Determination of Spatiotemporal Gait Parameters Using a Smartphone’s IMU in the Pocket: Threshold-Based and Deep Learning Approaches
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Equipment
2.3. Experimental Methods
2.3.1. Normal Gait
2.3.2. Patient Gait
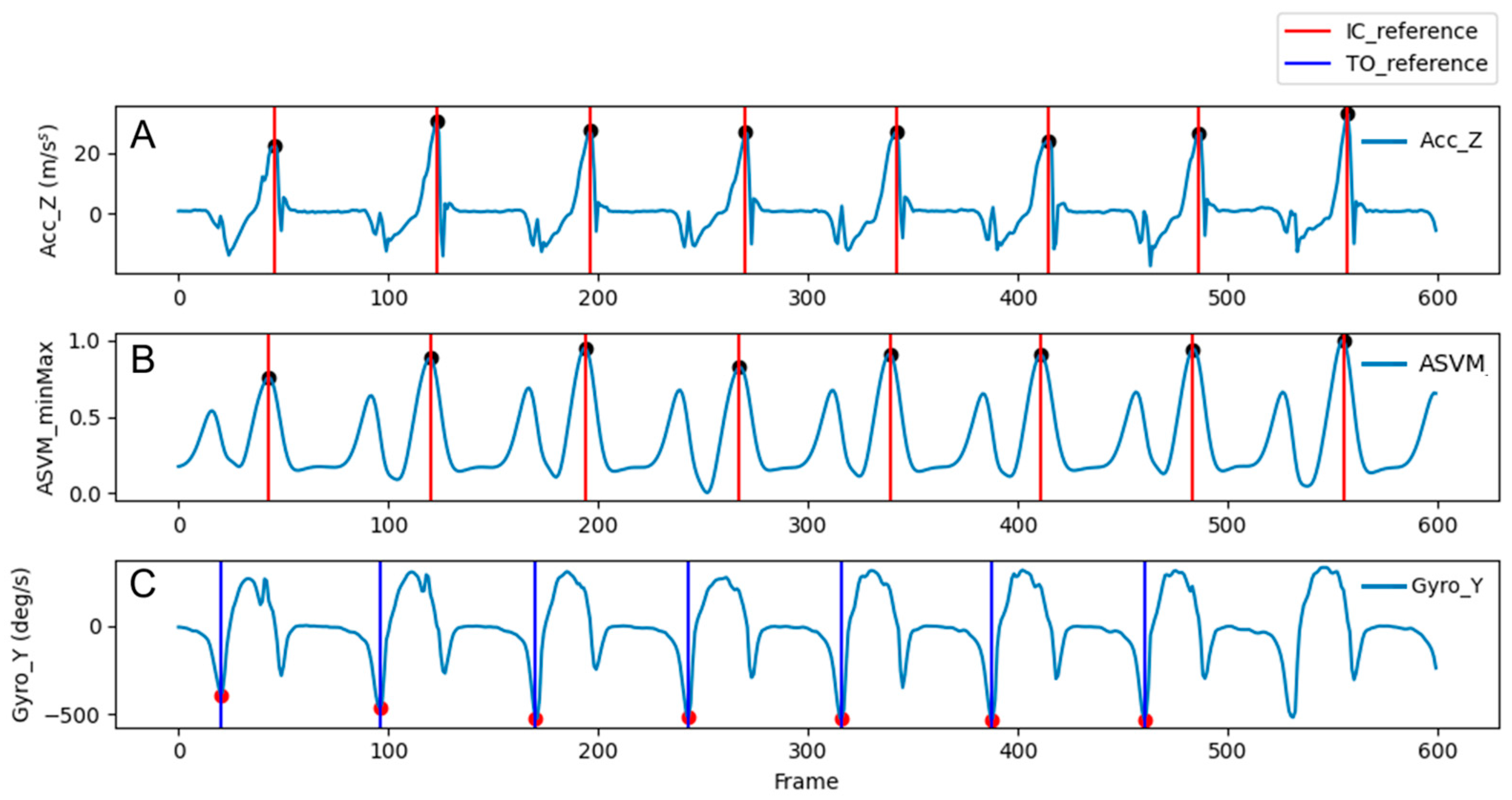
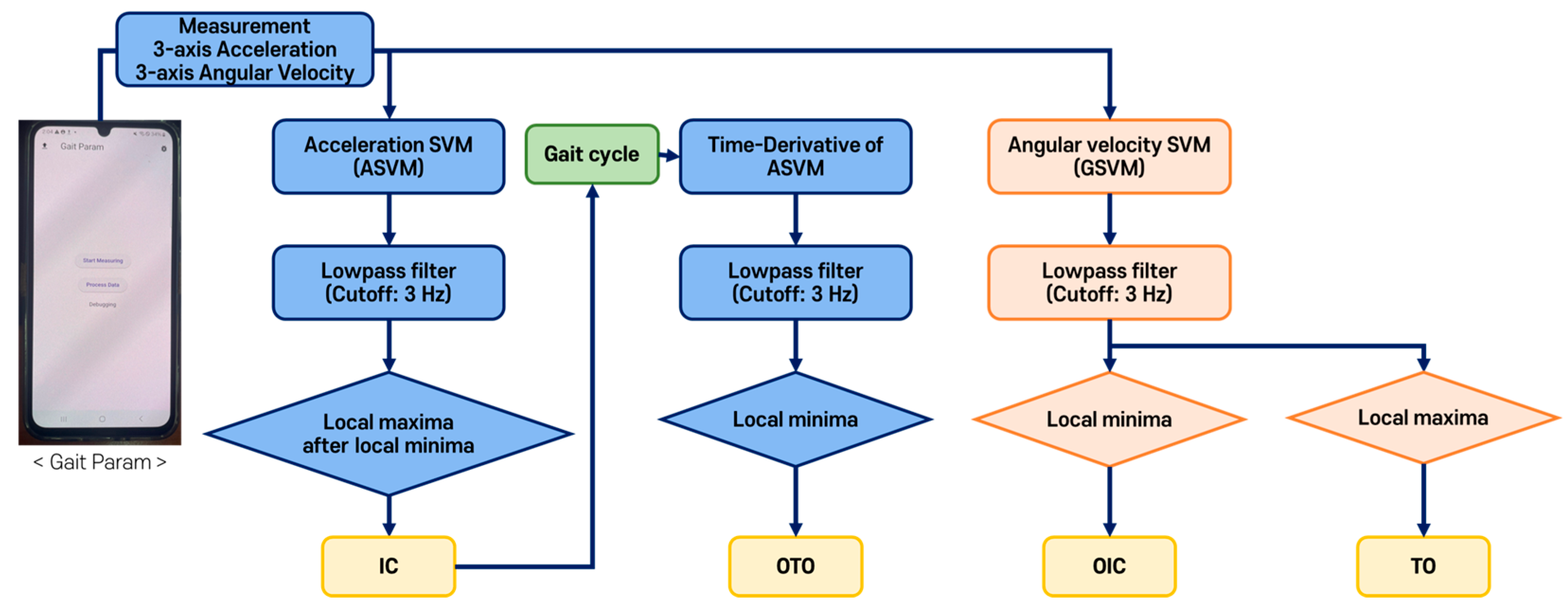
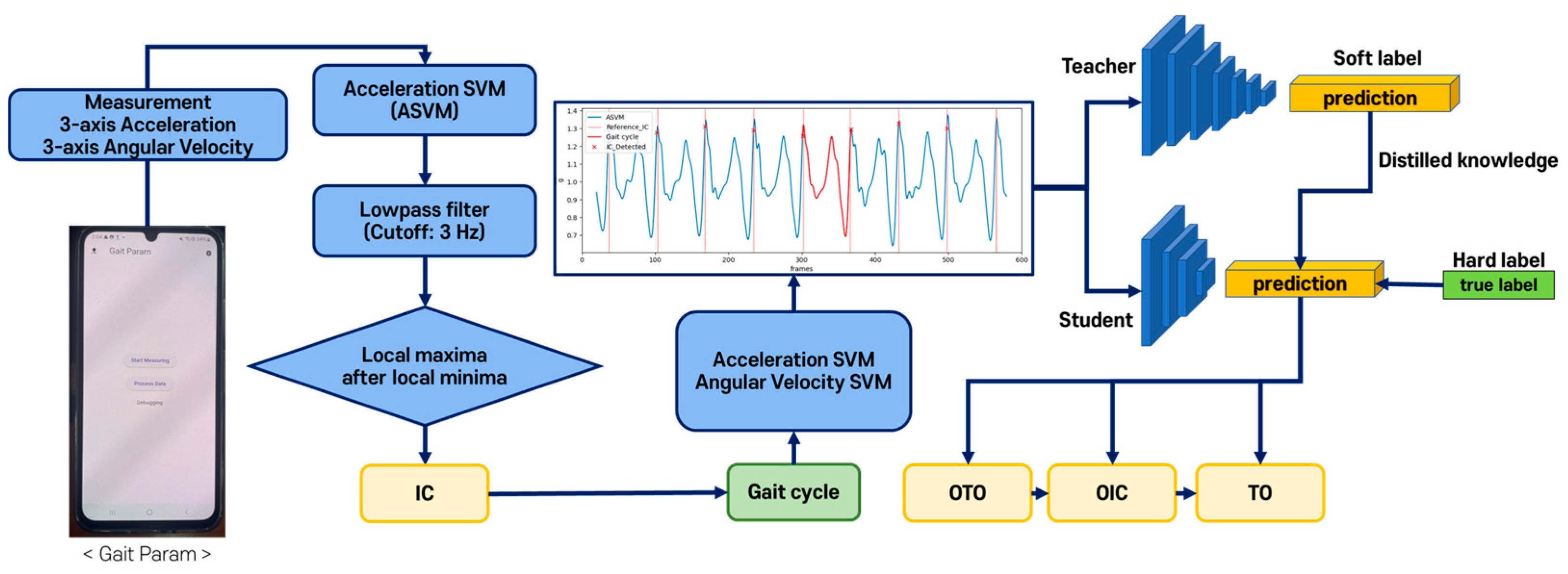
2.4. Gait Event and Gait Parameter Detection Algorithms
2.4.1. Reference Gait Events
2.4.2. Threshold-Based Gait Event Detection
2.4.3. DL-Based Gait Event Detection
2.4.4. Statistical Analysis
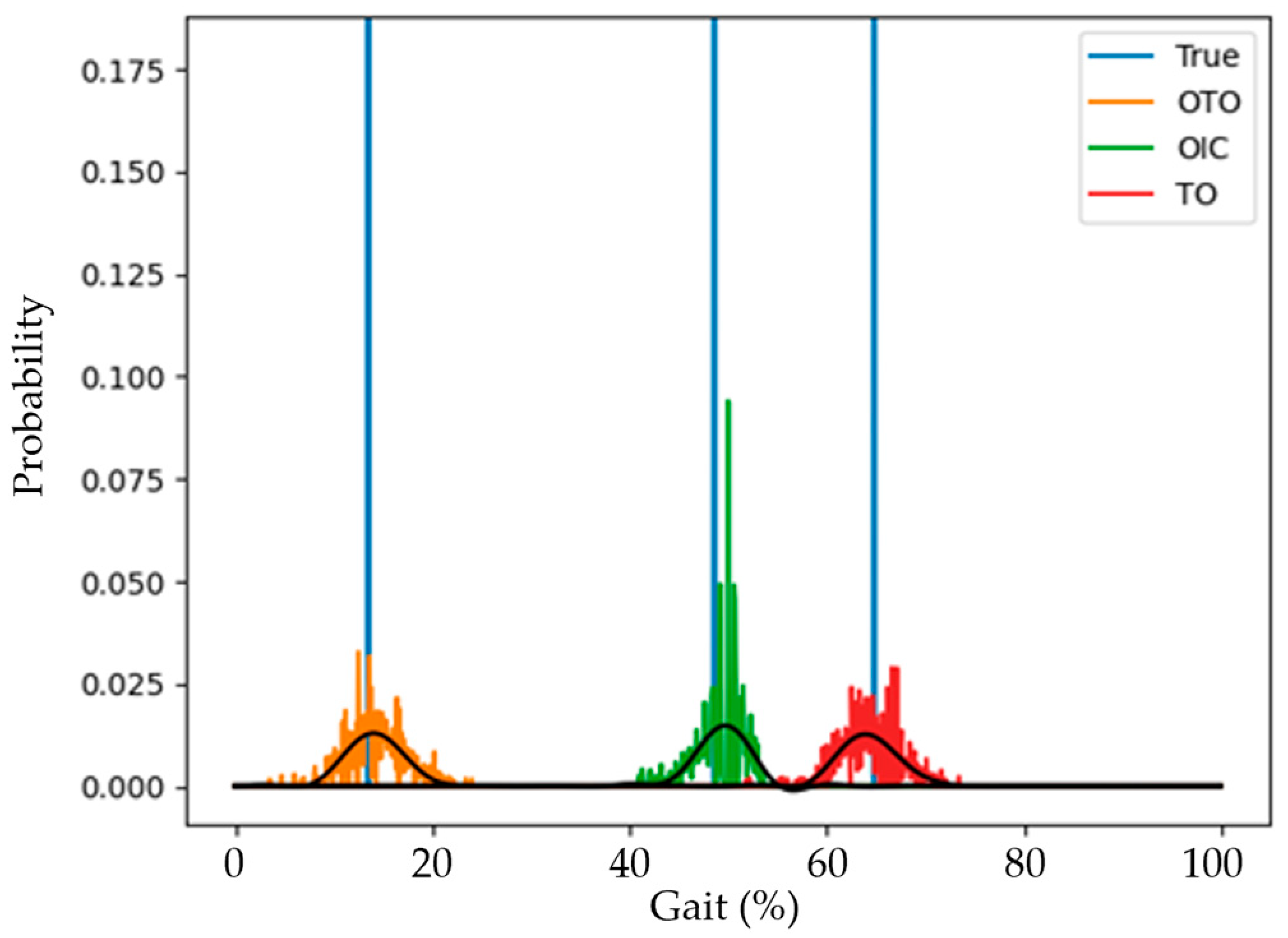
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pepa, L.; Verdini, F.; Spalazzi, L. Gait parameter and event estimation using smartphones. Gait Posture 2017, 57, 217–223. [Google Scholar] [CrossRef]
- Hamer, M.; Chida, Y. Walking and primary prevention: A meta-analysis of prospective cohort studies. Br. J. Sports Med. 2008, 42, 238–243. [Google Scholar] [CrossRef]
- Venegas-Sanabria, L.C.; Cavero-Redondo, I.; Martínez-Vizcaino, V.; Cano-Gutierrez, C.A.; Álvarez-Bueno, C. Effect of Multicomponent Exercise in Cognitive Impairment: A Systematic Review and Meta-Analysis. BMC Geriatr. 2022, 22, 617. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.; Davids, J.R. Gait analysis: Normal and pathological function. J. Pediatr. Orthop. 1992, 12, 815. [Google Scholar] [CrossRef]
- Yang, S.; Koo, B.; Lee, S.; Jang, D.-J.; Shin, H.; Choi, H.-J.; Kim, Y. Determination of Gait Events and Temporal Gait Parameters for Persons with a Knee–Ankle–Foot Orthosis. Sensors 2024, 24, 964. [Google Scholar] [CrossRef] [PubMed]
- Verghese, J.; Lipton, R.; Hall, C.; Kuslansky, G.; Katz, M.; Buschke, H. Abnormality of gait as a predictor for non-Alzheimer’s dementia. N. Engl. J. Med. 2002, 347, 1762–1768. [Google Scholar] [CrossRef] [PubMed]
- Asiri, F.Y.; Marchetti, G.F.; Ellis, J.L.; Otis, L.; Sparto, P.J.; Watzlaf, V.; Whitney, S.L. Predictors of functional and gait outcomes for persons poststroke undergoing home-based rehabilitation. J. Stroke Cerebrovasc. Dis. 2014, 23.7, 1856–1864. [Google Scholar] [CrossRef]
- Casartelli, N.C.; Item-Glatthorn, J.F.; Bizzini, M.; Leunig, M.; Maffiuletti, N.A. Differences in gait characteristics between total hip, knee, and ankle arthroplasty: A six-month postoperative comparison. BMC Musculoskelet. Disord. 2013, 14, 176. [Google Scholar] [CrossRef]
- Tanaka, S.; Kamiya, K.; Hamazaki, N.; Matsuzawa, R.; Nozaki, K.; Nakamura, T.; Yamashita, M.; Maekawa, E.; Noda, C.; Yamaoka-Tojo, M.; et al. Short-term change in gait speed and clinical outcomes in older patients with acute heart. failure. Circ. J. 2019, 83, 1860–1867. [Google Scholar] [CrossRef]
- Holden, M.K.; Gill, K.M.; Magliozzi, M.R.; Nathan, J.; Piehl-Baker, L. Clinical gait assessment in the neurologically impaired reliability and meaningfulness. Phys. Ther. 1984, 64, 35–40. [Google Scholar] [CrossRef]
- Kim, J.; Bae, M.N.; Lee, K.B.; Hong, S.G. Gait event detection algorithm based on smart insoles. ETRI J. 2020, 42, 46–53. [Google Scholar] [CrossRef]
- McCamley, J.; Donati, M.; Grimpampi, E.; Mazza, C. An enhanced estimate of initial contact and final contact instants of time using lower trunk inertial sensor data. Gait Posture 2012, 36, 316–318. [Google Scholar] [CrossRef]
- Maqbool, H.F.; Husman, M.A.B.; Awad, M.I.; Abouhossein, A.; Iqbal, N.; Dehghani-Sanij, A.A. A real-time gait event detection for lower limb prosthesis control and evaluation. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 1500–1509. [Google Scholar] [CrossRef] [PubMed]
- Ledoux, E. Inertial Sensing for Gait Event Detection and Transfemoral Prosthesis Control Strategy. IEEE Trans. Biomed. Eng. 2018, 65, 2704–2712. [Google Scholar] [CrossRef]
- Romijnders, R.; Warmerdam, E.; Hansen, C.; Welzel, J.; Schmidt, G.; Maetzler, W. Validation of IMU-based gait event detection during curved walking and turning in older adults and Parkinson’s Disease patients. J. Neuroeng. Rehabil. 2021, 18, 28. [Google Scholar] [CrossRef] [PubMed]
- Romijnders, R.; Warmerdam, E.; Hansen, C.; Schmidt, G.; Maetzler, W. A Deep Learning Approach for Gait Event Detection from a Single Shank-Worn IMU: Validation in Healthy and Neurological Cohorts. Sensors 2022, 22, 3859. [Google Scholar] [CrossRef]
- De Miguel-Fernández, J.; Salazar-Del Rio, M.; Rey-Prieto, M.; Bayón, C.; Guirao-Cano, L.; Font-Llagunes, J.M.; Lobo-Prat, J. Inertial sensors for gait monitoring and design of adaptive controllers for exoskeletons after stroke: A feasibility study. Front. Bioeng. Biotechnol. 2023, 11, 1208561. [Google Scholar] [CrossRef]
- Strick, J.A.; Farris, R.J.; Sawicki, J.T. A Novel Gait Event Detection Algorithm Using a Thigh-Worn Inertial Measurement Unit and Joint Angle Information. J. Biomech. Eng. 2024, 146, 044502. [Google Scholar] [CrossRef]
- Voisard, C.; de l’Escalopier, N.; Ricard, D.; Oudre, L. Automatic gait events detection with inertial measurement units: Healthy subjects and moderate to severe impaired patients. J. Neuroeng. Rehabil. 2024, 21, 104. [Google Scholar] [CrossRef]
- Gurchiek, R.D.; Garabed, C.P.; McGinnis, R.S. Gait event detection using a thigh-worn accelerometer. Gait Posture 2020, 80, 214–216. [Google Scholar] [CrossRef]
- Silsupadol, P.; Prupetkaew, P.; Kamnardsiri, T.; Lugade, V. Smartphone-Based Assessment of Gait During Straight Walking, Turning, and Walking Speed Modulation in Laboratory and Free-Living Environments. IEEE J. Biomed. Health Inform. 2019, 24, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.F.; Duke, K.; Gneezy, A.; Bos, M.W. Brain drain: The mere presence of one’s own smartphone reduces available cognitive capacity. J. Assoc. Consum. Res. 2017, 2, 140–154. [Google Scholar] [CrossRef]
- Redmayne, M. Where’s your phone? A survey of where women aged 15–40 carry their smartphone and related risk perception: A survey and pilot study. PLoS ONE 2017, 12, e0167996. [Google Scholar] [CrossRef]
- Zijlstra, W.; Hof, A.L. Assessment of spatio-temporal gait parameters from trunk accelerations during human walking. Gait Posture 2003, 18, 1–10. [Google Scholar] [CrossRef]
- González, R.C.; López, A.M.; Rodriguez-Uría, J.; Álvarez, D.; Alvarez, J.C. Real-time gait event detection for normal subjects from lower trunk accelerations. Gait Posture 2010, 31, 322–325. [Google Scholar] [CrossRef]
- Rampp, A.; Barth, J.; Schuelein, S.; Gaßmann, K.G.; Klucken, J.; Eskofier, B. Inertial sensor-based stride parameter calculation from gait sequences in geriatric patients. IEEE Trans. Biomed. Eng. 2015, 62, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Niswander, W.; Kontson, K. Evaluating the Impact of IMU Sensor Location and Walking Task on Accuracy of Gait Event Detection Algorithms. Sensors 2021, 21, 3989. [Google Scholar] [CrossRef] [PubMed]
- Salarian, A.; Russmann, H.; Vingerhoets, F.J.G.; Dehollaini, C.; Blanc, Y.; Burkhard, P.R.; Aminian, K. Gait assessment in Parkinson’s disease: Toward an ambulatory system for long-term monitoring. IEEE Trans. Biomed. Eng. 2004, 51, 1434–1443. [Google Scholar] [CrossRef]
- Winter, D.A.; Yack, H.J. EMG profiles during normal human walking: Stride-to-stride and inter-subject variability. Electroencephalogr. Clin. Neurophysiol. 1987, 67, 402–411. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Z.; Qiu, S.; Shen, Y.; Wang, J. IMU-based gait analysis for rehabilitation assessment of patients with gait disorders. In Proceedings of the 2017 4th International Conference on Systems and Informatics (ICSAI), Hangzhou, China, 11–13 November 2017; pp. 622–626. [Google Scholar]
- Hori, K.; Mao, Y.; Ono, Y.; Ora, H.; Hirobe, Y.; Sawada, H.; Inaba, A.; Orimo, S.; Miyake, Y. Inertial Measurement Unit-Based Estimation of Foot Trajectory for Clinical Gait Analysis. Front. Physiol. 2020, 10, 1530. [Google Scholar] [CrossRef]
- Iglesias, G.; Talavera, E.; González-Prieto, A.; Mozo, A.; Gómez-Canaval, S. Data Augmentation techniques in time series domain: A survey and taxonomy. Neural Comput. Appl. 2023, 35, 10123–10145. [Google Scholar] [CrossRef]
- Hinton, G.; Vinyals, O.; Dean, J. Distilling the knowledge in a neural network. arXiv 2015, arXiv:1503.02531. [Google Scholar]
- LeCun, Y.; Boser, B.; Denker, J.S.; Henderson, D.; Howard, R.E.; Hubbard, W.; Jackel, L.D. Backpropagation applied to handwritten zip code recognition. Neural Comput. 1989, 1, 541–551. [Google Scholar] [CrossRef]
- Huang, S.; Tang, J.; Dai, J.; Wang, Y. Signal Status Recognition Based on 1DCNN and Its Feature Extraction Mechanism Analysis. Sensors 2019, 19, 2018. [Google Scholar] [CrossRef]
- Hochreiter, S.; Schmidhuber, J. Long short-term memory. Neural Comput. 1997, 9, 1735–1780. [Google Scholar] [CrossRef]
- Caruana, R. Multitask learning. Mach. Learn. 1997, 28, 41–75. [Google Scholar] [CrossRef]
- Buda, M.; Maki, A.; Mazurowski, M.A. A systematic study of the class imbalance problem in convolutional neural networks. Neural Netw. 2018, 106, 249–259. [Google Scholar] [CrossRef]
- Vickery-Howe, D.M.; Bonanno, D.R.; Dascombe, B.J.; Drain, J.R.; Clarke, A.C.; Hoolihan, B.; Willy, R.W.; Middleton, K.J. Physiological, perceptual, and biomechanical differences between treadmill and overground walking in healthy adults: A systematic review and meta-analysis. J. Sports Sci. 2023, 41, 2088–2120. [Google Scholar] [CrossRef]
- Alton, F.; Baldey, L.; Caplan, S.; Morrissey, M.C. A kinematic comparison of overground andtreadmill walking. Clin. Biomech. 1998, 13, 434–440. [Google Scholar] [CrossRef]


| Patient No. | Height (cm) | Weight (kg) | Age (Years) | Sex | Type of Stroke | Onset (Months) | Side | FAC (Level) |
|---|---|---|---|---|---|---|---|---|
| P01 | 165 | 71 | 83 | M | Infarct | 14 | Right | 1 |
| P02 | 161 | 73 | 65 | F | Infarct | 45 | Left | 5 |
| P03 | 170 | 80 | 58 | M | Infarct | 106 | Right | 5 |
| P04 | 175 | 70 | 53 | M | Infarct | 32 | Right | 5 |
| P05 | 167 | 75 | 56 | M | Infarct | 38 | Right | 5 |
| P06 | 164 | 78 | 68 | M | Infarct | 8 | Right | 5 |
| P07 | 173 | 75 | 41 | M | Hemorrhage | 236 | Left | 5 |
| P08 | 169 | 70 | 77 | M | Hemorrhage | 260 | Right | 4 |
| P09 | 162 | 62 | 67 | M | Infarct | 67 | Right | 4 |
| P10 | 163 | 65 | 66 | M | Infarct | 167 | Left | 4 |
| P11 | 164 | 68 | 54 | F | Hemorrhage | 141 | Left | 5 |
| P12 | 156 | 48 | 35 | F | Hemorrhage | 287 | Right | 5 |
| P13 | 158 | 59 | 51 | F | Infarct | 116 | Left | 5 |
| Average | 165.1 | 69.1 | 60.7 | - | ||||
| SD | 5.2 | 8.2 | 13.1 | |||||
| Subjects | Algorithm | IC | OTO | OIC | TO | |
|---|---|---|---|---|---|---|
| Normal | Threshold-based | −0.3 ± 3.7% ** p < 0.01 [0.5, 2.0] | −0.1 ± 4.4% ** p < 0.01 [1.3, 4.8] | 0.4 ± 4.5% 0.09 [−0.1, 1.7] | 0.3 ± 4.3% ** p < 0.01 [1.0, 3.8] | |
| DL-based | Teacher | - | −0.2 ± 3.6% 0.56 [−0.7, 0.4] | 0.5 ± 3.1% * 0.04 [0.0, 0.9] | −0.2 ± 4.3% 0.52 [−0.8, 0.4] | |
| KD | 0.7 ± 2.7% ** p < 0.01 [0.3, 1.1] | −0.3 ± 2.4% 0.16 [−0.61, 0.1] | 1.9 ± 2.9% ** p< 0.01 [1.5, 2.3] | |||
| Patient | Threshold-based | 0.7 ± 3.6% * 0.02 [−1.0, −0.1] | −1.1 ± 5.9% 0.20 [−0.4, 1.8] | −0.4 ± 5.8% 0.32 [−0.64, 2.0] | −0.3 ± 6.4% 0.91 [−1.5, 1.3] | |
| DL-based | Teacher | - | −1.4 ± 6.8% 0.24 [−3.9, 1.0] | −3.9 ± 4.6% ** p < 0.01 [−5.6, −2.3] | −3.5 ± 5.2% ** p < 0.01 [−5.4, −1.6] | |
| KD | −0.8 ± 5.7% 0.44 [−2.8, 1.3] | −3.2 ± 5.6% ** p < 0.01 [−5.2, −1.2] | −2.6 ± 5.7% * 0.01 [−4.7, −0.6] | |||
| Threshold-Based | Gait Parameters | ||
|---|---|---|---|
| Cadence (steps/min) | Stride Length (cm) | ||
| Normal | Reference | 106.6 ± 11.4 | 126.8 ± 18.5 |
| Detected | 107.7 ± 11.9 | 125.6 ± 18.3 | |
| Patient | Reference | 96.9 ± 14.1 | 134.2 ± 22.5 |
| Detected | 96.9 ± 14.7 | 134.4 ± 22.6 | |
| Model | LR (%) | PSw (%) | SLS (%) | DLS (%) | Symmetry (%) | ||
|---|---|---|---|---|---|---|---|
| Normal | Threshold -based | Reference | 10.7 ± 3.3 | 12.8 ± 3.2 | 38.1 ± 2.4 | 23.5 ± 4.4 | 48.8 ± 2.4 |
| Detected | 10.5 ± 3.3 | 13.1 ± 3.3 | 38.0 ± 3.9 * | 23.6 ± 4.8 | 48.6 ± 3.2 | ||
| DL-based | Reference | 13.4 ± 1.8 | 17.0 ± 2.7 | 35.7 ± 3.1 | 30.5 ± 3.5 | 49.2 ± 2.5 | |
| Teacher | 13.6 ± 2.9 | 17.7 ± 4.3 | 35.1 ± 4.1 | 31.2 ± 5.4 | 48.7 ± 3.0 | ||
| KD | 12.8 ± 2.0 | 14.9 ± 1.3 | 36.6 ± 2.0 | 28.2 ± 2.9 | 49.4 ± 1.0 | ||
| Patient | Threshold -based | Reference | 17.0 ± 4.2 † | 17.0 ± 3.8 † | 28.8 ± 5.4 † | 34.0 ± 6.5 † | 45.8 ± 4.4 † |
| Detected | 17.2 ± 3.1 † | 17.5 ± 5.3 † | 28.0 ± 8.0 † | 34.2 ± 7.0 † | 45.2 ± 8.1 † | ||
| DL-based | Reference | 17.7 ± 5.1 † | 19.9 ± 3.0 † | 27.1 ± 4.0 † | 37.5 ± 5.4 † | 44.7 ± 4.8 † | |
| Teacher | 19.1 ± 4.6 † | 19.4 ± 3.7 † | 29.6 ± 4.3 † | 40.2 ± 5.9 † | 48.7 ± 3.1 † | ||
| KD | 18.5 ± 3.2 † | 19.3 ± 5.9 † | 29.5 ± 3.5 † | 40.7 ± 5.9 † | 48.0 ± 2.3 † |
| [12] | [15] | [13] | [14] | [20] | [5] | This Study | |||
|---|---|---|---|---|---|---|---|---|---|
| Threshold | Teacher | ||||||||
| Sensor position | Trunk | Shank | Shank | Shank | Thigh | Thigh | |||
| Sampling rate (Hz) | 100 | 200 | 100 | 500 | 128 | 60 | 50 | ||
| IC | Error rate (%) | 2.0 | −1.7 ± 0.6 | −0.1 ± 0.6 | −0.3 ± 3.7 | ||||
| Error time (ms) | 6 ± 24 | −11 ± 57 | 11 ± 18 | 39 ± 28 | −1 ± 8 | −8.3 ± 67 | |||
| TO | Error rate (%) | 3.0 | −1.8 ± 0.6 | −0.2 ± 1.9 | 0.3 ± 4.3 | −0.2 ± 4.3 | |||
| Error time (ms) | −29 ± 26 | 2 ± 31 | −8 ± 35 | 28 ± 28 | −3 ± 24 | 9.9 ± 70.7 | −0.4 ± 5.4 | ||
| OIC | Error rate (%) | 1.0 ± 1.3 | 0.4 ± 4.5 | 0.2 ± 2.7 | |||||
| OTO | Error rate (%) | −1.1 ± 2.9 | −0.1 ± 4.4 | 0.7 ± 3.0 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Park, C.; Ha, E.; Hong, J.; Kim, S.H.; Kim, Y. Determination of Spatiotemporal Gait Parameters Using a Smartphone’s IMU in the Pocket: Threshold-Based and Deep Learning Approaches. Sensors 2025, 25, 4395. https://doi.org/10.3390/s25144395
Lee S, Park C, Ha E, Hong J, Kim SH, Kim Y. Determination of Spatiotemporal Gait Parameters Using a Smartphone’s IMU in the Pocket: Threshold-Based and Deep Learning Approaches. Sensors. 2025; 25(14):4395. https://doi.org/10.3390/s25144395
Chicago/Turabian StyleLee, Seunghee, Changeon Park, Eunho Ha, Jiseon Hong, Sung Hoon Kim, and Youngho Kim. 2025. "Determination of Spatiotemporal Gait Parameters Using a Smartphone’s IMU in the Pocket: Threshold-Based and Deep Learning Approaches" Sensors 25, no. 14: 4395. https://doi.org/10.3390/s25144395
APA StyleLee, S., Park, C., Ha, E., Hong, J., Kim, S. H., & Kim, Y. (2025). Determination of Spatiotemporal Gait Parameters Using a Smartphone’s IMU in the Pocket: Threshold-Based and Deep Learning Approaches. Sensors, 25(14), 4395. https://doi.org/10.3390/s25144395






