Can a Commercially Available Smartwatch Device Accurately Measure Nighttime Sleep Outcomes in Individuals with Knee Osteoarthritis and Comorbid Insomnia? A Comparison with Home-Based Polysomnography
Abstract
Highlights
- Fitbit Sense demonstrates high accuracy and sensitivity for detecting sleep in individuals with knee osteoarthritis and insomnia.
- The device shows limited ability to differentiate quiet wakefulness from sleep and less precision in classifying specific sleep stages.
- Fitbit Sense can be a useful complementary tool for monitoring general sleep duration, timing, and regularity in this population.
- Sleep stage and fragmentation data should be interpreted with caution, as agreement with polysomnography decreases under more disrupted sleep conditions.
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measurements
2.2.1. Procedure and Data Collection
2.2.2. Data Processing
- Fitbit Sense Sleep Outcomes and Processing
- PSG Sleep Outcomes, Processing, and Scoring
2.3. Data Selection
2.4. Statistical Analysis
2.4.1. Descriptives
2.4.2. Discrepancy Analysis and Bland–Altman Plot
2.4.3. EBE Analysis
2.4.4. Sensitivity Analysis
3. Results
3.1. Characteristics of Participants
3.2. Fitbit Sense–PSG Comparison
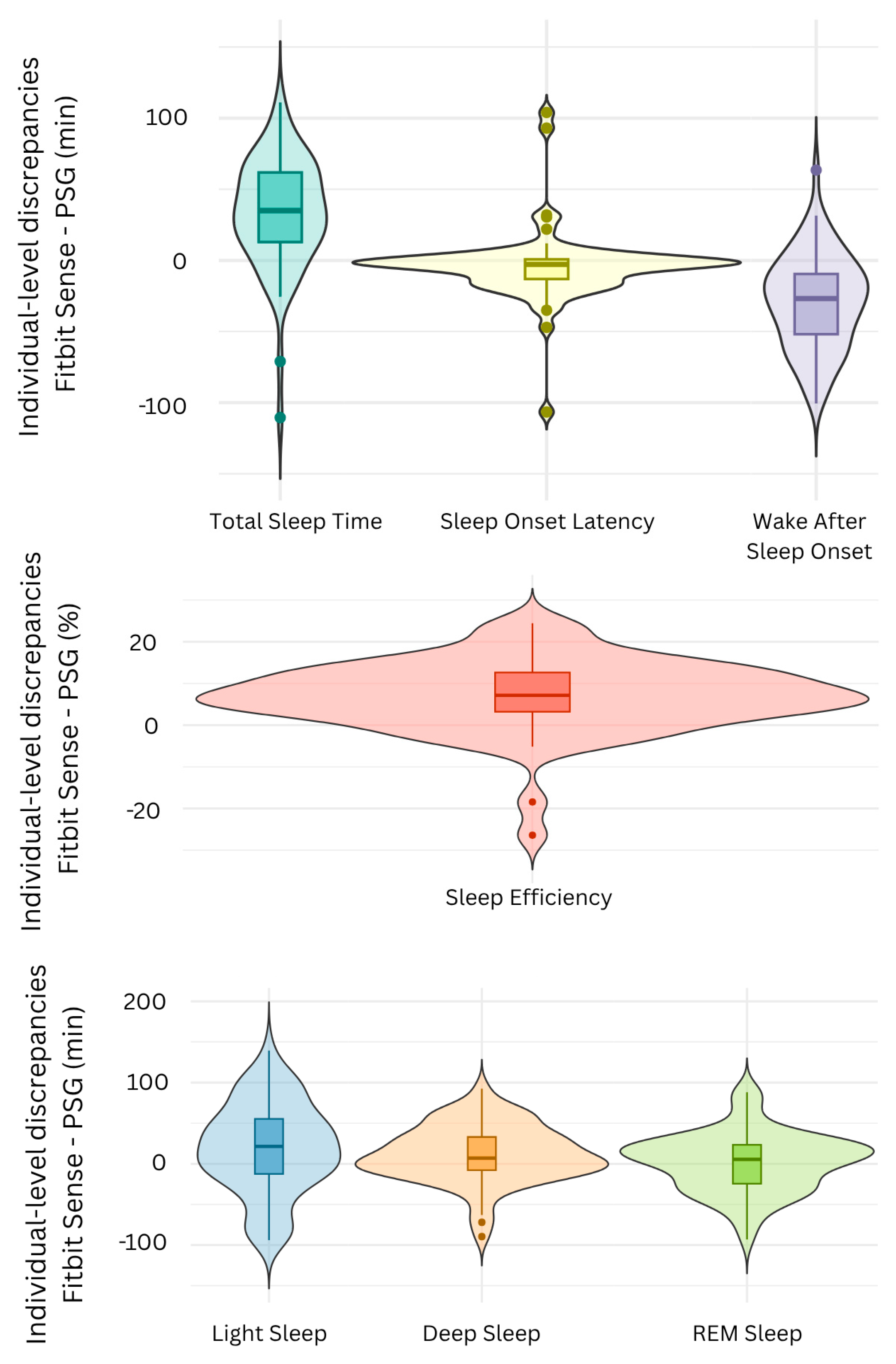
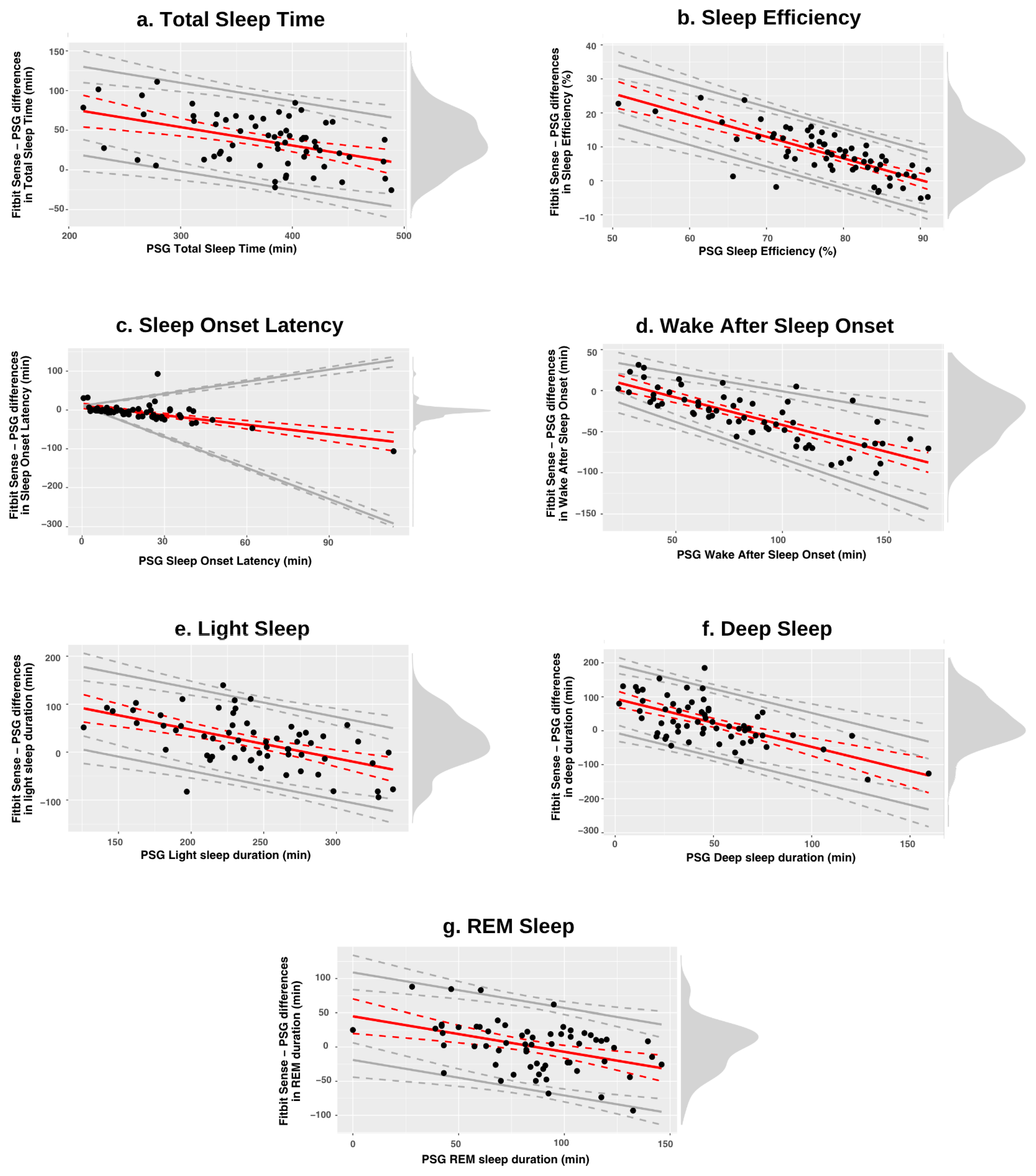
3.2.1. Discrepancy Analysis
3.2.2. Epoch-by-Epoch Analysis
- Wake Detection
- Light Sleep
- Deep Sleep
- REM Sleep
3.3. Sensitivity Analyses
4. Discussion
4.1. Clinical Implications
4.2. Future Research
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OA | Osteoarthritis |
| PSG | Polysomnography |
| CST | Consumer sleep tracking |
| RCT | Randomized controlled trial |
| TIB | Time in bed |
| EBE | Epoch-by-epoch |
| EEG | Electroencephalography |
| EOG | Electrooculography |
| EMG | Electromyography |
| TST | Total sleep time |
| SE | Sleep efficiency |
| SOL | Sleep onset latency |
| WASO | Wake after sleep onset |
| SD | Standard deviation |
| IQR | Interquartile range |
| LOAs | Limits of agreement |
| PABAK | Prevalence-adjusted bias-adjusted kappa |
| NRS | Numeric Rating Scale |
| KOOS | Knee Disability and Osteoarthritis Outcome Score |
| BPI | Brief Pain Inventory |
| CSI | Central Sensitization Inventory |
| HADS | Hospital Anxiety and Depression Scale |
| PSQI | Pittsburgh Sleep Quality Index |
| ISI | Insomnia Severity Index |
| BFS | Brugmann Fatigue Scale |
| ESS | Epworth Sleepiness Scale |
| AHI | Apnea–Hypopnea Index |
| PLMSI | Periodic Limb Movement Sleep Index |
| REM | Rapid-eye-movement |
| CI | Confidence interval |
References
- Husak, A.J.; Bair, M.J. Chronic Pain and Sleep Disturbances: A Pragmatic Review of Their Relationships, Comorbidities, and Treatments. Pain Med. 2020, 21, 1142–1152. [Google Scholar] [CrossRef]
- Steinmetz, J.D.; Culbreth, G.T.; Haile, L.M.; Rafferty, Q.; Lo, J.; Fukutaki, K.G.; Cruz, J.A.; Smith, A.E.; Vollset, S.E.; Brooks, P.M. Global, regional, and national burden of osteoarthritis, 1990–2020 and projections to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e508–e522. [Google Scholar] [CrossRef]
- Thorlund, J.B.; Skou, S.T.; Roos, E.M.; Grønne, D.T.; Vægter, H.B. 264—How common is insomnia among patients with knee and hip osteoarthritis? A cross-sectional study using data from the Good Life with osteoArthritis in Denmark (GLA:D®) register. Osteoarthr. Cartil. 2024, 32, S194. [Google Scholar] [CrossRef]
- Jacob, L.; Smith, L.; Konrad, M.; Kostev, K. Association between sleep disorders and osteoarthritis: A case–control study of 351,932 adults in the UK. J. Sleep Res. 2021, 30, e13367. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; McCurry, S.M.; Belza, B.; Dobra, A.; Buchanan, D.T.; Vitiello, M.V.; Von Korff, M. Effects of osteoarthritis pain and concurrent insomnia and depression on health care use in a primary care population of older adults. Arthritis Care Res. 2019, 71, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.S.; Hughes, J.M.; Coffman, C.J.; Jeffreys, A.S.; Ulmer, C.S.; Oddone, E.Z.; Bosworth, H.B.; Yancy, W.S.; Allen, K.D. Prevalence of and characteristics associated with insomnia and obstructive sleep apnea among veterans with knee and hip osteoarthritis. BMC Musculoskelet. Disord. 2018, 19, 79. [Google Scholar] [CrossRef]
- Silva, A.; Mello, M.T.; Serrão, P.R.; Luz, R.P.; Ruiz, F.; Bittencourt, L.R.; Tufik, S.; Mattiello, S.M. Influence of obstructive sleep apnea in the functional aspects of patients with osteoarthritis. J. Clin. Sleep Med. 2018, 14, 265–270. [Google Scholar] [CrossRef]
- Woolhead, G.; Gooberman-Hill, R.; Dieppe, P.; Hawker, G. Night pain in hip and knee osteoarthritis: A focus group study. Arthritis Care Res. 2010, 62, 944–949. [Google Scholar] [CrossRef]
- Jung, J.H.; Seok, H.; Choi, S.J.; Bae, J.; Lee, S.H.; Lee, M.H.; Kim, J.-H.; Song, G.G. The association between osteoarthritis and sleep duration in Koreans: A nationwide cross-sectional observational study. Clin. Rheumatol. 2018, 37, 1653–1659. [Google Scholar] [CrossRef]
- Park, H.-M.; Kwon, Y.-J.; Kim, H.-S.; Lee, Y.-J. Relationship between sleep duration and osteoarthritis in middle-aged and older women: A nationwide population-based study. J. Clin. Med. 2019, 8, 356. [Google Scholar] [CrossRef]
- Ni, J.; Zhou, W.; Cen, H.; Chen, G.; Huang, J.; Yin, K.; Sui, C. Evidence for causal effects of sleep disturbances on risk for osteoarthritis: A univariable and multivariable Mendelian randomization study. Osteoarthr. Cartil. 2022, 30, 443–450. [Google Scholar] [CrossRef]
- Whibley, D.; Braley, T.J.; Kratz, A.L.; Murphy, S.L. Transient Effects of Sleep on Next-Day Pain and Fatigue in Older Adults With Symptomatic Osteoarthritis. J. Pain 2019, 20, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Yeung, W.K.; Morgan, K.; Mckenna, F. Comparison of sleep structure and psychometric profiles in patients with fibromyalgia, osteoarthritis and healthy controls. J. Sleep Res. 2018, 27, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Doherty, M.; Smith, J. Elusive’alpha-delta’sleep in fibromyalgia and osteoarthritis. Ann. Rheum. Dis. 1993, 52, 245. [Google Scholar] [CrossRef]
- Leigh, T.; Hindmarch, I.; Bird, H.; Wright, V. Comparison of sleep in osteoarthritic patients and age and sex matched healthy controls. Ann. Rheum. Dis. 1988, 47, 40–42. [Google Scholar] [CrossRef] [PubMed]
- Spira, A.P.; Runko, V.T.; Finan, P.H.; Kaufmann, C.N.; Bounds, S.C.; Liu, L.; Buenaver, L.F.; McCauley, L.M.; Ancoli-Israel, S.; Smith, M.T. Circadian rest/activity rhythms in knee osteoarthritis with insomnia: A study of osteoarthritis patients and pain-free controls with insomnia or normal sleep. Chronobiol. Int. 2015, 32, 242–247. [Google Scholar] [CrossRef][Green Version]
- Chronic Pain (Primary and Secondary) in Over 16s: Assessment of All Chronic Pain and Management of Chronic Primary Pain; NICE Guideline NG193; National Institute for Health and Care Excellence (NICE): London, UK, 2021.
- Edwards, R.R.; Schreiber, K.L.; Dworkin, R.H.; Turk, D.C.; Baron, R.; Freeman, R.; Jensen, T.S.; Latremoliere, A.; Markman, J.D.; Rice, A.S. Optimizing and accelerating the development of precision pain treatments for chronic pain: IMMPACT review and recommendations. J. Pain 2023, 24, 204–225. [Google Scholar] [CrossRef]
- Lim, D.C.; Najafi, A.; Afifi, L.; Bassetti, C.L.; Buysse, D.J.; Han, F.; Högl, B.; Melaku, Y.A.; Morin, C.M.; Pack, A.I. The need to promote sleep health in public health agendas across the globe. Lancet Public Health 2023, 8, e820–e826. [Google Scholar] [CrossRef]
- de Zambotti, M.; Goldstein, C.; Cook, J.; Menghini, L.; Altini, M.; Cheng, P.; Robillard, R. State of the science and recommendations for using wearable technology in sleep and circadian research. Sleep 2023, 47, zsad325. [Google Scholar] [CrossRef]
- Smith, M.T.; McCrae, C.S.; Cheung, J.; Martin, J.L.; Harrod, C.G.; Heald, J.L.; Carden, K.A. Use of Actigraphy for the Evaluation of Sleep Disorders and Circadian Rhythm Sleep-Wake Disorders: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2018, 14, 1231–1237. [Google Scholar] [CrossRef]
- Yuan, H.; Hill, E.A.; Kyle, S.D.; Doherty, A. A systematic review of the performance of actigraphy in measuring sleep stages. J. Sleep Res. 2024, 33, e14143. [Google Scholar] [CrossRef] [PubMed]
- Redline, S.; Purcell, S.M. Sleep and Big Data: Harnessing data, technology, and analytics for monitoring sleep and improving diagnostics, prediction, and interventions—An era for Sleep-Omics? Sleep 2021, 44, zsab107. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, R.G.; Czarnecki, P.; Howard, J.; Jacelon, C.S.; Marquard, J. Usability Experience of a Personal Sleep Monitoring Device to Self-manage Sleep Among Persons 65 Years or Older With Self-reported Sleep Disturbances. CIN Comput. Inform. Nurs. 2022, 40, 598–605. [Google Scholar] [CrossRef]
- Brückner, S.; Sadare, O.; Fesl, S.; Scheibe, M.; Lang, C.; Gilbert, S. Attitudes of healthcare professionals and researchers toward wearable and app derived patient generated health data. npj Digit. Med. 2025, 8, 186. [Google Scholar] [CrossRef]
- Garbarino, S.; Bragazzi, N.L. Revolutionizing sleep health: The emergence and impact of personalized sleep medicine. J. Pers. Med. 2024, 14, 598. [Google Scholar] [CrossRef]
- Slitzky, M.; Yong, R.J.; Bianco, G.L.; Emerick, T.; Schatman, M.E.; Robinson, C.L. The future of pain medicine: Emerging technologies, treatments, and education. J. Pain Res. 2024, 17, 2833–2836. [Google Scholar] [CrossRef]
- Scott, H.; Lechat, B.; Manners, J.; Lovato, N.; Vakulin, A.; Catcheside, P.; Eckert, D.J.; Reynolds, A.C. Emerging applications of objective sleep assessments towards the improved management of insomnia. Sleep Med. 2023, 101, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Menghini, L.; Cellini, N.; Goldstone, A.; Baker, F.C.; De Zambotti, M. A standardized framework for testing the performance of sleep-tracking technology: Step-by-step guidelines and open-source code. Sleep 2021, 44, zsaa170. [Google Scholar] [CrossRef]
- Schyvens, A.-M.; Peters, B.; Van Oost, N.C.; Aerts, J.-M.; Masci, F.; Neven, A.; Dirix, H.; Wets, G.; Ross, V.; Verbraecken, J. A performance validation of six commercial wrist-worn wearable sleep-tracking devices for sleep stage scoring compared to polysomnography. Sleep Adv. 2025, 6, zpaf021. [Google Scholar] [CrossRef]
- Chinoy, E.D.; Cuellar, J.A.; Huwa, K.E.; Jameson, J.T.; Watson, C.H.; Bessman, S.C.; Hirsch, D.A.; Cooper, A.D.; Drummond, S.P.; Markwald, R.R. Performance of seven consumer sleep-tracking devices compared with polysomnography. Sleep 2021, 44, zsaa291. [Google Scholar] [CrossRef]
- Kahawage, P.; Jumabhoy, R.; Hamill, K.; de Zambotti, M.; Drummond, S.P. Validity, potential clinical utility, and comparison of consumer and research-grade activity trackers in insomnia disorder I: In-lab validation against polysomnography. J. Sleep Res. 2020, 29, e12931. [Google Scholar] [CrossRef]
- Moreno-Pino, F.; Porras-Segovia, A.; López-Esteban, P.; Artés, A.; Baca-García, E. Validation of Fitbit Charge 2 and Fitbit Alta HR Against Polysomnography for Assessing Sleep in Adults With Obstructive Sleep Apnea. J. Clin. Sleep Med. 2019, 15, 1645–1653. [Google Scholar] [CrossRef]
- Doheny, E.P.; Renerts, K.; Braun, A.; Werth, E.; Baumann, C.; Baumgartner, P.; Morgan-Jones, P.; Busse, M.; Lowery, M.M.; Jung, H.H. Assessment of Fitbit Charge 4 for sleep stage and heart rate monitoring against polysomnography and during home monitoring in Huntington’s disease. J. Clin. Sleep Med. 2024, 20, 1163–1171. [Google Scholar] [CrossRef]
- Ogasawara, M.; Takeshima, M.; Kosaka, S.; Imanishi, A.; Itoh, Y.; Fujiwara, D.; Yoshizawa, K.; Ozaki, N.; Nakagome, K.; Mishima, K. Exploratory validation of sleep-tracking devices in patients with psychiatric disorders. Nat. Sci. Sleep 2023, 15, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.D.; Prairie, M.L.; Plante, D.T. Utility of the Fitbit Flex to evaluate sleep in major depressive disorder: A comparison against polysomnography and wrist-worn actigraphy. J. Affect. Disord. 2017, 217, 299–305. [Google Scholar] [CrossRef]
- Labie, C.; Runge, N.; Mairesse, O.; Nijs, J.; Malfliet, A.; Verschueren, S.; Van Assche, D.; de Vlam, K.; Luyten, F.; Bilterys, T.; et al. Integration of Cognitive Behavioral Therapy for Insomnia in Best-Practice Care for Patients With Knee Osteoarthritis and Insomnia: A Randomized Controlled Trial Protocol. Phys. Ther. 2023, 104, pzad181. [Google Scholar] [CrossRef]
- Association, W.M. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar]
- Depner, C.M.; Cheng, P.C.; Devine, J.K.; Khosla, S.; De Zambotti, M.; Robillard, R.; Vakulin, A.; Drummond, S.P. Wearable technologies for developing sleep and circadian biomarkers: A summary of workshop discussions. Sleep 2020, 43, zsz254. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.; Asch, E.; Bloch, D.; Bole, G.; Borenstein, D.; Brandt, K.; Christy, W.; Cooke, T.D.; Greenwald, R.; Hochberg, M.; et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986, 29, 1039–1049. [Google Scholar] [CrossRef]
- Sleep-Wake Disorders. In DSM-5-TR, 5th ed.; Text Revision (DSM-5-TR) ed.; American Psychiatric Association Publishing: Washington, DC, USA, 2022. [CrossRef]
- Haghayegh, S.; Khoshnevis, S.; Smolensky, M.H.; Diller, K.R.; Castriotta, R.J. Accuracy of wristband Fitbit models in assessing sleep: Systematic review and meta-analysis. J. Med. Internet Res. 2019, 21, e16273. [Google Scholar] [CrossRef]
- Fitbit, I. Available online: https://www.fitbit.com/sg/sense (accessed on 4 May 2025).
- Berry, R.B.; Brooks, R.; Gamaldo, C.E.; Harding, S.M.; Marcus, C.; Vaughn, B.V. The AASM Manual for the Scoring of Sleep and Associated Events; Rules, Terminology and Technical Specifications; American Academy of Sleep Medicine: Darien, IL, USA, 2012. [Google Scholar]
- Lee, T.; Cho, Y.; Cha, K.S.; Jung, J.; Cho, J.; Kim, H.; Kim, D.; Hong, J.; Lee, D.; Keum, M. Accuracy of 11 wearable, nearable, and airable consumer sleep trackers: Prospective multicenter validation study. JMIR Mhealth Uhealth 2023, 11, e50983. [Google Scholar] [CrossRef]
- Ong, J.L.; Golkashani, H.A.; Ghorbani, S.; Wong, K.F.; Chee, N.I.; Willoughby, A.R.; Chee, M.W. Selecting a sleep tracker from EEG-based, iteratively improved, low-cost multisensor, and actigraphy-only devices. Sleep Health 2024, 10, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.E.; Kim, H.S.; Lee, S.W.; Bae, K.-H.; Baek, Y.H. Validation of fitbit inspire 2TM against polysomnography in adults considering adaptation for use. Nat. Sci. Sleep 2023, 15, 59–67. [Google Scholar] [CrossRef]
- Haghayegh, S.; Khoshnevis, S.; Smolensky, M.H.; Diller, K.R.; Castriotta, R.J. Performance assessment of new-generation Fitbit technology in deriving sleep parameters and stages. Chronobiol. Int. 2020, 37, 47–59. [Google Scholar] [CrossRef]
- Grandner, M.A.; Bromberg, Z.; Hadley, A.; Morrell, Z.; Graf, A.; Hutchison, S.; Freckleton, D. Performance of a multisensor smart ring to evaluate sleep: In-lab and home-based evaluation of generalized and personalized algorithms. Sleep 2023, 46, zsac152. [Google Scholar] [CrossRef] [PubMed]
- Robbins, R.; Weaver, M.D.; Sullivan, J.P.; Quan, S.F.; Gilmore, K.; Shaw, S.; Benz, A.; Qadri, S.; Barger, L.K.; Czeisler, C.A. Accuracy of Three Commercial Wearable Devices for Sleep Tracking in Healthy Adults. Sensors 2024, 24, 6532. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-G.; Kang, J.M.; Ko, K.-P.; Park, S.-C.; Mariani, S.; Weng, J. Validity of a commercial wearable sleep tracker in adult insomnia disorder patients and good sleepers. J. Psychosom. Res. 2017, 97, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Feige, B.; Baglioni, C.; Spiegelhalder, K.; Hirscher, V.; Nissen, C.; Riemann, D. The microstructure of sleep in primary insomnia: An overview and extension. Int. J. Psychophysiol. 2013, 89, 171–180. [Google Scholar] [CrossRef]
- Riemann, D.; Espie, C.A.; Altena, E.; Arnardottir, E.S.; Baglioni, C.; Bassetti, C.L.; Bastien, C.; Berzina, N.; Bjorvatn, B.; Dikeos, D. The European Insomnia Guideline: An update on the diagnosis and treatment of insomnia 2023. J. Sleep Res. 2023, 32, e14035. [Google Scholar] [CrossRef]
- Baron, K.G.; Abbott, S.; Jao, N.; Manalo, N.; Mullen, R. Orthosomnia: Are Some Patients Taking the Quantified Self Too Far? J. Clin. Sleep Med. 2017, 13, 351–354. [Google Scholar] [CrossRef] [PubMed]
- The Academy of Medical Sciences. Multimorbidity: A Priority for Global Health Research; Academy of Medical Sciences: London, UK, 2018. [Google Scholar]
- Swain, S.; Kamps, A.; Runhaar, J.; Dell’Isola, A.; Turkiewicz, A.; Robinson, D.; Strauss, V.; Mallen, C.; Kuo, C.-F.; Coupland, C. Comorbidities in osteoarthritis (ComOA): A combined cross-sectional, case–control and cohort study using large electronic health records in four European countries. BMJ Open 2022, 12, e052816. [Google Scholar] [CrossRef] [PubMed]
- Lujan, M.R.; Perez-Pozuelo, I.; Grandner, M.A. Past, present, and future of multisensory wearable technology to monitor sleep and circadian rhythms. Front. Digit. Health 2021, 3, 721919. [Google Scholar] [CrossRef]
- Koerber, D.; Khan, S.; Shamsheri, T.; Kirubarajan, A.; Mehta, S. Accuracy of heart rate measurement with wrist-worn wearable devices in various skin tones: A systematic review. J. Racial Ethn. Health Disparities 2023, 10, 2676–2684. [Google Scholar] [CrossRef] [PubMed]

| Demographics | Mean (SD)/N | Median (IQR) | Range/Percentage | ||
|---|---|---|---|---|---|
| Age, years | 61.0 (8.2) | 63 (13.5) | 45–78 | ||
| Female | 32 | 60.4 | |||
| BMI, kg/m2 | 24.7 (2.6) | 20.2–30.5 | |||
| White-Caucasian | 52 | 98.1 | |||
| Postsecondary education | 46 | 86.8 | |||
| Currently working | 23 | 43.4 | |||
| Lives alone | 17 | 30.1 | |||
| Smoking | 5 | 9.3 | |||
| Baseline characteristics | |||||
| Pain duration, years | 9.7 (9.7) | 5 (12.7) | 0.8–45 | ||
| Pain NRS | 5.2 (1.9) | 5 (3) | 2–9 | ||
| Pain at night | 7.0 | 86.5 | |||
| KOOS pain | 54.2 (14.2) | 13.9–91.7 | |||
| KOOS Function | 62.6 (13.9) | 63.2 (13.2) | 23.5–94.1 | ||
| KOOS Quality of life | 39.3 (15.7) | 0–81.3 | |||
| ≥1 Comorbidities | 45 | 84.9 | |||
| BPI Severity | 5 (2.1) | 5 (2.3) | 1.3–14 | ||
| BPI Interference | 3.8 (1.8) | 3.6 (3.3) | 1–7.4 | ||
| CSI | 38 (12.7) | 12–68 | |||
| HADS Anxiety | 6.3 (3.3) | 6 (4) | 2–15 | ||
| Moderate to severe symptoms | 6 | 12 | |||
| HADS Depression | 5.2 (3.7) | 4.5 (6) | 0–16 | ||
| Moderate to severe symptoms | 4 | 8 | |||
| Sleep characteristics | |||||
| Sleep problem duration, years | 11.1 (11.0) | 8 (14.5) | 0.6–54 | ||
| Sleep medication use | 12 | 22.6 | |||
| ISI | 15.8 (4.1) | 17 (4) | 5–24 | ||
| No Insomnia | 2 | 3.9 | |||
| Subclinical insomnia | 15 | 29.4 | |||
| Clinical insomnia, moderate | 31 | 60.8 | |||
| Clinical insomnia, severe | 3 | 5.9 | |||
| PSQI | 10.2 (2.9) | 10 (2) | 4–17 | ||
| Poor sleep quality (PSQI > 5) | 49 | 96.1 | |||
| BFS Mental Fatigue | 29.6 (23.6) | 25 (41.7) | 0–83.3 | ||
| BFS Physical Fatigue | 34 (21.9) | 33.3 (33.3) | 0–83.3 | ||
| ESS | 8.4 (4.1) | 8 (4) | 1–20 | ||
| Excessive daytime sleepiness (ESS > 10) | 17 | 32 | |||
| AHI (h−1) | 9 (9.7) | 5.1 (9) | 0.5–45.7 | ||
| Moderate (AHI ≥ 15) | 10 | 16.1 | |||
| Severe (AHI ≥ 30) | 3 | 4.8 | |||
| PLMSI (h−1) | 8.5 (18) | 1.3 (7) | 0–100.2 | ||
| Moderate (PLMSI ≥ 25) | 5 | 8.1 | |||
| Severe (PLMSI ≥ 50) | 2 | 1.2 | |||
| Mean (SD) | Median (IQR) | Range | |
|---|---|---|---|
| TIB (min) | 477 (60) | 482 (81) | 318–593 |
| TST (min) | 373 (63) | 387 (76) | 213–489 |
| SE (%) | 78 (8) | 79 (11) | 51–91 |
| SOL (min) | 20 (18) | 17 (20) | 1–114 |
| WASO (min) | 84 (37) | 80 (52) | 23–169 |
| Light | |||
| Duration (min) | 241 (50) | 241 (61) | 126–339 |
| Percentage | 65 (10) | 66 (13) | 36–85 |
| Deep | |||
| Duration (min) | 47 (30) | 45 (38) | 2–160 |
| Percentage | 13 (8) | 11 (10) | 1–41 |
| REM | |||
| Duration (min) | 85 (30) | 86 (38) | 0–146 |
| Percentage | 22 (6) | 23 (7) | 0–34 |
| Mean Bias ± SD (95% CI) | Mean Absolute Difference | Mean Standard Error (95% CI) | |
|---|---|---|---|
| TST (min) | 37.05 ± 32.04 (28.97, 45.13) | 40.81 | 0.52 (39.86–41.85) |
| SE (%) | 7.89 ± 6.95 (6.13, 9.65) | 8.65 | 0.11 (8.42–8.88) |
| SOL (min) | −5.8 ± 22.5 (−11.52, −0.08) | 13.1 | 0.4 (12.3–13.8) |
| WASO (min) | −31.23 ± 31.45 (−39.22, −23.24) | 35.79 | 0.37 (35.05–36.53) |
| Light (min) | 22.29 ± 53.17 (8.74, 35.84) | 45.63 | 0.67 (44.28–46.98) |
| Deep (min) | 13.74 ± 33.23 (5.34, 22.14) | 40.81 | 0.44 (39.93–41.69) |
| REM (min) | 1.02 ± 36.13 (−10.15, 12.19) | 28.08 | 0.43 (27.21–28.95) |
| Fitbit Sense Mean, ±SD | PSG Mean, ±SD | Proportional Bias, 95% CI | LOA Lower, 95% CI | LOA Upper, 95% CI | |
|---|---|---|---|---|---|
| TST (min) | 409.90 ± 56.40 | 372.85 ± 63.34 | 123.37 − 0.23 × ref b0 = [79.46, 167.28], b1 = [−0.35, −0.12] | bias − 55.83 bias − [49.46, 64.79] | bias + 55.83 bias + [49.46, 64.79] |
| SE (%) | 85.87 ± 5.41 | 77.99 ± 8.35 | 57.54 − 0.64 × ref b0 = [46.69, 68.4], b1 = [−0.78, −0.5] | bias − 8.78 bias − [6.96, 10.98] | bias + 8.78 bias + [6.96, 10.98] |
| SOL (min) | 14.52 ± 17.53 | 20.34 ± 17.77 | 10.75 − 0.82 × ref b0 = [4.02, 17.49], b1 = [−1.07, −0.56] | bias − ref × 1.85 bias − ref × [1.7, 1.93] | bias + ref × 1.85 bias + ref × [1.7, 1.93] |
| WASO (min) | 53.00 ± 22.67 | 84.23 ± 37.50 | 25.12 − 0.67 × ref b0 = [13.09, 37.16], b1 = [−0.8, −0.54] | bias − 2.46 (7.64 + 0.09 × ref) c0 = [0.91, 14.36], c1 = [0.02, 0.16] | bias + 2.46 (7.64 + 0.09 × ref) c0 = [0.91, 14.36], c1 = [0.02, 0.16] |
| Light (min) | 263.27 ± 48.35 | 240.98 ± 49.70 | 166.66 − 0.6 × ref b0 = [110.36, 222.96], b1 = [−0.83, −0.37] | bias − 86.34 bias − [71.84, 103.48] | bias + 86.34 bias + [71.84, 103.48] |
| Deep (min) | 60.90 ± 26.93 | 47.16 ± 30.10 | 47.19 − 0.71 × ref b0 = [34.99, 59.38], b1 = [−0.93, −0.49] | bias − 49.92 bias − [41.52, 59.66] | bias + 49.92 bias + [41.52, 59.66] |
| REM (min) | 85.72 ± 35.64 | 84.70 ± 30.01 | 45.03 − 0.52 × ref b0 = [19.85, 70.21], b1 = [−0.8, −0.24] | bias − 63.88 bias − [55.04, 75.85] | bias + 63.88 bias + [55.04, 75.85] |
| PSG Stage | Fitbit Sense Wake | Fitbit Sense Light | Fitbit Sense Deep | Fitbit Sense REM |
|---|---|---|---|---|
| Wake | 0.51 (0.15) [0.47, 0.55] | 0.37 (0.15) [0.33, 0.4] | 0.02 (0.04) [0.01, 0.03] | 0.11 (0.1) [0.08, 0.13] |
| Light | 0.04 (0.03) [0.04, 0.05] | 0.73 (0.10) [0.7, 0.75] | 0.13 (0.07) [0.11, 0.15] | 0.10 (0.07) [0.08, 0.12] |
| Deep | 0.02 (0.06) [0.01, 0.03] | 0.46 (0.25) [0.39, 0.52] | 0.49 (0.27) [0.42, 0.55] | 0.03 (0.08) [0.01, 0.05] |
| REM | 0.04 (0.05) [0.02, 0.05] | 0.33 (0.25) [0.26, 0.39] | 0.03 (0.06) [0.02, 0.05] | 0.60 (0.27) [0.54, 0.67] |
| Stage | Accuracy | Sensitivity | Specificity | PPV | NPV | Kappa | PABAK |
|---|---|---|---|---|---|---|---|
| Wake | 85.76 (5.49) [84.44, 87.17] | 50.96 (15.46) [47.14, 54.72] | 95.95 (2.90) [95.27, 96.71] | 76.91 (14.22) [73.49, 80.51] | 87.05 (7.08) [85.3, 88.82] | 0.51 (0.13) [0.48, 0.55] | 0.72 (0.11) [0.69, 0.74] |
| Light | 67.22 (7.85) [65.33, 69.19] | 72.50 (9.98) [70.05, 75.07] | 62.62 (13.32) [59.36, 65.93] | 66.51 (11.98) [63.62, 69.50] | 68.87 (10.70) [66.18, 71.48] | 0.34 (0.15) [0.31, 0.38] | 0.34 (0.16) [0.31, 0.38] |
| Deep | 87.65 (4.57) [86.55, 88.82] | 48.75 (27.24) [42.04, 55.61] | 91.76 (4.41) [90.70, 92.86] | 39.65 (25.94) [33.23, 45.94] | 94.44 (4.91) [93.32, 95.73] | 0.34 (0.23) [0.28, 0.39] | 0.75 (0.09) [0.73, 0.78] |
| REM | 85.23 (6.02) [83.75, 86.74] | 60.41 (26.51) [53.82, 67.12] | 90.79 (5.27) [89.54, 92.15] | 56.62 (18.09) [52.29, 61.15] | 91.39 (6) [89.94, 92.91] | 0.47 (0.23) [0.41, 0.53] | 0.70 (0.12) [0.67, 0.74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labie, C.; Runge, N.; Goossens, Z.; Mairesse, O.; Nijs, J.; Malfliet, A.; Van Assche, D.; de Vlam, K.; Menghini, L.; Verschueren, S.; et al. Can a Commercially Available Smartwatch Device Accurately Measure Nighttime Sleep Outcomes in Individuals with Knee Osteoarthritis and Comorbid Insomnia? A Comparison with Home-Based Polysomnography. Sensors 2025, 25, 4813. https://doi.org/10.3390/s25154813
Labie C, Runge N, Goossens Z, Mairesse O, Nijs J, Malfliet A, Van Assche D, de Vlam K, Menghini L, Verschueren S, et al. Can a Commercially Available Smartwatch Device Accurately Measure Nighttime Sleep Outcomes in Individuals with Knee Osteoarthritis and Comorbid Insomnia? A Comparison with Home-Based Polysomnography. Sensors. 2025; 25(15):4813. https://doi.org/10.3390/s25154813
Chicago/Turabian StyleLabie, Céline, Nils Runge, Zosia Goossens, Olivier Mairesse, Jo Nijs, Anneleen Malfliet, Dieter Van Assche, Kurt de Vlam, Luca Menghini, Sabine Verschueren, and et al. 2025. "Can a Commercially Available Smartwatch Device Accurately Measure Nighttime Sleep Outcomes in Individuals with Knee Osteoarthritis and Comorbid Insomnia? A Comparison with Home-Based Polysomnography" Sensors 25, no. 15: 4813. https://doi.org/10.3390/s25154813
APA StyleLabie, C., Runge, N., Goossens, Z., Mairesse, O., Nijs, J., Malfliet, A., Van Assche, D., de Vlam, K., Menghini, L., Verschueren, S., & De Baets, L. (2025). Can a Commercially Available Smartwatch Device Accurately Measure Nighttime Sleep Outcomes in Individuals with Knee Osteoarthritis and Comorbid Insomnia? A Comparison with Home-Based Polysomnography. Sensors, 25(15), 4813. https://doi.org/10.3390/s25154813







