Highlights
What are the main findings?
- Eyeblink indices are potentially useful biomarkers for the effects of electroacupuncture and other neuromodulatory interventions.
- Significant autonomic correlates for some eyeblink parameters and EEG measures appear likely.
What is the implication of the main finding?
- Traditionally viewed artefacts, such as eyeblink measures, may themselves possess diagnostic and research value.
- Transcutaneous electroacupuncture stimulation can modulate brain activity and autonomic function in a time- and frequency-dependent manner.
Abstract
This study investigates the effects of transcutaneous electroacupuncture stimulation (TEAS) on eyeblink rate, EEG, and heart rate variability (HRV), emphasising whether eyeblink data—often dismissed as artefacts—can serve as useful physiological markers. Sixty-six participants underwent four TEAS sessions with different stimulation frequencies (2.5, 10, 80, and 160 pps, with 160 pps as a low-amplitude sham). EEG, ECG, PPG, and respiration data were recorded before, during, and after stimulation. Using non-parametric statistical analyses, including Friedman’s test, Wilcoxon, Conover–Iman, and bootstrapping, the study found significant changes across eyeblink, EEG, and HRV measures. Eyeblink laterality, particularly at 2.5 and 10 pps, showed strong frequency-specific effects. EEG power asymmetry and spectral centroids were associated with HRV indices, and 2.5 pps stimulation produced the strongest parasympathetic HRV response. Blink rate correlated with increased sympathetic and decreased parasympathetic activity. Baseline HRV measures, such as lower heart rate, predicted participant dropout. Eyeblinks were analysed using BLINKER software (v. 1.1.0), and additional complexity and entropy (‘CEPS-BLINKER’) metrics were derived. These measures were more predictive of adverse reactions than EEG-derived indices. Overall, TEAS modulates multiple physiological markers in a frequency-specific manner. Eyeblink characteristics, especially laterality, may offer valuable insights into autonomic function and TEAS efficacy in neuromodulation research.
1. Introduction
Transcutaneous electroacupuncture or electrical ‘acupoint’ stimulation (TEAS) is used increasingly in clinical practice as a non-invasive alternative to electroacupuncture using needles. Most common applications are for the alleviation of pain, nausea and vomiting, postoperative recovery, and in vitro fertilisation [1].
Eye movements such as blinking are usually considered as contaminating artefacts in electroencephalography (EEG) research that should be removed to enable better analysis of brain function. However, eyeblink characteristics may also be useful indicators in themselves. Blink rate (frequency), for example, typically between 10 and 25 blinks per minute at rest, may increase with fatigue, stress, anxiety, or nervousness, but may decrease in Parkinson’s disease or during tasks that require intense concentration or cognitive effort [2]. Other eyeblink parameters are provided as output from the easy-to-use MATLAB® software package BLINKER [3], including several variants of duration, peak maximal values, and ‘amplitude–velocity ratios’ [4]. These are listed in the accompanying online Supplementary Material and described in more detail on the GitHub BLINKER page [5]. Versions R2024a and 1.1.0 were used for MATLAB® and BLINKER, respectively.
A novel index that we introduce here is ‘best’ blink laterality, referring to the tendency for blinks to occur more frequently in one eye compared to the other (detailed explanation below, based on Kleifges et al. (2017) [3]). We found little previously published material on eyeblink laterality prior to BLINKER, other than a study indicating that eyelid (blink) asymmetries decrease with increasing experimental trigeminal stimulation but are in general stable over several months [6], together with several studies on ‘pre-pulse inhibition’ (PPI) of the startle response [7]. In one of these, Braff et al. cited previous research in which blink reflex magnitude was found to be greater for the right than for the left eye in the general population [8]. PPI itself was also noted to be greater in the right eye in healthy individuals, and particularly in smokers, but this difference was reduced in patients with schizophrenia [9]. Nicotine is well known to affect blink rate, presumably via a dopamine-mediated mechanism [10].
In 2013, aware that eyeblink rate (EBR) may be an indirect marker of striatal dopamine activity [10,11,12] and inversely correlated with parasympathetic activity [13,14], we presented a preliminary report based on four small pilot studies (N participants = 1 to 12) [15]. Our findings suggested that EBR increased more with electroacupuncture (EA), i.e., electrical stimulation applied using needle electrodes, than with gentle manual acupuncture (MA), that EBR increased more with 20 min than 5 min of EA, and that EBR decreased following EA. These results were all statistically encouraging, with p-values < 0.001 for Wilcoxon signed-rank and binomial tests. A finding that did not attain statistical significance was that EBR was usually greater for transcutaneous EA stimulation (TEAS)—i.e., transcutaneous electrical nerve stimulation (TENS) applied to acupuncture points—than for MA. In addition, we observed differences in EBR that failed to reach statistical significance with stimulation frequency in cycles per second (2.5 Hz or 10 Hz), and that EBR appeared to increase more with 20 min than with 5 min of EA.
Here, we analyse eyeblink data from a larger and more recent study on TEAS alone (2015–2016, N = 66, of whom 41 were women and 25 were men), comparing the effects of different stimulation frequencies in four sessions spaced a week or more apart. We also extended the range of indices provided by BLINKER by using their time series as input for CEPS, an open access MATLAB® software package for the analysis of complexity and entropy in physiological signals [16,17]. We then compared effect sizes for the effects of TEAS frequency, session order, and time within sessions on the original and extended BLINKER measures, other EEG-derived indices, and different measures of heart rate variability (HRV) and pulse rate variability (PRV).
On the supposition that there might be a central ‘frequency following effect’ in response to peripheral rhythmical stimulation, such as TEAS [18], with low-frequency stimulation enhancing low-frequency (delta, theta) EEG power and so tending to relax, and high-frequency stimulation increasing high-frequency (beta, gamma) EEG power and so tending to stimulate [19], our original study objective was to explore the effects of different frequencies of TEAS, rather than of stimulation amplitude [20]. Since then, at least one research group has explored this possibility, even writing of ‘theta and gamma electroacupuncture’ (stimulation at 6 Hz and 40 Hz, respectively) [21,22]. Another group noted that low-frequency (but not high-frequency) median nerve stimulation (TENS) enhanced occipital EEG alpha power spectral density (PSD) and reduced frontal Beta1 PSD in patients with generalised anxiety disorder [23]. In contrast, other researchers found that 100 Hz TENS applied locally to the forearm at either side of a pressure cuff used to induce ischaemic pain reduced the increase in gamma power that occurred when the cuff was inflated [24].
In 2021, we analysed the data from our 66 study participants in a conference presentation extending the range of autonomic measures associated with heart rate variability, in which we categorised the HRV measures output by the Kubios HRV software (Kubios Oy, Kuopio, Finland) as indicating either predominantly parasympathetic (‘PNS-like’) or sympathetic (‘SNS-like’) modulation, ‘ambivalent’ or ‘other’ effects. These same categories were also used to classify a variety of non-HRV measures derived from the electrocardiography (ECG), photoplethysmography (PPG), and respiration data collected concurrently [25]. Although it would be overly simplistic to consider the two main divisions of the autonomic nervous system, the parasympathetic and sympathetic, as functionally polar opposites [26], here, we extend this classification to a number of different EEG data types (see Appendix S4 in the Supplementary Material). As an aside, although PPG data were collected, pulse rate variability (PRV) was not analysed in detail independently (although similar in some respects, HRV and PRV provide different information on underlying physiological function [27]).
Our present objectives are as follows:
Objective 1. To consider whether time, stimulation frequency, or session order has a greater effect on eyeblink, EEG, and HRV indices/measures, and whether the eyeblink indices offer any advantage over the EEG and HRV measures in this regard.
Objective 2. To explore some possible parasympathetic/sympathetic associations of these measures, comparing strengths of association for the different eyeblink and EEG-derived data types.
Objective 3. To investigate whether these measures may be predictive of participant dropout or adverse reactions to stimulation.
2. Materials and Methods
2.1. Data Collection
EEG, ECG, and other data were recorded in the University of Hertfordshire’s physiotherapy laboratory, as described in detail elsewhere [28,29,30]. The University Health and Human Sciences Ethics Committee granted ethical approval (protocol number HSK/SF/UH/00124).
The study design was that participants should attend for four planned sessions, during each of which TEAS was applied to both hands over the acupuncture point hegu (LI4) and a point on the ulnar border of the same hand (so that current did not cross the midline of the body and did not flow through the arms and torso, and thus, in principle, should not affect the heart—or brain—directly). A different stimulation frequency was applied in each session—at 2.5, 10, 80, or 160 pulses per second (pps)—with the 160 pps frequency used as a ‘sham’ treatment.
To avoid repeated order effects, the different frequencies were applied in semi-randomised order: the sequence of sessions was first randomised, then checked to ensure no particular frequency was preponderant, and the sequence was amended if this was the case. For the active treatments, amplitude was increased until participants described it as ‘strong but comfortable’ [31], while for the sham treatment, amplitude was very low, with the amplitude control on the charge-balanced biphasic square-wave Equinox E-T388 stimulator (Equinox, Liverpool, UK) used being turned to zero once it had been felt initially by the participants, but with a flashing indicator lamp still visible (a procedure similar to that used previously in both clinical and experimental studies of transcutaneous electrical nerve stimulation (TENS) [32,33,34] and TEAS itself [35,36,37]). Participants were informed that they would habituate to the sham stimulation and would be unlikely to feel it after a short time. Nonetheless, only 19 (30.6%) reported after their sham session that they had been unable to feel anything at all during it.
On first arriving, the experimental procedure was explained to the study participants. As part of this, they were told: “We are not asking you to meditate or do anything out of the ordinary, but just to keep your eyes open and blinking as little as possible” (during the experiment, if participants were observed to blink rapidly, they were also asked by one of the researchers to slow their blinking down). Every attempt was made to keep the participants comfortable. Electrode caps were selected individually, in accordance with participant head size, to try and minimise any feelings of constriction or compression. Following the completion of some initial questionnaires and a numerical rating scale (NRS) for mood [29,38], the various electrodes and sensors were attached, including a 19-channel ECI (Electro-Cap International, Inc., Eaton, OH, USA) EEG cap (electrodes positioned according to the international 10/20 system, and with linked ears as reference and ground anterior to Fz), forearm ECG, fingertip photoplethysmography (PPG), finger temperature, respiration belt, and head movement sensors. Data were then recorded in eight consecutive 5 min time slots—baseline (Slot 1), stimulation (Slots 2–5), and post-stimulation (Slots 6–8). The NRS instrument was also completed following Slots 3 and 8.
2.2. Participants
Sixty-six participants were recruited over a period of 14 months as a convenience sample from university staff and students, and from other interested local contacts (including several established complementary health practitioners). This sample was expected to be typical of the general population.
2.3. Data Recording and Preprocessing
EEG data were recorded at 500 Hz using a Mitsar EEG-202 amplifier, and WinEEG software (v2.91.54) (Mitsar Ltd., St. Petersburg, Russia). Preprocessing steps and artefact removal are described elsewhere [30].
ECG data were recorded using the same equipment and software, with standard ECG electrodes (Kendall™ H124SG, Cardinal Health UK, Leeds, UK) positioned bilaterally on the volar surfaces of the forearms. In addition, respiration and head movement data were recorded using, respectively, an abdominal piezo crystal SleepSense respiration effort sensor belt (Scientific Laboratory Products, Elgin, IL, USA) and a head-mounted Pico Micro Movement Sensor (Unimed Electrodes, Farnham, Surrey, UK); these data were not analysed for the purposes of the current paper. ECG artefact correction and detrending (using the ‘smoothness priors’ method) were conducted using Kubios HRV software (Kubios HRV Standard, 3.3.0, Kuopio, Finland).
Finger temperature was recorded using a NeXus-10 physiological monitoring unit with its associated temperature sensor and BioTrace v2015B software (MindMedia BV, Herten, The Netherlands). The same unit and software were also used for recording bilateral PPG and a further channel of ECG (results not analysed here). Temperature was recorded at 32 Hz, recordings trimmed to remove obvious initial artefacts and then smoothed using the method available in the BioTrace software, but otherwise not preprocessed.
2.4. Measures Analysed
- 1.
- BLINKER
BLINKER considers as potential blinks only those events that last more than 50 milliseconds (ms) and are at least 50 ms apart. A single EEG (or electrooculogram, EOG) channel is then selected by the algorithm to provide the ‘best’ blinks, defined by the closeness of their actual trajectory to that of a ‘stereotypical’ blink, with correlation between them (Pearson’s R2) ≥ 0.98; for ‘good’ blinks, R2 ≥ 0.90 [3]. Parameters of the ‘stereotypical blink’ were not defined by Kleifges et al. [3], as appears to be the case for other studies in the literature in which stereotypical blinks are simply manually selected without further discussion (e.g., [39,40]). However, Kay Robbins, the communicating author of the original BLINKER study, has clarified that by ‘stereotypical blink’, the authors meant the ‘normal’ blink described by Johns [4], namely one with a single maximum whose derivative also has a single maximum, but followed by a single minimum [41]. More generally, it has been suggested that “all blinks of a test subject look pretty much alike, even among independent sessions. While blinks of different persons seem to look slightly different, they appear to be still highly correlated. Hence, it is possible to use a single prototype blink with the signals of different subjects” [42]. Miyakoshi et al. [43] made the interesting observation that physical effort to suppress blinks may indeed prevent the generation of stereotypical blink-induced EOG waveforms.
We considered several basic ‘families’ of measures output by BLINKER (for details, see Figure 1 here and Tables S1 and S2 in the Supplementary Material):
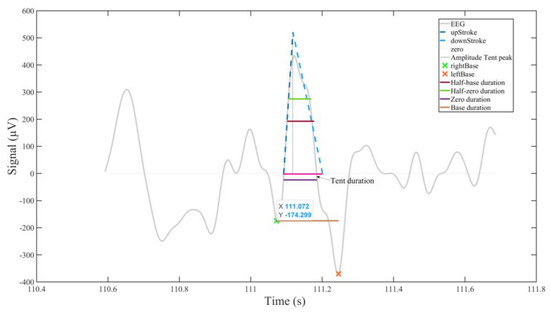
Figure 1.
Some eyeblink landmarks and the BLINKER outputs derived from them (based on [44], modified and reproduced with the author’s permission). Details of the ocular indices generated are provided in the Supplementary Material.
- (1)
- Duration: median, mean, and ‘mad’ (mean absolute deviation) blink durations (at blink base and zero levels, as well as at half-base and half-zero levels, together with ‘tent’ duration), with corresponding measures for ‘good’ blinks (goodMedian, GoodMean, etc.).
- (2)
- Blink rate (blinks per minute, BpM)—mean, or mean of ‘good’ blinks per minute—together with numbers of blinks and of ‘good’ blinks.
- (3)
- Amplitude–velocity ratios (nAVRB, nAVRT, and nAVRZ), estimated from intervals between peak maximum and right base, between tent peak and right tent line, and between peak maximum and right zero; correspondingly for pAVRB, pAVRT and pAVRZ and right blink markers.
In addition to this subset of 61 of the standard ocular indices provided by BLINKER, we also considered the ratio of left to right channels providing the best blinks (LRBR), as well as counts of left and right best blinks, resulting in 63 indices (see Supplementary Table S1). Furthermore, we fed the output of all the ocular indices from BLINKER for which this seemed appropriate to CEPS [16,17], to calculate a second layer defined as CEPS-BLINKER composite measures. Although less immediately interpretable in terms of physiology than the BLINKER indices, these would provide estimates of the complexity and variability/predictability of the ocular indices themselves. Given that the number of blinks in each 5 min recording was relatively small (see Section 3 below), only 34 of the CEPS measures could be used for this purpose, based on 30 of the original BLINKER indices, resulting in 34 × 30 or 1020 composite measures. Details are provided in Table S2 in the Supplementary Material.
For comparison purposes, as a convenience sample, a number of EEG power-based data types were also used, selected partly because their analysis was possible using the skills and resources available to us at the time, and on the basis of prior research indicating their potential usefulness for this project. Brief descriptions follow.
- 2.
- Median power for each 5 min recording, for all 228 channel (19) and band (12) combinations.
The EEG bands used were the five standard bands (delta, theta, alpha, etc.), together with Beta1 (15.0–18.0 Hz), Beta2 (18.0–25.0 Hz), Beta3 (25.0–35.0 Hz), the sensorimotor rhythm (SMR, 12.0–15.0 Hz), 2.0–20.0 Hz and 0.5–45.0 Hz, with one other (‘Infill1’, 12.0–19.0 Hz). EEG power is a usual starting point used in investigations of the effects of different interventions on the central nervous system. Prior research on EA frequency and EEG power can be found in the literature (e.g., [45,46]). As mentioned, the correlations between EEG features and HRV or stress measures have been researched by a number of authors [47,48,49,50,51].
- 3.
- Regional power ratios: posterior/anterior, left/right, central/outer ratios of absolute or relative power in 12 standard and ‘infill’ EEG bands—a total of 252 measures.
We initially considered these regional ratios as a means of differentiating between volume (outer) and neural (central/somatosensory) conduction effects [52] and then added the other regional ratios as well [53]. Changes in EEG laterality have been reported before in response to TEAS [54] and TENS [55]. To our knowledge, the posterior/anterior (P/A) ratio has not been used in acupuncture-related research before, and although EEG laterality may be affected by stress [56], the P/A and central/outer (C/O) ratios have not, to our knowledge, been considered in relation to HRV.
- 4.
- Eighteen median power asymmetries, for both symmetrical right/left electrodes and regions [Loge(Mdn Pwr Right) − Loge(Mdn Pwr Left)], and for posterior/anterior and peripheral/central regions, in the same bands as for median power, together with 6 narrow bands centred on frequencies of potential interest (stimulation frequencies or their sub-multiples) and a further 12 narrow bands centred on frequencies presumed unrelated to stimulation frequencies, with 18 absolute and relative powers in narrow bands—a total of 602 measures.
Frontal alpha band asymmetry is a frequently used measure in EEG research [57], with changes reported in response to acupuncture, for example [58]. In particular, frontal alpha asymmetry is commonly used in studies on stress [48,51]. Here, we assessed EEG asymmetries more generally, not just in the alpha band.
- 5.
- Median power asymmetry ratios [e.g., 2 × (Loge(Mdn Pwr Right) − Loge(Mdn Pwr Left))/(Loge(Mdn Pwr Right) + Loge(Mdn Pwr Left))], as for the asymmetries but omitting narrow bands centred on 120 Hz and the absolute and relative power bands—a total of 530 measures. We also considered power asymmetry ratios [59] as variant measures of asymmetry.
- 6.
- Cordance measures in various standard and non-standard EEG bands, for all electrodes, both non-normalised and calculated as a comparison using both log-normalised [60,61] and square-root-normalised algorithms [62], with bands created using Thomson multi-taper or continuous Morlet wavelet methods [63,64]—a total of 8148 measures.
Leuchter and colleagues have claimed that cordance, a measure they derived from averaged absolute and relative EEG power in a particular band measured from all or some of the electrode pairs used [65], “is potentially a more specific indicator of cerebral dysfunction than QEEG [i.e., quantitative EEG] power, since it has normal and abnormal indicator states” [66]. They also noted that greater EEG alpha represents the inhibition of brain activity, thereby reflecting a reduction in brain perfusion in the healthy [62]. Given that the frequency of TEAS or EA may affect circulation (e.g., [67]), we considered that cordance would, therefore, be a useful measure to use in the present study. To our knowledge, no studies on interactions between HRV and cordance have been published. One study on cordance and ‘dry needling’ (a modern, stripped-down version of traditional acupuncture) was located [68], but none on cordance and EA or TENS/TEAS.
- 7.
- Centroids: regional power and frequency centroids; electrode spectral centroids; finger temperature slope (over time). ‘x’ and ‘y’ coordinates of centroids of all electrodes, those on the left or right, anterior or posterior, central or peripheral regions of the cranium, together with spectral centroids for each of the 19 channels—a total of 48 measures.
Sulaiman et al. (2010) [69] used EEG spectral centroids for identifying stress, and Giannakakis et al. (2015) [70] found that EEG centroid frequencies in many channels did indeed appear to increase with stress. We therefore considered this method appropriate here and also used spatial centroids as another method of identifying particular brain regions that might be affected by different frequencies of TEAS.
Acute stress may trigger peripheral vasoconstriction, causing a rapid drop in skin temperature [71]. Skin temperature itself may increase or decrease in response to EA, depending on the individual [72]. Finger temperature slope was investigated here to explore the autonomic effects of different frequencies of TEAS and whether these were experienced as stressful or not. Low-frequency stimulation was expected to result in vasodilation rather than vasoconstriction [67].
- 8.
- Power in 14 1 Hz bins, either bands centred on frequencies of potential interest (stimulation frequencies or their sub-multiples) or centred on frequencies presumed unrelated to stimulation frequencies, with bands created using the methods mentioned above. Two different methods of independent component analysis (ICA) were used in the data preparation: Extended InfoMax [73] (1065 measures) and Adaptive Mixture ICA, or AMICA [74] (1063 measures). Preprocessing was either using the standard methods available in EEGLab v 2022.0 [75] and associated software, using a pipeline created by Paul Steinfath as described elsewhere [30], or a semi-automated machine learning method developed by Thea Radüntz (who kindly provided the results for us) [76]—a total of 2128 measures.
Our initial hypothesis [18] was that peripheral stimulation as applied in this study might induce a ‘frequency-following response’ (FFR) centrally, in the EEG. Using narrow frequency bins, we hoped to detect any resulting FFR. Given that correlations have been found between the HRV HFnu index and EEG Alpha2 (a narrow band between 10 and 12 Hz) [49], we also hoped to detect such correlations in our own data.
- 9.
- In addition, the ‘classical’ EEG Hjorth activity, complexity and mobility parameters [77,78] were computed for each electrode (228 measures), as well as Wackermann’s ‘global descriptor’ indices sigma, phi and omega [79] (median values at the start, middle, and end of each recording) (48 measures).
- 10.
- A further dataset consisted of the Wackermann descriptors for the 4 sec segments in each 5 min slot, with a repeat of the median values from the previous dataset, based on either Infomax or AMICA ICA (3188 measures).
- 11.
- Finally, HRV and PRV (pulse rate variability) indices computed using Kubios HRV software (Standard version 3.3.0) were included, for both the 5 min recordings (59 PRV and 59 HRV measures) and their 1 min segments (only a further 66, i.e., 2 × 33, measures, as 1 min segments were too short to compute some measures).
To our knowledge, neither the Hjorth parameters nor Wackerman’s global descriptors have been used in prior acupuncture research, nor have they been researched for their association with HRV measures. However, they do provide an overview of the EEG that we considered could be useful in this study.
In total, some 15,356 measures were available for analysis.
Data for all these measures will be made available by the corresponding author (DB, email duncan.banks@open.ac.uk), upon reasonable request. Some findings for the most salient measures are presented in Table 7 below, and in Tables S4, S8, and Appendix S4 Table S14 in the Supplementary Material.
2.5. Statistical Analysis
Analysis was conducted in various stages.
- (1)
- Slot Values:
Initially, 5 min values of the various measures were analysed, or their median values for each 5 min recording when more appropriate.
- (2)
- Slot Differences (values normalised with respect to baseline, within session):
To take into account differences at baseline (in Slot 1) that could clearly not be attributed to the effects of stimulation frequency, the authors decided to also examine normalised values, or differences between values in a given slot and in Slot 1.
Shapiro–Wilk tests were used (in both SPSS and R) for both values and differences to determine whether data were normally distributed or not. Preliminary tests for normality of distribution were initially also carried out in Excel using the Jarque–Bera test [80].
- 12.
- Given the large number of variables involved in this exploratory study, most would be expected to provide little, if any, useful information on any questions we might ask of the data. In an earlier project, we explored the effects of space and terrestrial weather conditions on EEG and ECG readings collected during TEAS sessions, investigating more than 7700 features using a method called highly comparative time series analysis [81]. Here, we used a different approach, making use of what we call ‘top slicing’ methods in order to reduce the number of measures considered to something more informative and manageable. Various methods of ‘top slicing’ were compared to focus on those measures most likely to show significant differences with stimulation frequency, session, or slot within session. These are discussed below, in the Results section. Friedman’s test for repeated measures was used for both values and differences (as a non-parametric equivalent of analysis of variance, or ANOVA), with Kendall’s W as a measure of effect size, and Wilcoxon and Conover–Iman matched-pair signed-rank tests for post hoc analysis.
- (3)
- Bootstrapping (random sampling with replacement):
To provide more robust results for the Friedman, Kendall, Wilcoxon, and Conover–Iman tests, ‘bootstrapping’ of 1000 randomly sampled sets of results was used [82], with results reported as medians and 95% confidence intervals.
- (4)
- Interactions between frequency, session, and slot within session were also tested for, using the simple rank test for interaction proposed by Hettmansperger and Elmore (2002) [83].
- (5)
- Other non-parametric tests were used as required, such as the Mann–Whitney U test (also known as the Wilcoxon rank-sum test) for differences between independent samples and Spearman’s rank correlation method for correlations between them. For the latter, confidence intervals were calculated using the method of Bonett and Wright [84].
- 13.
- Effect size: In addition to statistical significance (using p-values), effect size (ES) was computed using different methods, as appropriate for each test. For Mann–Whitney tests, Cohen’s r (or Z/√n) was used, where Z is the Z-score, and n is the total number of observations on which Z is based [85,86]; for Friedman and Conover tests, Kendall’s W (or coefficient of concordance) was used, linearly related to Friedman’s χ2 (W = χ2/n(k − 1), where k is the number of measurements per participant [86]. For Spearman’s rank correlations, the correlation coefficient itself (rho, ρ) was taken as a measure of ES [87]. For all three methods, the usual convention was adopted: 0.1 ≤ ES < 0.3 was considered ‘small’, 0.3 ≤ ES < 0.5 as ‘medium, and ≥ 0.5 as ‘large’.
The following software packages were used: IBM SPSS Statistics v. 28 (IBM, Armonk, NY, USA); RStudio 2024.04.0+735 “Chocolate Cosmos” Release for Windows (RStudio Team, 2020); and RStudio: Integrated Development for R. RStudio (PBC, Boston, MA, USA), with subsidiary packages for the Friedman, Kendall, Wilcoxon, and post hoc Conover tests (the latter amended by author NS).
When discussing statistical significance, the usual 5% level of significance was employed, except when otherwise specified, but we must be mindful of the fact that many comparisons can be carried out. In these circumstances, p-values should not be taken at face value and we have two options: (i) make adjustments to the p-values so that they can be interpreted in a traditional manner; (ii) treat the p-values merely as indications of the strength of evidence against the null hypothesis without any more formal interpretation. In the publication of most studies, option (i) would be favoured over option (ii). However, traditional methods for p-value adjustment (e.g., Bonferroni, Šidák) are only appropriate for situations where the multiple tests are independent of each other, and this is far from being the case here. For situations, such as the case in this paper, where the multiple tests are not independent, simulation methods can, in theory, be used to make adjustments (e.g., [88]). However, in the current context, with a very large number of highly correlated tests, it was not computationally feasible to undertake such simulations. As a result, option (i) for dealing with the multiple comparisons issue was not available, and we were obliged to use option (ii), taking the p-values as they were and applying appropriate scientific judgment and caution when drawing conclusions.
3. Results
3.1. Participant Demographics
Of the 66 participants recruited, 4 dropped out after only one session, and another only completed three sessions. Technical and other issues (such as a computer crash on one occasion and a participant ignoring the instruction to visit the toilet before a session, so that it had to be interrupted) led to incomplete data in a number of other cases. Complete datasets were obtained for 48 participants (18 men and 30 women, aged between 18 and 69). Their ages were not evenly distributed, and although more women (F) than men (M) took part in the study, this was not the case in all age groups, as shown in Figure S1 from the Supplementary Material.
The flowchart in Figure 2 summarises the above, from recruitment to statistical tests used.
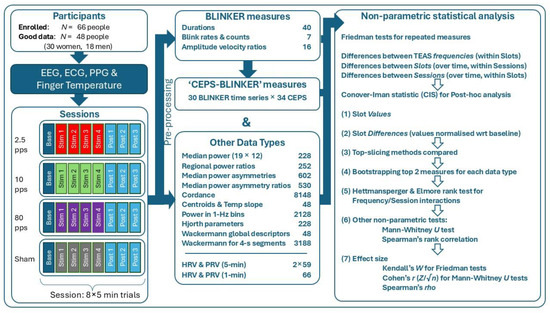
Figure 2.
Flowchart of methods used to acquire and analyse EEG, ECG, PPG, and finger temperature data. Background colours indicate the stimulation frequencies applied.
3.2. Normality of Distribution
Testing for the normality of the distribution of measure values using Shapiro–Wilk tests indicated that BLINKER, CEPS-BLINKER, and most of the other data types used were not normally distributed, except for some EEG Median power, median power asymmetry and asymmetry ratio results, justifying the use of non-parametric rather than parametric statistical tests for the remainder of the study.
3.3. Top Slicing Methods Used
We considered taking only those in the upper quartile, the top 10%, top 5%, top 1% or top 0.1% of values or counts of Friedman’s chi-square, Kendall’s W or the Conover–Iman statistic (CIS), counts of these three statistics greater than a certain threshold (e.g., 1, 2 or 3 for the Conover–Iman statistic), or simply the top 10 or top 2 measures—or even the single very top measure—for each data type. For example, differences in BLINKER measures between slots for each stimulation frequency (4 × 61 rows in the R output file) resulted in Spearman correlations between the results from the different top slicing methods with rho > 0.8 and p < 0.001 regardless of whether strengths of the correlations between the resulting 45 pairs of percentiles, counts and/or Kendall’s W were checked. Thus, in a way, it almost seems irrelevant which method of top slicing should be selected, whether these are based on percentages or counts greater than a certain threshold. We eventually settled on selecting the ‘top 10’ measures for each data type as a pragmatic way of proceeding. However, different methods could be used to select the ‘top 10’ measures for each data type, such as either including or excluding repetitions. In the former, the same measure may appear in several rows (e.g., for several frequencies when testing for differences in measures between slots for each stimulation frequency); in the latter, only for a single row in the R output. As an example, the ‘top 10’ BLINKER measures using each method when testing for differences in measures between slots for each stimulation frequency are shown in Table S3, with six measures occurring in both lists.
Results for Objective 1. Does time, stimulation frequency, or session order have a greater effect on eyeblink, EEG, and HRV indices/measures, and do the eyeblink indices offer any advantage over the EEG and HRV measures in this regard?
Counts of the ‘top 10’ measures in the results for Friedman’s chi-square or for Kendall’s W will be very much the same, given the linear relationship between the two statistics. The results for Kendall’s W are shown in graphical form in Figure 3, for all the data types shown in the study flowchart (Figure 2) taken together. For example, counts of differences between slots varied with stimulation frequency, being greatest at 2.5 pps (70+ out of 140 measures) and least for sham (only around 10 of 140 measures) (Figure 3A). Counts of differences between slots were also greatest in Session 1 (Figure 3B), whereas counts of differences between TEAS frequencies were greatest during stimulation (Slots 2–5) (Figure 3C). Note that counts of the ‘top 10’ measures are not the same as those of the 10-scoring measures, which will always be equal to 10.
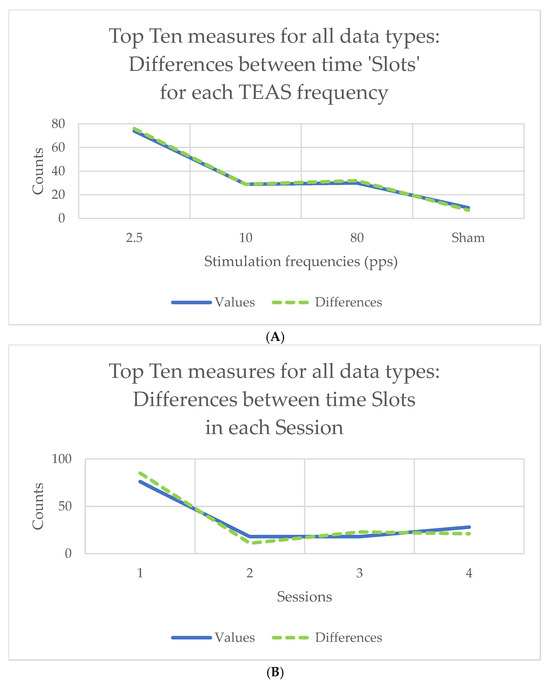
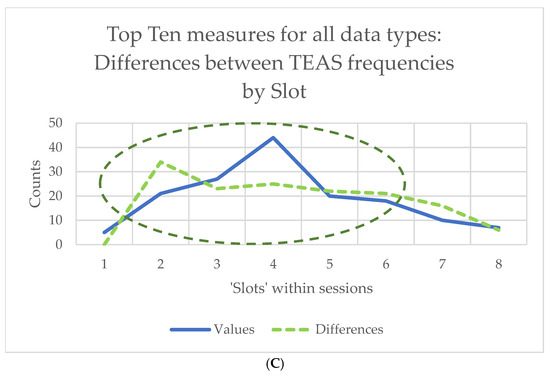
Figure 3.
Counts of differences for the 140 ‘top 10’ measures (10 for each of 14 data types): (A) between time ‘slots’ for the four stimulation frequencies used; (B) between time ‘slots’ in the four sessions attended; (C) counts of differences between stimulation frequencies over time, showing changes with session. Counts are shown for both slot values and differences of slot values from baseline, so the latter are zero in (C) (see above, Section 2.5, for explanation).
Those of the ‘top 10’ measures that occurred three times or more for both Values and Differences—i.e., for three or more comparisons (4 × Stimulation frequency within Slot, 8 × Slot within Session, etc., including 32 Slot × Stimulation frequency combinations for Values, but not for Differences)—are shown in Table S4 in the Supplementary Material.
Note that for 19 of the ‘top 10’ measures for all data types in Table S4, the effect size (Kendall’s W) was greater for differences, whereas this was only the case for 11 values. Considering only the single top-most measure for each data type, Kendall’s W for differences between stimulation frequencies within each slot was now more often greater for values (nine data types) than for differences (six data types). Corresponding results for Friedman’s Chi2 are shown in Figure 4. Note, too, that nearly all the effect sizes (median W) were small (0.1 ≤ ES < 0.3), if not very small. Incidentally, for all of the top 10 measures for ‘1 Hz bins’, W for both values and differences was >0.2 for differences between slots for each stimulation frequency, and also for differences between slots for each session, but not for the other value or difference comparisons. Maximum effect sizes were found for the 1 Hz bins, followed by median power (values: O2.5_P3, Wmax = 0.3023; differences: O5_P3, Wmax = 0.3258). These were for differences between stimulation frequencies within each slot, not differences between sessions within each slot. Corresponding results for Friedman’s Chi2 are shown in Figure 4. The results for the 1 Hz bins are provided in more detail in Appendix S1 in the Supplementary Material.
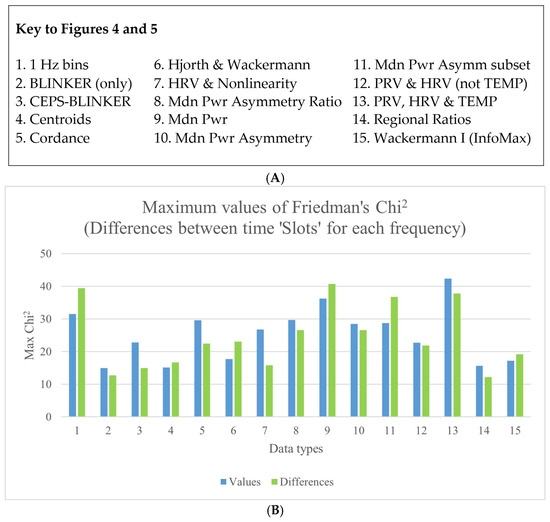
Figure 4.
(A) A key to explain the numbering system used on the X-axis. (B) Differences between stimulation frequencies within each slot, for the various data types—both values and differences.
More graphical results comparing values and differences (values normalised with respect to baseline within session) are included in the Supplementary Material, Appendix S2.
3.4. Comparing Maximum Effect Sizes for Differences Between Sessions and Differences Between Stimulation Frequencies, Both Within Slots
Figure 5 shows the maximum effect sizes for differences between sessions and differences between stimulation frequencies within slots.
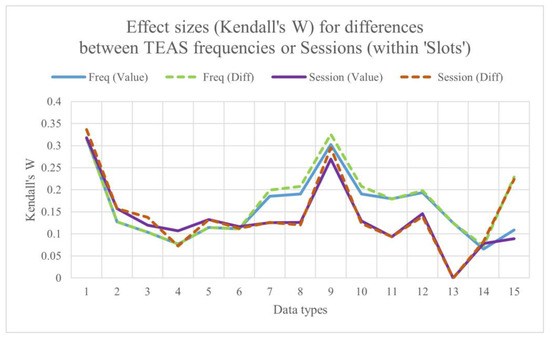
Figure 5.
Maximum effect sizes for differences between sessions and differences between stimulation frequencies, both within time ‘slots’.
Median W over all data types:
- Session by slot (Diffs): 0.129;
- Stimulation frequency by slot (Diffs): 0.179;
- Session by slot (values): 0.126;
- Stimulation frequency by slot (values): 0.127.
Overall, ES is marginally greater for differences than for values, and, more obviously, for differences between frequencies than for differences between sessions.
The lowest values of W are consistently for the regional ratios, followed by centroids, highest values for median power in 1 Hz bins and in the standard EEG bands. BLINKER and CEPS-BLINKER measures do not perform particularly well in differentiating between stimulation frequency and session. As might be predicted, finger temperature and HRV/PRV do not show a session order effect.
3.5. Differences over Time (Between Slots) Within Sessions
For the majority of data types considered (8 out of 11), the 99th percentile of Kendall’s W is greatest for changes over time (i.e., when comparing time slots within sessions) than when comparing results for the four different sessions or the four stimulation frequencies, as can be seen in Figure S6.
From Figure S6, it is clear that Kendall’s W, as a measure of effect size, was most commonly very small (<0.1), although >0.1 for five data types (including BLINKER) for time slot comparisons, and for the frequency and session comparisons for cordance only.
3.6. Post hoc Results, Using the Conover–Iman Statistic (CIS)
Turning now to the post hoc results, it is clear that findings for the Conover–Iman statistic follow a similar pattern to those for Kendall’s W (Figure S7). Furthermore, counts of the Conover–Iman statistic (CIS) > 3 (as well as of the 95th percentiles of the CIS) indicate that differences from baseline (Slot 1) increase with time, when all 63 BLINKER measures are considered together (Figure 6). Early changes (between Slots 1 and 3) appear to be greater for 80 pps stimulation, with a cumulative effect for all stimulation frequencies by Slot 5, although this is most consistently maintained only for 10 pps stimulation thereafter.
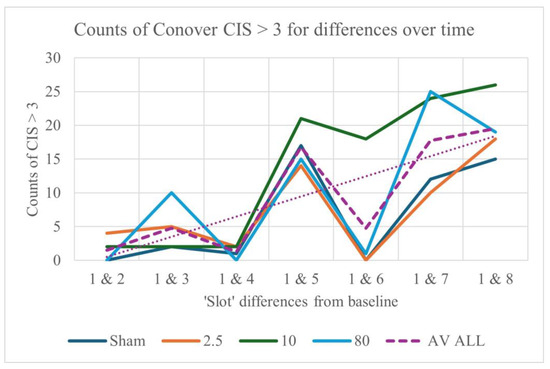
Figure 6.
Differences from baseline in counts of CIS > 3 for the different stimulation frequencies, for 63 BLINKER output measures. Median counts for all seven pairs of slots are shown in the inset box. The ‘average’ trendline is dotted.
Counts (and values) of the blink measures appear to increase more rapidly during 80 pps than 2.5 pps stimulation, with more of a consistently cumulative effect over time for 10 pps. The apparent pattern of increases only for every other slot is in part due to the cumulative effects of stimulation (in Slots 2–5) and a subsequent decrease post-stimulation (in Slot 6), together with a steady increase in CIS over time. An overview of CIS results for the BLINKER and CEPS-BLINKER measures that differentiate between slots at each stimulation frequency, between frequencies, and between sessions within each slot is provided in Table 1.

Table 1.
Values of CIS using different ‘top slicing’ methods (maxima, 90th and 95th percentiles, and upper quartiles), for differentiating between time slots at each stimulation frequency (28 comparisons), between frequencies within each time slot, and between sessions within each time slot (6 comparisons each). Median values are also shown.
From this top-level analysis, changes over time within sessions—whether for the BLINKER measures or for the CEPS measures derived from them—are greater than their differences with stimulation frequency, and greater than their differences over time between sessions. However, contrary to expectation, differences in the CEPS measures derived from the BLINKER indices are less over time (within sessions) than those for the BLINKER measures themselves. Nonetheless, the maximal values of the CEPS-BLINKER measures are indeed more than the maximal BLINKER measures themselves when comparing their values for the different stimulation frequencies, as well as for the different sessions.
3.7. Measures and Indices Most Affected by Stimulation Frequency, Time Slot, or Session Order
The ten CEPS measures and BLINKER data types from which they were derived that provided the greatest differentiation between stimulation frequencies, between times, and between sessions (the largest 95th percentiles of CIS) are shown in the Supplementary Materials, Tables S5 and S6.
When all data types are considered (1 Hz bins, HRV, etc.), the patterns of change from baseline are similar to those for the 63 BLINKER output measures shown in Figure 6 above (also see Figures S4 and S5)—namely, consistent increases from Slots 1, 2, 3 and 4 (before and during stimulation), with subsequent decreases from Slots 5, 6 and 7 (post-stimulation). The top two measures most affected, together with the Frequency, slot or session pair for which they showed maximum values of the Conover–Iman statistic, are listed in Table S8. Note that pairs are rarely consistent for the two measures. The most commonly occurring pairs, i.e., greatest differences, were time ‘Slots’ 2–7 (four occurrences), Sessions 1–4 (eight occurrences), and stimulation frequencies 2.5–80 pps (eight occurrences). The effects of stimulation amplitude were not so easy to disentangle (a brief summary of methods used and findings is provided in Appendix S3 in the Supplementary Material).
3.8. Bootstrapping Results
Because of the large number of variables/measures analysed in this study, it was not feasible to bootstrap results for them all. Instead, bootstrapping was only applied to the top two measures for each data type (as listed in Table S8 in the Supplementary Material). As expected, 95% confidence intervals were much narrower (i.e., more precise) for Kendall’s W (and Friedman’s Chi2) for the bootstrapped than the non-bootstrapped results. As an example, in Figure S2 the median values of W are plotted for differences between time ‘Slots’ at each of the four stimulation frequencies for EEG power at channel P8 in the 1 Hz EEG bin centred on 7.5 Hz (using the extended InfoMax algorithm for ICA and Thomson’s multi-taper method to create the band/bin). For the non-bootstrapped results, the upper confidence interval = 1, so it is not shown. Note that the median W is greater for the bootstrapped results (maximal for sham stimulation) and is less for the non-bootstrapped results (maximal for 10 pps).
3.9. Interactions Analysed Using the Hettmansperger–Elmore Test
Using the Hettmansperger–Elmore test, most of the different data types showed interactions between session and stimulation frequency, indicating that despite having allocated stimulation frequencies to sessions in a semi-randomised balanced order, there are still effects specific to combinations of sessions and stimulation frequencies present for unexplained reasons. Counts of p-values < 0.01 for the Hettmansperger–Elmore test were transformed into percentages of all counts for each data type (e.g., of 2128 for 1 Hz EEG median power bins, or of 63 for the EEG regional ratios). In general, the percentage counts were lower for the measure differences (values normalised with respect to baseline) than for the values themselves, but all were more than 70%. However, for three data types, significant interactions were found between session and time slot (rather than between session and stimulation frequency): MdnPwr_ASYMM_171 (86.0%), MdnPwr_ASYMM (79.2%), and MdnPwr (78.1%), although these were only for differences, not for values.
Results for Objective 2. Are there meaningful and significant associations between parasympathetic/sympathetic indices and the different eyeblink and EEG-derived data types?
Several HRV indices are widely accepted as indicating parasympathetic or sympathetic modulation of heart rate [89]. In a 2021 conference poster [25], we proposed extending this list, considering some indices to be ‘PNS-like’, others ‘SNS-like’, and a third group ‘ambivalent’, as shown in Table 2 (based on Tables in the online ‘Background Information’ for the poster). The measures listed are those included in the Kubios HRV software package.

Table 2.
‘Core’ measures considered as indicating parasympathetic (‘PNS-like’) or sympathetic (‘SNS-like’) modulation of heart rate, with a third group of ‘ambivalent’ measures, and others for which this autonomic classification did not seem relevant. The occupational medicine guideline list included as a comparison is from Sammito et al. (2024) [89] and does not consider the PNS, SNS, SI, HFlog and LFlog, SD2/SD1, and EDR (‘electrocardiogram derived respiration’) indices provided by Kubios HRV (so listed as ‘n/a’, not applicable).
Spearman correlations between 39 HRV indices and BLINKER measures were then investigated (including those in Table 2 and excluding 20 multiscale entropies in the output from recent versions of the Kubios HRV software). Spearman’s rho was computed before, during, and after stimulation at the four different frequencies. BLINKER measures considered were the amplitude–velocity ratios (nAVRZ and pAVRZ), blink rate (BpM), and the ratio of left to right channels providing the best blinks (LRBR). Correlations with the blink rate were consistently positive for the HRV SNS-like measures and negative for the PNS-like measures. Correlations with BLINKER durations, nAVRZ, and pAVRZ were less consistently associated with one or the other, and LRBR appeared almost uniformly PNS-like (except for in the 10 pps group at baseline). The results—excluding durations, durations, nAVRZ, and pAVRZ—are shown in Table 3.

Table 3.
Spearman correlations between Kubios HRV and BLINKER measures, with 0.2 < Spearman’s |rho| < 0.3 (if |rho| ≥ 0.3, as for several correlations with BpM, this is indicated). HRV indices in red are considered to be ‘SNS-like’, those in blue ‘PNS-like’, and those in italic type as ‘other’.
The following correlations with |rho| > 0.3 were also noted: (a) five negative between the standard deviation of good blink duration and various SNS-like measures (SI, HRmin, Hrmean, and SNS itself) and also five negative between ‘goodRatio’ (the ratio of good to all blinks) and various PNS-like measures (RMSSD/SD1, D2, NNxx, and pNNxx), all of these mostly at baseline and preceding sham stimulation; (b) 17 positive, of which 11 were between the standard deviation of good blink duration and PNS-like measures (3 of these at baseline and before sham stimulation and 8 post-stimulation at 2.5 pps). Correlations between the HRV indices and other data type measures are presented in the Supplementary Material, Appendix S4.
3.10. ‘Best’ Blink Laterality and Autonomic Modulation of HRV
For our study participants, many more ‘best’ blinks occurred on the left than on the right (1031 for channel Fp1, 735 for Fp2, 50 for F7, and 19 for F8, with 11 each for F3 and F4). This pattern was found consistently, irrespective of session (1 to 4), stimulation frequency (Sham, 2.5, 10, or 80 pps), and time slot (1 to 8). However, counts of left and right best blinks over time within sessions indicated that the ratio of these counts (LRBR) differed most significantly from the median during rather than before or after stimulation (Figure 7).
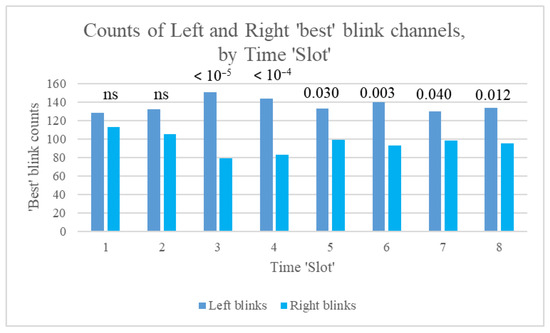
Figure 7.
Counts of left and right best blinks over time (Slots 1 to 8), showing p-values for the binomial test of difference in distribution between left and right blinks.
Furthermore, counts of left and right best blinks were most different for stimulation at 10 pps, followed by 2.5 pps, and were not significantly different for sham stimulation (Figure 8).
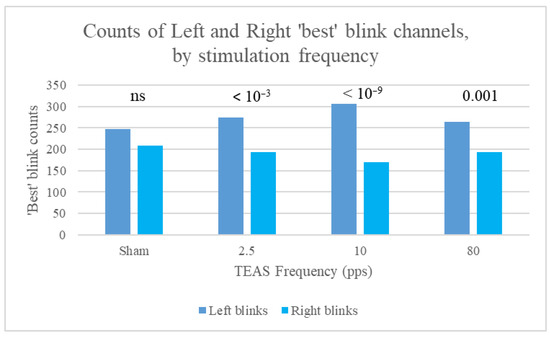
Figure 8.
Counts of left and right best blinks for the different stimulation frequencies (all slots taken together), for data split by stimulation frequency, with p-values for the binomial test of difference in distribution between left and right blinks. Note the lack of significance for sham stimulation.
In summary, differences in counts of left and right ‘best’ blinks did not achieve statistical significance at baseline (Slot 1) or at the start of stimulation (Slot 2). Count differences were largest during the middle 10 min of stimulation and then stabilised, with smaller p-values. When data were split by stimulation frequency, the binomial test yielded significant results during stimulation only for all frequencies, apart from 2.5 pps, for which differences remained significant immediately post-stimulation (Slot 6). As stated by Kleifges et al. (2017) [3], a continuing theme in research on ocular indices is that significant differences are found between individuals, but with consistent patterns in conditions such as perceived sleepiness. Here, we found that significant left blink dominance was found in 14 of our study participants, but significant right blink dominance only in 3 (significance assessed using the binomial test of difference in distribution between left and right blinks). Mann–Whitney tests were also conducted for the 39 Kubios HRV indices, using left or right laterality as the grouping variable. Significant differences for best blink laterality are shown in Table 4.

Table 4.
Counts of significant differences in 39 HRV indices between left (‘1’) and right (‘0’) laterality groups before, during, and after stimulation at the four different frequencies.
Note that left/right laterality patterns were significantly different, with p < 0.01 for 18 HRV measures only during stimulation at 2.5 pps.
Of these 18 measures, 7 could be classified with some confidence as ‘PNS-like’ (plus a further two with less confidence), 3 as ‘SNS-like’, and a further 6 as ‘other’ (Table 5). As described above, the effect size (ES) was calculated for each Mann–Whitney test as |Z|/√(n), where Z = the Z-score and n = the number of participants involved.

Table 5.
The eighteen measures for which left/right laterality patterns were significantly different, with p < 0.01 during stimulation at 2.5 pps. R > L indicates those measures which were greater for right laterality, and L > R those which were greater for left laterality. See list of abbreviations and our 2021 conference presentation [25] for further information.
Note that values of all three SNS-like measures in this Table were greater for right laterality, whereas of the PNS-like measures, this was the case only for SampEn, one of the two measures with least evidence for its PNS-like classification (the other being Correlation Dimension, D2). Post-stimulation, all frequencies taken together, only PNS was significantly different with laterality (p = 0.005, Z = −2.801, U = 49,611.5, N = 681). The median PNS was larger (−0.624) for left-dominant blink and smaller (−0.805) for right-dominant blink, in keeping with the results in the above table. However, SNS, SI, SD2/SD1, DFA α1, and several frequency-domain measures also differed with laterality exactly as in Table 5, although with less significance (p < 0.05 rather than 0.01). Regardless of p-values, for these comparisons, the effect sizes shown were all between 0.1 (‘small’) and 0.3 (‘moderate’) [90], a useful reminder that even small p-values do not always provide a complete picture.
3.11. Autonomic Associations for the Other Data Types
As noted above (Table 3), absolute values of Spearman’s rho for correlations between Kubios HRV and BLINKER measures were mostly between 0.2 and 0.3, for data split into baseline/stimulation/post-stimulation and by stimulation frequency. Very few values of rho lay outside that range. In contrast, for the spectral centroids in the ‘centroids’ data type, many values of |rho| were > 0.3. When only these were considered, the following patterns emerged:
Positive correlations with HRV measures only occurred for Baevsky’s SNS-like ‘Stress Index’ (SI) [91], while negative correlations were all either with PNS-like measures (HFabs, NNxx, RMSSD, etc.) or with ‘ambivalent’ measures such as SDHR or total power. Furthermore, these correlations occurred only at 6 of the 19 scalp electrodes, Cz, P7, P3, Pz, P4, and P8, with most at those on the midline (Cz and Pz).
Correlations (median values of |rho|) were greatest before stimulation, decreasing thereafter, and were also lower for sham than for the active stimulation frequencies.
Further analysis of correlations between the HRV indices and data type measures is presented in Appendix S4 in the Supplementary Material, ‘Autonomic Correlates with Other Data Types’. However, scatter plots did not suggest that the correlations found would be of any great use, practically, as they showed no obvious patterns, even for Spearman’s correlations with p < 0.001.
Results for Objective 3. Are measures predictive of participant dropout or adverse reactions to stimulation?
3.12. Dropout
At baseline (Session 1, Slot 1), Mann–Whitney tests indicated that measures of all data types, bar one, including BLINKER and CEPS-BLINKER, as well as questionnaire and scale scores, did not show significant differences between those who subsequently dropped out (6) and those who did not (60) (some reasons for dropping out are given in the next section). In contrast, eight HRV measures at baseline did appear to predict subsequent dropout, including DFA α2 with an effect size of 0.366 (p = 0.008), RR, and mean HR with ES 0.349 (p = 0.006). Heart rate was—perhaps surprisingly—lower at baseline in those who subsequently dropped out than in those who did not. However, the fact that multiple comparisons are being carried out should be considered when interpreting these findings, and caution applied.
3.13. Adverse Reactions
Participants were encouraged to report feeling uncomfortable at any time and were also asked to report any adverse reactions after taking part in the study. Six women and six men did so, whereas fifty participants did not. Physical reactions were mostly minor, including transient nausea, dizziness, or feeling tired, or general discomfort due to the room being too hot. Some participants found skin preparation for the EEG data collection uncomfortable, and nine participants indicated that wearing the EEG cap was itself uncomfortable, resulting in a transient headache for two. At least two reported pins and needles in the hands following electrical stimulation, but this did not last more than a few hours and was not experienced after all four sessions. Three stated that they felt faint towards the end of one session but nonetheless returned for further sessions without further problems. One participant who had previously suffered from a vestibular condition and had experienced a short spell of dizziness in the week prior to the study reported more lasting dizziness following stimulation to the hands and so withdrew from the study following their first session. Another who found ‘the whole situation stressful and unpleasant’ also withdrew after the first session. A third participant withdrew after the first session, without explanation, and a further participant did not attend for their fourth session. Two others also left the study early. Twelve participants were asked, during their sessions, if they could slow their blinking rate, and one attempted to do so without prompting. Eight of the twelve participants reported spontaneously that they found wearing the electrode cap uncomfortable, as did one other. Three participants experienced a headache, and three experienced tiredness following the sessions. One of the latter likened symptoms of general achiness and low mood to those of possible fibromyalgia experienced two years earlier and then dropped out of the study. Three others felt faint (two of them with accompanying nausea) but recovered and continued to take part.
However, again at baseline, no BLINKER measures predicted subsequent adverse effects, whereas 17 CEPS-BLINKER did so, with p < 0.01 and ES between 0.336 and 0.420 (Table 6). In contrast, no questionnaire results predicted adverse effects or dropout with this level of significance. However, a number of other data types, like CEPS-BLINKER, also appeared to predict subsequent adverse effects (Table 7).

Table 6.
CEPS-BLINKER measures that appear to predict subsequent adverse effects, using Mann–Whitney tests with p < 0.01. Median values of the measures and their interquartile ranges are shown in the final two columns, with the greater value for each measure in bold.

Table 7.
Other data type measures that appear to predict subsequent adverse effects, using Mann–Whitney tests with p < 0.01. Median values of the measures and their interquartile ranges are shown in the final two columns, with the greater absolute value for each measure in bold.
Note that the largest effect sizes (0.420) were both for Hjorth complexity variants of the standard BLINKER indices.
Note that both maximum and median effect sizes are greater for CEPS-BLINKER than for the other data types, and that the percentage of measures showing significant differences between those that appear to predict subsequent adverse effects and those that do not is also greater for CEPS-BLINKER.
4. Discussion
This study explored the effects of transcutaneous electroacupuncture stimulation (TEAS) on eyeblink rate and other parameters, electroencephalography (EEG), and heart rate variability (HRV). Using a range of non-parametric statistical methods, we analysed physiological responses to different TEAS frequencies, session orders, and time slots within sessions. For the majority of data types analysed, the test statistics and effect sizes were greater when comparing time slots within sessions than when comparing stimulation frequency or session order (see Table 1, Figures S6, S7, S23, S25, and S26). As shown in Figure 6, early changes (between Slots 1 and 3) appear to be greater for 80 pps stimulation (in line with reports on high-frequency TENS in the literature [31]), with a cumulative effect for all TEAS frequencies after 20 min of stimulation, although this was most consistently maintained only for 10 pps stimulation thereafter. Our key findings suggest that TEAS exerts significant influences on eyeblink parameters, EEG power spectra, and HRV indices. Notably, eyeblink measures, often disregarded as artefacts in EEG research, emerged as potential indicators of TEAS effects and autonomic modulation.
One of the novel contributions of this study is the identification of eyeblink parameters as useful physiological markers in acupuncture-related research. Blink rate (BpM) was found to correlate positively with HRV indices associated with sympathetic nervous system (SNS) activity and negatively with parasympathetic nervous system (PNS)-related indices. These correlations reinforce the hypothesis that eyeblink dynamics may reflect autonomic balance, particularly in response to TEAS. Additionally, the laterality of ‘best’ blinks (i.e., those whose trajectory was closest to that of a stereotypical blink) exhibited significant associations with autonomic modulation, particularly during 2.5 Hz stimulation. Right-dominant blink patterns were more strongly correlated with SNS-like measures, whereas left-dominant blink patterns were associated with PNS-like measures. These findings align with previous research suggesting that eyeblink asymmetries may be influenced by neurological or neurochemical states.
In recent years, a number of researchers have investigated correlations between EEG features and HRV or stress measures [47,48,51]. There is also increasing interest in using HRV itself as a biomarker of altered autonomic function. In one critical review, for example, negative affect (emotion or mood) was found to be associated with a reduction in parasympathetic activity as assessed using HRV [92]. Additionally, there is a growing body of literature on the effects of EA and TEAS on HRV (see, for example, the literature review in [93]). The authors of one study found that low-frequency EA (2.5 Hz) showed a measurable reduction in sympathetic stress with subsequent improvement in vagal tone, whereas medium-frequency EA (15 Hz) elicited changes in all HRV markers, but these failed to achieve statistical significance [94]. In an earlier study, 2 Hz EA appeared to increase vagal activity, whereas 15 Hz EA increased sympathetic activity [95]. Other researchers found a significant increase in the standard deviation of the normal-to-normal heart rate interval (SDNN), usually considered an index of parasympathetic modulation, following 120 Hz EA, with no such change following the 2 Hz EA group [96]. Such findings suggest inconsistencies in the literature that invite further investigation, particularly regarding a central FFR to peripheral stimulation such as TEAS. An incidental finding in the present study was that a more positive mood at baseline might be associated with a decrease in markers of SNS HRV modulation with stimulation (Supplementary Material, Appendix S4). This again suggests further avenues to explore.
Our results demonstrate that TEAS frequency significantly affects EEG power, particularly within the 1 Hz bins and median power asymmetries, but has less effect on more abstruse measures such as cordance or the Wackermann global descriptors for the 4 sec segments in each 5 min slot (Table S9). The maximum effect sizes were observed in EEG bands associated with sensory and cognitive processing, particularly at lower stimulation frequencies. This finding may support some prior research indicating that low-frequency electroacupuncture (EA) preferentially enhances vagal activity, whereas higher frequencies may have mixed or less pronounced effects, but provides little evidence in favour of—or against—a central (EEG) FFR in response to TEAS as a form of ‘brain entrainment’.
HRV analysis revealed that 2.5 Hz TEAS elicited the most significant autonomic changes, primarily increasing vagal tone while reducing sympathetic activity. These results align with other research suggesting that low-frequency stimulation modulates autonomic balance in a way that promotes relaxation and stress reduction [97]. Conversely, higher stimulation frequencies, particularly 80 pps, showed less consistent effects on HRV markers, further highlighting the need for a more nuanced understanding of frequency-dependent autonomic modulation.
The methodological approach employed in this study was largely exploratory, utilising non-parametric methods such as Friedman’s test, Kendall’s W, and bootstrapped Wilcoxon and Conover–Iman tests. Given the non-normal distribution of most measures, these approaches provided a robust means of detecting significant effects while reducing the risk of Type I (false positive) and Type II (false negative) errors. Effect size measures (Kendall’s W and Spearman’s rho) proved particularly valuable in quantifying physiological changes over time, across TEAS frequencies, and between sessions. However, one challenge in dealing with a large dataset encompassing over 15,000 measures was the need for ‘top slicing’ to identify the most relevant physiological markers. Various slicing methods produced consistent rankings, suggesting that our approach was reliable. Nonetheless, given the multiple comparisons issue, our results should be interpreted with caution, and future studies should consider refining statistical methods and feature selection criteria to enhance reproducibility and interpretability.
A key objective of this study was to determine whether physiological markers could predict participant dropout or adverse reactions to TEAS. Notably, baseline HRV measures, particularly DFA Alpha2 and mean heart rate, differentiated those who subsequently withdrew from those who completed all sessions. Additionally, CEPS-derived BLINKER measures outperformed standard EEG and HRV indices in predicting adverse effects, suggesting that complexity and entropy analyses of eyeblink parameters may serve as early indicators of discomfort or sensitivity to unfamiliar sensations such as those of electrical stimulation. Furthermore, it is somewhat easier to calculate CEPS-BLINKER measures than those derived from the EEG itself, which in general require time-consuming preprocessing. Indeed, some eyeblink measures could potentially be assessed from video rather than EEG recordings. It may therefore be worth considering the CEPS-BLINKER method of predicting adverse events in other research.
5. Conclusions
Firstly, for the majority of data types analysed, whether derived from eyeblink parameters, EEG measures or HRV indices, test statistics and effect sizes were greater for the effects of time (differences between baseline, stimulation and post-stimulation phases, for example) than for those of stimulation frequency or session order. Secondly, significant autonomic (PNS or SNS) correlates for some eyeblink parameters and EEG measures appear likely. Thirdly, HRV and CEPS-derived BLINKER measures at baseline were early indicators of subsequent adverse effects.
This study provides novel insights into the physiological effects of TEAS, highlighting the utility of some eyeblink parameters, measures based on EEG power asymmetries, and HRV indices in understanding acupuncture mechanisms. Our findings suggest that TEAS effects are frequency-dependent, with 2.5 Hz stimulation demonstrating the most consistent autonomic modulation. Eyeblink laterality and CEPS-BLINKER measures emerged as promising, though as yet underutilised, biomarkers of TEAS effects. Future research should focus on integrating these markers into broader frameworks of acupuncture efficacy and patient safety monitoring.
Eyeblink indices could serve as biomarkers for acupuncture effects, with HRV measures at baseline predicting participant dropout and adverse reactions better than EEG-derived indices. These results demonstrate that TEAS modulates brain activity and autonomic function in a frequency-dependent manner and that eyeblink measures, traditionally seen as artefacts, may hold diagnostic and research value. Given their strong correlation with autonomic indices here, further studies should validate their potential as non-invasive biomarkers in acupuncture and other neuromodulation research.
The differential effects of TEAS frequencies on HRV indices suggest that individualised frequency selection may optimise therapeutic outcomes, particularly for conditions involving autonomic dysregulation. Future research should explore whether TEAS-induced changes in EEG asymmetry correspond to clinical improvements in cognitive and affective disorders. Another avenue to explore is how data length affects different measures. Here, we used 5 min recordings. In parallel studies, time ‘Slots’ 1, 4, and 7 were subdivided into 10 s epochs, resulting in different shortlists of potentially useful measures [29,30], with further reports in preparation. It is anticipated that some measures may provide significant results at both timescales. A next step will be to compare results using the measures from the different shortlists to determine which approach is likely to be the most fruitful, or whether they are complementary.
Our findings suggest that CEPS-enhanced eyeblink analysis could be applied in clinical trials to predict and mitigate adverse responses to electroacupuncture or other neuromodulatory interventions.
Innovation and Limitations
The conclusions of this study are derived from analysing a plethora of measures drawn from a relatively small sample of 66 participants. Many of these measures have not been used before in acupuncture-related research, and indeed some data (e.g., that collected from head movement sensors) are only now being analysed. Data were recorded in less-than-perfect circumstances (varying lab temperature, external noise, occasional requests to slow down EBR), and effect sizes were often small, if not very small. Nonetheless, patterns of response were sufficiently uniform (often strikingly so) that we hope this study will encourage other researchers to develop and refine our findings using a larger sample of individuals and a smaller selection of measures derived from eyeblink, EEG, and ECG data.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/s25144468/s1.
Author Contributions
D.M.: conceptualisation, writing—original draft, investigation, writing—review and editing, data curation, formal analysis, funding acquisition, project administration, resources; T.S.: data curation, software, visualisation, conceptualisation, investigation, resources; P.S.: software; T.W.: data curation, methodology, conceptualisation, resources, project administration, supervision; N.S.: formal analysis, methodology, software, supervision; D.B.: writing—review and editing, project administration, resources. All authors have read and agreed to the published version of the manuscript.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article. A Small Project Grant for computer hardware was awarded (to D.M.) by the Acupuncture Association of Chartered Physiotherapists (UK) to enable the processing of the EEG and other data collected in our original study (22 February 2017).
Institutional Review Board Statement
This study involving human participants was reviewed and approved by the University of Hertfordshire’s Health and Human Sciences Ethics Committee (protocol number HSK/SF/UH/00124).
Informed Consent Statement
The participants provided their written informed consent to participate in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the corresponding author (DB, email duncan.banks@open.ac.uk), upon reasonable request.
Acknowledgments
We thank all those who participated in the experiments and the University of Hertfordshire for their support; Türker Tekin Ergüzel (Department of Computer Engineering, Üsküdar University, Istanbul, Türkiye) for sharing his Excel code for cordance; Thea Radüntz (Federal Institute for Occupational Safety and Health, Mental Health and Cognitive Capacity, 10317 Berlin, Germany) for processing our EEG data using her machine learning method of automated EEG artefact elimination; Kay Robbins for her elucidation of ‘stereotypical blink’; and Débora Pereira Salgado for permission to use Figure 1. We would like to acknowledge the commitment of our research assistants, Aistė Noreikaitė and Lidia Zaleczna, who both meticulously recorded the data, and Aistė for the hours she spent in addition on preprocessing and then processing ECG and PPG data to extract HRV (and PRV) measures using the Kubios HRV software package. We would also like to thank our partners and wives for their forbearance during the lengthy gestation of this article. Without them, it is unlikely that our hard work would have come to fruition.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
Abbreviations of BLINKER and CEPS terms are listed in Tables S2A and B in the Supplementary Material, with other abbreviations used in the Supplementary Material but not in the main paper also listed there.
Further abbreviations used in this paper:
| ECG/PPG | Measure or term | Abbreviation |
| Electroencephalography | ECG | |
| Photoplethysmography | PPG | |
| HRV | Measure or term | Abbreviation |
| Approximate entropy | ApEn | |
| Correlation dimension | D2 (or CorrD) | |
| Detrended fluctuation analysis alpha 1 (short-term fluctuation slope) | DFA α1 | |
| Detrended fluctuation analysis alpha 2 (long-term fluctuation slope) | DFA α2 | |
| Electrocardiogram-derived respiration | EDR | |
| Relative power in the high-frequency band | HF% | |
| Peak frequency in the high-frequency band | HF.Hz | |
| Absolute power in the high-frequency band | HFabs | |
| Log-transformed high-frequency power | HFlog | |
| High-frequency power in normalised units | HFnu | |
| Relative power in the low-frequency band | LF% | |
| Heart rate (maximum, mean, minimum) | HR. HRmax, HRmean, HRmin | |
| Absolute power in the low-frequency band | LFabs | |
| Log-transformed the low-frequency power | LFlog | |
| Low-frequency power in normalised units | LFnu | |
| Ratio of low and high-frequency band powers | LF/HF | |
| Number of successive RR interval pairs that differ by more than xx milliseconds (ms) | NNxx | |
| NNxx divided by the total number of RR intervals | pNNxx | |
| Parasympathetic nervous system | PNS | |
| Square root of mean squared differences between successive RR intervals (also = SD1) | RMSSD | |
| Sample entropy | SampEn | |
| Standard deviations of Poincaré plot perpendicular to (SD1) and along (SD2) line-of-identity | SD1, SD2 | |
| Ratio of SD2 and SD1 | SD2/SD1 | |
| Standard deviation of heart rate | SDHR | |
| Standard deviation of RR intervals | SDNN | |
| Stress Index | SI | |
| Sympathetic nervous system | SNS | |
| Triangular index | TI | |
| Triangular interpolation of NN interval histogram | TINN | |
| Sum of HF, LF, and very low-frequency power | TotPwr | |
| Nonlinear HRV measures | HRV_Nonlin | |
| Other HRV and PRV measures | HRV_PRV | |
| EEG | Measure or term | Abbreviation |
| EEG Channels in the 10–20 system, as in ‘SQzTInf_mT_Theta4_8_P4’ | Fp1, Fp2, F7, F3, Fz, F4, F8, T7 (T3), C3, Cz, C4, T8 (T4), P7 (T5), P3, Pz, P4, P8 (T6), O1, O2 | |
| Median power, as in ‘1 Hz_MdnPwr_bins’ or ‘Mdnpwr_ASYMM’ [or ASYMM_171, where ‘171’ refers to binning method used] | MdnPwr | |
| Asymmetry, as in ‘Mdnpwr_ASYMM’ | ASYMM | |
| Multi-taper, alternative to Fourier transform for time-frequency analysis (as in ‘[channel]_[stimulation frequency]_A_PS_mT’) | mT | |
| Variant of mT | mTR | |
| Other (frequency) | O | |
| 1 Hz bin centred on frequency, at channel | O[frequency]_Channel | |
| Theta band between 4 and 8 Hz | Theta4_8 | |
| AMICA | A | |
| Extended InfoMax | I | |
| Independent component analysis | ICA | |
| Wackermann omega at epoch number, AMICA | Omega_[number 1 to 75]_A | |
| Wackermann omega at epoch number, InfoMax | Omega_[number 1 to 75]_I | |
| Wackermann phi at epoch number, AMICA | Phi_[number 1 to 75]_A | |
| Wackermann phi at epoch number, InfoMax | Phi_[number 1 to 75]_I | |
| Hjorth and Wackermann measures (median Slot values) | Hjorth_Wack’mn | |
| DFA from measures of analysis of time series (MATS) software | DFA_MATS | |
| Data processed using Paul Steinfath’s pipeline | PS | |
| Cordance | Measure or term | Abbreviation |
| Normalised, as in ‘CorN_Alpha_P3Inf_mTR_ln’ | CorN | |
| Natural logarithm, as in ‘CorN_Alpha_P3Inf_mTR_ln’ | ln | |
| ‘score’ (??), as in ‘CorN_Alpha_P3Inf_mTR_s’ | s | |
| InfoMax ICA, as in ‘CorN_Alpha_P3Inf_mTR_ln’ or ‘SQzTInf_mT_Theta4_8_P4’ | Inf | |
| EEG band, as in ‘CorN_Alpha_P3Inf_mTR_ln’ | Delta, Theta, Alpha, Beta, Gamma | |
| Square-root normalised and Z-transformed, as in ‘SQzTInf_mT_Theta4_8_P4’ | SQzT | |
| Ocular | Measure or term | Abbreviation |
| Blinks per minute | BpM | |
| Temperature | Measure or term | Abbreviation |
| Temperature | TEMP | |
| Statistics | Measure or term | Abbreviation |
| Interquartile range | IQR | |
| Kendall’s coefficient of concordance, its maximum | W, Wmax |
References
- Szmit, M.; Krajewski, R.; Rudnicki, J.; Agrawal, S. Application and efficacy of transcutaneous electrical acupoint stimulation (TEAS) in clinical practice: A systematic review. Adv. Clin. Exp. Med. 2023, 32, 1063–1074. [Google Scholar] [CrossRef] [PubMed]
- Maffei, A.; Angrilli, A. Spontaneous eye blink rate: An index of dopaminergic component of sustained attention and fatigue. Int. J. Psychophysiol. 2018, 123, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Kleifges, K.; Bigdely-Shamlo, N.; Kerick, S.E.; Robbins, K.A. BLINKER: Automated extraction of ocular indices from EEG enabling large-scale analysis. Front. Neurosci. 2017, 11, 12. [Google Scholar] [CrossRef] [PubMed]
- Johns, M.W. The amplitude-velocity ratio of blinks: A new method for monitoring drowsiness. Sleep 2003, 26 (Suppl). [Google Scholar]
- Robbins, K.; EEG-Blinks. BLINKER: Automated Blink Detector for EEG. n.d. Available online: https://vislab.github.io/EEG-Blinks/ (accessed on 14 April 2025).
- Kassem, I.S.; Evinger, C. Asymmetry of blinking. Investig. Ophthalmol. Vis. Sci. 2006, 47, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Braff, D.L.; Geyer, M.A.; Swerdlow, N.R. Human studies of prepulse inhibition of startle: Normal subjects, patient groups, and pharmacological studies. Psychopharmacology 2001, 156, 234–258. [Google Scholar] [CrossRef] [PubMed]
- Hager, J.C.; Ekman, P. The asymmetry of facial actions is inconsistent with models of hemispheric specialization. Psychophysiology 1985, 22, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Cadenhead, K.S.; Swerdlow, N.R.; Shafer, K.M.; Diaz, M.; Braff, D.L. Modulation of the startle response and startle laterality in relatives of schizophrenic patients and in subjects with schizotypal personality disorder: Evidence of inhibitory deficits. Am. J. Psychiatry 2000, 157, 1660–1668. [Google Scholar] [CrossRef] [PubMed]
- Schröder, R.; Reuter, M.; Faßbender, K.; Plieger, T.; Poulsen, J.; Lui, S.S.Y.; Chan, R.C.K.; Ettinger, U. The role of the SLC6A3 3’ UTR VNTR in nicotine effects on cognitive, affective, and motor function. Psychopharmacology 2022, 239, 489–507. [Google Scholar] [CrossRef] [PubMed]
- Karson, C.N. Spontaneous eye-blink rates and dopaminergic systems. Brain 1983, 106 Pt 3, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.; Andresen, B.; Thom, E. Blinking, alpha brain waves and smoking in schizophrenia. Acta Psychiatr. Scand. 1993, 87, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Shioiri, T.; Hosoki, T.; Sakai, M.; Bando, T.; Someya, T. Blink rate variability in patients with panic disorder: New trial using audiovisual stimulation. Psychiatry Clin. Neurosci. 2002, 56, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Verhoeff, N.P.; Christensen, B.K.; Hussey, D.; Lee, M.; Papatheodorou, G.; Kopala, L.; Rui, Q.; Zipursky, R.B.; Kapur, S. Effects of catecholamine depletion on D2 receptor binding, mood, and attentiveness in humans: A replication study. Pharmacol. Biochem. Behav. 2003, 74, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Mayor, D.; Steffert, T. EEG and eyeblink response to different acupuncture modalities: Some preliminary results from four exploratory pilot studies. Poster presentation. In Proceedings of the 15th International Acupuncture Research Symposium, London South Bank University, London, UK, 2 March 2013; Available online: https://researchprofiles.herts.ac.uk/en/publications/eeg-and-eyeblink-response-to-different-acupuncture-modalities-som (accessed on 13 May 2025).
- Mayor, D.; Panday, D.; Kandel, H.K.; Steffert, T.; Banks, D. CEPS: An open access MATLAB Graphical User Interface (GUI) for the analysis of Complexity and Entropy in Physiological Signals. Entropy 2021, 23, 321. [Google Scholar] [CrossRef] [PubMed]
- Mayor, D.; Steffert, T.; Datseris, G.; Firth, A.; Panday, D.; Kandel, H.; Banks, D. Complexity and Entropy in Physiological Signals (CEPS): Resonance breathing rate assessed using measures of fractal dimension, heart rate asymmetry and permutation entropy. Entropy 2023, 25, 301. [Google Scholar] [CrossRef] [PubMed]
- Mayor, D.F. CNS resonances to peripheral stimulation: Is frequency important? J. AACP 2001, 29–63. [Google Scholar]
- Salansky, N.; Fedotchev, A.; Bondar, A. Responses of the nervous system to low frequency stimulation and EEG rhythms: Clinical implications. Neurosci. Biobehav. Rev. 1998, 22, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Mayor, D.F. Exploring amplitude in transcutaneous electroacupuncture stimulation (TEAS). In Proceedings of the AACP Leeds Conference, Principal Met Hotel, Leeds, UK, 13 October 2018. [Google Scholar]
- Xu, J.J.; Ren, M.; Zhao, J.J.; Wu, J.J.; Zhang, S.C.; Zhong, Y.B.; Xu, S.T.; Cao, Z.Y.; Zhou, Z.Q.; Li, Y.L.; et al. Effectiveness of theta and gamma electroacupuncture for post-stroke patients on working memory and electrophysiology: Study protocol for a double-center, randomized, patient- and assessor-blinded, sham-controlled, parallel, clinical trial. Trials 2020, 21, 910. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Xu, J.; Zhao, J.; Zhang, S.; Wang, W.; Xu, S.; Zhou, Z.; Chen, X.; Chen, S.; Li, Y.; et al. The modulation of working-memory performance using gamma-electroacupuncture and theta-electroacupuncture in healthy adults. Evid. Based Complement. Alternat Med. 2021, 2021, 2062718. [Google Scholar] [CrossRef] [PubMed]
- Al-Zamil, M.; Kulikova, N.G.; Minenko, I.A.; Shurygina, I.P.; Petrova, M.M.; Mansur, N.; Kuliev, R.R.; Blinova, V.V.; Khripunova, O.V.; Shnayder, N.A. Comparative analysis of high-frequency and low-frequency transcutaneous electrical stimulation of the right median nerve in the regression of clinical and neurophysiological manifestations of generalized anxiety disorder. J. Clin. Med. 2024, 13, 3026. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimian, M.; Razeghi, M.; Zamani, A.; Bagheri, Z.; Rastegar, K.; Motealleh, A. Does high frequency transcutaneous electrical nerve stimulation (TENS) affect EEG gamma band activity? J. Biomed. Phys. Eng. 2018, 8, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Mayor, D.; Panday, D.; Steffert, T.; Kandel, H.K.; Beyond, H.R.V. Extending the range of autonomic measures associated with heart rate variability: The effects of transcutaneous electroacupuncture (TEAS). Poster presentation. In Proceedings of the 22nd International Acupuncture Research Symposium, Virtual Event, 27 March 2021; Available online: https://researchprofiles.herts.ac.uk/en/publications/beyond-hrv-extending-the-range-of-autonomic-measures-associated-w (accessed on 13 May 2025).
- Agorastos, A.; Mansueto, A.C.; Hager, T.; Pappi, E.; Gardikioti, A.; Stiedl, O. Heart rate variability as a translational dynamic biomarker of altered autonomic function in health and psychiatric disease. Biomedicines 2023, 11, 1591. [Google Scholar] [CrossRef] [PubMed]
- Yuda, E.; Shibata, M.; Ogata, Y.; Ueda, N.; Yambe, T.; Yoshizawa, M.; Hayano, J. Pulse rate variability: A new biomarker, not a surrogate for heart rate variability. J. Physiol. Anthropol. 2020, 39, 21. [Google Scholar] [CrossRef] [PubMed]
- Uyulan, Ç.; Mayor, D.; Steffert, T.; Watson, T.; Banks, D. Classification of the central effects of transcutaneous electroacupuncture stimulation (TEAS) at different frequencies: A deep learning approach using wavelet packet decomposition with an entropy estimator. Appl. Sci. 2023, 13, 2703. [Google Scholar] [CrossRef]
- Vasei, T.; Gediya, H.; Ravan, M.; Santhanakrishnan, A.; Mayor, D.; Steffert, T. Investigating brain responses to transcutaneous electroacupuncture stimulation: A Deep Learning approach. Algorithms 2024, 17, 477. [Google Scholar] [CrossRef]
- Lopes Alves, R.; Zortea, M.; Mayor, D.; Watson, T.; Steffert, T. Effect of different frequencies of transcutaneous electrical acupoint stimulation (TEAS) on EEG source localization in healthy volunteers: A semi-randomized, placebo-controlled, crossover study. Brain Sci. 2025, 15, 270. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.I. Long-standing uncertainty about the clinical efficacy of Transcutaneous Electrical Nerve Stimulation (TENS) to relieve pain: A comprehensive review of factors influencing outcome. Medicina 2021, 57, 378. [Google Scholar] [CrossRef] [PubMed]
- Jarzem, P.F.; Harvey, E.J.; Arcaro, N.; Kaczorowski, J. Transcutaneous electrical nerve stimulation [TENS] for short-term treatment of low back pain–randomized double blind crossover study of sham versus conventional TENS. J. Musculoskelet. Pain. 2005, 13, 11–17. [Google Scholar] [CrossRef]
- Oosterhof, J.; Wilder-Smith, O.H.; de Boo, T.; Oostendorp, R.A.; Crul, B.J. The long-term outcome of transcutaneous electrical nerve stimulation in the treatment for patients with chronic pain: A randomized, placebo-controlled trial. Pain. Pract. 2012, 12, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Elboim-Gabyzon, M.; Andrawus Najjar, S.; Shtarker, H. Effects of transcutaneous electrical nerve stimulation (TENS) on acute postoperative pain intensity and mobility after hip fracture: A double-blinded, randomized trial. Clin. Interv. Aging 2019, 14, 1841–1850. [Google Scholar] [CrossRef] [PubMed]
- Meade, C.S.; Lukas, S.E.; McDonald, L.J.; Fitzmaurice, G.M.; Eldridge, J.A.; Merrill, N.; Weiss, R.D. A randomized trial of transcutaneous electric acupoint stimulation as adjunctive treatment for opioid detoxification. J. Subst. Abuse Treat. 2010, 38, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Hu, B.; He, S.; Li, X.; Gong, H.; Li, F.; Wang, Q. Transcutaneous Electrical acupoint stimulation accelerates the recovery of gastrointestinal function after Cesarean section: A randomized controlled trial. Evid. Based Complement. Alternat Med. 2018, 2018, 7341920. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Zhang, L.; Han, X.; Wei, L.; Fang, J.; Zhang, X.; Zhang, J.; Wang, H.; Zhou, Q.; Wang, C.; et al. Transcutaneous: A Multi Center Randomized electrical acupoint stimulation decreases the inci-dence of postoperative nausea and vomiting after laparoscopic non gastrointestinal surgery: A multi center randomized controlled trial. Front. Med. 2022, 9, 766244. [Google Scholar] [CrossRef] [PubMed]
- Mayor, D.; Steffert, T. Measuring mood: Relative sensitivity of numerical rating and Likert scales in the context of teaching electroacupuncture. Poster presentation. In Proceedings of the 18th ARRC International Acupuncture Research Symposium, King’s College, London, UK, 19 March 2016; Available online: https://researchprofiles.herts.ac.uk/en/publications/measuring-mood-relative-sensitivity-of-numerical-rating-and-liker? (accessed on 15 May 2025).
- Liu, C.C.; Ghosh Hajra, S.; Cheung, T.P.; Song, X.; D’Arcy, R.C. Spontaneous blinks activate the precuneus: Characterizing blink-related oscillations using magnetoencephalography. Front. Hum. Neurosci. 2017, 11, 489. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Ghosh Hajra, S.; Pawlowski, G.; Fickling, S.D.; Song, X.; D’Arcy, R.C.N. Differential neural processing of spontaneous blinking under visual and auditory sensory environments: An EEG investigation of blink-related oscillations. Neuroimage 2020, 218, 116879. [Google Scholar] [CrossRef] [PubMed]
- Robbins, K. (Department of Computer Science, University of Texas at San Antonio, San Antonio, TX, USA). Personal communication, 10 April 2003.
- Calliess, J.-P. Further Investigations on Unspoken Speech-Findings in an Attempt of Developing EEG-Based Word Recognition. Studienarbeit. Institut für Theoretische Informatik, Universität Karlsruhe 2006. Available online: https://www.cs.cmu.edu/~tanja/Papers/SA-Callies.pdf (accessed on 13 May 2025).
- Miyakoshi, M.; Jurgiel, J.; Dillon, A.; Chang, S.; Piacentini, J.; Makeig, S.; Loo, S.K. Modulation of frontal oscillatory power during blink suppression in children: Effects of premonitory urge and reward. Cereb. Cortex Commun. 2020, 1, tgaa046. [Google Scholar] [CrossRef] [PubMed]
- Salgado, D.P. Carga cognitiva e sua correlação com piscadas durante o uso de um simulador de cadeira de rodas [Cognitive Load and Its Correlation with Blinks During the Use of a Wheelchair Simulator]. Master’s Dissertation, Program in Biomedical Engineering, Faculty of Electrical Engineering, Federal University of Uberlândia, Uberlândia, Brasil, 2020. Available online: https://repositorio.ufu.br/bitstream/123456789/29909/3/CargaCognitivaCorrelacao.pdf (accessed on 13 May 2025).
- Lee, J.S.; Choi, Y.J.; Cho, S.H. Effect of electroacupuncture stimulation at different frequencies on brain waves. Acupunct. Electro-Ther. Res. 2019, 44, 11–22. [Google Scholar] [CrossRef]
- Witzel, T.; Napadow, V.; Kettner, N.W.; Vangel, M.G.; Hämäläinen, M.S.; Dhond, R.P. Differences in cortical response to acupressure and electroacupuncture stimuli. BMC Neurosci. 2011, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Aspiotis, V.; Miltiadous, A.; Kalafatakis, K.; Tzimourta, K.D.; Giannakeas, N.; Tsipouras, M.G.; Peschos, D.; Glavas, E.; Tzallas, A.T. Assessing electroencephalography as a stress indicator: A VR high-altitude scenario monitored through EEG and ECG. Sensors 2022, 22, 5792. [Google Scholar] [CrossRef] [PubMed]
- Attar, E.T.; Balasubramanian, V.; Subasi, E.; Kaya, M. Stress analysis based on simultaneous heart rate variability and EEG monitoring. IEEE J. Transl. Eng. Health Med. 2021, 9, 2700607. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, T.; Shiratori, H.; Amano, K. The relationship between alpha power and heart rate variability commonly seen in various mental states. PLoS ONE 2024, 19, e0298961. [Google Scholar] [CrossRef] [PubMed]
- Teo, J.T.; Johnstone, S.J.; Thomas, S.J. Use of portable devices to measure brain and heart activity during relaxation and comparative conditions: Electroencephalogram, heart rate variability, and correlations with self-report psychological measures. Int. J. Psychophysiol. 2023, 189, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Vanhollebeke, G.; De Smet, S.; De Raedt, R.; Baeken, C.; van Mierlo, P.; Vanderhasselt, M.A. The neural correlates of psychosocial stress: A systematic review and meta-analysis of spectral analysis EEG studies. Neurobiol. Stress. 2022, 18, 100452. [Google Scholar] [CrossRef] [PubMed]
- Napadow, V.; Makris, N.; Liu, J.; Kettner, N.W.; Kwong, K.K.; Hui, K.K. Effects of electroacupuncture versus manual acupuncture on the human brain as measured by fMRI. Hum. Brain Mapp. 2005, 24, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Mayor, D. Electroacupuncture (EA) and the EEG: An Unfinished Personal Journey, 2001–2022. From Simple Hypothesis to Artificial Intelligence (AI). Presentation. In Proceedings of the AACP Virtual Conference, Virtual, 21 May 2022; Available online: https://researchprofiles.herts.ac.uk/en/publications/electroacupuncture-ea-and-the-eeg-an-unfinished-personal-journey- (accessed on 15 May 2025).
- Sarosiek, I.; Song, G.; Sun, Y.; Sandoval, H.; Sands, S.; Chen, J.; McCallum, R.W. Central and peripheral effects of transcutaneous acupuncture treatment for nausea in patients with diabetic gastroparesis. J. Neurogastroenterol. Motil. 2017, 23, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Yüksel, M.; Ayaş, Ş.; Cabıoğlu, M.T.; Yılmaz, D.; Cabıoğlu, C. Quantitative data for transcutaneous electrical nerve stimulation and acupuncture effectiveness in treatment of fibromyalgia syndrome. Evid. Based Complement. Alternat Med. 2019, 2019, 9684649. [Google Scholar] [CrossRef] [PubMed]
- Tegeler, C.H.; Shaltout, H.A.; Tegeler, C.L.; Gerdes, L.; Lee, S.W. Rightward dominance in temporal high-frequency electrical asymmetry corresponds to higher resting heart rate and lower baroreflex sensitivity in a heterogeneous population. Brain Behav. 2015, 5, e00343. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.E.; Reznik, S.J.; Stewart, J.L.; Allen, J.J. Assessing and conceptualizing frontal EEG asymmetry: An updated primer on recording, processing, analyzing, and interpreting frontal alpha asymmetry. Int. J. Psychophysiol. 2017, 111, 98–114. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.A.; Choi, Y.; Cho, S.H. Acupuncture for attenuating frontal lobe α band asymmetry induced by anger: A pilot study. J. Pharmacopuncture 2023, 26, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.H.; Zhang, Y.M.; Fan, F.F.; Song, X.Y.; Liu, L. Functional role of frontal electroencephalogram alpha asymmetry in the resting state in patients with depression: A review. World J. Clin. Cases 2023, 11, 1903–1917. [Google Scholar] [CrossRef] [PubMed]
- Spronk, D.; Arns, M.; Barnett, K.J.; Cooper, N.J.; Gordon, E. An investigation of EEG, genetic and cognitive markers of treatment response to antidepressant medication in patients with major depressive disorder: A pilot study. J. Affect. Disord. 2011, 128, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Francis, A.M.; Bissonnette, J.N.; Hull, K.M.; Leckey, J.; Pimer, L.; Lawrence, M.A.; Berrigan, L.I.; Fisher, D.J. Measuring the attention networks and quantitative-electroencephalography correlates of attention in depression. Psychiatry Res. Neuroimaging 2023, 333, 111661. [Google Scholar] [CrossRef] [PubMed]
- Leuchter, A.F.; Uijtdehaage, S.H.; Cook, I.A.; O’Hara, R.; Mandelkern, M. Relationship between brain electrical activity and cortical perfusion in normal subjects. Psychiatry Res. 1999, 90, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Thomson, D.J. Spectrum estimation and harmonic analysis. Proc. IEEE 1982, 70, 1055–1096. [Google Scholar] [CrossRef]
- Grossman, A.; Morlet, J. Decomposition of Hardy functions into square integrable wavelets of constant shape. SIAM J. Math. Anal. 1984, 15, 723–736. [Google Scholar] [CrossRef]
- Cook, I.A.; Leuchter, A.F. Synaptic dysfunction in Alzheimer’s disease: Clinical assessment using quantitative EEG. Behav. Brain Res. 1996, 78, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Leuchter, A.F.; Cook, I.A.; Lufkin, R.B.; Dunkin, J.; Newton, T.F.; Cummings, J.L.; Mackey, J.K.; Walter, D.O. Cordance: A new method for assessment of cerebral perfusion and metabolism using quantitative electroencephalography. Neuroimage 1994, 1, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, V.; Gagliardi, G.; Ceccherelli, F.; Lovato, A. Effect of low- and high-frequency auricular stimulation with electro-acupuncture on cutaneous microcirculation: A cross-over study in healthy subjects. Medicines 2023, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Calvo, S.; Navarro, J.; Herrero, P.; Del Moral, R.; De Diego, C.; Marijuán, P.C. Electroencephalographic changes after application of dry needling [DNHS© technique] in two patients with chronic stroke. Myopain 2015, 23, 112–117. [Google Scholar] [CrossRef]
- Sulaiman, N.; Taib, M.N.; Aris, S.A.M.; Hamid, N.H.A.; Lias, S.; Murat, Z.H. Stress features identification from EEG signals using EEG Asymmetry & Spectral Centroids techniques. In Proceedings of the 2010 IEEE EMBS Conference on Bio-Medical Engineering and Sciences (IECBES), Kuala Lumpur, Malaysia, 30 November–2 December 2010; IEEE: New York, NY, USA, 2010; pp. 417–421. [Google Scholar] [CrossRef]
- Giannakakis, G.; Grigoriadis, D.; Tsiknakis, M. Detection of stress/anxiety state from EEG features during video watching. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; Volume 2015, pp. 6034–6037. [Google Scholar] [CrossRef]
- Herborn, K.A.; Graves, J.L.; Jerem, P.; Evans, N.P.; Nager, R.; McCafferty, D.J.; McKeegan, D.E. Skin temperature reveals the intensity of acute stress. Physiol. Behav. 2015, 152 Pt A, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Li, J.; Cao, X.D.; Feng, X.Y. Can electroacupuncture affect the sympathetic activity, estimated by skin temperature measurement? A functional MRI study on the effect of needling at GB 34 and GB 39 on patients with pain in the lower extremity. Acupunct. Electrother. Res. 2009, 34, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.W.; Girolami, M.; Sejnowski, T.J. Independent component analysis using an extended infomax algorithm for mixed subgaussian and super-gaussian sources. Neural Comput. 1999, 11, 417–441. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.H.; Pion-Tonachini, L.; Palmer, J.; Miyakoshi, M.; Makeig, S.; Jung, T.P. Modeling brain dynamic state changes with adaptive mixture independent component analysis. Neuroimage 2018, 183, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Radüntz, T.; Scouten, J.; Hochmuth, O.; Meffert, B. Automated EEG artifact elimination by applying machine learning algorithms to ICA-based features. J. Neural Eng. 2017, 14, 046004. [Google Scholar] [CrossRef] [PubMed]
- Hjorth, B. EEG analysis based on time domain properties. Electroencephalogr. Clin. Neurophysiol. 1970, 29, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Hjorth, B. The physical significance of time domain descriptors in EEG analysis. Electroencephalogr. Clin. Neurophysiol. 1973, 34, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Wackermann, J.; Allefeld, C. On the meaning and interpretation of global descriptors of brain electrical activity. Including a reply to X. Pei et al. Int. J. Psychophysiol. 2007, 64, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Jarque, C.M.; Bera, A.K. A test for normality of observations and regression residuals. Int. Stat. Rev./Rev. Int. Stat. 1987, 55, 163–172. [Google Scholar] [CrossRef]
- Spencer, N.H.; with Mayor, D.; Steffert, S.; Beggan, C.D. From a Berkshire Farm to a Fishing Expedition via Random Points. Presentation at the Royal Statistical Society, London, 29 March 2023. Available online: https://rss.org.uk/training-events/events/events-2023/sections/rss-business-industrial-section-from-a-berkshire-f/#fulleventinfo (accessed on 13 May 2025).
- Horowitz, J.L. Bootstrap methods in econometrics. Annu. Rev. Econ. 2019, 11, 193–224. [Google Scholar] [CrossRef]
- Hettmansperger, T.P.; Elmore, R. Tests for interaction in a two-way layout: Should they be included in a nonparametrics course? In Proceedings of the International Conference on Teaching Statistics (ICOTS 6), Cape Town, South Africa, 7–12 July 2002; p. 369. [Google Scholar]
- Bonett, D.G.; Wright, T.A. Sample size requirements for estimating Pearson, Kendall and Spearman correlations. Psychometrika 2000, 65, 23–28. [Google Scholar] [CrossRef]
- Pallant, J. SPSS Survival Manual. A Step by Step Guide to Data Analysis Using IBM SPSS v15, 3rd ed.; McGraw-Hill Open University Press: Maidenhead/Berks, UK, 2007. [Google Scholar]
- Tomczak, M.; Tomczak, E. The need to report effect size estimates revisited. An overview of some recommended measures of effect size. TRENDS Sport. Sci. 2014, 1, 19–25. [Google Scholar]
- Fern, E.F.; Monroe, K.B. Effect-size estimates: Issues and problems in interpretation. J. Consum. Res. 1996, 23, 89–105. [Google Scholar] [CrossRef]
- Spencer, N.H. Overcoming the multiple testing problem when testing randomness. J. R. Stat. Soc. C Appl. Stat. 2009, 58, 543–553. [Google Scholar] [CrossRef]
- Sammito, S.; Thielmann, B.; Klussmann, A.; Deußen, A.; Braumann, K.-M.; Böckelmann, I. Guideline for the application of heart rate and heart rate variability in occupational medicine and occupational health science. J. Occup. Med. Toxicol. 2024, 19, 15. [Google Scholar] [CrossRef] [PubMed]
- Karadimitriou, S.M.; Marshall, E. Mann-Whitney U Test (Non-Parametric Equivalent to Independent Samples t-Test). Statstutor Community Project, n.d. Available online: www.statstutor.ac.uk (accessed on 13 May 2025).
- Baevsky, R.M.; Chernikova, A.G. Heart rate variability analysis: Physiological foundations and main methods. Cardiometry 2017, 10, 66–76. [Google Scholar] [CrossRef]
- Gullett, N.; Zajkowska, Z.; Walsh, A.; Harper, R.; Mondelli, V. Heart rate var-iability (HRV) as a way to understand associations between the autonomic nervous system (ANS) and affective states: A critical review of the literature. Int. J. Psychophysiol. 2023, 192, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Steffert, T.; Mayor, D. The Fickleness of Data: Estimating the Effects of Different Aspects of Acupuncture Treatment on Heart Rate Variability (HRV). Initial Findings from Three Pilot Studies. Background Information to a Poster Presented at the 16th International Acupuncture Research Symposium, King’s College London, UK, 29 March 2014. Available online: https://researchprofiles.herts.ac.uk/en/publications/the-fickleness-of-data-estimating-the-effects-of-different-aspect (accessed on 13 May 2025).
- Armstrong, K.; Gokal, R.; Todorsky, W. Neuromodulating influence of two electroacupuncture treatments on heart rate variability, stress, and vagal activity. J. Altern. Complement. Med. 2020, 26, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.A.; Cheng, C.Y.; Lin, Y.W.; Li, T.C.; Liu, H.J.; Hsieh, C.L. The 2 Hz and 15 Hz electroacupuncture induced reverse effect on autonomic function in healthy adult using a heart rate variability analysis. J. Tradit. Complement. Med. 2011, 1, 51–56. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, J.H.; Kim, K.H.; Hong, J.W.; Lee, W.C.; Koo, S.T. Comparison of electroacupuncture frequency-related effects on heart rate variability in healthy volunteers: A randomized clinical trial. J. Acupunct. Meridian Stud. 2011, 4, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wu, Y.R.; Zhan, Z.; Pan, Y.H.; Jiang, J.F. State- and frequency-dependence in autonomic rebalance mediated by intradermal auricular electroacupuncture stimulation. Front. Neurosci. 2024, 18, 1367266. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).