Denoising Algorithm for High-Resolution and Large-Range Phase-Sensitive SPR Imaging Based on PFA
Abstract
1. Introduction
2. Principles
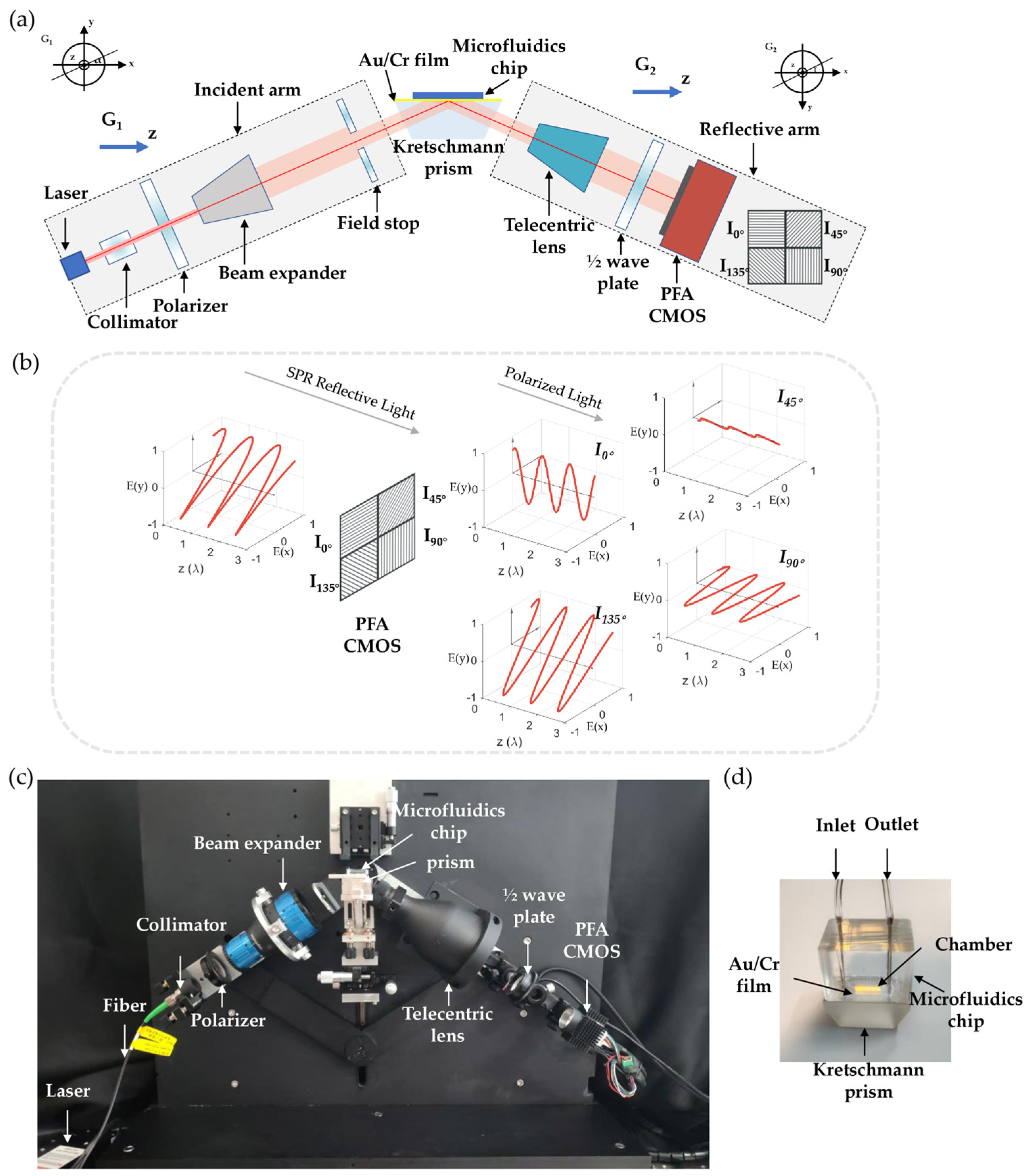
2.1. Quad-Polarization Filter Array Imaging Optical Configuration
2.2. Optical Path Modulation Model
3. Materials and Methods
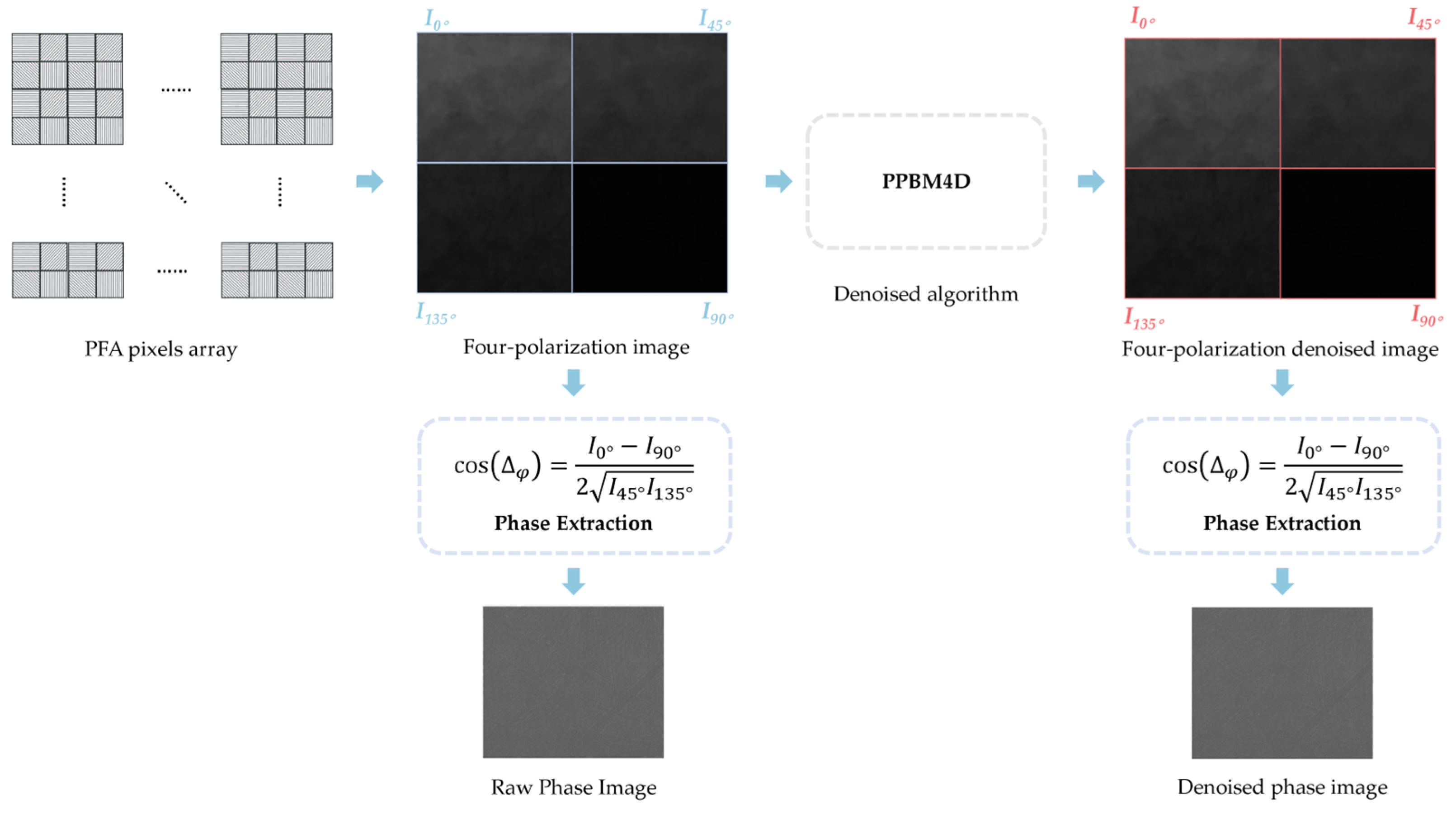
3.1. Description of the Denoising Problem
3.2. Brief Introduction to BM3D/BM4D Algorithms
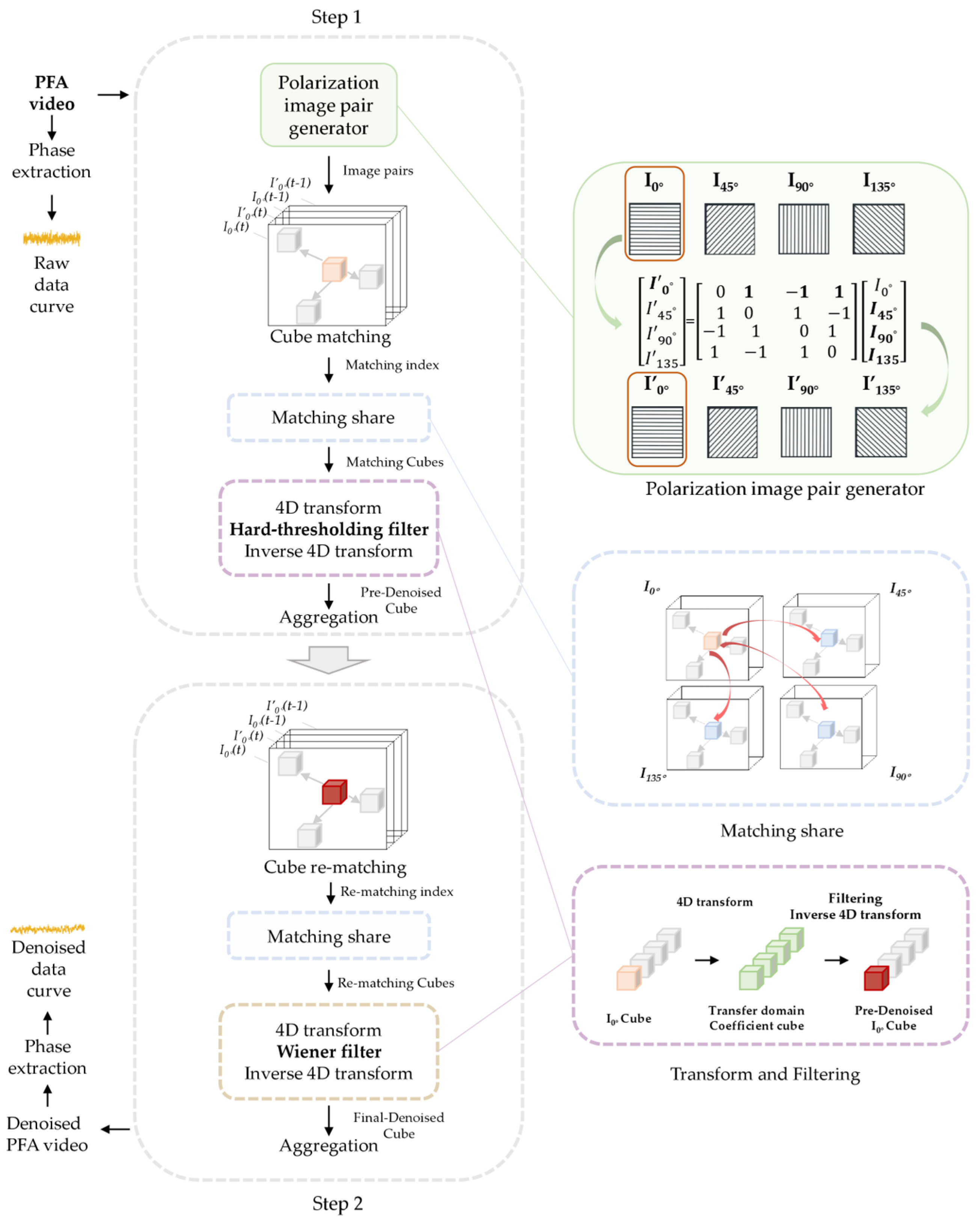
3.3. Brief Introduction to PPBM4D Algorithms
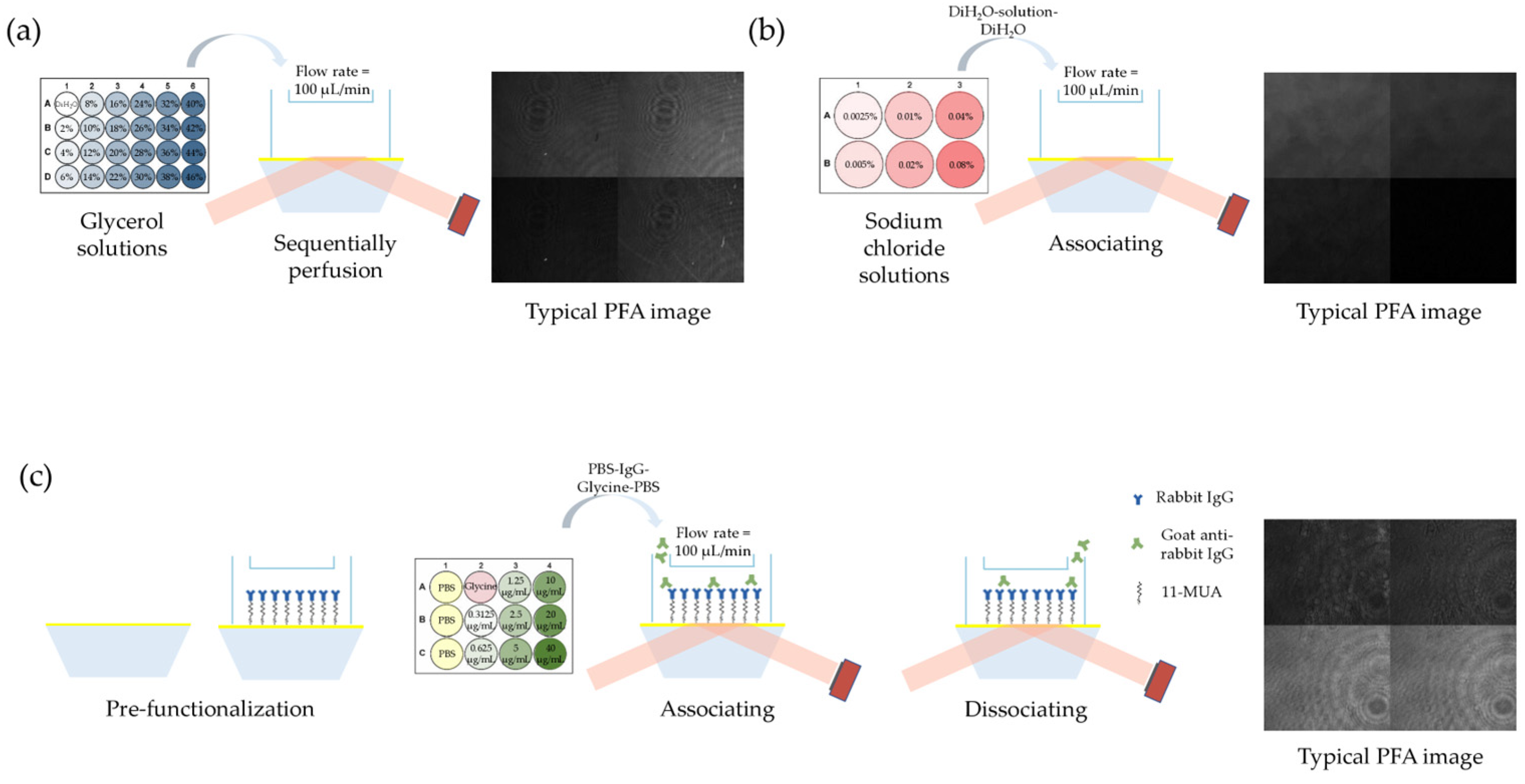
3.4. Reagents and Experimental Protocols
4. Results and Discussion
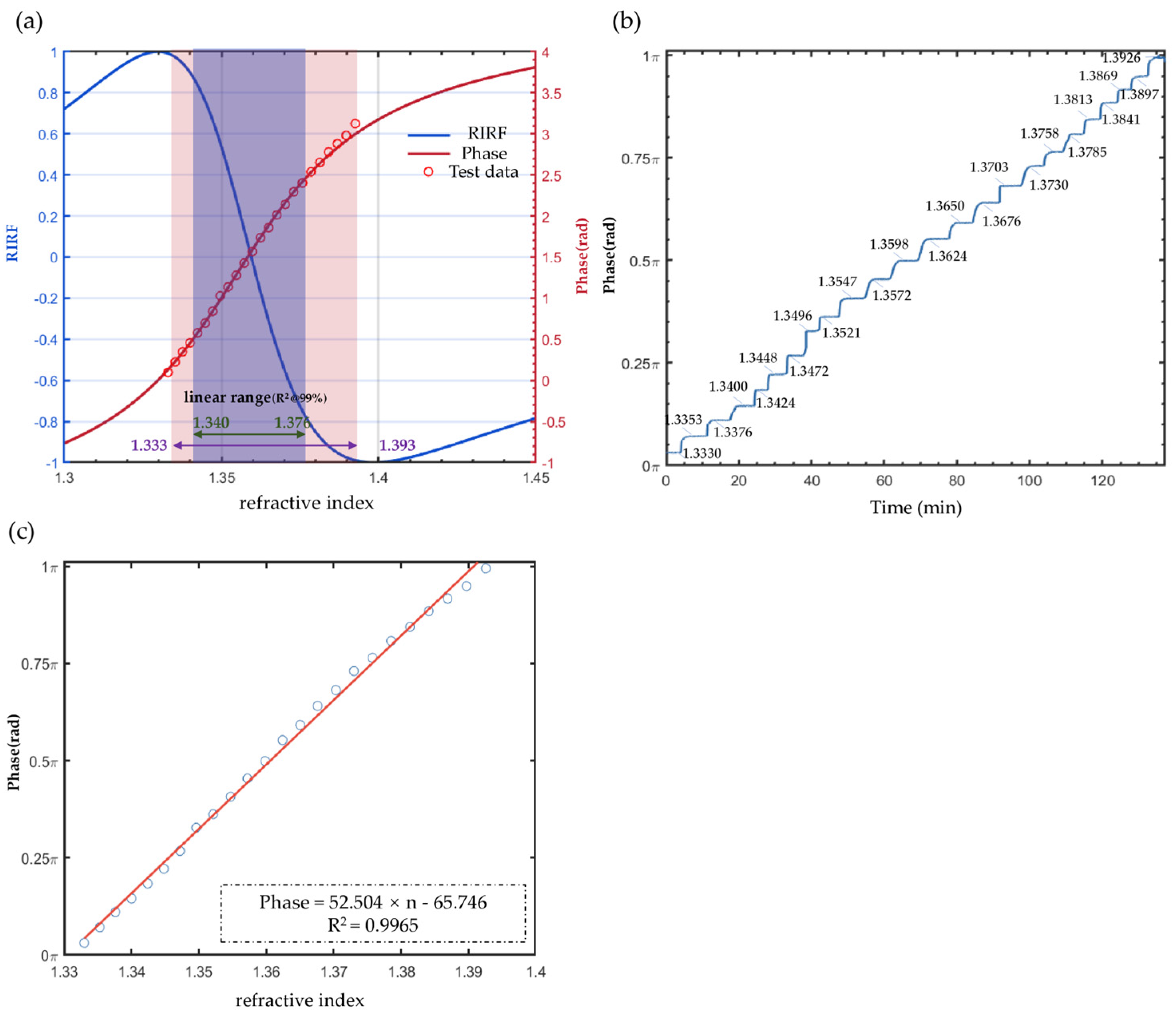
4.1. Measurement Range and Sensitivity Calibration
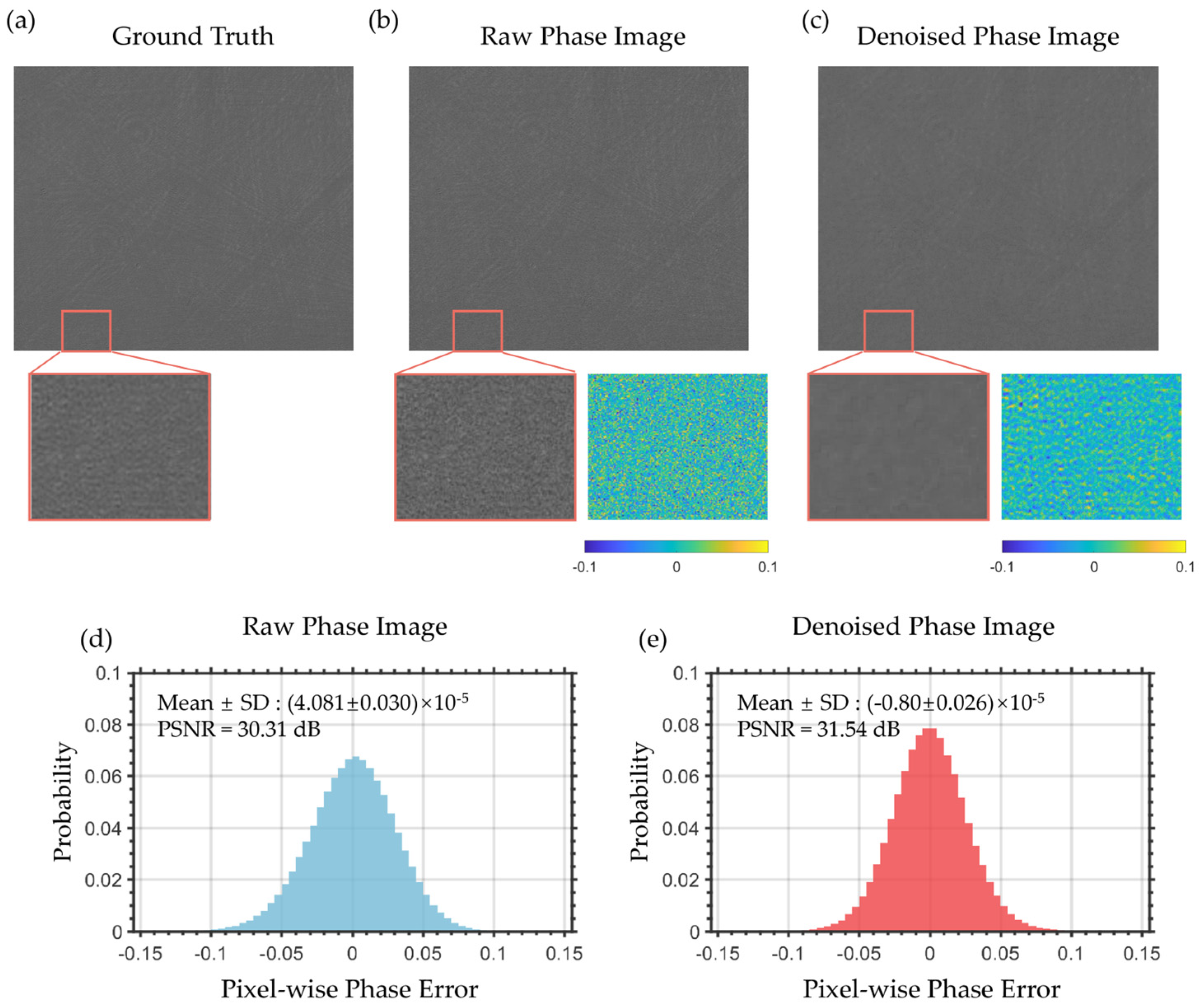
4.2. Enhancement of Refractive Index Resolution via PPBM4D
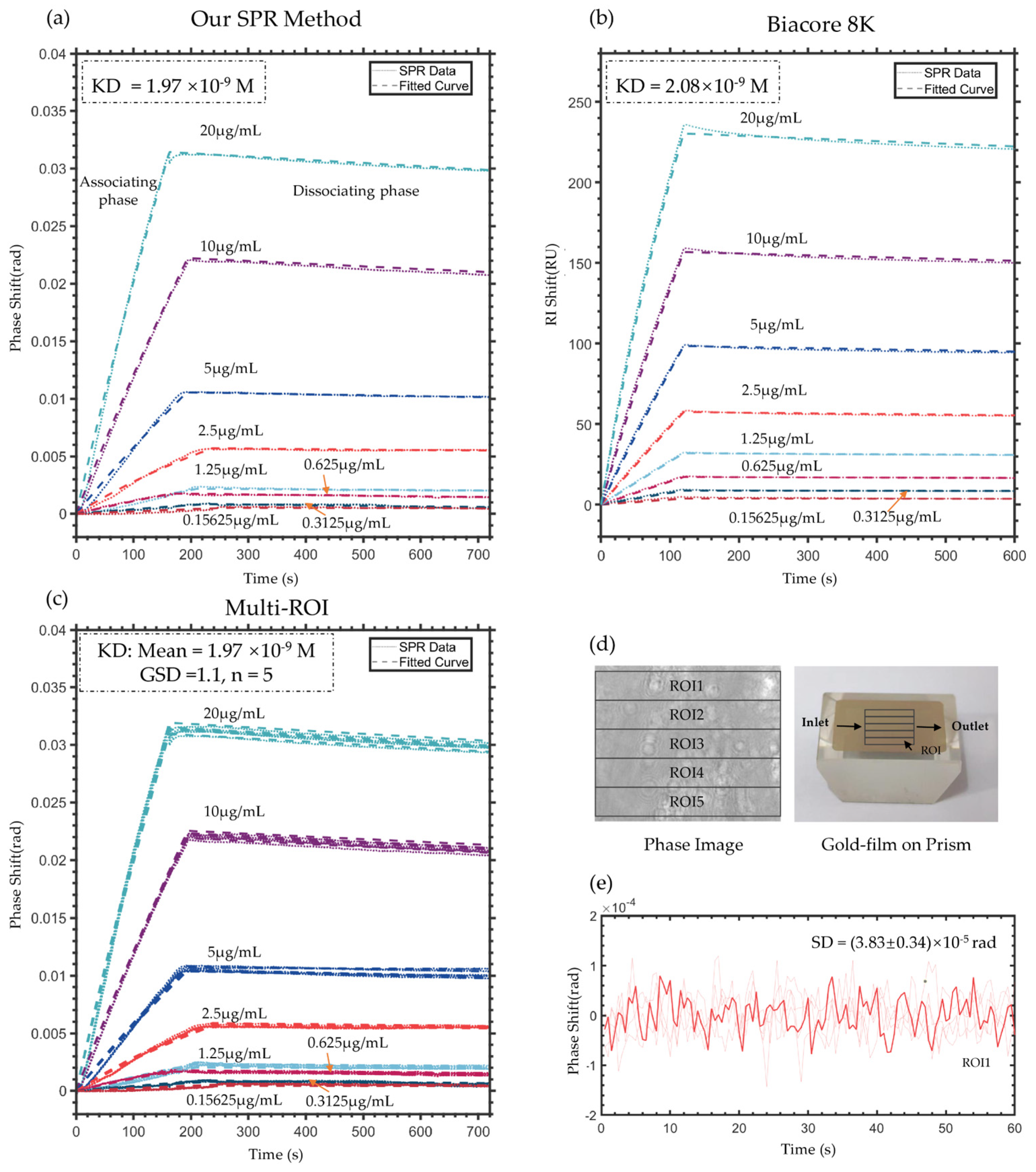
4.3. Validation of Protein Binding Kinetics Measurement
4.4. Discussion on Application Limitations and Future Work
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumari, A.; Yadav, A.; Singh, O.P.; Sharan, P. A Review Of Surface Plasmon Resonance (SPR) Technology In Biosensing: Innovations, Applications And Future Trends. J. Opt. 2024, 1–9. [Google Scholar] [CrossRef]
- Deng, S.; Wang, P.; Yu, X. Phase-Sensitive Surface Plasmon Resonance Sensors: Recent Progress and Future Prospects. Sensors 2017, 17, 2819. [Google Scholar] [CrossRef]
- Wang, D.; Loo, J.F.C.; Chen, J.; Yam, Y.; Chen, S.-C.; He, H.; Kong, S.K.; Ho, H.P. Recent Advances in Surface Plasmon Resonance Imaging Sensors. Sensors 2019, 19, 1266. [Google Scholar] [CrossRef]
- Wang, X.; Luo, X.; Wang, P. Temporally and spatially resolved SPR imaging of electrical double layer dynamics in electrolyte-gated transistors with ionic liquid. J. Mater. Chem. C 2024, 12, 9742–9752. [Google Scholar] [CrossRef]
- Huo, Z.; Li, Y.; Chen, B.; Zhang, W.; Yang, X.; Yang, X. Recent advances in surface plasmon resonance imaging and biological applications. Talanta 2022, 255, 124213. [Google Scholar] [CrossRef]
- Chen, R.; Li, Q.; Zeng, Y.; Sang, W.; Tang, S.; Jiao, Y.; Zhou, J.; Xu, Z.; Shao, Y.; Ying, M.; et al. Cellular analyses for label-free and rapid HER2-positive cancer diagnosis based on SPRi-modified with nanobody. Sensors Actuators B: Chem. 2023, 395. [Google Scholar] [CrossRef]
- Miyan, R.; Wang, X.; Zhou, J.; Zeng, Y.; Qu, J.; Ho, H.-P.; Zhou, K.; Gao, B.Z.; Chen, J.; Shao, Y.; et al. Phase interrogation surface plasmon resonance hyperspectral imaging sensor for multi-channel high-throughput detection. Opt. Express 2021, 29, 31418–31425. [Google Scholar] [CrossRef]
- Wong, C.L.; Olivo, M. Surface Plasmon Resonance Imaging Sensors: A Review. Plasmonics 2014, 9, 809–824. [Google Scholar] [CrossRef]
- Huang, Y.H.; Ho, H.P.; Wu, S.Y.; Kong, S.K. Detecting Phase Shifts in Surface Plasmon Resonance: A Review. Adv. Opt. Technol. 2012, 2012, 1–12. [Google Scholar] [CrossRef]
- Huang, Y.H.; Ho, H.P.; Wu, S.Y.; Kong, S.K.; Wong, W.W.; Shum, P. Phase sensitive SPR sensor for wide dynamic range detection. Opt. Lett. 2011, 36, 4092–4094. [Google Scholar] [CrossRef]
- Canning, J.; Qian, J.; Cook, K. Large dynamic range SPR measurements using a ZnSe prism. Photon- Sensors 2015, 5, 278–283. [Google Scholar] [CrossRef]
- Gul, B.; Ashraf, S.; Khan, S.; Nisar, H.; Ahmad, I. Cell refractive index: Models, insights, applications and future perspectives. Photodiagnosis Photodyn. Ther. 2021, 3, 102096. [Google Scholar] [CrossRef]
- Liang, X.J.; Liu, A.Q.; Lim, C.S.; Ayi, T.C.; Yap, P.H. Determining refractive index of single living cell using an integrated microchip. Sens. Actuators A Phys. 2007, 133, 349–354. [Google Scholar] [CrossRef]
- Ruemmele, J.A.; Golden, M.S.; Gao, Y.; Cornelius, E.M.; Anderson, M.E.; Postelnicu, L.; Georgiadis, R.M. Quantitative Surface Plasmon Resonance Imaging: A Simple Approach to Automated Angle Scanning. Anal. Chem. 2008, 80, 4752–4756. [Google Scholar] [CrossRef]
- El-Assar, M.; Taha, T.E.; El-Samie, F.E.A.; Fayed, H.A.; Aly, M.H. ZnSe-based highly-sensitive SPR biosensor for detection of different cancer cells and urine glucose levels. Opt. Quantum Electron. 2022, 55, 1–16. [Google Scholar] [CrossRef]
- Jain, S.; Choudhary, K.; Kumar, S. Photonic crystal fiber-based SPR sensor for broad range of refractive index sensing applications. Opt. Fiber Technol. 2022, 73. [Google Scholar] [CrossRef]
- Li, M.-C.; Chen, K.-R.; Kuo, C.-C.; Lin, Y.-X.; Su, L.-C. A Simple Phase-Sensitive Surface Plasmon Resonance Sensor Based on Simultaneous Polarization Measurement Strategy. Sensors 2021, 21, 7615. [Google Scholar] [CrossRef]
- He, H.; Cao, M.; Gao, Y.; Zheng, P.; Yan, S.; Zhong, J.-H.; Wang, L.; Jin, D.; Ren, B. Noise learning of instruments for high-contrast, high-resolution and fast hyperspectral microscopy and nanoscopy. Nat. Commun. 2024, 15, 1–12. [Google Scholar] [CrossRef]
- Liu, C.; Zeng, X.; An, Z.; Yang, Y.; Eisenbaum, M.; Gu, X.; Jornet, J.M.; Dy, G.K.; Reid, M.E.; Gan, Q.; et al. Sensitive Detection of Exosomal Proteins via a Compact Surface Plasmon Resonance Biosensor for Cancer Diagnosis. ACS Sensors 2018, 3, 1471–1479. [Google Scholar] [CrossRef]
- Peng, W.; Liu, Y.; Fang, P.; Liu, X.; Gong, Z.; Wang, H.; Cheng, F. Compact surface plasmon resonance imaging sensing system based on general optoelectronic components. Opt. Express 2014, 22, 6174–6185. [Google Scholar] [CrossRef]
- Batista, J.C.S.; Costa, M.V.S.; Oliveira, L.C. Smart Noise Reduction in SPR Sensors Response Using Multiple-ANN Design. IEEE Sensors J. 2020, 21, 4517–4524. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Y.; Xu, J.; Zhang, Y.; Liao, T. Post-experimental denoising and background subtraction of surface plasmon resonance images for better quantification. Chemom. Intell. Lab. Syst. 2012, 114, 56–63. [Google Scholar] [CrossRef]
- Scarano, S.; Scuffi, C.; Mascini, M.; Minunni, M. Surface plasmon resonance imaging (SPRi)-based sensing: A new approach in signal sampling and management. Biosens. Bioelectron. 2010, 26, 1380–1385. [Google Scholar] [CrossRef]
- Nizamov, S.; Scherbahn, V.; Mirsky, V.M. Self-referencing SPR-sensor based on integral measurements of light intensity reflected by arbitrarily distributed sensing and referencing spots. Sensors Actuators B: Chem. 2015, 207, 740–747. [Google Scholar] [CrossRef]
- Piliarik, M.; Homola, J. Self-referencing SPR imaging for most demanding high-throughput screening applications. Sensors Actuators B: Chem. 2008, 134, 353–355. [Google Scholar] [CrossRef]
- Wang, D.; Ding, L.; Zhang, W.; Luo, Z.; Ou, H.; Zhang, E.; Yu, X. A high-throughput surface plasmon resonance biosensor based on differential interferometric imaging. Meas. Sci. Technol. 2012, 23. [Google Scholar] [CrossRef]
- Kolosova, O.S.; Efremenko, Y.; Laurinavichyute, V.K.; Nizamov, S.; Petrushenko, S.I.; Mirsky, V.M. Poly-3-thienylboronic Acid Nanoparticles: Synthesis, Characterization, and Interaction with Saccharides Studied at the Level of Individual Nanoparticles. ACS Appl. Nano Mater. 2024, 7, 11120–11135. [Google Scholar] [CrossRef]
- Wang, S.; Shan, X.; Patel, U.; Huang, X.; Lu, J.; Li, J.; Tao, N. Label-free imaging, detection, and mass measurement of single viruses by surface plasmon resonance. Proc. Natl. Acad. Sci. 2010, 107, 16028–16032. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, X.; Zhou, J.; Miyan, R.; Qu, J.; Ho, H.-P.; Zhou, K.; Gao, B.Z.; Shao, Y.; Ho, A. Phase interrogation SPR sensing based on white light polarized interference for wide dynamic detection range. Opt. Express 2020, 28, 3442–3450. [Google Scholar] [CrossRef]
- Chang, Y.-F.; Wang, Y.-C.; Huang, T.-Y.; Li, M.-C.; Chen, S.-Y.; Lin, Y.-X.; Su, L.-C.; Lin, K.-J. AI integration into wavelength-based SPR biosensing: Advancements in spectroscopic analysis and detection. Anal. Chim. Acta 2025, 1341, 343640. [Google Scholar] [CrossRef]
- Dabov, K.; Foi, A.; Katkovnik, V.; Egiazarian, K. Image Denoising by Sparse 3-D Transform-Domain Collaborative Filtering. IEEE Trans. Image Process. 2007, 16, 2080–2095. [Google Scholar] [CrossRef]
- Maggioni, M.; Boracchi, G.; Foi, A.; Egiazarian, K. Video Denoising, Deblocking, and Enhancement Through Separable 4-D Nonlocal Spatiotemporal Transforms. IEEE Trans. Image Process. 2012, 21, 3952–3966. [Google Scholar] [CrossRef]
- Miao, S.; Fan, C.; Wen, G.; Dong, X. Image denoising and enhancement strategy based on polarization detection of space targets. Appl. Opt. 2022, 61, 904–918. [Google Scholar] [CrossRef]
- Liu, H.; Li, X.; Wang, Z.; Huang, Y.; Zhai, J.; Hu, H. Review of polarimetric image denoising. Adv. Imaging 2024, 1, 022001. [Google Scholar] [CrossRef]
- Ji, L.; Chen, Y.; Yuan, Y.J. Investigation of surface plasmon resonance phenomena by finite element analysis and Fresnel calculation. Sensors Actuators B: Chem. 2014, 198, 82–86. [Google Scholar] [CrossRef]
- Kurihara, K.; Suzuki, K. Theoretical Understanding of an Absorption-Based Surface Plasmon Resonance Sensor Based on Kretchmann’s Theory. Anal. Chem. 2002, 74, 696–701. [Google Scholar] [CrossRef]
- Surana, P.; Madhani, N.; Gopalakrishnan, T. A Comparative Study on the Recent Smart Mobile Phone Processors. In Proceedings of the 2020 7th International Conference on Smart Structures and Systems (ICSSS), Chennai, India, 23–24 July 2020; pp. 1–3. [Google Scholar]
- Liu, H.; Li, X.; Cheng, Z.; Liu, T.; Zhai, J.; Hu, H. Pol2Pol: Self-supervised polarimetric image denoising. Opt. Lett. 2023, 48, 4821–4824. [Google Scholar] [CrossRef]
- Deng, S.; Wang, P.; Liu, S.; Zhao, T.; Xu, S.; Guo, M.; Yu, X. A Novel Microfluidic Flow Rate Detection Method Based on Surface Plasmon Resonance Temperature Imaging. Sensors 2016, 16, 964. [Google Scholar] [CrossRef]
- Zhao, Y.; Deng, Z.-Q.; Hu, H.-F. Fiber-Optic SPR Sensor for Temperature Measurement. IEEE Trans. Instrum. Meas. 2015, 64, 3099–3104. [Google Scholar] [CrossRef]
- Hou, R.; Wang, Z.; Diamond, J.; Zheng, Z.; Zhu, J.; Wang, Z.; Chu, B. A quantitative evaluation model of denoising methods for surface plasmon resonance imaging signal. Sensors Actuators B: Chem. 2011, 160, 951–956. [Google Scholar] [CrossRef]
- Hauseman, Z.J.; Stauffer, F.; Beyer, K.S.; Mollé, S.; Cavicchioli, E.; Marchand, J.-R.; Fodor, M.; Viscomi, J.; Dhembi, A.; Katz, S.; et al. Targeting the SHOC2–RAS interaction in RAS-mutant cancers. Nature 2025, 642, 232–241. [Google Scholar] [CrossRef]
- Dunn, M.E.; Kithcart, A.; Kim, J.H.; Ho, A.J.-H.; Franklin, M.C.; Hernandez, A.R.; de Hoon, J.; Botermans, W.; Meyer, J.; Jin, X.; et al. Agonist antibody to guanylate cyclase receptor NPR1 regulates vascular tone. Nature 2024, 633, 654–661. [Google Scholar] [CrossRef]
- di Toma, A.; Brunetti, G.; Colapietro, P.; Ciminelli, C. High-Resolved Near-Field Sensing by Means of Dielectric Grating With a Box-Like Resonance Shape. IEEE Sensors J. 2024, 24, 6045–6053. [Google Scholar] [CrossRef]
- Torrijos-Morán, L.; Griol, A.; García-Rupérez, J. Bimodal Waveguide Sensors Enabled by Subwavelength Grating Structures. In OSA Advanced Photonics Congress (AP) 2020 (IPRSN); Optica Publishing Group: Washington, DC, USA, 2020. [Google Scholar]
- Aydin, D.; Barnes, J.A.; Loock, H.-P. In-fiber interferometry sensors for refractive index. Appl. Phys. Rev. 2023, 10, 011307. [Google Scholar] [CrossRef]
- Wu, Q.; Li, L.; Zhang, Y.; Ming, X.; Feng, N. Measurement methods, influencing factors and applications of intercellular receptor-ligand binding kinetics in diseases. Prog. Biophys. Mol. Biol. 2024, 194, 43–54. [Google Scholar] [CrossRef]
- Pu, Z.H.; Deng, S.J.; Luo, X.Y.; Wang, X.L.; Wang, P. Long-term Real-time In Situ Cell Chemosensitivity Analysis with Label-free SPRcellyzer. Surf. Plasmon Reson. (SPR) Protoc. Ebook 2025, Bio-101, e1011029. [Google Scholar] [CrossRef]
- Bolduc, O.R.; Pelletier, J.N.; Masson, J.-F. SPR Biosensing in Crude Serum Using Ultralow Fouling Binary Patterned Peptide SAM. Anal. Chem. 2010, 82, 3699–3706. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H.; Zhang, H.; Zhang, N.; Zheng, X.; Li, W.; Qiu, X.; Yu, D. Development of a portable multiplexed instrument for multi-proteins detection in human urine using surface plasmon resonance. Sensors Actuators B: Chem. 2022, 369. [Google Scholar] [CrossRef]
- Sankiewicz, A.; Romanowicz, L.; Pyc, M.; Hermanowicz, A.; Gorodkiewicz, E. SPR imaging biosensor for the quantitation of fibronectin concentration in blood samples. J. Pharm. Biomed. Anal. 2018, 150, 1–8. [Google Scholar] [CrossRef]

| Method | Standard Deviation (SD) of Phase (Rad) | Resolution of Refractive Index (RIU) |
|---|---|---|
| Raw data | (6.14 ± 0.39) ×10−5 | (3.51 ± 0.22) × 10−6 |
| BM3D | (6.54 ± 0.09) ×10−5 | (3.73 ± 0.05) × 10−6 |
| BM4D | (4.33 ± 0.18) × 10−5 | (2.47 ± 0.10) × 10−6 |
| Our method | (2.64 ± 0.19) × 10−5 | (1.51 ± 0.11) × 10−6 |
| Method | Number of Frame | SD of Phase (Rad) | Resolution of Refractive Index (RIU) |
|---|---|---|---|
| PPBM4D | 1 | (4.66 ± 0.23) × 10−5 | (2.66 ± 0.13) × 10−6 |
| PPBM4D (our method) | 2 | (2.64 ± 0.19) × 10−5 | (1.51 ± 0.11) × 10−6 |
| PPBM4D | 3 | (2.63 ± 0.13) × 10−5 | (1.51 ± 0.07) × 10−6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pu, Z.; Wang, X.; Chen, W.; Liu, Z.; Wang, P. Denoising Algorithm for High-Resolution and Large-Range Phase-Sensitive SPR Imaging Based on PFA. Sensors 2025, 25, 4641. https://doi.org/10.3390/s25154641
Pu Z, Wang X, Chen W, Liu Z, Wang P. Denoising Algorithm for High-Resolution and Large-Range Phase-Sensitive SPR Imaging Based on PFA. Sensors. 2025; 25(15):4641. https://doi.org/10.3390/s25154641
Chicago/Turabian StylePu, Zihang, Xuelin Wang, Wanwan Chen, Zhexian Liu, and Peng Wang. 2025. "Denoising Algorithm for High-Resolution and Large-Range Phase-Sensitive SPR Imaging Based on PFA" Sensors 25, no. 15: 4641. https://doi.org/10.3390/s25154641
APA StylePu, Z., Wang, X., Chen, W., Liu, Z., & Wang, P. (2025). Denoising Algorithm for High-Resolution and Large-Range Phase-Sensitive SPR Imaging Based on PFA. Sensors, 25(15), 4641. https://doi.org/10.3390/s25154641





