Does tDCS Enhance Complex Motor Skill Acquisition? Evidence from a Golf-Putting Task
Abstract
Highlights
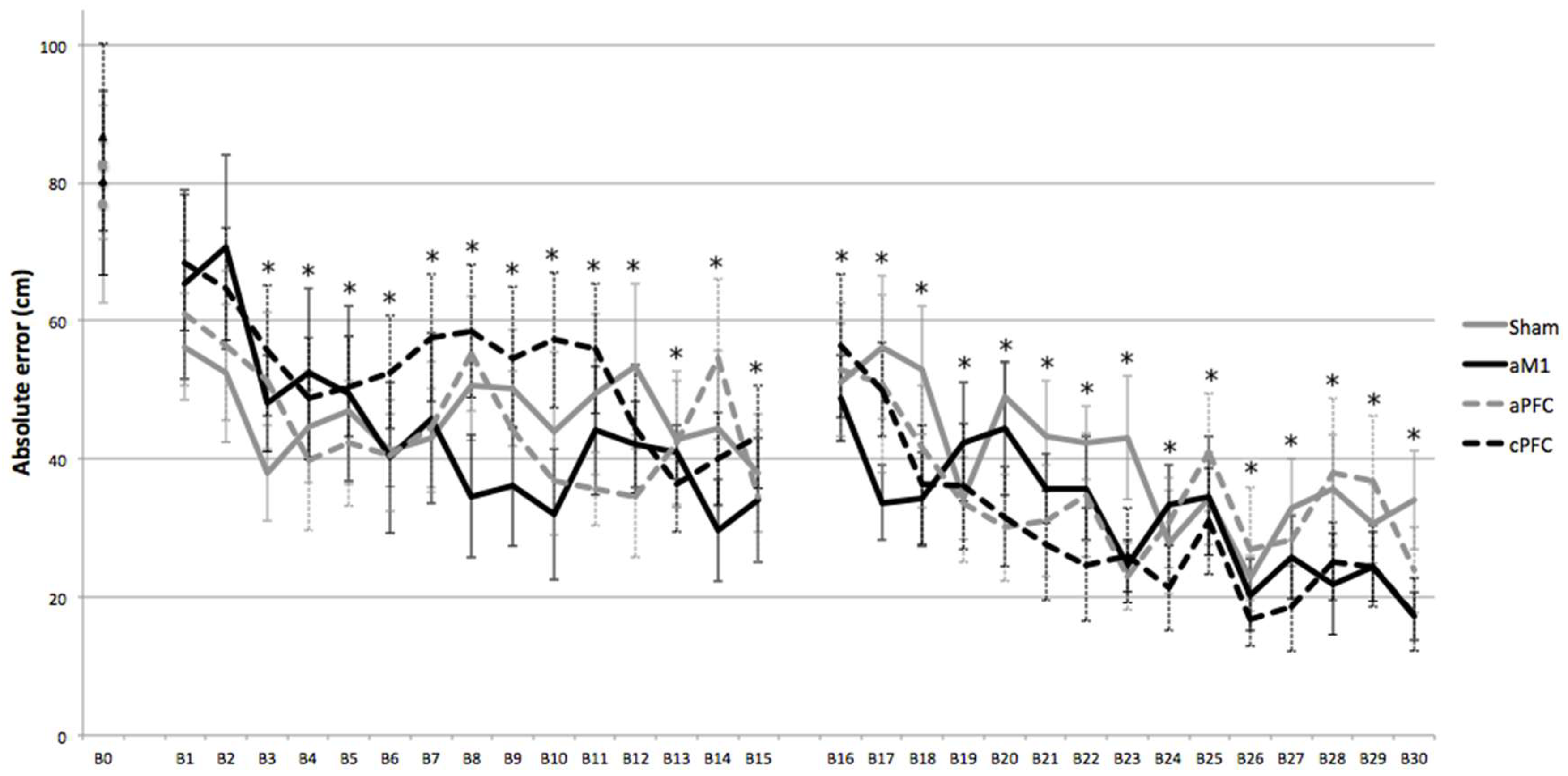
- Repeated practice of a complex motor skill (golf-putting) led to significant performance improvements in novice individuals, regardless of the transcranial direct current stimulation (tDCS) condition.
- tDCS over the motor and prefrontal cortex did not enhance performance in the learning of a complex motor skill (golf-putting) among novice individuals.
- These findings suggest that motor practice alone can drive learning of complex motor skills in novices, and the role of tDCS may depend on task complexity and individual variability.
- This study contributes to a better understanding of how non-invasive brain stimulation interacts with full-body motor tasks, offering valuable insights for future sports neuroscience research.
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Procedure
2.3. Golf-Putting Task
2.4. tDCS
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| tDCS | Transcranial direct current stimulation |
| M1 | Motor cortex |
| PFC | Prefrontal cortex |
| TMS | Transcranial magnetic stimulation |
| aM1 | Anodal M1 |
| eb0 | Baseline absolute error |
References
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000, 527, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Priori, A.; Berardelli, A.; Rona, S.; Accornero, N.; Manfredi, M. Polarization of the human motor cortex through the scalp. Neuroreport 1998, 9, 2257–2260. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Bamugaddam, A.; Alasheikh, M.; Alhassan, T.; Alhaidar, S.; Almutairi, A.K.; Alfaleh, M.; Al-Regaiey, K.; Al Zahrani, S.S.; Albaiji, B.A.; et al. Anodal transcranial direct current stimulation (tDCS) over the primary motor cortex (M1) enhances motor response inhibition and visual recognition memory. Med. Sci. Monit. Basic Res. 2022, 28, e934180. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, S.; Koeda, M.; Ikeda, Y.; Hama, T.; Funayama, T.; Akiyama, T.; Arakawa, R.; Tateno, A.; Suzuki, H.; Okubo, Y. Effects of anodal transcranial direct current stimulation on implicit motor learning and language-related brain function: An fMRI study. Psychiatry Clin. Neurosci. 2021, 75, 200–207. [Google Scholar] [CrossRef]
- Summers, J.J.; Kang, N.; Cauraugh, J.H. Does transcranial direct current stimulation enhance cognitive and motor functions in the ageing brain? A systematic review and meta- analysis. Ageing Res. Rev. 2016, 25, 42–54. [Google Scholar] [CrossRef]
- Yamamoto, S.; Ishii, D.; Ishibashi, K.; Kohno, Y. Transcranial direct current stimulation of the dorsolateral prefrontal cortex modulates cognitive function related to motor execution during sequential task: A randomized control study. Front. Hum. Neurosci. 2022, 16, 890963. [Google Scholar] [CrossRef]
- Angius, L.; Mauger, A.R.; Hopker, J.; Pascual-Leone, A.; Santarnecchi, E.; Marcora, S.M. Bilateral extracephalic transcranial direct current stimulation improves endurance performance in healthy individuals. Brain Stimul. 2018, 11, 108–117. [Google Scholar] [CrossRef]
- Banissy, M.J.; Muggleton, N.G. Transcranial direct current stimulation in sports training: Potential approaches. Front. Hum. Neurosci. 2013, 7, 129. [Google Scholar] [CrossRef]
- Grosprêtre, S.; Grandperrin, Y.; Nicolier, M.; Gimenez, P.; Vidal, C.; Tio, G.; Haffen, E.; Bennabi, D. Effect of transcranial direct current stimulation on the psychomotor, cognitive, and motor performances of power athletes. Sci. Rep. 2021, 11, 9731. [Google Scholar] [CrossRef]
- Nielsen, J.B.; Cohen, L.G. The Olympic brain. Does corticospinal plasticity play a role in acquisition of skills required for high-performance sports? J. Physiol. 2008, 586, 65–70. [Google Scholar] [CrossRef]
- Cogiamanian, F.; Marceglia, S.; Ardolino, G.; Barbieri, S.; Priori, A. Improved isometric force endurance after transcranial direct current stimulation over the human motor cortical areas. Eur. J. Neurosci. 2007, 26, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, R.; Okano, A.; Gurgel, J.; Porto, F.; Cunha, F.; Massaferri, R.; Farinatti, P. Motor cortex tDCS does not improve strength performance in healthy subjects. Mot. Rev. De Educ. Física 2015, 21, 185–193. [Google Scholar] [CrossRef]
- Tanaka, S.; Sandrini, M.; Cohen, L.G. Modulation of motor learning and memory formation by non-invasive cortical stimulation of the primary motor cortex. Neuropsychol. Rehabil. 2011, 21, 650–675. [Google Scholar] [CrossRef] [PubMed]
- Alix-Fages, C.; García-Ramos, A.; Calderón-Nadal, G.; Colomer-Poveda, D.; Romero-Arenas, S.; Fernández-del-Olmo, M.; Márquez, G. Anodal transcranial direct current stimulation enhances strength training volume but not the force–velocity profile. Eur. J. Appl. Physiol. 2020, 120, 1881–1891. [Google Scholar] [CrossRef]
- Garner, C.T.; Dykstra, R.M.; Hanson, N.J.; Miller, M.G. Transcranial direct current stimulation with the halo sport does not improve performance on a three-minute, high intensity cycling test. Int. J. Exerc. Sci. 2021, 14, 962–970. [Google Scholar] [CrossRef]
- Huang, L.; Deng, Y.; Zheng, X.; Liu, Y. Transcranial direct current stimulation with halo sport enhances repeated sprint cycling and cognitive performance. Front. Physiol. 2019, 10, 118. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Schauenburg, A.; Lang, N.; Liebetanz, D.; Exner, C.; Paulus, W.; Tergau, F. Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. J. Cogn. Neurosci. 2003, 15, 619–626. [Google Scholar] [CrossRef]
- Antal, A.; Nitsche, M.A.; Kincses, T.Z.; Kruse, W.; Hoffmann, K.P.; Paulus, W. Facilitation of visuo-motor learning by transcranial direct current stimulation of the motor and extrastriate visual areas in humans. Eur. J. Neurosci. 2004, 19, 2888–2892. [Google Scholar] [CrossRef]
- Reis, J.; Schambra, H.M.; Cohen, L.G.; Buch, E.R.; Fritsch, B.; Zarahn, E.; Krakauer, J.W. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc. Natl. Acad. Sci. USA 2009, 106, 1590–1595. [Google Scholar] [CrossRef]
- Krakauer, J.W.; Mazzoni, P. Human sensorimotor learning: Adaptation, skill, and beyond. Curr. Opin. Neurobiol. 2011, 21, 636–644. [Google Scholar] [CrossRef]
- Reis, J.; Robertson, E.; Krakauer, J.W.; Rothwell, J.; Marshall, L.; Gerloff, C.; Cohen, L.G. Consensus: “Can tDCS and TMS enhance motor learning and memory formation?”. Brain Stimul. 2008, 1, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.F.; Yeung, A.Y.; Poolton, J.M.; Lee, T.M.; Leung, G.K.; Masters, R.S. Cathodal Transcranial Direct Current Stimulation Over Left Dorsolateral Prefrontal Cortex Area Promotes Implicit Motor Learning in a Golf Putting Task. Brain Stimul. 2015, 8, 784–786. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.J.; Wilson, M.R.; Buckingham, G.; Vine, S.J. No effect of transcranial direct current stimulation of frontal, motor or visual cortex on performance of a self-paced visuomotor skill. Psychol. Sport. Exerc. 2019, 43, 368–373. [Google Scholar] [CrossRef]
- Parma, J.O.; Profeta, V.L.D.S.; Andrade, A.G.P.D.; Lage, G.M.; Apolinário-Souza, T. TDCS of the primary motor cortex: Learning the absolute dimension of a complex motor task. J. Mot. Behav. 2020, 53, 431–444. [Google Scholar] [CrossRef]
- Mizuguchi, N.; Katayama, T.; Kanosue, K. The effect of cerebellar transcranial direct current stimulation on a throwing task depends on individual level of task performance. Neuroscience 2018, 371, 119–125. [Google Scholar] [CrossRef]
- Suzuki, K.; Suzuki, T.; Ono, Y. Effect of middletemporal tDCS stimulation on dance-game exercise performance. Trans. Jpn. Soc. Med. Biol. Eng. 2017, 55, 503–505. [Google Scholar]
- Moreira, A.; Moscaleski, L.; Machado, D.G.D.S.; Bikson, M.; Unal, G.; Bradley, P.S.; Cevada, T.; da Silva, F.T.G.; Baptista, A.F.; Morya, E.; et al. Transcranial direct current stimulation during a prolonged cognitive task: The effect on cognitive and shooting performances in professional female basketball players. Ergonomics 2023, 66, 492–505. [Google Scholar] [CrossRef]
- Molero-Chamizo, A.; Alameda Bailén, J.R.; Garrido Béjar, T.; García López, M.; Jaén Rodríguez, I.; Gutiérrez Lérida, C.; Rivera-Urbina, G.N. Poststimulation time interval-dependent effects of motor cortex anodal tDCS on reaction-time task performance. Cogn. Affect. Behav. Neurosci. 2018, 18, 167–175. [Google Scholar] [CrossRef]
- Horvath, J.C.; Carter, O.; Forte, J.D. No significant effect of transcranial direct current stimulation (tDCS) found on simple motor reaction time comparing 15 different simulation protocols. Neuropsychologia 2016, 91, 544–552. [Google Scholar] [CrossRef]
- Vergallito, A.; Feroldi, S.; Pisoni, A.; Romero Lauro, L.J. Inter-Individual Variability in tDCS Effects: A Narrative Review on the Contribution of Stable, Variable, and Contextual Factors. Brain Sci. 2022, 12, 522. [Google Scholar] [CrossRef]
- Weightman, M.; Brittain, J.S.; Hall, A.; Miall, C.; Jenkinson, N. Timing is everything: Event-related transcranial direct current stimulation improves motor adaptation. Brain Stimul. 2022, 15, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Ericsson, K.A.; Lehmann, A.C. Expert and exceptional performance: Evidence of maximal adaptation to task constraints. Annu. Rev. Psychol. 1996, 47, 273–305. [Google Scholar] [CrossRef] [PubMed]
- Dayan, E.; Cohen, L.G. Neuroplasticity subserving motor skill learning. Neuron 2011, 72, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Doyon, J.; Benali, H. Reorganization and plasticity in the adult brain during learning of motor skills. Curr. Opin. Neurobiol. 2005, 15, 161–167. [Google Scholar] [CrossRef]
- Kim, T.; Kim, H.; Wright, D.L. Improving consolidation by applying anodal transcranial direct current stimulation at primary motor cortex during repetitive practice. Neurobiol. Learn. Mem. 2021, 178, 107365. [Google Scholar] [CrossRef]
- Iezzi, E.; Suppa, A.; Conte, A.; Agostino, R.; Nardella, A.; Berardelli, A. Theta-burst stimulation over primary motor cortex degrades early motor learning. Eur. J. Neurosci. 2010, 31, 585–592. [Google Scholar] [CrossRef]
- Karni, A.; Meyer, G.; Jezzard, P.; Adams, M.M.; Turner, R.; Ungerleider, L.G. Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature 1995, 377, 155–158. [Google Scholar] [CrossRef]
- Marinelli, L.; Quartarone, A.; Hallett, M.; Frazzitta, G.; Ghilardi, M.F. The many facets of motor learning and their relevance for Parkinson’s disease. Clin. Neurophysiol. 2017, 128, 1127–1141. [Google Scholar] [CrossRef]
- Rivera-Urbina, G.N.; Molero-Chamizo, A.; Nitsche, M.A. Discernible effects of tDCS over the primary motor and posterior parietal cortex on different stages of motor learning. Brain Struct. Funct. 2022, 227, 1115–1131. [Google Scholar] [CrossRef]
- Hamzei, F.; Ritter, A.; Güllmar, D. Implicit Motor Learning Under Anodal or Cathodal tDCS During fMRI Induces Partially Distinct Network Responses. Eur. J. Neurosci. 2025, 61, e70053. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Luft, C.D.B.; Zioga, I.; Banissy, M.J.; Bhattacharya, J. Relaxing learned constraints through cathodal tDCS on the left dorsolateral prefrontal cortex. Sci. Rep. 2017, 7, 2916. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kaminski, E.; Hoff, M.; Sehm, B.; Taubert, M.; Conde, V.; Steele, C.J.; Ragert, P. Effect of transcranial direct current stimulation (tDCS) during complex whole body motor skill learning. Neurosci. Lett. 2013, 552, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Ungerleider, L.G.; Doyon, J.; Karni, A. Imaging brain plasticity during motor skill learning. Neurobiol. Learn. Mem. 2002, 78, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Hashemirad, F.; Fitzgerald, P.B.; Zoghi, M.; Jaberzadeh, S. Single-Session Anodal tDCS with Small-Size Stimulating Electrodes Over Frontoparietal Superficial Sites Does Not Affect Motor Sequence Learning. Front. Hum. Neurosci. 2017, 11, 153. [Google Scholar] [CrossRef]
- Minarik, T.; Sauseng, P.; Dunne, L.; Berger, B.; Sterr, A. Effects of anodal transcranial direct current stimulation on visually guided learning of grip force control. Biology 2015, 4, 173–186. [Google Scholar] [CrossRef]
- Guerra, A.; Lopez-Alonso, V.; Cheeran, B.; Suppa, A. Solutions for managing variability in non-invasive brain stimulation studies. Neurosci. Lett. 2017, 719, 133332. [Google Scholar] [CrossRef]
- Guerra, A.; Lopez-Alonso, V.; Cheeran, B.; Suppa, A. Variability in non-invasive brain stimulation studies: Reasons and results. Neurosci. Lett. 2017, 719, 133330. [Google Scholar] [CrossRef]
- Lopez-Alonso, V.; Cheeran, B.; Rio-Rodriguez, D.; Fernandez-Del-Olmo, M. Inter-individual Variability in Response to Non-invasive Brain Stimulation Paradigms. Brain Stimul. 2014, 7, 372–380. [Google Scholar] [CrossRef]
- Wiethoff, S.; Hamada, M.; Rothwell, J.C. Variability in Response to Transcranial Direct Current Stimulation of the Motor Cortex. Brain Stimul. 2014, 7, 468–475. [Google Scholar] [CrossRef]
- Li Voti, P.; Conte, A.; Suppa, A.; Iezzi, E.; Bologna, M.; Aniello, M.S.; Berardelli, A. Correlation between cortical plasticity, motor learning and BDNF genotype in healthy subjects. Exp. Brain Res. 2011, 212, 91–99. [Google Scholar] [CrossRef]
- Lopez-Alonso, V.; Cheeran, B.; Fernandez-del-Olmo, M. Relationship Between Non-invasive Brain Stimulation-induced Plasticity and Capacity for Motor Learning. Brain Stimul. 2015, 8, 1209–1219. [Google Scholar] [CrossRef] [PubMed]
- Bortoletto, M.; Pellicciari, M.C.; Rodella, C.; Miniussi, C. The interaction with task-induced activity is more important than polarization: A tDCS study. Brain Stimul. 2015, 8, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Saucedo Marquez, C.M.; Zhang, X.; Swinnen, S.P.; Meesen, R.; Wenderoth, N. Task-specific effect of transcranial direct current stimulation on motor learning. Front. Hum. Neurosci. 2013, 7, 333. [Google Scholar] [CrossRef] [PubMed]
- Batsikadze, G.; Moliadze, V.; Paulus, W.; Kuo, M.F.; Nitsche, M.A. Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. J. Physiol. 2013, 591, 1987–2000. [Google Scholar] [CrossRef]
- Hardwick, R.M.; Rottschy, C.; Miall, R.C.; Eickhoff, S.B. A quantitative meta-analysis and review of motor learning in the human brain. Neuroimage 2013, 67, 283–297. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Doemkes, S.; Karakose, T.; Antal, A.; Liebetanz, D.; Lang, N.; Paulus, W. Shaping the effects of transcranial direct current stimulation of the human motor cortex. J. Neurophysiol. 2007, 97, 3109–3117. [Google Scholar] [CrossRef]
- Jancke, L.; Koeneke, S.; Hoppe, A.; Rominger, C.; Hanggi, J. The architecture of the golfer’s brain. PLoS ONE 2009, 4, e4785. [Google Scholar] [CrossRef]
- Kearney, P. A distal focus of attention leads to superior performance on a golf putting task. Int. J. Sport. Exerc. Psychol. 2015, 13, 104–120. [Google Scholar] [CrossRef]
- Wulf, G.; Lauterbach, B.; Toole, T. The learning advantages of an external focus of attention in golf. Res. Q. Exerc. Sport. 1999, 70, 120–126. [Google Scholar] [CrossRef]
- Ishikura, T. Reduced relative frequency of knowledge of results without visual feedback in learning a golf-putting task. Percept. Mot. Skills 2008, 106, 225–233. [Google Scholar] [CrossRef]
- Keogh, J.W.; Hume, P.A. Evidence for biomechanics and motor learnig research improving golf performance. Sports Biomech. 2012, 11, 288–309. [Google Scholar] [CrossRef] [PubMed]
- Milton, J.; Solodkin, A.; Hlustik, P.; Small, S.L. The mind of expert motor performance is cool and focused. Neuroimage 2007, 35, 804–813. [Google Scholar] [CrossRef] [PubMed]
- Munzert, J.; Maurer, H.; Reiser, M. Verbal-motor attention-focusing instructions influence kinematics and performance on a golf-putting task. J. Mot. Behav. 2014, 46, 309–318. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez-Alonso, V.; López-Bermúdez, G.; Pagaduan, J.C.; Sánchez-Molina, J.A. Does tDCS Enhance Complex Motor Skill Acquisition? Evidence from a Golf-Putting Task. Sensors 2025, 25, 4297. https://doi.org/10.3390/s25144297
Lopez-Alonso V, López-Bermúdez G, Pagaduan JC, Sánchez-Molina JA. Does tDCS Enhance Complex Motor Skill Acquisition? Evidence from a Golf-Putting Task. Sensors. 2025; 25(14):4297. https://doi.org/10.3390/s25144297
Chicago/Turabian StyleLopez-Alonso, Virginia, Gabriel López-Bermúdez, Jeffrey Cayaban Pagaduan, and Jose Andrés Sánchez-Molina. 2025. "Does tDCS Enhance Complex Motor Skill Acquisition? Evidence from a Golf-Putting Task" Sensors 25, no. 14: 4297. https://doi.org/10.3390/s25144297
APA StyleLopez-Alonso, V., López-Bermúdez, G., Pagaduan, J. C., & Sánchez-Molina, J. A. (2025). Does tDCS Enhance Complex Motor Skill Acquisition? Evidence from a Golf-Putting Task. Sensors, 25(14), 4297. https://doi.org/10.3390/s25144297





