Research on the Impact of Shot Selection on Neuromuscular Control Strategies During Basketball Shooting
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Subjects
2.2. Experimental Instruments
2.2.1. Three-Dimensional Infrared Motion Capture System
2.2.2. Three-Dimensional Force Platform
2.2.3. Wireless Surface Electromyography (sEMG) Collection System
2.3. Experimental Procedure
2.4. Movement Phases
2.5. Data Processing
2.5.1. Spinal Segment Motor Output
2.5.2. Muscle Synergy Extraction
2.6. Statistical Analysis
3. Results
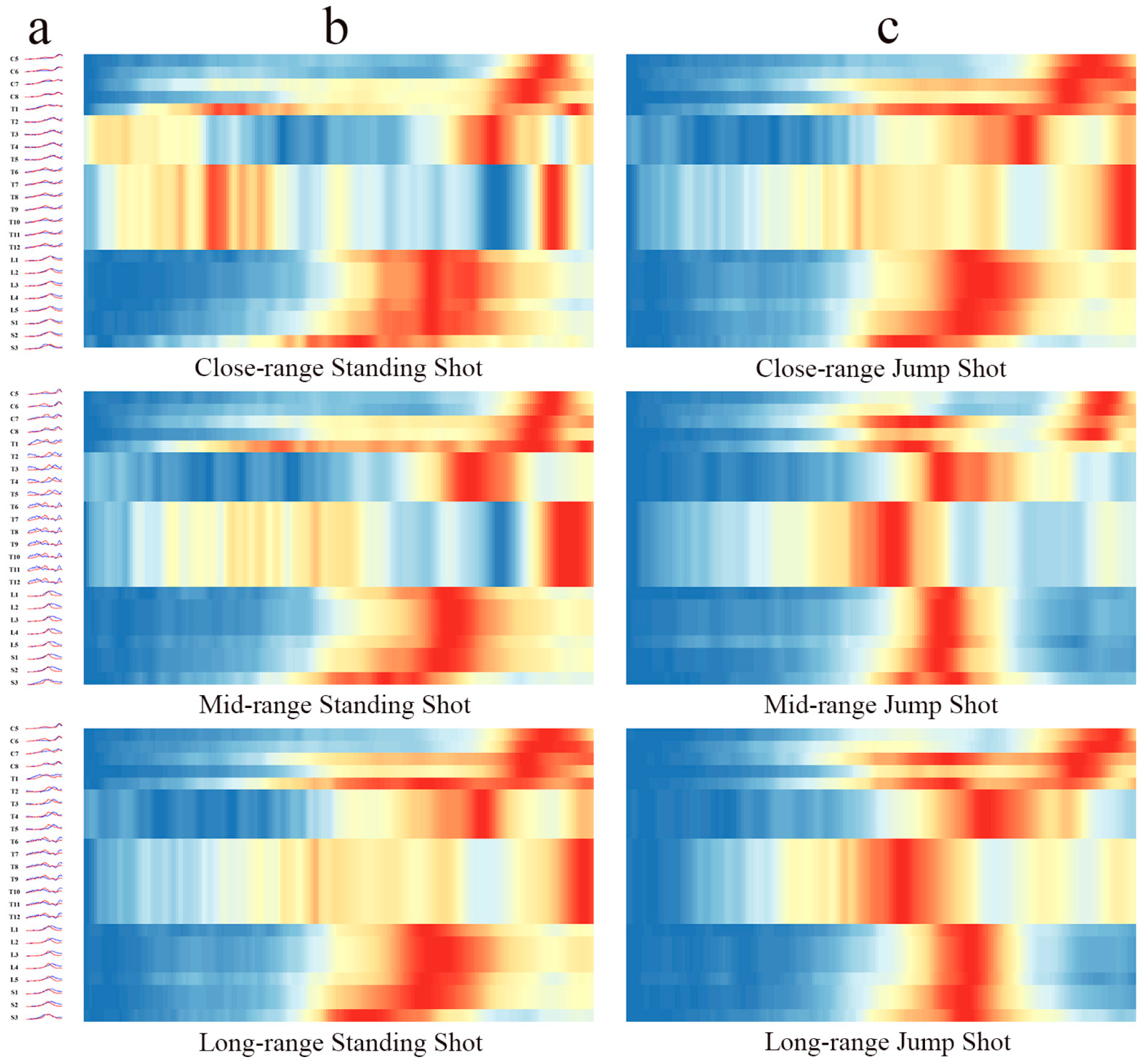
3.1. Spinal Segment Motor Output Characteristics
3.2. Muscle Synergy Characteristics
4. Discussion
- (1)
- Differences in spinal output amplitudes across phases: Previous studies have focused on the number and spatial distribution of muscle synergies (e.g., d’Avella et al., 2003) [11], without linking these observations to spinal-level regulatory mechanisms. Our findings reveal distinct neural control demands for standing versus jump shots: standing shots require early postural stabilization, resulting in greater activation of spinal segments C5–S3 during the TP phase compared to jump shots, whereas jump shots emphasize explosive power, exhibiting significantly higher activation amplitudes in later segments (e.g., L3–S3; p < 0.01), thus demonstrating different force-generation patterns. This conclusion aligns with the spinal–muscle dynamic coupling model proposed by Pan et al. (2023) [18], which suggests that movement strategies optimize intermuscular coordination by modulating activation patterns encoded at the spinal level.
- (2)
- Shot distance adaptability: As shooting distance increases, the proportion of combined synergies decreases significantly, consistent with the movement efficiency optimization hypothesis proposed by Santuz et al. (2017) [21]. In long-distance shooting, athletes enhance movement accuracy and energy utilization efficiency by reducing reliance on redundant synergy modules. This effect is particularly pronounced in jump shots and parallels findings by Zhang et al. (2021) in archery [25].
- (3)
- Functional differentiation of synergy modules: Unlike Li et al. (2019) [2], who focused on upper-limb joint kinematics, this study uncovers significant functional differences in muscle synergy modules between standing and jump shots. For example, Synergy 1 during the TP phase exhibited widespread lower-limb activation, aligning with Konno et al. (2020) [6], both emphasizing lower-limb extension as the critical force-generation component in shooting. In contrast, Synergy 2 in standing shots (TP→RP transition) was dominated by lower-limb muscle activation, whereas jump shots at the same stage relied more heavily on core muscles (e.g., rectus abdominis and erector spinae). This divergence arises from distinct mechanical demands: standing shots depend on sustained lower-limb support for balance and stability, whereas jump shots require rapid core muscle contraction to facilitate smooth lift-off and force transmission. These findings also corroborate Bizzi et al.’s (2005) theory that muscle synergies adapt dynamically to varying task demands [13].
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
| TLA | Three-letter acronym |
| LD | Linear dichroism |
References
- Yang, Z.; Mi, J.; Liu, H. Research Progress of Motion Characteristics of Basketball Shooting. China Sport Sci. 2016, 36, 79–90. [Google Scholar]
- Li, N.; Ma, X.; Zhou, Y. Research Status and Comments on Technical Characteristics of Single-Handed Shoulder Shooting. J. Guangzhou Sport Univ. 2019, 39, 94–100. [Google Scholar]
- Fan, P.; Yang, Z.; Wang, T.; Li, J.; Kim, Y.; Kim, S. Neuromuscular Control Strategies in Basketball Shooting: Distance-Dependent Analysis of Muscle Synergies. J. Sports Sci. Med. 2024, 23, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Bennett, H.J.; Taylor, J.; Valenzuela, K.A.; Haegele, J.A. Coordination variability during running in adolescents with autism spectrum disorder. Autism Int. J. Res. Pract. 2021, 26, 1201–1215. [Google Scholar] [CrossRef] [PubMed]
- Botsi, V.; Bourdas, D.I.; Travlos, A.K.; Bakirtzoglou, P.; Gofas, D.C.; Ktistakis, I.E.; Zacharakis, E. Comparative Analysis of 2-Point Jump Shot and Free Throw Kinematics in High- and Low-Level U18 Male Basketball Players. J. Funct. Morphol. Kinesiol. 2024, 9, 278. [Google Scholar] [CrossRef]
- Konno, K.; Itaya, A.; Kizuka, T.; Ono, S. The effects of pressure on anticipatory postural adjustments during a jump shot in basketball. Gait Posture 2024, 114, 215–221. [Google Scholar] [CrossRef]
- Matsunaga, N.; Oshikawa, T. Muscle synergy during free throw shooting in basketball is different between scored and missed shots. Front. Sports Act. Living 2022, 4, 990925. [Google Scholar] [CrossRef]
- Okubo, H.; Hubbard, M. Kinematic Differences between Set- and Jump-Shot Motions in Basketball. Proceedings 2018, 2, 201. [Google Scholar] [CrossRef]
- Bezodis, N.E.; Willwacher, S.; Salo, A.I.T. The Biomechanics of the Track and Field Sprint Start: A Narrative Review. Sports Med. 2019, 49, 1345–1364. [Google Scholar] [CrossRef]
- Bai, X.; Zhu, Y.; Huo, H. Effects of Exercise-induced Fatigue on Synergy of Foot and Ankle Muscles during Running. Chin. J. Sports Med. 2023, 42, 191–200. [Google Scholar]
- d’Avella, A.; Saltiel, P.; Bizzi, E. Combinations of muscle synergies in the construction of a natural motor behavior. Nat. Neurosci. 2003, 6, 300–308. [Google Scholar] [CrossRef]
- Bizzi, E.; Cheung, V.C.K. The Neural Origin of Muscle Synergies. Front. Comput. Neurosci. 2013, 7, 51. [Google Scholar] [CrossRef]
- d’Avella, A.; Bizzi, E. Shared and specific muscle synergies in natural motor behaviors. Proc. Natl. Acad. Sci. USA 2005, 102, 3076–3081. [Google Scholar] [CrossRef]
- Cui, C.; Miu, H.; Liang, T.; Liu, X.; Liu, X. Analysis of muscle synergy and muscle functional network at different walking speeds based on surface electromyographic signal. J. Biomed. Eng. 2023, 40, 938–944. [Google Scholar]
- Pan, Z.; Liu, L.; Li, X.; Ma, Y. The Influence of Experience on Neuromuscular Control of the Body When Cutting at Different Angles. J. Mot. Behav. 2023, 55, 11–12. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Liu, L.; Sun, Y.; Su, R.; Ma, Y. Research on Effect of Motor Experience on Neuromuscular Control Strategies during Sprint Starts. China Sport Sci. Technol. 2024, 60, 3–10. [Google Scholar]
- Matsunaga, N.; Kaneoka, K. Comparison of Modular Control during Smash Shot between Advanced and Beginner Badminton Players. Appl. Bionics Biomech. 2018, 2018, 6592357. [Google Scholar] [CrossRef]
- Pan, Z.; Liu, L.; Li, X.; Ma, Y. Characteristics of muscle synergy and anticipatory synergy adjustments strategy when cutting in different angles. Gait Posture 2023, 107, 114–120. [Google Scholar] [CrossRef]
- Lee, D.D.; Seung, H.S. Learning the parts of objects by non-negative matrix factorization. Nature 1999, 401, 788–791. [Google Scholar] [CrossRef]
- Cappellini, G.; Ivanenko, Y.P.; Martino, G.; MacLellan, M.J.; Sacco, A.; Morelli, D.; Lacquaniti, F. Immature spinal locomotor output in children with Cerebral Palsy. Front. Physiol. 2016, 7, 478. [Google Scholar] [CrossRef]
- Santuz, A.; Ekizos, A.; Janshen, L.; Baltzopoulos, V.; Arampatzis, A. The Influence of Footwear on the Modular Organization of Running. Front. Physiol. 2017, 8, 958. [Google Scholar] [CrossRef] [PubMed]
- Debra, C. Muscles: Testing and Function with Posture and Pain, ed 5 (with Primal Anatomy CD-ROM). Phys. Ther. 2006, 86, 304–305. [Google Scholar]
- La Scaleia, V.; Ivanenko, Y.P.; Zelik, K.E.; Lacquaniti, F. Spinal motor outputs during step-to-step transitions of diverse human gaits. Front. Hum. Neurosci. 2014, 8, 305. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Peng, Y.; Hao, Z.; Liu, Y.; Wang, X.; Wang, J. Research Progress and Prospect of Muscle Synergies Theory for Redundancy Control of Complex Human Movement. China Sport Sci. 2020, 40, 63–72. [Google Scholar]
- Zhang, B.; Xu, C.; Zhou, X.; Luo, D. Application of Muscle Synergy Hypothesis in Sport Skill Analysis—An Example of Archery. China Sport Sci. 2021, 41, 70–78. [Google Scholar]
- Hou, X.; Peng, C. Robust Nonnegative Matrix Factorization with Local Similarity Learning. J. Data Acquis. Process. 2023, 38, 1125–1141. [Google Scholar]
- Liu, W.; Deng, X.; Liu, D.; Liu, Y. Block Sparse Symmetric Nonnegative Matrix Factorization Based on Constrained Graph Regularization. Comput. Sci. 2023, 50, 89–97. [Google Scholar]



| Research Sources | Brief Research Content | Brief Research Findings |
|---|---|---|
| Fan et al. (2024) [3] | Differentiating changes in lower-limb synergy patterns between near- and far-distance shots using muscle synergies. | Shooting distance does not change the number of muscle synergies; however, it alters their activation timing and muscle involvement patterns. |
| Botsi et al. (2024) [5] | Comparing shot entry angle (EA), release time (RT), and shooting accuracy. | Jump shots exhibit larger entry angles (closer to the ideal 45°), whereas standing shots achieve higher accuracy. |
| Konno et al. (2024) [6] | Analyzing anticipatory postural adjustments. | Defensive perturbations disrupt anticipatory postural control mechanisms prior to jump shots, revealing the dynamic control system’s sensitivity in response. |
| Matsunaga et al. (2022) [7] | Analyzing differences in muscle synergy patterns between successful and unsuccessful shots. | Successful shots involve four muscle synergy modules, whereas missed shots involve only three. |
| Okubo et al. (2018) [8] | Analyzing the influence of vertical shoulder velocity and acceleration on ball release. | Jump shots require greater vertical fingertip acceleration to generate backspin; shoulder elevation can compensate for this additional demand. |
| Movement Phase | Definition | Biomechanical Markers |
|---|---|---|
| Setup Phase, SP | Pre-shot ball-holding phase | Between ball acquisition and the onset of center-of-mass descent |
| Transformation Phase, TP | Descent of the body’s center of mass [6] | The initial decrease in shoulder marker height and the instant when vertical ground reaction force exceeded 15 N [6] |
| Rhythmical Phase, RP | The process of simultaneous lower-limb extension and upper-limb ball propulsion | The onset of the propulsive phase is defined as the moment when the flexion angle begins to increase. Furthermore, a vertical ground reaction force below 15 N marks the onset of the jump shot’s propulsive phase [6] |
| Ball Release Phase, BP | Release of the basketball from the athlete’s hands | The wrist joint attains its minimum flexion angle for the first time during the propulsive phase |
| Shooting Condition | Total Shots | Successful Shots | Shooting Accuracy | p |
|---|---|---|---|---|
| Close-range Standing Shot | 140 | 117 | 83.6% | 0.229 |
| Close-range Jump Shot | 140 | 108 | 77.1% | |
| Mid-range Standing Shot | 140 | 99 | 70.7% * | 0.020 |
| Mid-range Jump Shot | 140 | 93 | 66.4% | |
| Long-range Standing Shot | 140 | 80 | 57.1% * | <0.001 |
| Long-range Jump Shot | 140 | 61 | 43.6% |
| Close-Range Standing Shot | Close-Range Jump Shot | Mid-Range Standing Shot | Mid-Range Jump Shot | Long-Range Standing Shot | Long-Range Jump Shot | |
|---|---|---|---|---|---|---|
| Minimum Number of Synergies | 4.45 ± 0.58 | 4.82 ± 0.57 | 4.45 ± 0.58 | 4.59 ± 0.65 | 4.45 ± 0.50 | 4.41 ± 0.65 |
| Reconstruction Quality (VAF) | 0.94 ± 0.02 | 0.93 ± 0.02 | 0.94 ± 0.02 | 0.94 ± 0.02 | 0.95 ± 0.01 | 0.95 ± 0.02 |
| Combined Synergy Proportion (%) | 20.4% | 25.4% | 18.3% * | 18.8% * | 12.2% * | 17.7% * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Q.; Wu, S.; Zhang, J.; Pan, Z.; Kang, Z.; Ma, Y. Research on the Impact of Shot Selection on Neuromuscular Control Strategies During Basketball Shooting. Sensors 2025, 25, 4104. https://doi.org/10.3390/s25134104
Zhou Q, Wu S, Zhang J, Pan Z, Kang Z, Ma Y. Research on the Impact of Shot Selection on Neuromuscular Control Strategies During Basketball Shooting. Sensors. 2025; 25(13):4104. https://doi.org/10.3390/s25134104
Chicago/Turabian StyleZhou, Qizhao, Shiguang Wu, Jiashun Zhang, Zhengye Pan, Ziye Kang, and Yunchao Ma. 2025. "Research on the Impact of Shot Selection on Neuromuscular Control Strategies During Basketball Shooting" Sensors 25, no. 13: 4104. https://doi.org/10.3390/s25134104
APA StyleZhou, Q., Wu, S., Zhang, J., Pan, Z., Kang, Z., & Ma, Y. (2025). Research on the Impact of Shot Selection on Neuromuscular Control Strategies During Basketball Shooting. Sensors, 25(13), 4104. https://doi.org/10.3390/s25134104






