional Biosensing Platforms
- Faster response times, which are crucial in dynamic clinical settings such as intensive care or surgery [12];
- Higher clinical relevance of measurements, particularly for drugs or metabolites that exhibit compartmentalization (e.g., plasma vs. interstitial fluid) [73];
- Integration with delivery systems (e.g., infusion pumps, stents), enabling closed-loop therapies [49].
3. Disease Diagnostics
3.1. Detection of Disease Biomarkers
3.2. Infection Diagnostics
4. Modern Approaches to Drug Therapy Monitoring and Systems
4.1. Drug Therapy Monitoring
4.2. Drug Delivery Systems
4.3. Examples of Intravascular Sensor Implementations
5. Micro- and Nanotechnology in Intravascular Biosensors
5.1. Stents and Medical Implants
5.2. Nanoparticles in Imaging
- Miniaturization to avoid vascular occlusion and enable deployment via standard catheter systems. Recent reviews emphasize that implantable sensors must be dramatically miniaturized down to sub-millimeter form factors to avoid disrupting the blood flow or damaging vessel walls [148].
- Long-term biocompatibility, requiring advanced antifouling coatings (e.g., PEG, zwitterionic hydrogels). Chronic implantation often results in biofouling and immune encapsulation, degrading sensor performance. Antifouling surfaces such as zwitterionic polymer brushes have been shown to reduce protein adsorption by over 99% and preserve sensitivity in serum for >15 days [149].
- Reliable wireless data transmission from within deep vasculature to external receivers. Deep-tissue telemetry faces challenges related to signal attenuation and power constraints. Reviews note that implantable antennas and optical or RF-based wireless links require careful architectural design to ensure reliability [150].
- Energy autonomy, such as harvesting energy from blood flow or inductive coupling. Techniques like inductive coupling or ultrasound-based wireless power transfer are highlighted as feasible but limited by tissue depth and alignment requirements [151].
- Regulatory hurdles, especially for devices placed in high-risk cardiovascular sites. Implantable devices must meet stringent biocompatibility, sterilization, and safety standards, creating barriers for commercialization [80].
6. Cancer Diagnosis and Treatment
6.1. Biosensor Technologies and Their Diagnostic Applications
6.2. Application of Nanotechnology in Cancer Diagnosis and Monitoring
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haleem, A.; Javaid, M.; Singh, R.P.; Suman, R.; Rab, S. Biosensors Applications in Medical Field: A Brief Review. Sens. Int. 2021, 2, 100100. [Google Scholar] [CrossRef]
- Castillo-Henríquez, L.; Brenes-Acuña, M.; Castro-Rojas, A.; Cordero-Salmerón, R.; Lopretti-Correa, M.; Vega-Baudrit, J.R. Biosensors for the Detection of Bacterial and Viral Clinical Pathogens. Sensors 2020, 20, 6926. [Google Scholar] [CrossRef]
- Salcedo-Arancibia, F.; Gutiérrez, M.; Chavoya, A. Design, Modeling and in Silico Simulation of Bacterial Biosensors for Detecting Heavy Metals in Irrigation Water for Precision Agriculture. Heliyon 2024, 10, e35050. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Baidya, A.; Banerjee, T.; Kumar, A.; Sarkar, A.; Sar, S.; Ghosh, N. Biosensors for Cholesterol Monitoring. In Applications of Biosensors in Healthcare; Academic Press: Cambridge, MA, USA, 2025; Volume 3, pp. 283–298. [Google Scholar] [CrossRef]
- Su, H.; Yan, J.; Yan, X.; Zhao, Q.; Liao, C.; Li, N.; Wang, X. Highly Sensitive Standardized Toxicity Biosensors for Rapid Water Quality Warning. Bioresour. Technol. 2024, 406, 130985. [Google Scholar] [CrossRef]
- Thakur, A.; Kumar, A. Exploring the Potential of Ionic Liquid-Based Electrochemical Biosensors for Real-Time Biomolecule Monitoring in Pharmaceutical Applications: From Lab to Life. Results Eng. 2023, 20, 101533. [Google Scholar] [CrossRef]
- Wahab, M.R.A.; Palaniyandi, T.; Ravi, M.; Viswanathan, S.; Baskar, G.; Surendran, H.; Gangadharan, S.G.D.; Rajendran, B.K. Biomarkers and Biosensors for Early Cancer Diagnosis, Monitoring and Prognosis. Pathol. Res. Pract. 2023, 250, 154812. [Google Scholar] [CrossRef] [PubMed]
- Biosensors Market USD 69.67 Billion by 2034. Available online: https://www.novaoneadvisor.com/report/biosensors-market?utm_source= (accessed on 3 May 2025).
- Mayol, B.; Qubbaj, I.Z.; Nava-Granados, J.; Vasquez, K.; Keene, S.T.; Sempionatto, J.R. Aptamer and Oligonucleotide-Based Biosensors for Health Applications. Biosensors 2025, 15, 277. [Google Scholar] [CrossRef]
- Wang, J. Electrochemical Glucose Biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef]
- Yan, L.; Yang, Y.; Zhang, W.; Chen, X. Advanced Materials and Nanotechnology for Drug Delivery. Adv. Mater. 2014, 26, 5533–5540. [Google Scholar] [CrossRef]
- Rodbard, D. Continuous Glucose Monitoring: A Review of Successes, Challenges, and Opportunities. Diabetes Technol. Ther. 2016, 18, S2-3–S2-13. [Google Scholar] [CrossRef]
- Chandra, P. Personalized Biosensors for Point-of-Care Diagnostics: From Bench to Bedside Applications. Nanotheranostics 2023, 7, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Brazaca, L.C.; Sempionatto, J.R. The Application of Biosensors in Precision Medicine. In Biosensors in Precision Medicine; Elsevier: Amsterdam, The Netherlands, 2024; pp. 133–162. [Google Scholar]
- Fu, J.; Gao, Q.; Li, S. Application of Intelligent Medical Sensing Technology. Biosensors 2023, 13, 812. [Google Scholar] [CrossRef]
- Li, W.; Long, Y.; Yan, Y.; Xiao, K.; Wang, Z.; Zheng, D.; Leal-Junior, A.; Kumar, S.; Ortega, B.; Marques, C.; et al. Wearable Photonic Smart Wristband for Cardiorespiratory Function Assessment and Biometric Identification. Opto-Electron. Adv. 2025, 8, 240254-1–240254-21. [Google Scholar] [CrossRef]
- Yoo, E.-H.; Lee, S.-Y. Glucose Biosensors: An Overview of Use in Clinical Practice. Sensors 2010, 10, 4558–4576. [Google Scholar] [CrossRef]
- Kumar, S.; Iadicicco, A.; Kim, S.; Tosi, D.; Marques, C. Introduction to the Feature Issue: Advances in Optical Biosensors for Biomedical Applications. Biomed. Opt. Express 2024, 15, 3183. [Google Scholar] [CrossRef]
- Youssef, K.; Ullah, A.; Rezai, P.; Hasan, A.; Amirfazli, A. Recent Advances in Biosensors for Real Time Monitoring of PH, Temperature, and Oxygen in Chronic Wounds. Mater. Today Bio 2023, 22, 100764. [Google Scholar] [CrossRef]
- Pereira, A.C.; Sales, M.G.F.; Rodrigues, L.R. Biosensors for Rapid Detection of Breast Cancer Biomarkers. In Advanced Biosensors for Health Care Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 71–103. [Google Scholar] [CrossRef]
- Wu, K.; Saha, R.; Su, D.; Krishna, V.D.; Liu, J.; Cheeran, M.C.-J.; Wang, J.-P. Magnetic Immunoassays: A Review of Virus and Pathogen Detection Before and Amidst the Coronavirus Disease-19 (COVID-19). ACS Appl. Nano Mater. 2020, 3, 9560–9580. [Google Scholar] [CrossRef]
- Pohanka, M. Overview of Piezoelectric Biosensors, Immunosensors and DNA Sensors and Their Applications. Materials 2018, 11, 448. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Das, P.K.; Bhethanabotla, V.R. Surface Acoustic Waves in Biosensing Applications. Sens. Actuators Rep. 2021, 3, 100041. [Google Scholar] [CrossRef]
- Wang, L.; Sipe, D.M.; Xu, Y.; Lin, Q. A MEMS Thermal Biosensor for Metabolic Monitoring Applications. J. Microelectromech. Syst. 2008, 17, 318–327. [Google Scholar] [CrossRef]
- Boateng, E.B.; Ampofo, A.G. A Glimpse into the Future: Modelling Global Prevalence of Hypertension. BMC Public Health 2023, 23, 1906. [Google Scholar] [CrossRef]
- Lee, H.; Hong, Y.J.; Baik, S.; Hyeon, T.; Kim, D.H. Enzyme-Based Glucose Sensor: From Invasive to Wearable Device. Adv. Healthc. Mater. 2018, 7, 1701150. [Google Scholar] [CrossRef]
- Vadgama, P. Monitoring with in Vivo Electrochemical Sensors: Navigating the Complexities of Blood and Tissue Reactivity. Sensors 2020, 20, 3149. [Google Scholar] [CrossRef] [PubMed]
- Bobrowski, T.; Schuhmann, W. Long-Term Implantable Glucose Biosensors. Curr. Opin. Electrochem. 2018, 10, 112–119. [Google Scholar] [CrossRef]
- Wu, J.; Liu, H.; Chen, W.; Ma, B.; Ju, H. Device Integration of Electrochemical Biosensors. Nat. Rev. Bioeng. 2023, 1, 346–360. [Google Scholar] [CrossRef]
- Lucisano, J.Y.; Routh, T.L.; Lin, J.T.; Gough, D.A. Glucose Monitoring in Individuals with Diabetes Using a Long-Term Implanted Sensor/Telemetry System and Model. IEEE Trans. Biomed. Eng. 2017, 64, 1982–1993. [Google Scholar] [CrossRef]
- Strasma, P.J.; Finfer, S.; Flower, O.; Hipszer, B.; Kosiborod, M.; Macken, L.; Sechterberger, M.; Van Der Voort, P.H.J.; DeVries, J.H.; Joseph, J.I. Use of an Intravascular Fluorescent Continuous Glucose Sensor in ICU Patients. J. Diabetes Sci. Technol. 2015, 9, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Crane, B.C.; Barwell, N.P.; Gopal, P.; Gopichand, M.; Higgs, T.; James, T.D.; Jones, C.M.; Mackenzie, A.; Mulavisala, K.P.; Paterson, W. The Development of a Continuous Intravascular Glucose Monitoring Sensor. J. Diabetes Sci. Technol. 2015, 9, 751–761. [Google Scholar] [CrossRef]
- Hemdan, M.; Ali, M.A.; Doghish, A.S.; Mageed, S.S.A.; Elazab, I.M.; Khalil, M.M.; Mabrouk, M.; Das, D.B.; Amin, A.S. Innovations in Biosensor Technologies for Healthcare Diagnostics and Therapeutic Drug Monitoring: Applications, Recent Progress, and Future Research Challenges. Sensors 2024, 24, 5143. [Google Scholar] [CrossRef]
- Estela, C. Blood Glucose Levels. Am. J. Grad. Math. 2011, 3, 12. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Wu, Y.; Lei, Y. Microneedle-Based Glucose Monitoring: A Review from Sampling Methods to Wearable Biosensors. Biomater. Sci. 2023, 11, 5727–5757. [Google Scholar] [CrossRef] [PubMed]
- Rivera, K.R.; Pozdin, V.A.; Young, A.T.; Erb, P.D.; Wisniewski, N.A.; Magness, S.T.; Daniele, M. Integrated Phosphorescence-Based Photonic Biosensor (IPOB) for Monitoring Oxygen Levels in 3D Cell Culture Systems. Biosens. Bioelectron. 2019, 123, 131–140. [Google Scholar] [CrossRef]
- Zhong, W.; Ji, Z.; Sun, C. A Review of Monitoring Methods for Cerebral Blood Oxygen Saturation. Healthcare 2021, 9, 1104. [Google Scholar] [CrossRef]
- Yang, M.-T. Multimodal Neurocritical Monitoring. Biomed. J. 2020, 43, 226–230. [Google Scholar] [CrossRef]
- Roldán, M.; Kyriacou, P.A. Near-Infrared Spectroscopy (NIRS) in Traumatic Brain Injury (TBI). Sensors 2021, 21, 1586. [Google Scholar] [CrossRef]
- Toffaletti, J.G.; Rackley, C.R. Monitoring Oxygen Status. In Advances in Clinical Chemistry; Elsevier: Amsterdam, The Netherlands, 2016; pp. 103–124. [Google Scholar]
- World Health Organization. Clinical Management of COVID-19: Interim Guidance; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Schierenbeck, F.; Nijsten, M.W.N.; Franco-Cereceda, A.; Liska, J. Introducing Intravascular Microdialysis for Continuous Lactate Monitoring in Patients Undergoing Cardiac Surgery: A Prospective Observational Study. Crit. Care 2014, 18, R56. [Google Scholar] [CrossRef]
- Ho, K.K.Y.; Peng, Y.W.; Ye, M.; Tchouta, L.; Schneider, B.; Hayes, M.; Toomasian, J.; Cornell, M.; Rojas-Pena, A.; Charpie, J.; et al. Evaluation of an Anti-Thrombotic Continuous Lactate and Blood Pressure Monitoring Catheter in an In Vivo Piglet Model Undergoing Open-Heart Surgery with Cardiopulmonary Bypass. Chemosensors 2020, 8, 56. [Google Scholar] [CrossRef]
- Setogawa, N.; Ohbe, H.; Matsui, H.; Yasunaga, H. Amputation After Endovascular Therapy With and Without Intravascular Ultrasound Guidance: A Nationwide Propensity Score–Matched Study. Circ. Cardiovasc. Interv. 2023, 16, e012451. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Jia, P.; Liu, J.; Ren, Q.; An, G.; Liang, T.; Xiong, J. MEMS-Based Reflective Intensity-Modulated Fiber-Optic Sensor for Pressure Measurements. Sensors 2020, 20, 2233. [Google Scholar] [CrossRef]
- Park, J.; Seo, B.; Jeong, Y.; Park, I. A Review of Recent Advancements in Sensor-Integrated Medical Tools. Adv. Sci. 2024, 11, 2307427. [Google Scholar] [CrossRef]
- Tang, C.; Liu, Z.; Li, L. Mechanical Sensors for Cardiovascular Monitoring: From Battery-Powered to Self-Powered. Biosensors 2022, 12, 651. [Google Scholar] [CrossRef]
- Javaid, S.; Zeadally, S.; Fahim, H.; He, B. Medical Sensors and Their Integration in Wireless Body Area Networks for Pervasive Healthcare Delivery: A Review. IEEE Sens. J. 2022, 22, 3860–3877. [Google Scholar] [CrossRef]
- Hoare, D.; Bussooa, A.; Neale, S.; Mirzai, N.; Mercer, J. The Future of Cardiovascular Stents: Bioresorbable and Integrated Biosensor Technology. Adv. Sci. 2019, 6, 1900856. [Google Scholar] [CrossRef] [PubMed]
- Chircov, C.; Grumezescu, A.M. Microelectromechanical Systems (MEMS) for Biomedical Applications. Micromachines 2022, 13, 164. [Google Scholar] [CrossRef]
- Hou, S.; Zhang, A.; Su, M. Nanomaterials for Biosensing Applications. Nanomaterials 2016, 6, 58. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The Rise of Graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Huang, H.; Su, S.; Wu, N.; Wan, H.; Wan, S.; Bi, H.; Sun, L. Graphene-Based Sensors for Human Health Monitoring. Front. Chem. 2019, 7, 399. [Google Scholar] [CrossRef]
- Medintz, I.L.; Uyeda, H.T.; Goldman, E.R.; Mattoussi, H. Quantum Dot Bioconjugates for Imaging, Labelling and Sensing. Nat. Mater. 2005, 4, 435–446. [Google Scholar] [CrossRef]
- Matea, C.T.; Mocan, T.; Tabaran, F.; Pop, T.; Mosteanu, O.; Puia, C.; Iancu, C.; Mocan, L. Quantum Dots in Imaging, Drug Delivery and Sensor Applications. Int. J. Nanomed. 2017, 12, 5421–5431. [Google Scholar] [CrossRef]
- Napi, M.L.M.; Sultan, S.M.; Ismail, R.; How, K.W.; Ahmad, M.K. Electrochemical-Based Biosensors on Different Zinc Oxide Nanostructures: A Review. Materials 2019, 12, 2985. [Google Scholar] [CrossRef] [PubMed]
- Wallace, G.Q.; Lagugné-Labarthet, F. Advancements in Fractal Plasmonics: Structures, Optical Properties, and Applications. Analyst 2018, 144, 13–30. [Google Scholar] [CrossRef]
- Ingawale, D.S.; Iyer, D.N. Precision Health: Exploring Biosensors in Hypertension Management. In Futuristic Trends in Medical Sciences; Iterative International Publisher, Selfypage Developers Pvt Ltd.: Chikmagalur, India, 2024; Volume 3, pp. 224–236. [Google Scholar]
- Ojha, M.K.; Wadhwani, S.; Wadhwani, A.K.; Shukla, A. Automatic Detection of Arrhythmias from an ECG Signal Using an Auto-Encoder and SVM Classifier. Phys. Eng. Sci. Med. 2022, 45, 665–674. [Google Scholar] [CrossRef]
- Singh, S.; Kuschner, W.G.; Lighthall, G. Perioperative Intravascular Fluid Assessment and Monitoring: A Narrative Review of Established and Emerging Techniques. Anesthesiol. Res. Pract. 2011, 2011, 231493. [Google Scholar] [CrossRef]
- Dong, T.; Zhu, W.; Yang, Z.; Matos Pires, N.M.; Lin, Q.; Jing, W.; Zhao, L.; Wei, X.; Jiang, Z. Advances in Heart Failure Monitoring: Biosensors Targeting Molecular Markers in Peripheral Bio-Fluids. Biosens. Bioelectron. 2024, 255, 116090. [Google Scholar] [CrossRef] [PubMed]
- Crnich, C.J.; Maki, D.G. The Promise of Novel Technology for the Prevention of Intravascular Device–Related Bloodstream Infection. I. Pathogenesis and Short-Term Devices. Clin. Infect. Dis. 2002, 34, 1232–1242. [Google Scholar] [CrossRef]
- Shuvo, M.M.H.; Titirsha, T.; Amin, N.; Islam, S.K. Energy Harvesting in Implantable and Wearable Medical Devices for Enduring Precision Healthcare. Energies 2022, 15, 7495. [Google Scholar] [CrossRef]
- Jin, X.; Liu, C.; Xu, T.; Su, L.; Zhang, X. Artificial Intelligence Biosensors: Challenges and Prospects. Biosens. Bioelectron. 2020, 165, 112412. [Google Scholar] [CrossRef]
- Singh, R.; Bathaei, M.J.; Istif, E.; Beker, L. A Review of Bioresorbable Implantable Medical Devices: Materials, Fabrication, and Implementation. Adv. Healthc. Mater. 2020, 9, 2000790. [Google Scholar] [CrossRef]
- Ji, C.; Jiang, T.; Liu, L.; Zhang, J.; You, L. Continuous Glucose Monitoring Combined with Artificial Intelligence: Redefining the Pathway for Prediabetes Management. Front. Endocrinol. 2025, 16, 1571362. [Google Scholar] [CrossRef] [PubMed]
- Flynn, C.D.; Chang, D.; Flynn, C.D.; Chang, D. Artificial Intelligence in Point-of-Care Biosensing: Challenges and Opportunities. Diagnostics 2024, 14, 1100. [Google Scholar] [CrossRef]
- Omar, R.; Saliba, W.; Khatib, M.; Zheng, Y.; Pieters, C.; Oved, H.; Silberman, E.; Zohar, O.; Hu, Z.; Kloper, V.; et al. Biodegradable, Biocompatible, and Implantable Multifunctional Sensing Platform for Cardiac Monitoring. ACS Sens. 2024, 9, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Cai, A.; Xu, T.; Zhang, X. Artificial Intelligence Biosensors for Continuous Glucose Monitoring. Interdiscip. Mater. 2023, 2, 290–307. [Google Scholar] [CrossRef]
- Chen, X.; Manshaii, F.; Tioran, K.; Wang, S.; Zhou, Y.; Zhao, J.; Yang, M.; Yin, X.; Liu, S.; Wang, K. Wearable Biosensors for Cardiovascular Monitoring Leveraging Nanomaterials. Adv. Compos. Hybrid. Mater. 2024, 7, 97. [Google Scholar] [CrossRef]
- Alam, F.; Ashfaq Ahmed, M.; Jalal, A.H.; Siddiquee, I.; Adury, R.Z.; Hossain, G.M.M.; Pala, N. Recent Progress and Challenges of Implantable Biodegradable Biosensors. Micromachines 2024, 15, 475. [Google Scholar] [CrossRef]
- Alyami, A.M.; Kirimi, M.T.; Neale, S.L.; Mercer, J.R. Implantable Biosensors for Vascular Diseases: Directions for the Next Generation of Active Diagnostic and Therapeutic Medical Device Technologies. Biosensors 2025, 15, 147. [Google Scholar] [CrossRef]
- Li, J.; Centurion, F.; Chen, R.; Gu, Z. Intravascular Imaging of Atherosclerosis by Using Engineered Nanoparticles. Biosensors 2023, 13, 319. [Google Scholar] [CrossRef] [PubMed]
- Hosain, M.N.; Kwak, Y.S.; Lee, J.; Choi, H.; Park, J.; Kim, J. IoT-Enabled Biosensors for Real-Time Monitoring and Early Detection of Chronic Diseases. Phys. Act. Nutr. 2024, 28, 60–69. [Google Scholar] [CrossRef]
- Ma, C.; Matin Nazar, A.; Moradi, A.H.; Goharian, H.; Mao, G.; Yari, M.; Ji, X.; Dong, S. Advanced Triboelectric Nanogenerator Sensing Technologies for High-Efficiency Cardiovascular Monitoring. Energy Technol. 2025, 13, 2401863. [Google Scholar] [CrossRef]
- Manoharan Nair Sudha Kumari, S.; Thankappan Suryabai, X. Sensing the Future–Frontiers in Biosensors: Exploring Classifications, Principles, and Recent Advances. ACS Omega 2024, 9, 48918–48987. [Google Scholar] [CrossRef]
- Smith, J.L.; Rice, M.J. Why Have So Many Intravascular Glucose Monitoring Devices Failed? J. Diabetes Sci. Technol. 2015, 9, 782. [Google Scholar] [CrossRef]
- Aberer, F.; Theiler-Schwetz, V.; Ziko, H.; Hausegger, B.; Wiederstein-Grasser, I.; Hochfellner, D.A.; Eller, P.; Tomberger, G.; Ellmerer, M.; Mader, J.K.; et al. Accuracy and Stability of an Arterial Sensor for Glucose Monitoring in a Porcine Model Using Glucose Clamp Technique. Sci. Rep. 2020, 10, 6604. [Google Scholar] [CrossRef]
- Van Steen, S.C.J.; Rijkenberg, S.; Limpens, J.; Van Der Voort, P.H.J.; Hermanides, J.; DeVries, J.H. The Clinical Benefits and Accuracy of Continuous Glucose Monitoring Systems in Critically Ill Patients—A Systematic Scoping Review. Sensors 2017, 17, 146. [Google Scholar] [CrossRef] [PubMed]
- Yogev, D.; Goldberg, T.; Arami, A.; Tejman-Yarden, S.; Winkler, T.E.; Maoz, B.M. Current State of the Art and Future Directions for Implantable Sensors in Medical Technology: Clinical Needs and Engineering Challenges. APL Bioeng. 2023, 7, 031506. [Google Scholar] [CrossRef]
- Chaum, E.; Lindner, E. A “Smart” Biosensor-Enabled Intravascular Catheter and Platform for Dynamic Delivery of Propofol to “Close the Loop” for Total Intravenous Anesthesia. Mil. Med. 2021, 186, 370–377. [Google Scholar] [CrossRef]
- Moore, T.J.; Moody, A.S.; Payne, T.D.; Sarabia, G.M.; Daniel, A.R.; Sharma, B. In Vitro and In Vivo SERS Biosensing for Disease Diagnosis. Biosensors 2018, 8, 46. [Google Scholar] [CrossRef]
- Lv, F.; Qiu, T.; Liu, L.; Ying, J.; Wang, S. Recent Advances in Conjugated Polymer Materials for Disease Diagnosis. Small 2016, 12, 696–705. [Google Scholar] [CrossRef]
- Bayes-Genis, A.; Docherty, K.F.; Petrie, M.C.; Januzzi, J.L.; Mueller, C.; Anderson, L.; Bozkurt, B.; Butler, J.; Chioncel, O.; Cleland, J.G.F.; et al. Practical Algorithms for Early Diagnosis of Heart Failure and Heart Stress Using NT-ProBNP: A Clinical Consensus Statement from the Heart Failure Association of the ESC. Eur. J. Heart Fail. 2023, 25, 1891–1898. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, J.E.; Stratton, C.W.; Persing, D.H.; Tang, Y.-W. Forty Years of Molecular Diagnostics for Infectious Diseases. J. Clin. Microbiol. 2022, 60, e02446-21. [Google Scholar] [CrossRef]
- Devulapally, P.R.; Bürger, J.; Mielke, T.; Konthur, Z.; Lehrach, H.; Yaspo, M.-L.; Glökler, J.; Warnatz, H.-J. Simple Paired Heavy- and Light-Chain Antibody Repertoire Sequencing Using Endoplasmic Reticulum Microsomes. Genome Med. 2018, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Morales, A.J.; Ramírez-Vallejo, E.; Alvarado-Arnez, L.E.; Paniz-Mondolfi, A.; Zambrano, L.I.; Ko, A.I. Fatal Zika Virus Disease in Adults: A Critical Reappraisal of an under-Recognized Clinical Entity. Int. J. Infect. Dis. 2019, 83, 160–162. [Google Scholar] [CrossRef]
- Khan, S.; Barve, K.H.; Kumar, M.S. Recent Advancements in Pathogenesis, Diagnostics and Treatment of Alzheimer’s Disease. Curr. Neuropharmacol. 2020, 18, 1106–1125. [Google Scholar] [CrossRef] [PubMed]
- Twarowski, B.; Herbet, M. Inflammatory Processes in Alzheimer’s Disease—Pathomechanism, Diagnosis and Treatment: A Review. Int. J. Mol. Sci. 2023, 24, 6518. [Google Scholar] [CrossRef]
- Porsteinsson, A.P.; Isaacson, R.S.; Knox, S.; Sabbagh, M.N.; Rubino, I. Diagnosis of Early Alzheimer’s Disease: Clinical Practice in 2021. J. Prev. Alzheimers Dis. 2021, 8, 371–386. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Wang, Q.; Li, Q.; Cui, S.; Chen, K.; Gu, T.; Li, S.; Bai, P. Novel Hyperbranched Rolling Circle Amplification-Driven Aptasensor for Ultrasensitive and Multiplex Biomarkers Detection of Alzheimer’s Disease. Sens. Actuators B Chem. 2025, 438, 137816. [Google Scholar] [CrossRef]
- Wilkins, E.; Atanasov, P. Glucose Monitoring: State of the Art and Future Possibilities. Med. Eng. Phys. 1996, 18, 273–288. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Zhang, F.; Zhang, C.; Zheng, L.-R.; Yang, J. The Biomarkers for Acute Myocardial Infarction and Heart Failure. Biomed. Res. Int. 2020, 2020, 2018035. [Google Scholar] [CrossRef]
- Pepys, M.B.; Hirschfield, G.M. C-Reactive Protein: A Critical Update. J. Clin. Investig. 2003, 111, 1805–1812. [Google Scholar] [CrossRef]
- Ng, S.B.; Turner, E.H.; Robertson, P.D.; Flygare, S.D.; Bigham, A.W.; Lee, C.; Shaffer, T.; Wong, M.; Bhattacharjee, A.; Eichler, E.E.; et al. Targeted Capture and Massively Parallel Sequencing of 12 Human Exomes. Nature 2009, 461, 272–276. [Google Scholar] [CrossRef]
- Mardis, E.R. The Impact of Next-Generation Sequencing Technology on Genetics. Trends Genet. 2008, 24, 133–141. [Google Scholar] [CrossRef]
- Wekalao, J.; Prasad Srinivasan, G.; Patel, S.K.; Ahmed Al-zahrani, F. Optimization of Graphene-Based Biosensor Design for Haemoglobin Detection Using the Gradient Boosting Algorithm for Behaviour Prediction. Measurement 2025, 239, 115452. [Google Scholar] [CrossRef]
- Yadav, S.; Jangra, R.; Sharma, B.R.; Sharma, M. Current Advancement in Biosensing Techniques for Determination of Alanine Aminotransferase and Aspartate Aminotransferase—A Mini Review. Process Biochem. 2022, 114, 71–76. [Google Scholar] [CrossRef]
- Wu, Y.; Liang, R.; Chen, W.; Wang, C.; Xing, D. The Development of Biosensors for Alkaline Phosphatase Activity Detection Based on a Phosphorylated DNA Probe. Talanta 2024, 270, 125622. [Google Scholar] [CrossRef]
- Wells, P.K.; Smutok, O.; Guo, Z.; Alexandrov, K.; Katz, E. Fluorometric Biosensing of α-Amylase Using an Artificial Allosteric Biosensor Immobilized on Nanostructured Interface. Talanta 2023, 255, 124215. [Google Scholar] [CrossRef]
- Manolov, D.E.; Röcker, C.; Hombach, V.; Nienhaus, G.U.; Torzewski, J. Plasmonic Optical Biosensors for Detecting C-Reactive Protein: A Review. Micromachines 2020, 11, 895. [Google Scholar] [CrossRef]
- Lee, T.; Ahn, J.H.; Choi, J.; Lee, Y.; Kim, J.M.; Park, C.; Jang, H.; Kim, T.H.; Lee, M.H. Development of the Troponin Detection System Based on the Nanostructure. Micromachines 2019, 10, 203. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, S.; Ebrahimi, F.; Saeni, A.; Kheiri, H.; Shamsara, M. CRISPR-Cas Based Biosensors as Innovative Platforms for Diagnosis of Human Papilloma Virus Infection. Microchem. J. 2025, 210, 112991. [Google Scholar] [CrossRef]
- Hasöksüz, M.; Kiliç, S.; Saraç, F. Coronaviruses and SARS-CoV-2. Turk. J. Med. Sci. 2020, 50, 549–556. [Google Scholar] [CrossRef]
- Borst, A.; Box, A.T.A.; Fluit, A.C. False-Positive Results and Contamination in Nucleic Acid Amplification Assays: Suggestions for a Prevent and Destroy Strategy. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 289–299. [Google Scholar] [CrossRef]
- Jolany Vangah, S.; Katalani, C.; Boone, H.A.; Hajizade, A.; Sijercic, A.; Ahmadian, G. CRISPR-Based Diagnosis of Infectious and Noninfectious Diseases. Biol. Proced. Online 2020, 22, 22. [Google Scholar] [CrossRef]
- He, R.-R.; Yue, G.-L.; Dong, M.-L.; Wang, J.-Q.; Cheng, C. Sepsis Biomarkers: Advancements and Clinical Applications—A Narrative Review. Int. J. Mol. Sci. 2024, 25, 9010. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Singh, A.; Dhaka, P.; Singh, A.; Agarwala, P.; Sharma, K.; Bhargava, A.; Bhatia, S.; Launey, T.; Kaushik, R.; et al. A Label-Free Gold Nanoparticles Functionalized Peptide Dendrimer Biosensor for Visual Detection of Breakthrough Infections in COVID-19 Vaccinated Patients. Sens. Biosensing Res. 2025, 47, 100718. [Google Scholar] [CrossRef]
- Li, P.; Lee, G.H.; Kim, S.Y.; Kwon, S.Y.; Kim, H.R.; Park, S. From Diagnosis to Treatment: Recent Advances in Patient-Friendly Biosensors and Implantable Devices. ACS Nano 2021, 15, 1960–2004. [Google Scholar] [CrossRef] [PubMed]
- Meneghello, A.; Tartaggia, S.; Alvau, M.D.; Polo, F.; Toffoli, G. Biosensing Technologies for Therapeutic Drug Monitoring. Curr. Med. Chem. 2017, 25, 4354–4377. [Google Scholar] [CrossRef]
- Wang, Q.; Li, S.; Chen, J.; Yang, L.; Qiu, Y.; Du, Q.; Wang, C.; Teng, M.; Wang, T.; Dong, Y. A Novel Strategy for Therapeutic Drug Monitoring: Application of Biosensors to Quantify Antimicrobials in Biological Matrices. J. Antimicrob. Chemother. 2023, 78, 2612–2629. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.W.; Yang, R.Y.; Wu, M.J.; Yu, Z.W.; Han, J.W.; Zhang, C.Z.; Huang, P.F.; Liu, A.L.; Liu, M.M. Therapeutic Drug Monitoring of Methotrexate by Disposable SPCE Biosensor for Personalized Medicine. Anal. Chim. Acta 2025, 1335, 343473. [Google Scholar] [CrossRef] [PubMed]
- Chellal, W.; Metarfi, Y.; Ben Khadda, Z.; Hoummani, H.; Berrady, R.; Achour, S. The Interest of Therapeutic and Pharmacological Drug Monitoring of Methotrexate: A Systematic Review. Semin. Oncol. 2025, 52, 152342. [Google Scholar] [CrossRef]
- Eskiköy Bayraktepe, D.; Yıldız, C.; Yazan, Z. The Development of Electrochemical DNA Biosensor Based on Poly-l-Methionine and Bimetallic AuPt Nanoparticles Coating: Picomolar Detection of Imatinib and Erlotinib. Talanta 2023, 257, 124361. [Google Scholar] [CrossRef]
- Chen, J.; Alberi, L.; Pétermann, Y.; Buclin, T.; Guidi, M.; Carrara, S. Imatinib Detection by Memristive Biosensors for Therapeutic Drug Monitoring. Biosens. Bioelectron. X 2025, 24, 100620. [Google Scholar] [CrossRef]
- Qin, S.N.; Xie, H.H.; Cao, Y.J.; Wan, T.; Feng, L.; Salminen, K.; Sun, J.J. Construction of an Electrochemical Aptamer-Based Sensors for Rapid Quantification of the Anticancer Drug Imatinib in Blood to Improve Drug Bioavailability at Microdoses. Int. J. Biol. Macromol. 2024, 282, 137325. [Google Scholar] [CrossRef]
- McKeating, K.S.; Aubé, A.; Masson, J.F. Biosensors and Nanobiosensors for Therapeutic Drug and Response Monitoring. Analyst 2016, 141, 429–449. [Google Scholar] [CrossRef]
- Habet, S. Narrow Therapeutic Index Drugs: Clinical Pharmacology Perspective. J. Pharm. Pharmacol. 2021, 73, 1285–1291. [Google Scholar] [CrossRef]
- Scholten, K.; Meng, E. A Review of Implantable Biosensors for Closed-Loop Glucose Control and Other Drug Delivery Applications. Int. J. Pharm. 2018, 544, 319–334. [Google Scholar] [CrossRef]
- Psotta, C.; Cirovic, S.; Gudmundsson, P.; Falk, M.; Mandal, T.; Reichhart, T.; Leech, D.; Ludwig, R.; Kittel, R.; Schuhmann, W.; et al. Continuous Ex Vivo Glucose Sensing in Human Physiological Fluids Using an Enzymatic Sensor in a Vein Replica. Bioelectrochemistry 2023, 152, 8–10. [Google Scholar] [CrossRef]
- Mage, P.L.; Ferguson, B.S.; Maliniak, D.; Ploense, K.L.; Kippin, T.E.; Soh, H.T. Closed-Loop Control of Circulating Drug Levels in Live Animals. Nat. Biomed. Eng. 2017, 1, 0070. [Google Scholar] [CrossRef]
- Weber, S.; Tombelli, S.; Giannetti, A.; Trono, C.; O’Connell, M.; Wen, M.; Descalzo, A.B.; Bittersohl, H.; Bietenbeck, A.; Marquet, P.; et al. Immunosuppressant Quantification in Intravenous Microdialysate—Towards Novel Quasi-Continuous Therapeutic Drug Monitoring in Transplanted Patients. Clin. Chem. Lab. Med. 2021, 59, 935–945. [Google Scholar] [CrossRef]
- Moonla, C.; Goud, K.Y.; Teymourian, H.; Tangkuaram, T.; Ingrande, J.; Suresh, P.; Wang, J. An Integrated Microcatheter-Based Dual-Analyte Sensor System for Simultaneous, Real-Time Measurement of Propofol and Fentanyl. Talanta 2020, 218, 121205. [Google Scholar] [CrossRef] [PubMed]
- Ezike, T.C.; Okpala, U.S.; Onoja, U.L.; Nwike, C.P.; Ezeako, E.C.; Okpara, O.J.; Okoroafor, C.C.; Eze, S.C.; Kalu, O.L.; Odoh, E.C.; et al. Advances in Drug Delivery Systems, Challenges and Future Directions. Heliyon 2023, 9, e17488. [Google Scholar] [CrossRef] [PubMed]
- Cicha, I.; Priefer, R.; Severino, P.; Souto, E.B.; Jain, S. Biosensor-Integrated Drug Delivery Systems as New Materials for Biomedical Applications. Biomolecules 2022, 12, 1198. [Google Scholar] [CrossRef] [PubMed]
- Thankathuraipandian, S.; Greenleaf, W.; Kyani, A.; Tomlinson, T.; Balasingh, B.; Ross, E.; Pathak, Y. Development of a Remote Therapeutic Monitoring Platform: Applications for Movement Disorders. Sci. Rep. 2024, 14, 29837. [Google Scholar] [CrossRef]
- Ngoepe, M.; Choonara, Y.E.; Tyagi, C.; Tomar, L.K.; du Toit, L.C.; Kumar, P.; Ndesendo, V.M.K.; Pillay, V. Integration of Biosensors and Drug Delivery Technologies for Early Detection and Chronic Management of Illness. Sensors 2013, 13, 7680–7713. [Google Scholar] [CrossRef]
- Lee, H.J.; Choi, N.; Yoon, E.S.; Cho, I.J. MEMS Devices for Drug Delivery. Adv. Drug Deliv. Rev. 2018, 128, 132–147. [Google Scholar] [CrossRef]
- Jeong, J.W.; McCall, J.G.; Shin, G.; Zhang, Y.; Al-Hasani, R.; Kim, M.; Li, S.; Sim, J.Y.; Jang, K.I.; Shi, Y.; et al. Wireless Optofluidic Systems for Programmable In Vivo Pharmacology and Optogenetics. Cell 2015, 162, 662–674. [Google Scholar] [CrossRef]
- Diabetes Technology Society. Pre-Meeting Workshops Agenda. J. Diabetes Sci. Technol. 2012, 6, 453–461. [Google Scholar] [CrossRef]
- Fanelli, A.; Ghezzi, D. Transient Electronics: New Opportunities for Implantable Neurotechnology. Curr. Opin. Biotechnol. 2021, 72, 22–28. [Google Scholar] [CrossRef]
- Shen, L.; Wang, P.; Ke, Y. DNA Nanotechnology-Based Biosensors and Therapeutics. Adv. Healthc. Mater. 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jiang, Q.; Wang, Y.; Ding, B. Biomedical Applications of DNA-Based Molecular Devices. Adv. Healthc. Mater. 2019, 8, 2002205. [Google Scholar] [CrossRef]
- Ramesh, M.; Janani, R.; Deepa, C.; Rajeshkumar, L. Nanotechnology-Enabled Biosensors: A Review of Fundamentals, Design Principles, Materials, and Applications. Biosensors 2022, 13, 40. [Google Scholar] [CrossRef] [PubMed]
- Guk, K.; Han, G.; Lim, J.; Jeong, K.; Kang, T.; Lim, E.-K.; Jung, J. Evolution of Wearable Devices with Real-Time Disease Monitoring for Personalized Healthcare. Nanomaterials 2019, 9, 813. [Google Scholar] [CrossRef] [PubMed]
- Tovar-Lopez, F.J. Recent Progress in Micro- and Nanotechnology-Enabled Sensors for Biomedical and Environmental Challenges. Sensors 2023, 23, 5406. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Stone, G.W.; Ormiston, J.; Kastrati, A. Coronary Balloon Angioplasty, Stents, and Scaffolds. Lancet 2017, 390, 781–792. [Google Scholar] [CrossRef]
- Hauser, L.J.; Turner, J.H.; Chandra, R.K. Trends in the Use of Stents and Drug-Eluting Stents in Sinus Surgery. Otolaryngol. Clin. North. Am. 2017, 50, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Decker, R.E.; Lamantia, Z.E.; Emrick, T.S.; Figueiredo, M.L. Sonodelivery in Skeletal Muscle: Current Approaches and Future Potential. Bioengineering 2020, 7, 107. [Google Scholar] [CrossRef]
- Beach, M.A.; Nayanathara, U.; Gao, Y.; Zhang, C.; Xiong, Y.; Wang, Y.; Such, G.K. Polymeric Nanoparticles for Drug Delivery. Chem. Rev. 2024, 124, 5505–5616. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Stafford, G.R.; Beauchamp, C.; Kim, S.A. Development of a Dental Implantable Temperature Sensor for Real-Time Diagnosis of Infectious Disease. Sensors 2020, 20, 3953. [Google Scholar] [CrossRef]
- Ardakani, A.B.; Nayeri, M.; Nasirizadeh, N.; Ostovari, F.; Seifati, S.M. Development of an Electrochemical Biosensor Based on MoSe2 Nanoparticles and AuNPs Modified Silicon Wafer for Measuring Lung Cancer Biomarker, MiR-21. Microchem. J. 2025, 213, 113805. [Google Scholar] [CrossRef]
- Cui, F.; Chen, W.; Wang, P.; Fan, J.; Si, D.; Ma, Q.; Shi, J.; He, Y. Gold Metallene-Based ECL Biosensor to Detect MiRNA-126 for Coronary Artery Calcification Diagnosis. Biosens. Bioelectron. 2025, 271, 116993. [Google Scholar] [CrossRef]
- Chen, T.; Sheng, A.; Hu, Y.; Mao, D.; Ning, L.; Zhang, J. Modularization of Three-Dimensional Gold Nanoparticles/Ferrocene/Liposome Cluster for Electrochemical Biosensor. Biosens. Bioelectron. 2019, 124–125, 115–121. [Google Scholar] [CrossRef]
- Najdian, A.; Beiki, D.; Abbasi, M.; Gholamrezanezhad, A.; Ahmadzadehfar, H.; Amani, A.M.; Ardestani, M.S.; Assadi, M. Exploring Innovative Strides in Radiolabeled Nanoparticle Progress for Multimodality Cancer Imaging and Theranostic Applications. Cancer Imaging 2024, 24, 127. [Google Scholar] [CrossRef]
- Ou, F.-S.; Michiels, S.; Shyr, Y.; Adjei, A.A.; Oberg, A.L. Biomarker Discovery and Validation: Statistical Considerations. J. Thorac. Oncol. 2021, 16, 537–545. [Google Scholar] [CrossRef]
- Sharma, V.; Dutta, S.; Roy, R.K.; Manna, S.; Choudhury, S.M.; Patra, G.K. Highly Sensitive Benzildihydrazone-N,N’-Bis(2-Hydroxy-4-Diethylamino-1-Formylbenzene) Stabilized ZnS Nanoparticles as Potential Optical Chemosensors for Hg2+ Ions: Anticancer Activity and Biosensor Imaging. J. Mol. Struct. 2025, 1329, 141398. [Google Scholar] [CrossRef]
- He, R.; Liu, H.; Niu, Y.; Zhang, H.; Genin, G.M.; Xu, F. Flexible Miniaturized Sensor Technologies for Long-Term Physiological Monitoring. NPJ Flex. Electron. 2022, 6, 20. [Google Scholar] [CrossRef]
- Hu, Y.; Liang, B.; Fang, L.; Ma, G.; Yang, G.; Zhu, Q.; Chen, S.; Ye, X. Antifouling Zwitterionic Coating via Electrochemically Mediated Atom Transfer Radical Polymerization on Enzyme-Based Glucose Sensors for Long-Time Stability in 37 °C Serum. Langmuir 2016, 32, 11763–11770. [Google Scholar] [CrossRef] [PubMed]
- Boulogeorgos, A.A.A.; Trevlakis, S.E.; Chatzidiamantis, N.D. Optical Wireless Communications for In-Body and Transdermal Biomedical Applications. IEEE Commun. Mag. 2021, 59, 119–125. [Google Scholar] [CrossRef]
- Haerinia, M.; Shadid, R. Wireless Power Transfer Approaches for Medical Implants: A Review. Signals 2020, 1, 209–229. [Google Scholar] [CrossRef]
- Zhu, R.; Avsievich, T.; Popov, A.; Bykov, A.; Meglinski, I. In Vivo Nano-Biosensing Element of Red Blood Cell-Mediated Delivery. Biosens. Bioelectron. 2021, 175, 112845. [Google Scholar] [CrossRef]
- Dixit, C.K.; Kadimisetty, K.; Otieno, B.A.; Tang, C.; Malla, S.; Krause, C.E.; Rusling, J.F. Electrochemistry-Based Approaches to Low Cost, High Sensitivity, Automated, Multiplexed Protein Immunoassays for Cancer Diagnostics. Analyst 2016, 141, 536–547. [Google Scholar] [CrossRef]
- Mendoza, A.; Torrisi, D.M.; Sell, S.; Cady, N.C.; Lawrence, D.A. Grating Coupled SPR Microarray Analysis of Proteins and Cells in Blood from Mice with Breast Cancer. Analyst 2016, 141, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, Z.; Zhou, S.; Jiang, L.P.; Zhu, J.J. An Electrochemical-TUNEL Method for Sensitive Detection of Apoptotic Cells. Analyst 2016, 141, 567–569. [Google Scholar] [CrossRef]
- Yockell-Lelièvre, H.; Bukar, N.; Toulouse, J.L.; Pelletier, J.N.; Masson, J.F. Naked-Eye Nanobiosensor for Therapeutic Drug Monitoring of Methotrexate. Analyst 2016, 141, 697–703. [Google Scholar] [CrossRef]
- García de Arquer, F.P.; Talapin, D.V.; Klimov, V.I.; Arakawa, Y.; Bayer, M.; Sargent, E.H. Semiconductor Quantum Dots: Technological Progress and Future Challenges. Science 2021, 373, eaaz8541. [Google Scholar] [CrossRef]
- Wagner, M.K.; Li, F.; Li, J.; Li, X.F.; Le, X.C. Use of Quantum Dots in the Development of Assays for Cancer Biomarkers. Anal. Bioanal. Chem. 2010, 397, 3213–3224. [Google Scholar] [CrossRef] [PubMed]
- Iannazzo, D.; Espro, C.; Celesti, C.; Ferlazzo, A.; Neri, G. Smart Biosensors for Cancer Diagnosis Based on Graphene Quantum Dots. Cancers 2021, 13, 3194. [Google Scholar] [CrossRef] [PubMed]

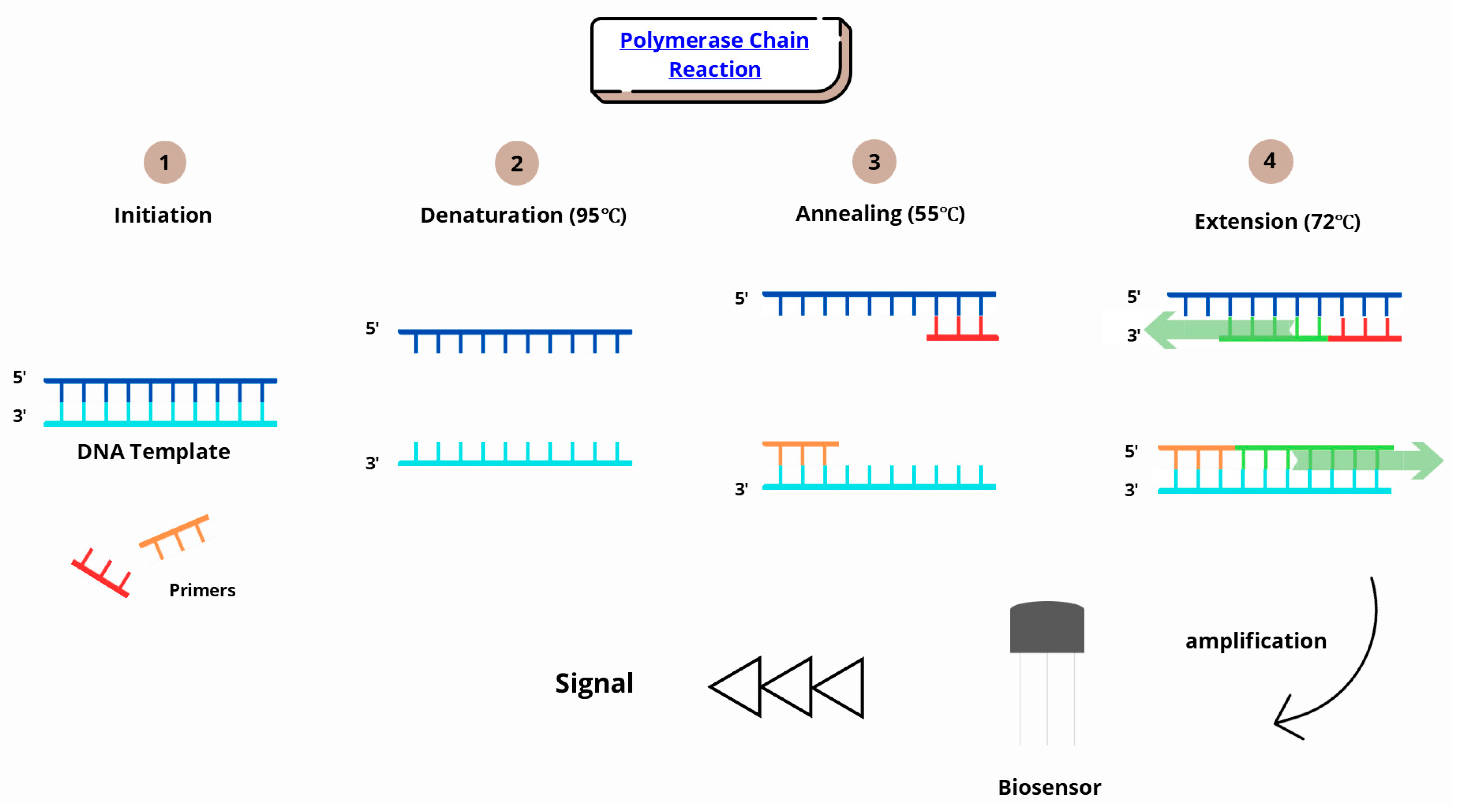

| Type of Biosensor | Applications | Advantages | Disadvantages | Refs. |
|---|---|---|---|---|
| Electrochemical | Glucose and blood pressure monitoring | High sensitivity, broad applicability | Sensitivity to chemical interferences | [10,17] |
| Optical | Oxygen saturation measurement, biomarker detection | Safety, non-invasiveness | Limited long-term durability | [18,19] |
| Magnetic | Pathogen detection, cancer biomarker, immunoassays | High specificity, no optical background interference | Requires external magnet setups, limited commercial use | [20,21] |
| Acoustic (SAW, QCM) | Virus identification, small molecule and toxin sensing | Label-free, real-time, high sensitivity | Sensitive to environmental conditions and mechanical vibrations | [22,23] |
| Thermal | Enzyme activity, small molecule sensing | Simple readout, label-free | Low sensitivity, affected by ambient temperature | [24] |
| Technology | Applications | Advantages | Examples | Refs. |
|---|---|---|---|---|
| Microelectromechanical Systems (MEMS) | Monitoring pressure, glucose, heart rate | Miniaturization, high sensitivity | Real-time monitoring in implants | [50] |
| Nanomaterials | Biocompatible coatings, biomarker detection, surface modification | Reduced thrombosis, precision, biocompatibility | Nanoparticles in stents and biosensors | [51] |
| Graphene and Carbon Nanotubes | Detection of low analyte concentrations | High surface area, conductivity | Electrochemical sensors in diagnostics | [52,53] |
| Quantum Dots | Fluorescence, cancer diagnostics | High sensitivity, multifunctionality | Imaging diagnostics and biomarker sensors | [54,55] |
| Metal Oxide Nanostructures | Enzyme sensors, electrochemical detection | Catalytic activity, chemical stability | ZnO nanorods, TiO2 thin films | [56] |
| Fractal Nanostructures | Surface enhancement, optical signal amplification | Increased active surface area | Fractal gold nanoarrays | [57] |
| Feature | Intravascular Biosensors | Subcutaneous/Wearable Biosensors | Refs. |
|---|---|---|---|
| Access to biomarkers | Direct and continuous access to blood plasma | Indirect via interstitial fluid; delayed correlation | [76,77] |
| Measurement lag | Minimal lag (seconds) | Significant delay (minutes) due to diffusion | [78,79] |
| Response time | Rapid sampling suitable for ICU/surgery settings | Slower response not optimal for acute care | [77] |
| Clinical relevance | Plasma-level accuracy, suitable for dynamic drug/metabolite monitoring | Moderate/correlated to interstitial changes | [79] |
| Biocompatibility requirements | Very high—must minimize clotting, inflammation, biofouling | Moderate level for skin contact | [77] |
| Thrombosis/infection risk | Elevated risk if coatings/materials are suboptimal | Lower risk, mainly surface exposure | [80] |
| Integration potential | Compatible with catheters, closed-loop pumps, stent-integrated systems | Primarily diagnostic, limited actuation capabilities | [81] |
| Maintenance/calibration | Challenging in vivo drift, difficult recalibration | Easier; patient-controlled recalibration possible | [80] |
| Biomarker | Diagnostic Applications | Detection Methods | Advantages | Refs. |
|---|---|---|---|---|
| Glucose | Diabetes monitoring | Electrochemical biosensors | Fast and accurate detection | [17,92] |
| Troponin | Detection of myocardial damage | Immunosensors | High specificity for the heart | [93] |
| C-reactive protein (CRP) | Diagnosing inflammation and infections | Biochemical tests, biosensors | Rapid inflammation detection | [94] |
| Genetic mutations (DNA/RNA) | Cancer diagnostics, genetic disorders | NGS, PCR, genomic analyses | Therapy personalization, early diagnostics | [95,96] |
| Hemoglobin detection | Fast and reliable blood test, tracking medical disorders, such as anemia | Biosensor grounded on metasurfaces | High sensitivity, achieving a peak value of 267 GHzRIU−1 | [97] |
| Alanine aminotransferase (ALT), aspartate aminotransferase (AST) | Diagnosis of heart failure and liver injury, as well as various tissues in the organism | Working electrode altered with nanomaterials | Opportunity to monitor, among others, liver conditions | [98] |
| Alkaline phosphatase (ALP) | Detection of diseases of bone and hepatic dysfunction | Phosphorylated DNA probe | High sensitivity of detecting | [99] |
| α-Amylase | Detecting acute pancreatitis and psychological stress | Fluorescent biosensor arrays | Accurate determination of α-amylase concentrations in serum and saliva | [100] |
| Drug | Disease/Condition | Type of Biosensor | Details/Outcomes | Refs. |
|---|---|---|---|---|
| Vancomycin | Severe bacterial infections | Fluorescence-based biosensor | Monitors drug levels in real time, reducing risks of nephrotoxicity and ototoxicity. Allows precise dosing adjustments. | [33] |
| Insulin | Diabetes mellitus | Electrochemical glucose biosensor | Continuous monitoring and real-time insulin delivery to maintain glucose control. | [119,120] |
| Chemotherapy drugs (e.g., doxorubicin) | Cancer | Electrochemical biosensor | This feedback-loop system enables precise, patient-specific dosing of drugs within narrow therapeutic windows. | [121] |
| Immunosuppressants (e.g., cyclosporine) | Transplant medicine | Optical biosensor | It combines the potential of microdialysis with an optical immunosensor in the therapeutic drug monitoring of immunosuppressants. | [122] |
| Propofol | Total intravenous anesthesia | Electrochemical measurement using biosensor-enabled catheter | This biosensor enables the detection of the propofol present in blood, and it is characterized by the accuracy, specificity, and high stability of the emitted signal. | [81] |
| Propofol and fentanyl | Anesthesia | Electrochemical sensor | Real-time monitoring of the concentrations of both propofol and fentanyl simultaneously throughout surgical operations using a dual-analyte microcatheter-based system. | [123] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudłacik-Kramarczyk, S.; Kieres, W.; Przybyłowicz, A.; Ziejewska, C.; Marczyk, J.; Krzan, M. Recent Advances in Micro- and Nano-Enhanced Intravascular Biosensors for Real-Time Monitoring, Early Disease Diagnosis, and Drug Therapy Monitoring. Sensors 2025, 25, 4855. https://doi.org/10.3390/s25154855
Kudłacik-Kramarczyk S, Kieres W, Przybyłowicz A, Ziejewska C, Marczyk J, Krzan M. Recent Advances in Micro- and Nano-Enhanced Intravascular Biosensors for Real-Time Monitoring, Early Disease Diagnosis, and Drug Therapy Monitoring. Sensors. 2025; 25(15):4855. https://doi.org/10.3390/s25154855
Chicago/Turabian StyleKudłacik-Kramarczyk, Sonia, Weronika Kieres, Alicja Przybyłowicz, Celina Ziejewska, Joanna Marczyk, and Marcel Krzan. 2025. "Recent Advances in Micro- and Nano-Enhanced Intravascular Biosensors for Real-Time Monitoring, Early Disease Diagnosis, and Drug Therapy Monitoring" Sensors 25, no. 15: 4855. https://doi.org/10.3390/s25154855
APA StyleKudłacik-Kramarczyk, S., Kieres, W., Przybyłowicz, A., Ziejewska, C., Marczyk, J., & Krzan, M. (2025). Recent Advances in Micro- and Nano-Enhanced Intravascular Biosensors for Real-Time Monitoring, Early Disease Diagnosis, and Drug Therapy Monitoring. Sensors, 25(15), 4855. https://doi.org/10.3390/s25154855






