1. Introduction
The optimization and fine-tuning of therapy in response to the progression of Parkinson’s disease (PD) necessitate in-hospital diagnostic assessments and evaluations by means of clinical rating scales [
1,
2]. The overdosing of dopaminergic drugs often evokes levodopa-induced dyskinesia (LID), an uncomfortable side effect that is characterized by uncontrolled, involuntary muscle movements of all body parts [
3]. While many symptoms associated with PD may be more effectively identified through observational means, dyskinesias in the upper limbs hold significant potential for detection using wrist-worn sensors. The direct recording of health parameters provides vital insights into the ongoing progression of motor symptoms. Unlike a clinician who can only assess a snapshot of a moment, body-mounted systems are worn continuously throughout the day, offering a temporal recording of events. The acquired data is confined to the specific body part where the sensor is worn, yet it is tailored precisely to its designated tasks, thereby facilitating the capture of therapy-dependent motor fluctuations [
4,
5]. Subsequently, wearable devices such as smartwatches provide accessible monitoring aids, enabling the recording and tracking of movement data for understanding motor symptom variations and optimizing therapy plans [
6,
7,
8].
Wearables offer potential benefits for observing daily fluctuations in a hospital setting, allowing an expert-guided labeling of symptoms. While various sensor-driven approaches for capturing tremor and bradykinesia have been explored, there is a limited focus on dyskinesia detection despite its potential as a key biomarker for therapy responses [
9,
10]. Alongside other reports, studies conducted by Hssayeni et al. and Pfister et al. aim to assess dyskinesia in a free-living environment to be able to monitor the disease condition at home [
11,
12]. Hssayeni et al. seeks to estimate dyskinesia using accelerometer data during daily activities, reporting a Pearson correlation within the range of 0.70 to 0.84. In a similar vein, Pfister et al. reports the capability to detect dyskinesias in a free-living environment, achieving a sensitivity/specificity of 0.64/0.89. Acknowledging the importance of understanding the symptoms impact on everyday activities, there is clinical relevance in observing and estimating its occurrence during a hospital stay. When patients visit the clinic, neurologists adjust dopaminergic pharmaceuticals, leading to the frequent appearance of LID [
3,
13]. Monitoring these side effects during hospital admission could provide insights into the symptomatic fluctuations of patients, potentially aiding in the determination of the optimal drug dosage for individuals and supporting clinicians in addressing LIDs before patients are discharged. Sieberts et al. conducted a study referred to as the DREAM Challenge, which aligns with this suggestion by identifying biomarkers linked to tremor, bradykinesia, and dyskinesia in PD. Utilizing public datasets, the DREAM Challenge aimed to predict the severity of PD symptoms, resulting in an AUPR (area under the precision-recall curve) of 0.48 specifically for dyskinesia [
9].
Subsequently, we encourage the implementation of a novel monitoring setup specifically designed for dyskinesias emerging during hospital admission. Additionally, we propose an evaluation of the generalizability of movement data from publicly accessible datasets to our internally collected hospital data.
Thus, this paper employs two innovative approaches for movement data representation: one is a purely semantic technique utilizing principal component analysis (PCA) in combination with biomechanical feature extraction, and the other is an automatic, serial feature representation. Following the training of these methods on publicly available datasets from the Michael J. Fox Foundation (MJFF) [
14], our objective was to assess the performance of the resulting models on our own collected movement data and thus the models’ generalizability. To fulfill our objective, we formulated two intents: (1) investigating the impact of various movement data representations on model performance and (2) evaluating the generalizability of machine learning (ML) models from publicly available datasets [
14] onto the PACMAN (Parkinson’s Clinical Movement Assessment) dataset.
3. Results
3.1. The Patient Cohort
Our paper incorporated a total of 27 patients from the LRS dataset, spanning an age range of 50 to 84 years, with an average age of 67 years (±9 years). We included an average of 51 dyskinesia measurements (±9 measurements) per patient. Additionally, we included 24 patients from CIS-PD, with an average age of 63 years (±10 years), ranging from 36 to 75 years. Here, we used 12 dyskinesia measurements (±3 measurements) per patient on average. Finally, our in-clinic data collection contributed 25 patients, with an average age of 65 years (±8 years) and a range from 49 to 84 years. The PACMAN dataset incorporates an average of 3 dyskinesia measurements (±2 measurements) per patient.
3.2. Data Integrity
The presented data sources originate from distinct sites and were designed for different purposes. Nevertheless, they share comparability in terms of sensor type, demographics, neurological assessments, and disease-related intention of recording. The populations depicted in all MJFF studies fall within the same age range and undergo recurring hospital care for therapy adjustments. The types of sensors employed are consistent, as all studies integrate accelerometers, with both CIS-PD and PACMAN even utilizing Apple Watch devices.
Given that the MJFF studies involve a considerable number of ambulatory accelerometer recordings without precise physician annotations, our exclusive reliance on supervised labels was necessary to achieve our goal of identifying the most accurate representation of movement data for dyskinesia detection in a clinical setting. Consequently, all retained movement data and its corresponding labels are derived from hospital admittance and adhere to UPDRS standards.
3.3. Performance on Training Datasets
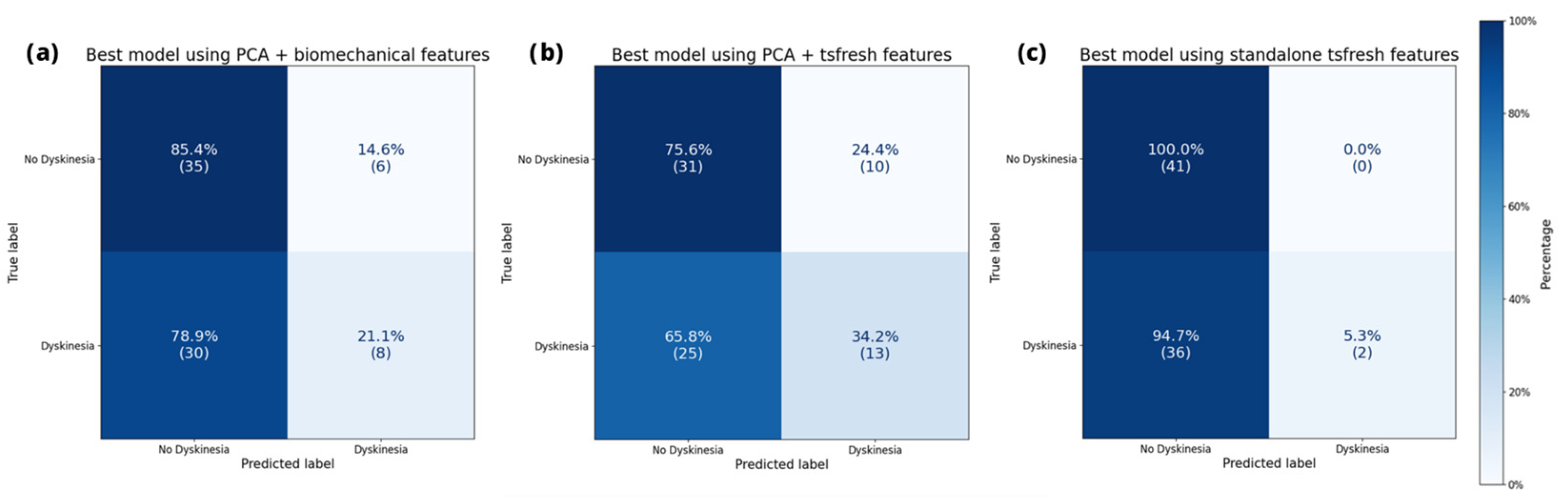
Figure 2 illustrates the confusion matrix of the leading model for each data representation on the MJFF studies. This visualization simultaneously presents the assigned dyskinesia label and the model’s prediction. Hence, the confusion matrix provides a reliable means to identify instances when a model incorrectly labeled a class. All depicted models adequately identified the absence of dyskinesia. However, they encountered challenges in accurately recognizing dyskinesia.
When it comes to the full evaluation of performance, we ranked the predictive outcomes for each representation by the unweighted F
1 score. Here, we achieved peak performances of 0.68 with both data representations, employing solely automatically extracted features and the automatically extracted features on the one-dimensional representation. The semantic representation using PCA and biomechanical features yielded a slightly lower performance, registering 0.63 for unweighted F
1. Accuracies of all three extraction methods were as high as 0.89 for each representation. The top five models per representation are listed in
Table 1,
Table 2 and
Table 3.
Neither the purely semantic technique, nor the automatic feature extraction, alone or combined, achieved a significant difference compared to each other. Across all performed combinations, the average unweighted F1 yielded scores of 0.53 ± 0.05, 0.57 ± 0.08, and 0.57 ± 0.08 for PCA with biomechanical features, PCA with feature extraction, and standalone feature extraction, respectively (mean ± standard deviation).
3.4. Generalization into Clinical Setting
While the performance on the public datasets though cross validations might approximate the generalizability on unseen data, we further tested this claim by utilizing the novel clinical data collected for this study. Application of the best 10 models per representation method on the PACMAN validation set yielded nuanced findings. The confusion matrix of the best model per representation, depicted in
Figure 3, shows a similar classification pattern as on the MJFF studies.
The top-performing representation for unweighted F
1 achieved a score of 0.53, utilizing PCA in conjunction with automatically extracted features. Following this was the semantic approach with a score of 0.48, and the standalone automatically extracted features ranked the lowest with a score of 0.40. The top five outcomes per representation of our generalization efforts are detailed in
Table 4,
Table 5 and
Table 6, including all the parameters employed.
Overall, the three methods yielded average unweighted F1 scores of 0.42 ± 0.04, 0.39 ± 0.06, and 0.36 ± 0.02 for PCA with biomechanical features, PCA with automatically extracted features, and automatically extracted features alone, respectively (mean ± standard deviation). Performances of the purely semantic representation were significantly higher than the automated technique (p < 0.001).
4. Discussion
This paper explores ML models for movement data representation, utilizing two distinct approaches and their combination. We first determined the top-performing models on the MJFF datasets and then evaluated their performance on our collected test dataset. Thereby, we were focusing on the impact of movement data representations and the generalizability of our resulting models for dyskinesia detection as our central hypotheses.
The results demonstrate how a dimensional reduction in movement data informed by the nature of the task has a positive impact on performance. Irrespective of whether combined with semantic or automatic extraction methods, the top 10 performing models incorporate this transformation when applied on our PACMAN dataset. The optimal performance is observed when the transformation is combined with automatic features. Nevertheless, the transformation combined with biomechanical features yielded comparable performance. This finding supports the idea that human-interpretable features enhance the ability of ML techniques to generalize across movement datasets. This finding confirms that semantically grounded preprocessing can serve as a safeguard against overfitting while supporting interpretability, a crucial factor in clinical implementation. The models using unselected features from a single automatic feature extraction generally show better results during training on the MJFF datasets but fail on our collected PACMAN data. Likely due to overfitting, it almost entirely fails to detect dyskinesia labels and assigns only two labels correctly. This underlines the importance of aligning feature representations with domain knowledge to improve robustness in real-world deployment.
Comparing the two approaches in detail, we observe that automated feature extraction (tsfresh) offers a wide array of statistical descriptors that may capture subtle signal characteristics, which contributes to strong performance on the structured and relatively homogeneous MJFF dataset. However, this approach appears to be less robust when applied to the clinically diverse PACMAN dataset, suggesting high sensitivity to variability in sensor noise, wearing conditions, and patient behavior. In contrast, the semantic representation consistently yields more stable results, even with limited and heterogeneous data. This suggests that the semantic approach not only improves interpretability for clinicians but also enhances robustness against real-world variability, which is critical for generalization across datasets. Therefore, while automated features may excel in high-data or controlled settings, semantically grounded representations prove more effective in noisy, low-data clinical environments, where reliability and explainability are essential.
Further, our analysis aligns closely with the performance reported in the DREAM Challenge, which aimed to identify LIDs on MJFF datasets resulting in an AUPR of 0.48. However, the latter study did not evaluate distinct data representations nor test generalization on an independent dataset [
9]. Previously, the mentioned studies by Hssayeni et al. and Pfister et al. report higher performances in dyskinesia detection, but their non-clinical setups are incomparable in terms of the sensor types used or a non-standardized assessment of dyskinesia [
11,
12]. Moreover, almost all the presented papers reveal distinct metrics, which are incomparable to each other. As each metric analysis is favorable for the individual intention, these metrics are a challenge to evaluate in terms of comparability. To this end, we chose the F
1 score as the principal metric for performance evaluation, as it balances precision and recall, two properties particularly important in the clinical context where both false positives and false negatives can impact therapeutic decisions.
While there is currently no universally accepted threshold for the F1 score in clinical ML applications, our observed values (MJFF: 0.68; PACMAN: 0.53) indicate a level of consistency and reliability that supports potential real-world use.
In order to lay the groundwork for ML generalization to work, standardization is required. First, the evaluative framework of studies working with supervised ML on movement data should use comparable and clinically relevant metrics. Analyses of medical data must account for class-specific performance, as predictions for each class need to be evaluated separately. Overall accuracy, for example, is insufficient in clinical settings, as it can obscure the detection of relevant disease phenotypes, particularly in imbalanced datasets. Secondly, the application of standardized neurological assessments, such as the suggested UPDRS, should be used. While some publications evaluate activities of daily living [
11], these activities are not sufficiently reproducible and only play a minor role in therapeutic adjustments. These assessments lay the foundation of the task-specific data labeling and thus are essential for generalizability.
On the contrary, the data collection approach presented in this paper provides a unique opportunity to acquire standardized clinical assessments of movement distortions over a two-week period per patient. The dense data quality obtained per patient facilitates the detection of dyskinesias in a hospital setting enabling early identification of LIDs for medication adjustments before the patient is discharged.
Regarding clinical implementation of the presented movement data methodology, the generalizability of the fully semantic representation suggests great opportunities for future applications of wearables to detect LIDs during clinical stays. Our primary assumption, that the periodic alternation between supination and pronation of the hand are well projectable by a simple dimensional reduction, turns out to be valid. Adhering to clinical expertise and translating it directly into straightforward data representations suitable for machine learning algorithms significantly influences the final performance outcomes. Although real-time analysis was not the focus of this study, the short inference time of our trained models suggests feasibility for future real-time applications, such as adaptive therapy monitoring during inpatient stays. The dimensionally reduced representations demonstrated better generalization on the PACMAN dataset compared to the automatic features, which overfitted on the MJFF dataset. This supports the value of embedding domain knowledge into preprocessing pipelines, particularly when data availability is limited, a common challenge in real-world clinical contexts. The results on the PACMAN dataset further suggest the enormous potential for a combination of both techniques. Combining task-specific semantic dimensional reduction with automatic feature extraction may offer the best of both worlds, as this hybrid approach performed best on the PACMAN dataset. Both techniques are rooted in the assumption that the UPDRS examination, a clinically validated neurological scoring standard for over 30 years, provides a robust foundation for interpreting dyskinesia. Accordingly, the semantic pipeline partially mimics a clinician’s process of evaluating movement patterns. A multidisciplinary approach between clinical expertise and data science is imperative for a successful application of this technology into routine.
However, this technology has its limitations for the detection of dyskinesia due to its unreliable predictive power. The relatively small size of our PACMAN dataset constrains the statistical power of our findings and likely contributes to variability in model performance. This study was designed as an empirical step towards estimating minimal data requirements under clinical constraints, but future work must include larger, statistically powered datasets. Particularly, an expanded data collection of clinically annotated measurements is essential for tracking LIDs and assisting clinicians in optimizing therapy. Perhaps even synthetically generated movement data could provide a foundation to train and optimize models without the extensive need of gathering patients. Furthermore, comparability between datasets and standardization plays a pivotal role for the generalizability and its coherent ability to detect dyskinesias, as stated previously. Also, it remains unclear if other neurological assessments that are diagnostically relevant for the dyskinesia detection can be projected by semantic knowledge. This suggestion is also undermined by the smartwatch’s limitation to detect movement of fingers or the hand. Nevertheless, the outcomes of this study demonstrate potential for the development of robust decision-support systems grounded in semantic principles, particularly in the analysis of clinically recorded movement data.