Does a SPR-Based Cell-Based Assay Provide Reliable Results on the Toxicity and Efficacy of Antiviral Drugs?
Abstract
Highlights
- A SPR-based cell-based assay provides accurate data on drug cytotoxicity and antiviral efficacy.
- Grating-based SPR requires sequential signal measurements of SPR slides removed from the medium at fixed hours after seeding, which successfully replaces continuous-flow SPR detection.
- Exploiting the key advantage of grating-based SPR—tuning the excitation wavelength of the resonance from the visible to the near-infrared region—to detect cell morphological changes with increased accuracy and sensitivity;
- Speed of detection: The SPR assay detects the precursors of biochemical reactions in cells before 48 h, which are detected by the MTT assay at 96 h.
Abstract
1. Introduction
2. Materials and Methods
2.1. SPR Slides
2.2. SPR Measurement and Setup
2.3. Cells and Viruses
2.4. Drug Preparations
2.5. SPR-Based Cell Assay
2.6. MTT Assay for Cytotoxicity Evaluation
2.7. MTT-Based Antiviral Assays
- Control cells (not infected with virus and not treated with compounds)—0.2 mL of supporting cell culture medium (2% FBS) were added to the wells designated for cell control;
- Virus control (virus-infected and compound-untreated cells)—0.1 mL of supporting cell culture medium with the appropriate virus concentration were added to the wells designated for virus control;
- Cells exposed to the compounds—infected with a virus and treated with different concentrations of the compounds studied—0.1 mL of the dilutions of the substances prepared in advance.
2.8. Cell Coverage Experiment
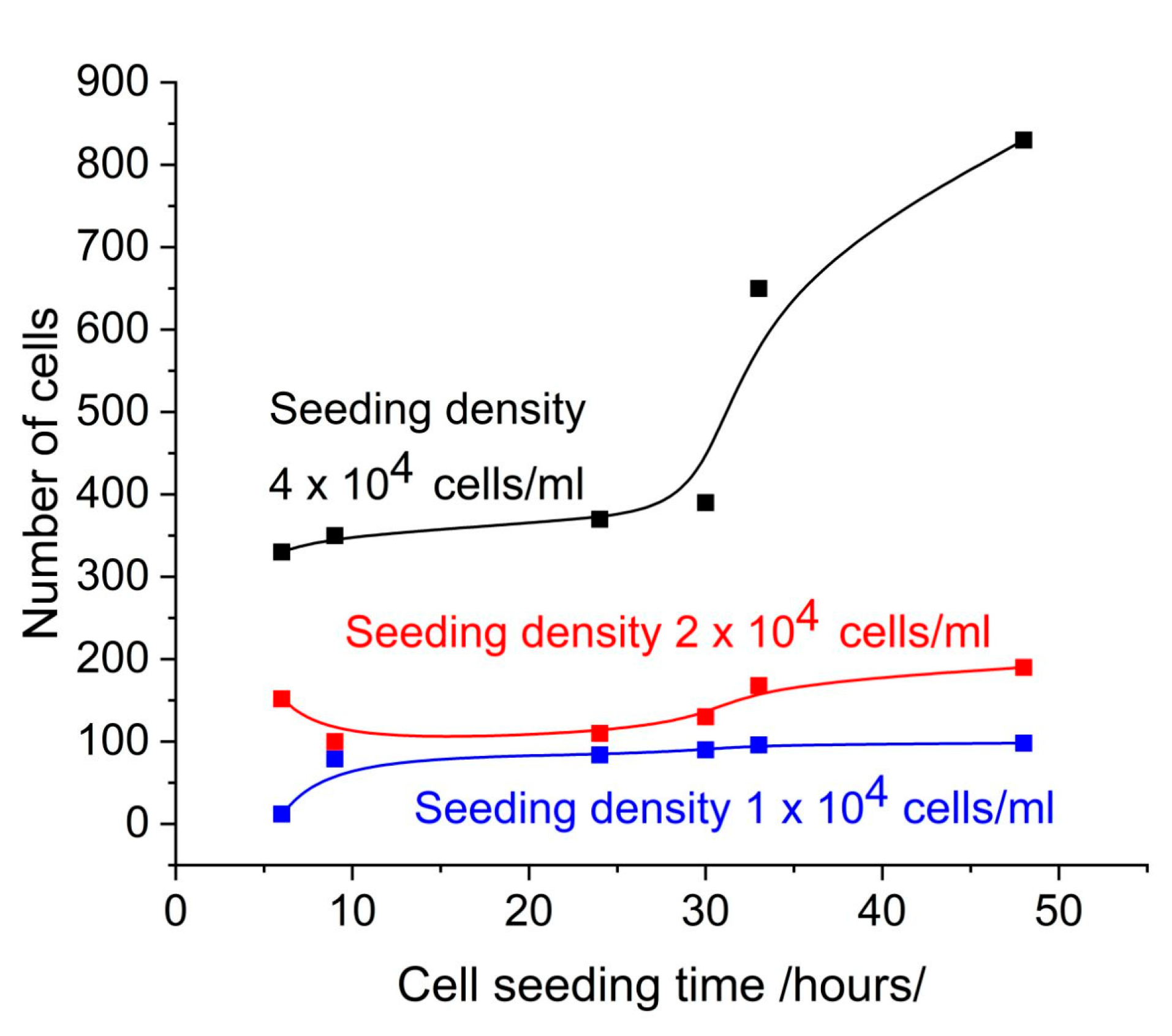
2.9. Microscope, Cell Number and Confluence
3. Results
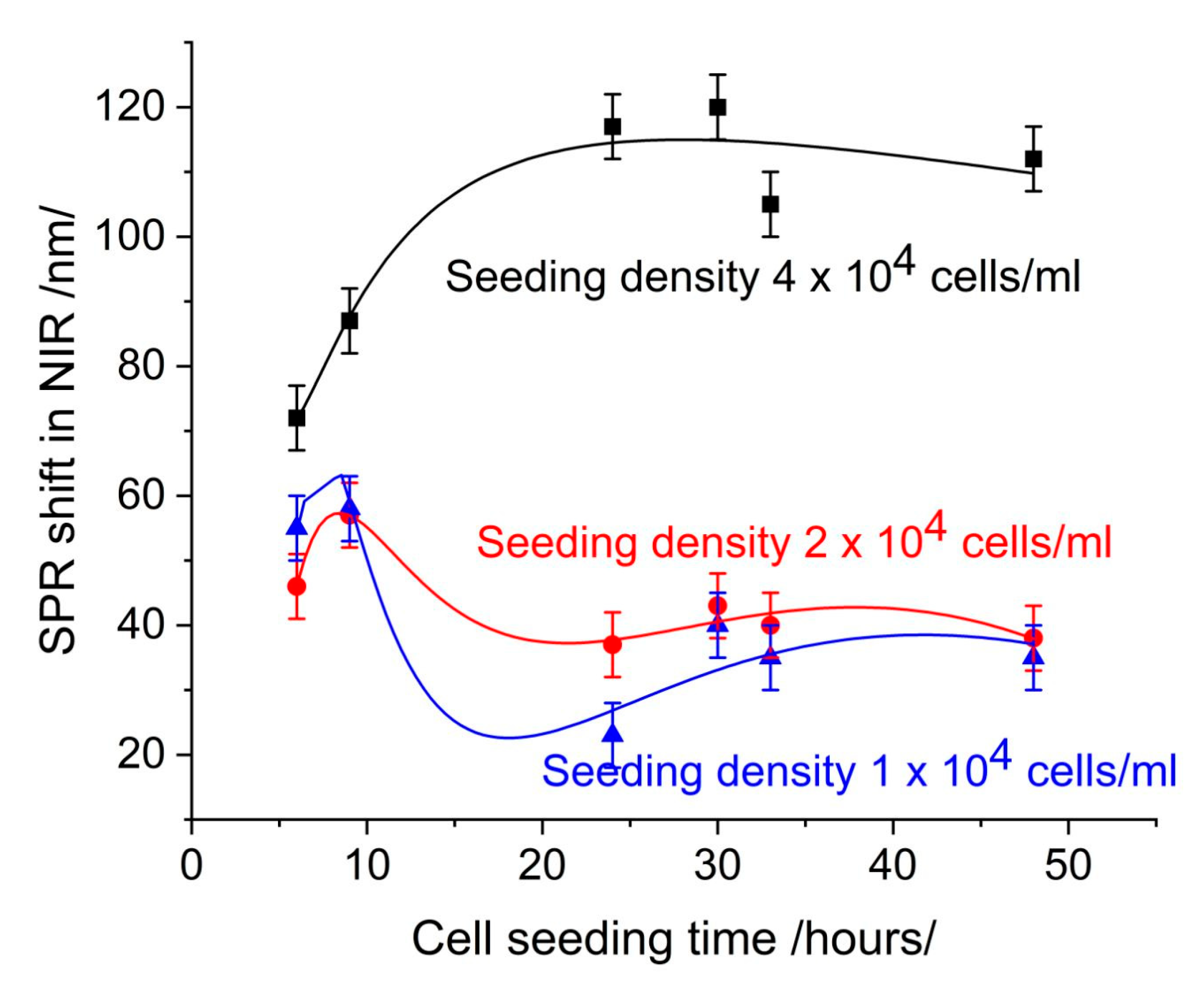
3.1. Cell Control: SPR Signal, Confluence, and Cell Coverage
3.2. SPR-Based Evaluation of Cytotoxicity
3.3. MTT-Based Evaluation of Cytotoxicity
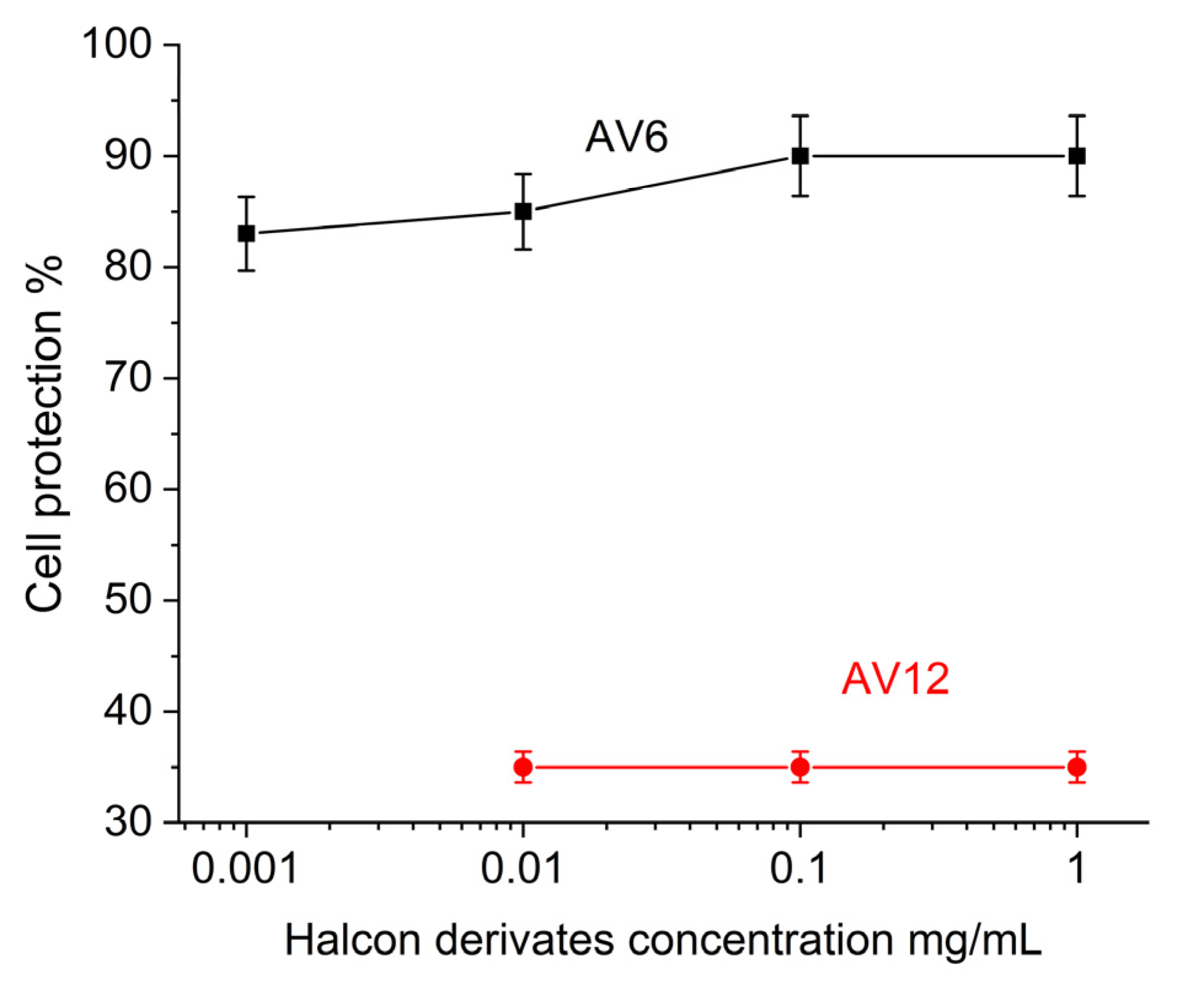
3.4. SPR-Based Evaluation of Inhibitory Effect
3.5. MTT-Based Evaluation of Antviral Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SPR | Surface plasmon resonance |
| MTT | Methyl thiazolyl tetrazolium |
| NCIPD | National Centre of Infectious and Parasitic Diseases |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| FBS | Fetal bovine serum |
| HCoV-229E | Human coronavirus type 229E |
| TCID | Tissue culture infectious dose |
| DMSO | Dimethyl sulfoxide |
| OD | Optical density |
| ELISA | Enzyme-linked immunosorbent assay |
| MNC | Maximum nontoxic concentration |
| CC | Cell control |
| CC50 | Drug concentration that induce visible morphological changes in 50% of the cells |
| AI | Artificial intelligence |
| RMSD | Root mean square deviation |
| VC | Viral control |
References
- Singh, P. SPR biosensors: Historical perspectives and current challenges. Sens. Actuators B Chem. 2016, 229, 110–130. [Google Scholar] [CrossRef]
- Capelli, D.; Scognamiglio, V.; Montanari, R. Surface plasmon resonance technology: Recent advances, applications and experimental cases. TrAC Trends Anal. Chem. 2023, 163, 117079. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, A.; Singh, O.P.; Sharan, P. A review of surface plasmon resonance (SPR) technology in biosensing: Innovations, applications and future trends. J. Opt. 2024, 1–9. [Google Scholar] [CrossRef]
- Méjard, R.; Griesser, H.; Thierry, B. Optical biosensing for label-free cellular studies. TrAC Trends Anal. Chem. 2014, 53, 178–186. [Google Scholar] [CrossRef]
- Abadian, P.N.; Kelley, C.; Goluch, E. Cellular analysis and detection using surface plasmon resonance techniques. Anal. Chem. 2014, 86, 2799–2812. [Google Scholar] [CrossRef]
- Yanase, Y.; Hiragun, T.; Ishii, K.; Kawaguchi, T.; Yanase, T.; Kawai, M.; Sakamoto, K.; Hide, M. Surface Plasmon Resonance for Cell-Based Clinical Diagnosis. Sensors 2014, 14, 4948–4959. [Google Scholar] [CrossRef]
- Hide, M.; Tsutsui, T.; Sato, H.; Nishimura, T.; Morimoto, K.; Yamamoto, S.; Yoshizato, K. Real-Time analysis of ligand-induced cell surface and intracellular reactions of living mast cells using a surface plasmon resonance-based biosensor. Anal. Biochem. 2002, 302, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Yanase, Y.; Suzuki, H.; Tsutsui, T.; Hiragun, T.; Kameyoshi, Y.; Hide, M. The SPR signal in living cells reflects changes other than the area of adhesion and the formation of cell constructions. Biosens. Bioelectron. 2007, 22, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Chabot, V.; Miron, Y.; Charette, P.; Grandbois, M. Identification of the molecular mechanisms in cellular processes that elicit a surface plasmon resonance (SPR) response using simultaneous surface plasmon-enhanced fluorescence (SPEF) microscopy. Biosens. Bioelectron. 2013, 50, 125–131. [Google Scholar] [CrossRef]
- Pezzotti, G. Raman spectroscopy in cell biology and microbiology. J. Raman Spectrosc. 2021, 52, 2348–2443. [Google Scholar] [CrossRef]
- Rai, Y.; Pathak, R.; Kumari, N.; Sah, D.K.; Pandey, S.; Kalra, N.; Soni, R.; Dwarakanath, B.S.; Bhatt, A.N. Mitochondrial biogenesis and metabolic hyperactivation limits the application of MTT assay in the estimation of radiation induced growth inhibition. Sci. Rep. 2018, 8, 1531. [Google Scholar] [CrossRef]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The MTT Assay: Utility, Limitations, Pitfalls, and Interpretation in Bulk and Single-Cell Analysis. Int. J. Mol. Sci. 2021, 22, 12827. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, Y.; Takeaki, O.; Moritoshi, S. Methods of analysis for chemicals that promote/disrupt cellular signaling. Anal. Sci. 2002, 18, 503–516. [Google Scholar] [CrossRef]
- Hiroshi, N.; Kosaihira, A.; Shibata, J.; Ona, T. Development of signaling echo method for cell-based quantitative efficacy evaluation of anti-cancer drugs in apoptosis without drug presence using high-precision surface plasmon resonance sensing. Anal. Sci. 2010, 26, 529–534. [Google Scholar] [CrossRef]
- Michaelis, S.; Wegener, J.; Robelek, R. Label-free monitoring of cell-based assays: Combining impedance analysis with SPR for multiparametric cell profiling. Biosens. Bioelectron. 2013, 49, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Genova-Kalou, P.; Dyankov, G.; Marinov, R.; Mankov, V.; Belina, E.; Kisov, H.; Strijkova-Kenderova, V.; Kantardjiev, T. SPR-Based Kinetic Analysis of the Early Stages of Infection in Cells Infected with Human Coronavirus and Treated with Hydroxychloroquine. Biosensors 2021, 11, 251. [Google Scholar] [CrossRef]
- Régis, M.; Thierry, B. Systematic study of the surface plasmon resonance signals generated by cells for sensors with different characteristic lengths. PLoS ONE 2014, 9, e107978. [Google Scholar] [CrossRef]
- Lee, K.; Hou, H.; Shi, X.; You, M.; Pan, M.; Matsuo, Y.; Cheng, J.; Misawa, H.; Wei, P. Aluminum-Coated Nanoridge Arrays with Dual Evanescent Wavelengths for Real-Time and Label-Free Cellular Analysis. J. Phys. Chem. C 2024, 128, 3384–3392. [Google Scholar] [CrossRef]
- Pasquereau, S.; Galais, M.; Bellefroid, M.; Angona, I.P.; Morot-Bizot, S.; Ismaili, L.; Lint, C.V.; Herbein, G. Ferulic acid derivatives block coronaviruses HCoV-229E and SARS-CoV-2 replication in vitro. Sci. Rep. 2022, 12, 20309. [Google Scholar] [CrossRef]
- Zaharieva, M.M.; Genova-Kalou, P.; Dincheva, I.; Badjakov, I.; Krumova, S.; Enchev, V.; Najdenski, H.; Markova, N. Anti-Herpes Simplex virus and antibacterial activities of Graptopetalum paraguayense E. Walther leaf extract: A pilot study. Biotechnol. Biotechnol. Equip. 2019, 33, 1251–1259. [Google Scholar] [CrossRef]
- Tapani, V.; Granqvist, N.; Hallila, S.; Raviña, M.; Yliperttula, M. Elucidating the signal responses of multi-parametric surface plasmon resonance living cell sensing: A comparison between optical modeling and drug–MDCKII cell interaction measurements. PLoS ONE 2013, 8, e72192. [Google Scholar] [CrossRef]
- Dyankov, G.; Genova-Kalou, P.; Eftimov, T.; Ghaffari, S.S.; Mankov, V.; Kisov, H.; Veselinov, P.; Hikova, E.; Malinowski, N. Binding of SARS-CoV-2 structural proteins to hemoglobin and myoglobin studied by SPR and DR LPG. Sensors 2023, 23, 3346. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genova-Kalou, P.; Hikova, E.; Kereziev, T.; Kolev, P.; Mankov, V.; Veselinov, P.; Valkov, T.; Dyankov, G. Does a SPR-Based Cell-Based Assay Provide Reliable Results on the Toxicity and Efficacy of Antiviral Drugs? Sensors 2025, 25, 3905. https://doi.org/10.3390/s25133905
Genova-Kalou P, Hikova E, Kereziev T, Kolev P, Mankov V, Veselinov P, Valkov T, Dyankov G. Does a SPR-Based Cell-Based Assay Provide Reliable Results on the Toxicity and Efficacy of Antiviral Drugs? Sensors. 2025; 25(13):3905. https://doi.org/10.3390/s25133905
Chicago/Turabian StyleGenova-Kalou, Petia, Evdokiya Hikova, Todor Kereziev, Petar Kolev, Vihar Mankov, Petar Veselinov, Trifon Valkov, and Georgi Dyankov. 2025. "Does a SPR-Based Cell-Based Assay Provide Reliable Results on the Toxicity and Efficacy of Antiviral Drugs?" Sensors 25, no. 13: 3905. https://doi.org/10.3390/s25133905
APA StyleGenova-Kalou, P., Hikova, E., Kereziev, T., Kolev, P., Mankov, V., Veselinov, P., Valkov, T., & Dyankov, G. (2025). Does a SPR-Based Cell-Based Assay Provide Reliable Results on the Toxicity and Efficacy of Antiviral Drugs? Sensors, 25(13), 3905. https://doi.org/10.3390/s25133905




