A Comparative Study of Optical Sensing Methods for Colourimetric Bio/Chemical Detection: Cost, Scale, and Performance
Abstract
Highlights
- Robust Comparison: We evaluated three optical sensing approaches for bio/chemical determination using a pH indicator.
- Superior Performance: The PEDD method showed superior resolution, accuracy, sensitivity, and detection limit results.
- Ratiometric Analysis: LED photometry outperformed spectrophotometry and imaging in all key metrics.
- Versatile Solution: The PEDD approach emerged as a versatile and high-performance solution for precise sensing.
- Valuable Insights: Our comprehensive evaluation offers valuable insights for optimal optical sensing methods.
- Enables low-cost, high-performance sensing for industrial use.
- Facilitates scale-up for decentralised and autonomous systems.
- Reduces dependence on complex lab-based instrumentation.
- Informs method selection for application-specific needs.
- Accelerates translation of sensing research into practice.
- Highlights tradeoffs between accuracy, cost, and complexity.
Abstract
1. Introduction
2. Materials and Methods
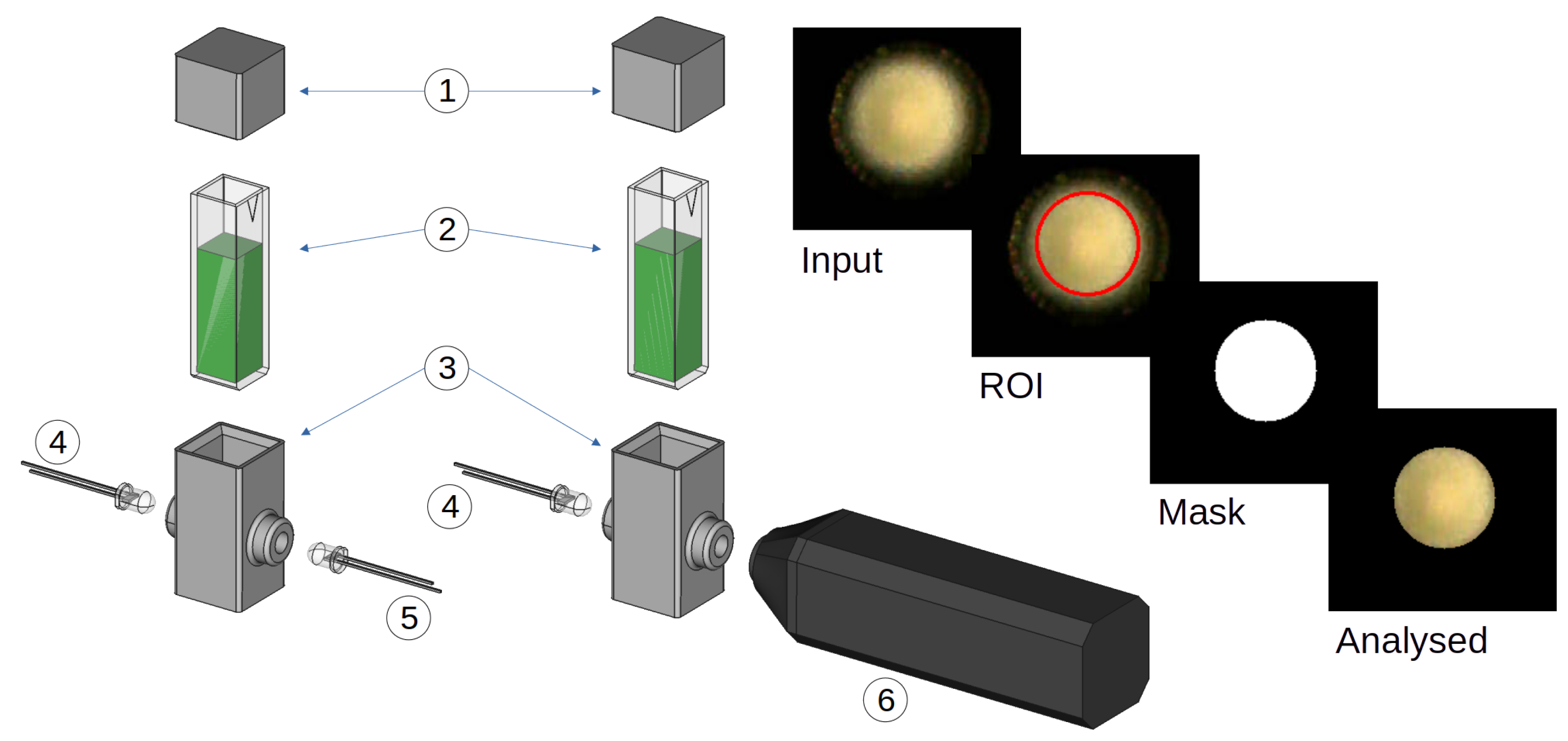
2.1. Experimental Overview
2.2. Sample Preparation
2.3. Reference Measurements
2.4. Optical Analysis Set Up
2.5. LED Measurements (PEDD Approach)
| Listing 1. PEDD charge/discharge firmware. |
 |
2.6. Imaging Analysis
| Spatial Resolution: | 180 × 160 (W × H); |
| Frame rate: | 15 fps; |
| Colour space: | sRGB; |
| Codec: | 24 bits RGB (RV24)-Uncompressed; |
| Container: | Audio Video Interleave (AVI). |
2.7. Comparative Analysis of the Three Approaches
| : | Final value; |
| : | Initial value; |
| : | Centre; |
| : | Exponential constant. |
3. Results and Discussion
3.1. Spectrophotometry
3.2. LED Photometry
3.3. Imaging Analysis
3.4. Comparison
3.4.1. Dynamic Range and Resolution
3.4.2. Accuracy: p Estimation
3.4.3. Sensitivity
3.4.4. Limit of Detection
3.4.5. Ranking
3.4.6. Experimental Error Analysis and Limitations
- Spectrophotometer measurements relied on manufacturer-calibrated devices. Their variability is primarily system-dependent and consistent with other spectrophotometric studies. Additionally, we conducted a baseline while performing measurements in triplicate.
- Imaging-based readings were taken with commercially available RGB cameras, where reflections from cuvette walls were identified as a potential error source. To mitigate this, only the central image region was analysed, as described in the methods.
- Photometric PEDD-based sensing is inherently sensitive to electromagnetic interference due to the low-current photodiode design. As such, all PEDD measurements were conducted within a grounded Faraday cage to minimise electrical noise—particularly relevant for field or industrial deployment scenarios.
3.5. Further Discussion
3.5.1. Photosensor
3.5.2. Cost
3.5.3. Implementation
3.5.4. Specificity vs. Generalisation
3.5.5. Imaging
3.5.6. Bio/Chemical Sensing
3.6. Applicability of Bromocresol Green and Methodological Versatility
3.6.1. Microfluidics
3.6.2. Industrial Relevance and the Need for Comparative Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PEDD | Paired Emitter–Detector Diode |
| LED | Light-Emitting Diode |
| IoT | Internet of Things |
| BCG | Bromocresol green |
| CAD | Computer-Aided Design |
| nm | nanometer |
| RGB | red, green, and blue |
| CIE | Commission Internationale de l’éclairage |
| HCl | Hydrochloric acid |
| KOH | Potassium hydroxide |
| mg | milligram |
| mL | millilitre |
| mm | millimetre |
| UV-Vis | Untraviolet–Visible |
| CAD | Computer-Aided Design |
| ABS | Acrylonitrile Butadiene Styrene |
| GND | Ground |
| IO | Input/Optpuut |
| ROI | Region of Interest |
| p | Acid Dissociation Constant |
| FSD | Full-Scale Deflection |
| %RE | Percentage Relative Error |
References
- Kassal, P.; Steinberg, M.D.; Steinberg, I.M. Wireless chemical sensors and biosensors: A review. Sens. Actuators B Chem. 2018, 266, 228–245. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, M. Optical biosensors for environmental monitoring: Recent advances and future perspectives in bacterial detection. Environ. Res. 2023, 236, 116826. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Faber, H.; Khosla, A.; Anthopoulos, T.D. 3D printed electrochemical devices for bio-chemical sensing: A review. Mater. Sci. Eng. R Rep. 2023, 156, 100754. [Google Scholar] [CrossRef]
- Matzeu, G.; Florea, L.; Diamond, D. Advances in wearable chemical sensor design for monitoring biological fluids. Sens. Actuators B Chem. 2015, 211, 403–418. [Google Scholar] [CrossRef]
- Senapati, M.; Singhal, S.; Gupta, A.K.; Sonowal, D.; Mishra, P.; Sahu, P.P. Bio/chemical sensors and microsensors involved in meat industry. In Biosensors in Food Safety and Quality; CRC Press: Boca Raton, FL, USA, 2022; pp. 159–175. [Google Scholar]
- Rabbani, M.; Hoque, M.E.; Mahbub, Z.B. Chapter 7-Nanosensors in biomedical and environmental applications: Perspectives and prospects. In Nanofabrication for Smart Nanosensor Applications; Pal, K., Gomes, F., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 163–186. [Google Scholar] [CrossRef]
- Diamond, D. Peer Reviewed: Internet-Scale Sensing. Anal. Chem. 2004, 76, 278A–286A. [Google Scholar] [CrossRef]
- Gupta, B.; Shrivastav, A.; Usha, S. Optical Sensors for Biomedical Diagnostics and Environmental Monitoring; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Nybo, M.; Cadamuro, J.; Cornes, M.P.; Rioja, R.G.; Grankvist, K. Sample transportation—An overview. Diagnosis 2019, 6, 39–43. [Google Scholar] [CrossRef]
- Mohan, A.; Rajendran, V.; Mishra, R.K.; Jayaraman, M. Recent advances and perspectives in sweat based wearable electrochemical sensors. TrAC Trends Anal. Chem. 2020, 131, 116024. [Google Scholar] [CrossRef]
- Ali, S.; Glass, T.; Parr, B.; Potgieter, J.; Alam, F. Low Cost Sensor with IoT LoRaWAN Connectivity and Machine Learning-Based Calibration for Air Pollution Monitoring. IEEE Trans. Instrum. Meas. 2021, 70, 5500511. [Google Scholar] [CrossRef]
- Mondal, S.; Subramaniam, C. Point-of-Care, Cable Type Electrochemical Sensor with Ultrahigh Sensitivity for Detection of Metabolites in Human Perspiration. ECS Meet. Abstr. 2020, MA2020-01, 1928. [Google Scholar] [CrossRef]
- Liu, X.; Guo, J.; Li, Y.; Wang, B.; Yang, S.; Chen, W.; Wu, X.; Guo, J.; Ma, X. SERS substrate fabrication for biochemical sensing: Towards point-of-care diagnostics. J. Mater. Chem. B 2021, 9, 8378–8388. [Google Scholar] [CrossRef]
- Murray, R. Challenges in Environmental Analytical Chemistry. Anal. Chem. 2010, 82, 1569. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhuang, J.; Wei, G. Recent advances in the design of colorimetric sensors for environmental monitoring. Environ. Sci. Nano 2020, 7, 2195–2213. [Google Scholar] [CrossRef]
- Umapathi, R.; Sonwal, S.; Lee, M.J.; Mohana Rani, G.; Lee, E.S.; Jeon, T.J.; Kang, S.M.; Oh, M.H.; Huh, Y.S. Colorimetric based on-site sensing strategies for the rapid detection of pesticides in agricultural foods: New horizons, perspectives, and challenges. Coord. Chem. Rev. 2021, 446, 214061. [Google Scholar] [CrossRef]
- Mayer, M.; Baeumner, A.J. A Megatrend Challenging Analytical Chemistry: Biosensor and Chemosensor Concepts Ready for the Internet of Things. Chem. Rev. 2019, 119, 7996–8027. [Google Scholar] [CrossRef]
- Amos, M.; Dittrich, P.; McCaskill, J.; Rasmussen, S. Biological and Chemical Information Technologies. Procedia Comput. Sci. 2011, 7, 56–60. [Google Scholar] [CrossRef]
- Perez de Vargas Sansalvador, I.M.; Fay, C.D.; Cleary, J.; Nightingale, A.M.; Mowlem, M.C.; Diamond, D. Autonomous reagent-based microfluidic pH sensor platform. Sens. Actuators B Chem. 2016, 225, 369–376. [Google Scholar] [CrossRef]
- Thomas, O.; Causse, J. Chapter 3-From spectra to qualitative and quantitative results. In UV-Visible Spectrophotometry of Waters and Soils, 3rd ed.; Thomas, O., Burgess, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 59–94. [Google Scholar] [CrossRef]
- Bachmann, L.M.; Miller, W.G. Chapter 7-Spectrophotometry. In Contemporary Practice in Clinical Chemistry, 4th ed.; Clarke, W., Marzinke, M.A., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 119–133. [Google Scholar] [CrossRef]
- Fernandes, G.M.; Silva, W.R.; Barreto, D.N.; Lamarca, R.S.; Lima Gomes, P.C.F.; Flávio da S Petruci, J.; Batista, A.D. Novel approaches for colorimetric measurements in analytical chemistry—A review. Anal. Chim. Acta 2020, 1135, 187–203. [Google Scholar] [CrossRef]
- Fay, C.D.; Nattestad, A. Advances in Optical Based Turbidity Sensing Using LED Photometry (PEDD). Sensors 2022, 22, 254. [Google Scholar] [CrossRef]
- Tymecki, L.; Brodacka, L.; Rozum, B.; Koncki, R. UV-PEDD photometry dedicated for bioanalytical uses. Analyst 2009, 134, 1333–1337. [Google Scholar] [CrossRef]
- O’Toole, M.; Lau, K.T.; Shazmann, B.; Shepherd, R.; Nesterenko, P.N.; Paull, B.; Diamond, D. Novel integrated paired emitter-detector diode (PEDD) as a miniaturized photometric detector in HPLC. Analyst 2006, 131, 938–943. [Google Scholar] [CrossRef]
- Bui, T.H.; Thangavel, B.; Sharipov, M.; Chen, K.; Shin, J.H. Smartphone-Based Portable Bio-Chemical Sensors: Exploring Recent Advancements. Chemosensors 2023, 11, 468. [Google Scholar] [CrossRef]
- Fan, Y.; Li, J.; Guo, Y.; Xie, L.; Zhang, G. Digital image colorimetry on smartphone for chemical analysis: A review. Measurement 2021, 171, 108829. [Google Scholar] [CrossRef]
- Balbach, S.; Jiang, N.; Moreddu, R.; Dong, X.; Kurz, W.; Wang, C.; Dong, J.; Yin, Y.; Butt, H.; Brischwein, M.; et al. Smartphone-based colorimetric detection system for portable health tracking. Anal. Methods 2021, 13, 4361–4369. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Koh, Y.G.; Lee, W. Smartphone-based colorimetric analysis of structural colors from pH-responsive photonic gel. Sens. Actuators B Chem. 2021, 345, 130359. [Google Scholar] [CrossRef]
- Alberti, G.; Zanoni, C.; Magnaghi, L.R.; Biesuz, R. Disposable and Low-Cost Colorimetric Sensors for Environmental Analysis. Int. J. Environ. Res. Public Health 2020, 17, 8331. [Google Scholar] [CrossRef]
- Venkatesan, M.; Veeramuthu, L.; Liang, F.C.; Chen, W.C.; Cho, C.J.; Chen, C.W.; Chen, J.Y.; Yan, Y.; Chang, S.H.; Kuo, C.C. Evolution of electrospun nanofibers fluorescent and colorimetric sensors for environmental toxicants, pH, temperature, and cancer cells—A review with insights on applications. Chem. Eng. J. 2020, 397, 125431. [Google Scholar] [CrossRef]
- Samanta, T.; Shunmugam, R. Colorimetric and fluorometric probes for the optical detection of environmental Hg(ii) and As(iii) ions. Mater. Adv. 2021, 2, 64–95. [Google Scholar] [CrossRef]
- Ali, Z.; Ullah, R.; Tuzen, M.; Ullah, S.; Rahim, A.; Saleh, T.A. Colorimetric sensing of heavy metals on metal doped metal oxide nanocomposites: A review. Trends Environ. Anal. Chem. 2023, 37, e00187. [Google Scholar] [CrossRef]
- Kaur, B.; Kumar, S.; Kaushik, B.K. Novel Wearable Optical Sensors for Vital Health Monitoring Systems—A Review. Biosensors 2023, 13, 181. [Google Scholar] [CrossRef]
- Kazanskiy, N.L.; Butt, M.A.; Khonina, S.N. Recent Advances in Wearable Optical Sensor Automation Powered by Battery versus Skin-like Battery-Free Devices for Personal Healthcare—A Review. Nanomaterials 2022, 12, 334. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, K.; Xu, H.; Li, T.; Jin, Q.; Cui, D. Recent developments in sensors for wearable device applications. Anal. Bioanal. Chem. 2021, 413, 6037–6057. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liu, Z.; Feng, S.; Gao, Z.; Chen, R.; Cai, G.; Bian, S. Wearable Microfluidic Sweat Chip for Detection of Sweat Glucose and pH in Long-Distance Running Exercise. Biosensors 2023, 13, 157. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, B.; Liao, Y.C. Microfluidic wearable electrochemical sweat sensors for health monitoring. Biomicrofluidics 2022, 16, 051501. [Google Scholar] [CrossRef] [PubMed]
- Di Nonno, S.; Ulber, R. Smartphone-based optical analysis systems. Analyst 2021, 146, 2749–2768. [Google Scholar] [CrossRef]
- Pohanka, M. Colorimetric hand-held sensors and biosensors with a small digital camera as signal recorder, a review. Rev. Anal. Chem. 2020, 39, 20–30. [Google Scholar] [CrossRef]
- Fay, C.D.; Nattestad, A. Optical Measurements Using LED Discharge Photometry (PEDD Approach): Critical Timing Effects Identified & Corrected. IEEE Trans. Instrum. Meas. 2022, 71, 1–9. [Google Scholar] [CrossRef]
- Fay, C.D.; Nattestad, A. LED PEDD Discharge Photometry: Effects of Software Driven Measurements for Sensing Applications. Sensors 2022, 22, 1526. [Google Scholar] [CrossRef]
- Fay, C.D.; Wu, L. Critical importance of RGB color space specificity for colorimetric bio/chemical sensing: A comprehensive study. Talanta 2024, 266, 124957. [Google Scholar] [CrossRef]
- Diamond, D.; Hanratty, V.C. Spreadsheet Applications in Chemistry Using Microsoft Excel, 1st ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1997. [Google Scholar]
- O’Toole, M.; Lau, K.T.; Diamond, D. Photometric detection in flow analysis systems using integrated PEDDs. Talanta 2005, 66, 1340–1344. [Google Scholar] [CrossRef]
- Levenberg, K. A method for the solution of certain non-linear problems in least squares. Q. Appl. Math. 1944, 2, 164–168. [Google Scholar] [CrossRef]
- Marquardt, D.W. An Algorithm for Least-Squares Estimation of Nonlinear Parameters. J. Soc. Ind. Appl. Math. 1963, 11, 431–441. [Google Scholar] [CrossRef]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental Algorithms for Scientific Computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Bradski, G. The OpenCV Library. Dr. Dobb’s J. Softw. Tools 2000, 25, 120–125. Available online: https://drdobbs.com/open-source/the-opencv-library/184404319 (accessed on 19 June 2025).
- Hipp, R.D. SQLite, version 3.31.1; 2020. Available online: https://www.sqlite.org/index.html (accessed on 19 June 2025).
- Harris, C.R.; Millman, K.J.; van der Walt, S.J.; Gommers, R.; Virtanen, P.; Cournapeau, D.; Wieser, E.; Taylor, J.; Berg, S.; Smith, N.J.; et al. Array programming with NumPy. Nature 2020, 585, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.D. Matplotlib: A 2D graphics environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Rumble, J. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Diamond, D.; Lau, K.T.; Brady, S.; Cleary, J. Integration of analytical measurements and wireless communications—Current issues and future strategies. Talanta 2008, 75, 606–612. [Google Scholar] [CrossRef]
- de Vargas-Sansalvador, I.P.; Fay, C.; Phelan, T.; Fernández-Ramos, M.; Capitán-Vallvey, L.; Diamond, D.; Benito-Lopez, F. A new light emitting diode–light emitting diode portable carbon dioxide gas sensor based on an interchangeable membrane system for industrial applications. Anal. Chim. Acta 2011, 699, 216–222. [Google Scholar] [CrossRef]
- Krauss, S.T.; Nauman, A.Q.; Garner, G.T.; Landers, J.P. Color manipulation through microchip tinting for colorimetric detection using hue image analysis. Lab Chip 2017, 17, 4089–4096. [Google Scholar] [CrossRef]
- Cantrell, K.; Erenas, M.M.; de Orbe-Payá, I.; Capitán-Vallvey, L.F. Use of the Hue Parameter of the Hue, Saturation, Value Color Space As a Quantitative Analytical Parameter for Bitonal Optical Sensors. Anal. Chem. 2010, 82, 531–542. [Google Scholar] [CrossRef]
- Curto, V.F.; Fay, C.; Coyle, S.; Byrne, R.; O’Toole, C.; Barry, C.; Hughes, S.; Moyna, N.; Diamond, D.; Benito-Lopez, F. Real-time sweat pH monitoring based on a wearable chemical barcode micro-fluidic platform incorporating ionic liquids. Sens. Actuators B Chem. 2012, 171–172, 1327–1334. [Google Scholar] [CrossRef]
- Fay, C.; Lau, K.T.; Beirne, S.; Ó Conaire, C.; McGuinness, K.; Corcoran, B.; O’Connor, N.E.; Diamond, D.; McGovern, S.; Coleman, G.; et al. Wireless aquatic navigator for detection and analysis (WANDA). Sens. Actuators B Chem. 2010, 150, 425–435. [Google Scholar] [CrossRef]
- Zhao, V.X.T.; Wong, T.I.; Zheng, X.T.; Tan, Y.N.; Zhou, X. Colorimetric biosensors for point-of-care virus detections. Mater. Sci. Energy Technol. 2020, 3, 237–249. [Google Scholar] [CrossRef] [PubMed]
- de Araujo, W.R.; Lukas, H.; Torres, M.D.T.; Gao, W.; de la Fuente-Nunez, C. Low-Cost Biosensor Technologies for Rapid Detection of COVID-19 and Future Pandemics. ACS Nano 2024, 18, 1757–1777. [Google Scholar] [CrossRef] [PubMed]
- Piriya, V.S.A.; Joseph, P.; Daniel, S.C.G.K.; Lakshmanan, S.; Kinoshita, T.; Muthusamy, S. Colorimetric sensors for rapid detection of various analytes. Mater. Sci. Eng. C 2017, 78, 1231–1245. [Google Scholar] [CrossRef]
- Huang, C.W.; Lin, C.; Nguyen, M.K.; Hussain, A.; Bui, X.T.; Ngo, H.H. A review of biosensor for environmental monitoring: Principle, application, and corresponding achievement of sustainable development goals. Bioengineered 2023, 14, 58–80. [Google Scholar] [CrossRef]
- Wang, W.; You, Y.; Gunasekaran, S. LSPR-based colorimetric biosensing for food quality and safety. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5829–5855. [Google Scholar] [CrossRef]
- Zhu, W.; Li, L.; Zhou, Z.; Yang, X.; Hao, N.; Guo, Y.; Wang, K. A colorimetric biosensor for simultaneous ochratoxin A and aflatoxins B1 detection in agricultural products. Food Chem. 2020, 319, 126544. [Google Scholar] [CrossRef]
- Dester, E.; Kao, K.; Alocilja, E.C. Detection of Unamplified E. coli O157 DNA Extracted from Large Food Samples Using a Gold Nanoparticle Colorimetric Biosensor. Biosensors 2022, 12, 274. [Google Scholar] [CrossRef]
- Torres-Molina, M.A.; Erenas, M.M.; Ortega Munoz, M.; Capitan Vallvey, L.F.; Perez de Vargas Sansalvador, I.M. Biocompatible sensors for ammonia gas detection. Talanta 2025, 281, 126916. [Google Scholar] [CrossRef]
- Fernández-Ramos, M.D.; Pageo-Cabrera, M.; Capitán-Vallvey, L.F.; Pérez de Vargas-Sansalvador, I.M. Inkjet-printed O2 gas sensors in intelligent packaging. Analyst 2021, 146, 3177–3184. [Google Scholar] [CrossRef]
- Perez de Vargas-Sansalvador, I.M.; Erenas, M.M.; Martínez-Olmos, A.; Mirza-Montoro, F.; Diamond, D.; Capitan-Vallvey, L.F. Smartphone based meat freshness detection. Talanta 2020, 216, 120985. [Google Scholar] [CrossRef] [PubMed]
- Perez de Vargas-Sansalvador, I.M.; Fay, C.; Fernez-Ramos, M.D.; Diamond, D.; Benito-Lopez, F.; Capitán-Vallvey, L.F. LED-LED portable oxygen gas sensor. Anal. Bioanal. Chem. 2012, 404, 2851–2858. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Cui, Y.; Cai, Z.; Li, L. Applications of smartphone-based colorimetric biosensors. Biosens. Bioelectron. X 2022, 11, 100173. [Google Scholar] [CrossRef]
- Kim, S.; Lee, Y.; Kim, J.Y.; Yang, J.H.; Kwon, H.J.; Hwang, J.Y.; Moon, C.; Jang, J.E. Color-sensitive and spectrometer-free plasmonic sensor for biosensing applications. Biosens. Bioelectron. 2019, 126, 743–750. [Google Scholar] [CrossRef]
- Wu, L.; Manchanda, A.; Gupta, V.; Paull, B. Graphene Oxide-Functionalized Thread-Based Electrofluidic Approach for DNA Hybridization. ACS Omega 2023, 8, 13569–13577. [Google Scholar] [CrossRef]
- Głowacka, J.; Wiśniewska, A.; Koncki, R.; Strzelak, K. Photometric flow system for the determination of serum lactate dehydrogenase activity. Talanta 2023, 265, 124817. [Google Scholar] [CrossRef]
- Seetasang, S.; Kaneta, T. Development of a miniaturized photometer with paired emitter-detector light-emitting diodes for investigating thiocyanate levels in the saliva of smokers and non-smokers. Talanta 2019, 204, 586–591. [Google Scholar] [CrossRef]
- Ziółkowski, R.; Oszwałdowski, S.; Kopyra, K.K.; Zacharczuk, K.; Zasada, A.A.; Malinowska, E. Toward Fluorimetric-Paired-Emitter-Detector-Diode test for Bacillus anthracis DNA based on graphene oxide. Microchem. J. 2020, 154, 104592. [Google Scholar] [CrossRef]
- Yang, W.; Fei, J.; Xu, W.; Jiang, H.; Sakran, M.; Hong, J.; Zhu, W.; Zhou, X. A biosensor based on the biomimetic oxidase Fe3O4@MnO2 for colorimetric determination of uric acid. Colloids Surfaces Biointerfaces 2022, 212, 112347. [Google Scholar] [CrossRef]
- Theint, H.; Walsh, J.; Wong, S.; Voon, K.; Shitan, M. Development of an optical biosensor for the detection of Trypanosoma evansi and Plasmodium berghei. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 218, 348–358. [Google Scholar] [CrossRef]
- Hidayat, M.A.; Maharani, D.A.; Purwanto, D.A.; Kuswandi, B.; Yuwono, M. Simple and Sensitive Paper-based Colorimetric Biosensor for Determining Total Polyphenol Content of the Green Tea Beverages. Biotechnol. Bioprocess Eng. 2020, 25, 255–263. [Google Scholar] [CrossRef]
- Abilevitch, L.; Otorgust, G.; Mizrahi, L.; Dotan, A.; Amir, E. Flexible colorimetric sensor for ammonia detection based on polyurethane and bromocresol green. Polym. Bull. 2025, 82, 1531–1550. [Google Scholar] [CrossRef]
- Bonthula, S.; Devarajan, S.; Maurya, M.R.; Al-Maadeed, S.; Maalej, R.; Chaari, M.Z.; Sadasivuni, K.K. Advancing Rapid Arsenic (III) Detection Through Device-Integrated Colorimetry. Chem. Afr. 2024, 7, 4381–4391. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, H.; Chen, X.; Li, W.; Zhang, J.; Wang, M.; Zhang, J.; Wang, S.; Liu, Y. Glucose-metabolism-triggered colorimetric sensor array for point-of-care differentiation and antibiotic susceptibility testing of bacteria. Food Chem. 2024, 438, 137983. [Google Scholar] [CrossRef]
- Block, D.R.; Cotten, S.W.; Franke, D.; Mbughuni, M.M. Comparison of Five Common Analyzers in the Measurement of Chemistry Analytes in an Authentic Cohort of Body Fluid Specimens. Am. J. Clin. Pathol. 2022, 158, 47–61. [Google Scholar] [CrossRef]
- Bonanno, L.M.; DeLouise, L.A. Whole blood optical biosensor. Biosens. Bioelectron. 2007, 23, 444–448. [Google Scholar] [CrossRef]
- Shaila, M.; Pai, G.P.; Shetty, P. Salivary protein concentration, flow rate, buffer capacity and pH estimation: A comparative study among young and elderly subjects, both normal and with gingivitis and periodontitis. J. Indian Soc. Periodontol. 2013, 17, 42–46. [Google Scholar] [CrossRef]
- Raj, M.; Moon, J.M.; Goyal, R.N.; Park, D.S.; Shim, Y.B. Simultaneous detection of ATP metabolites in human plasma and urine based on palladium nanoparticle and poly(bromocresol green) composite sensor. Biosens. Bioelectron. 2019, 126, 758–766. [Google Scholar] [CrossRef]
- Choi, J.; Bandodkar, A.J.; Reeder, J.T.; Ray, T.R.; Turnquist, A.; Kim, S.B.; Nyberg, N.; Hourlier-Fargette, A.; Model, J.B.; Aranyosi, A.J.; et al. Soft, Skin-Integrated Multifunctional Microfluidic Systems for Accurate Colorimetric Analysis of Sweat Biomarkers and Temperature. ACS Sens. 2019, 4, 379–388. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.F.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef]
- Wu, L.; Beirne, S.; Cabot, J.M.; Paull, B.; Wallace, G.G.; Innis, P.C. Fused filament fabrication 3D printed polylactic acid electroosmotic pumps. Lab Chip 2021, 21, 3338–3351. [Google Scholar] [CrossRef] [PubMed]
- Man, Y.; Li, A.; Li, B.; Liu, J.; Pan, L. A microfluidic colorimetric immunoassay for sensitive detection of altenariol monomethyl ether by UV spectroscopy and smart phone imaging. Anal. Chim. Acta 2019, 1092, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Kong, T.; You, J.B.; Zhang, B.; Nguyen, B.; Tarlan, F.; Jarvi, K.; Sinton, D. Accessory-free quantitative smartphone imaging of colorimetric paper-based assays. Lab Chip 2019, 19, 1991–1999. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.H.; Park, C.; Jeon, J.; Park, S. Three-Dimensional Paper-Based Microfluidic Analysis Device for Simultaneous Detection of Multiple Biomarkers with a Smartphone. Biosensors 2020, 10, 187. [Google Scholar] [CrossRef]
- Soares, S.; Fernandes, G.M.; Rocha, F.R. Smartphone-based digital images in analytical chemistry: Why, when, and how to use. TrAC Trends Anal. Chem. 2023, 168, 117284. [Google Scholar] [CrossRef]
- Wu, J.; Ahmad, W.; Zhang, J.; Wei, W.; Yu, J.; Zhang, W.; Chen, Q.; Ouyang, Q. Ratiometric upconversion-luminescence in-situ sampling aptasensing platform integrated with smartphone-based device for visual detection of 17β-estradiol. Sens. Actuators B Chem. 2023, 390, 133999. [Google Scholar] [CrossRef]
- Czugala, M.; Fay, C.; O’Connor, N.E.; Corcoran, B.; Benito-Lopez, F.; Diamond, D. Portable integrated microfluidic analytical platform for the monitoring and detection of nitrite. Talanta 2013, 116, 997–1004. [Google Scholar] [CrossRef]
- Chen, L.; Yang, C.; Xiao, Y.; Yan, X.; Hu, L.; Eggersdorfer, M.; Chen, D.; Weitz, D.; Ye, F. Millifluidics, microfluidics, and nanofluidics: Manipulating fluids at varying length scales. Mater. Today Nano 2021, 16, 100136. [Google Scholar] [CrossRef]
- Placer, L.; Lavilla, I.; Pena-Pereira, F.; Bendicho, C. A 3D microfluidic paper-based analytical device with smartphone-assisted colorimetric detection for iodine speciation in seaweed samples. Sens. Actuators B Chem. 2023, 377, 133109. [Google Scholar] [CrossRef]
- Fay, C.D.; Wu, L. Cost-Effective 3D Printing of Silicone Structures Using an Advanced Intra-Layer Curing Approach. Technologies 2023, 11, 179. [Google Scholar] [CrossRef]

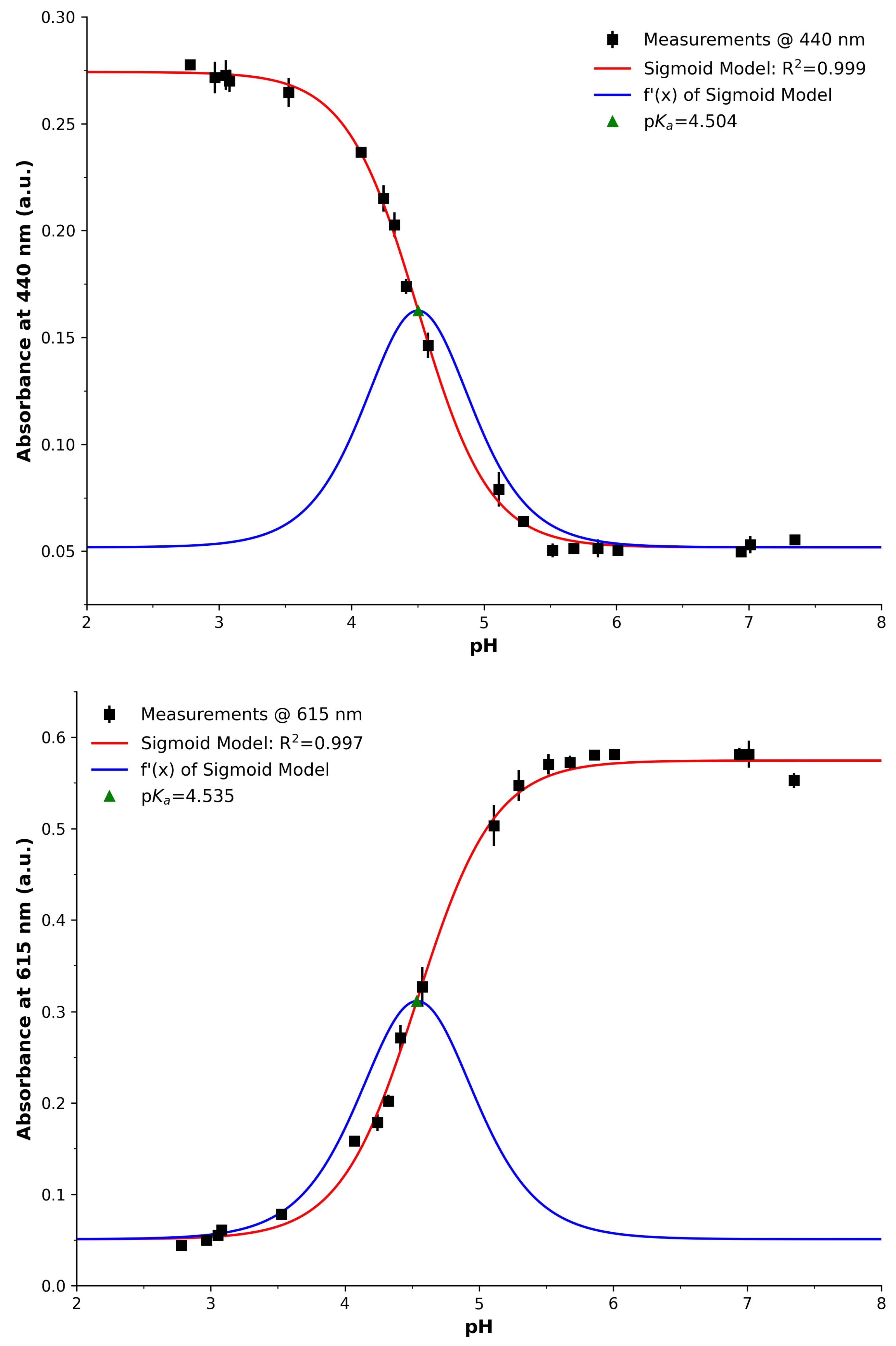
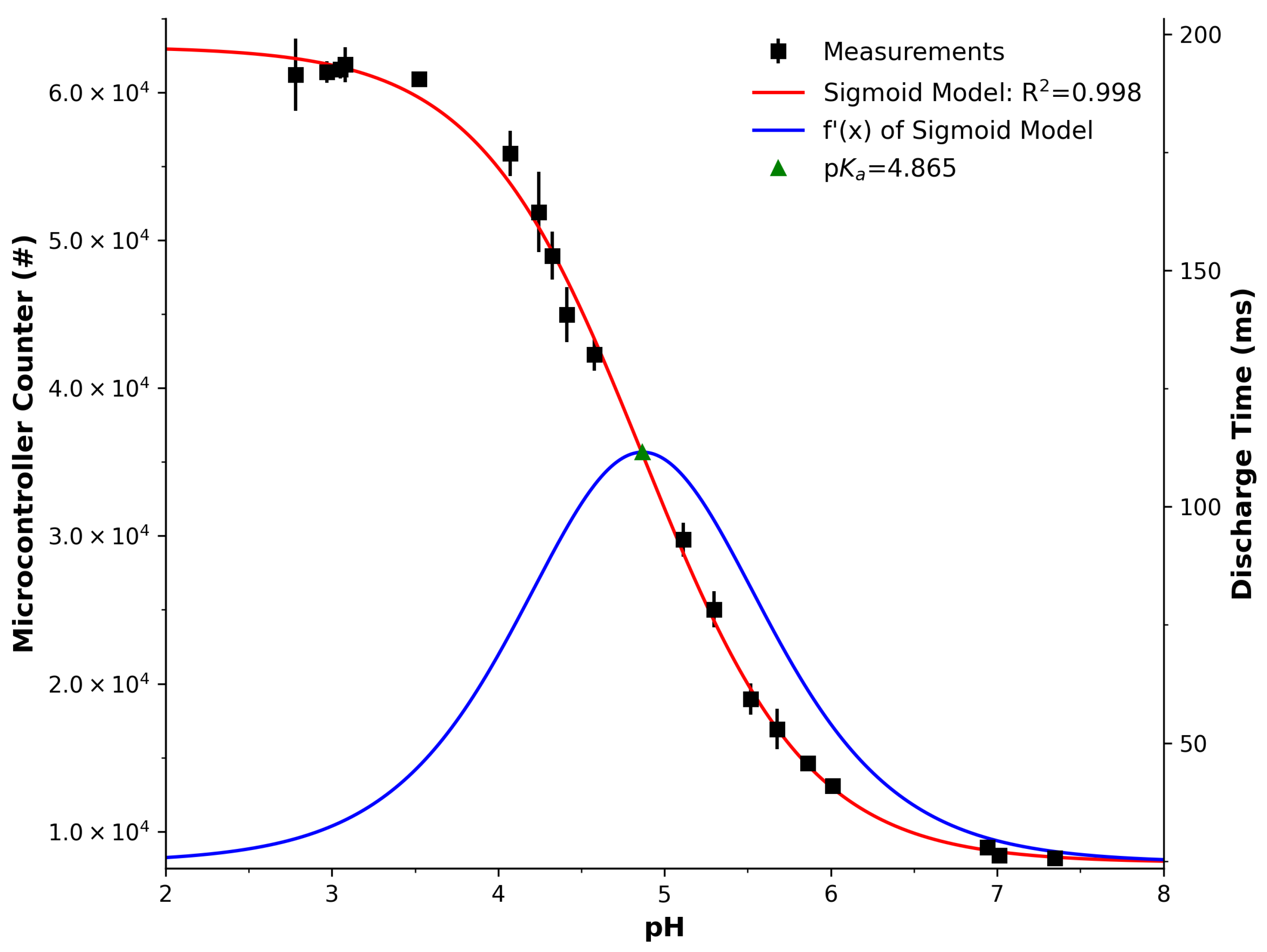

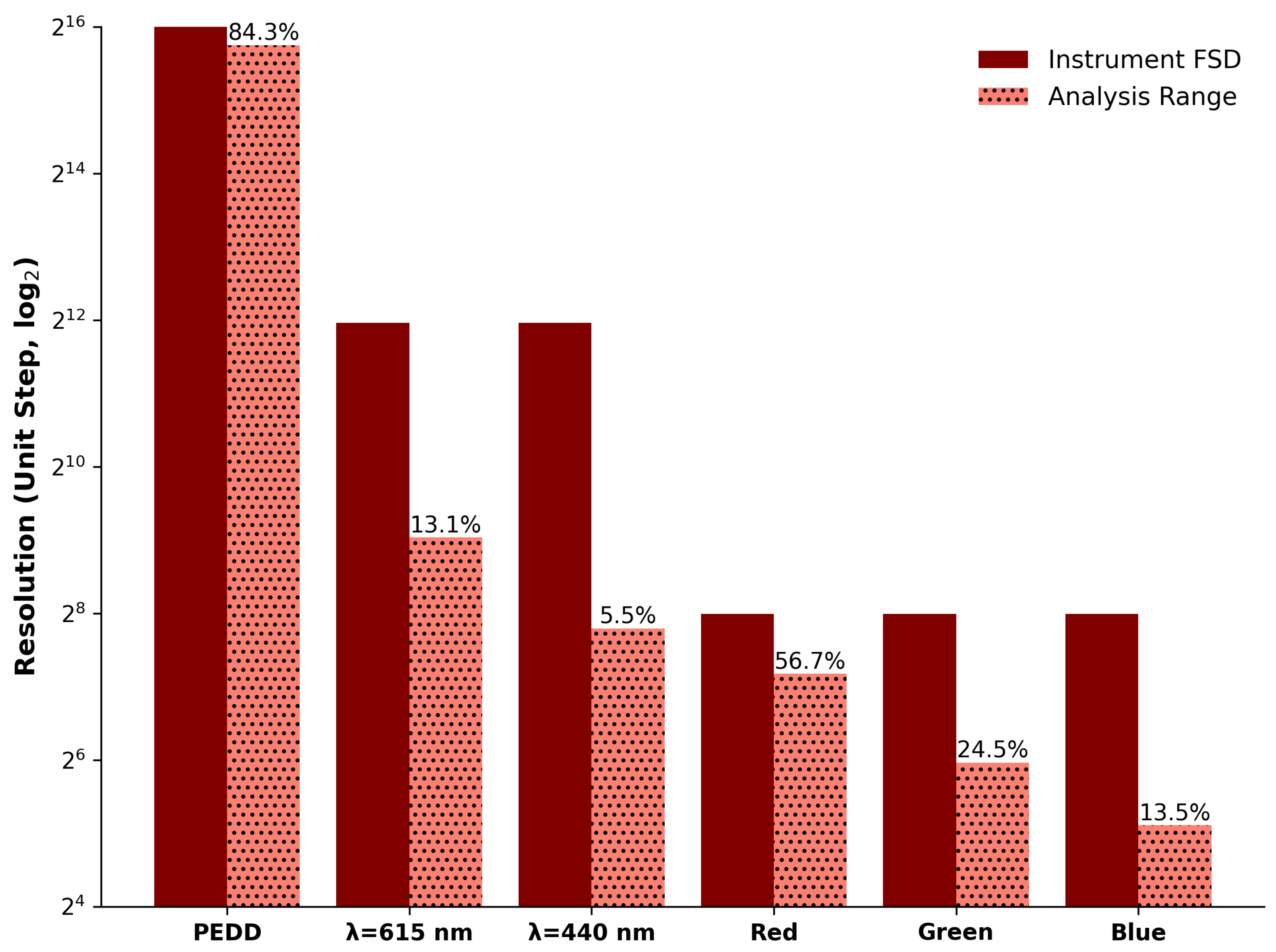
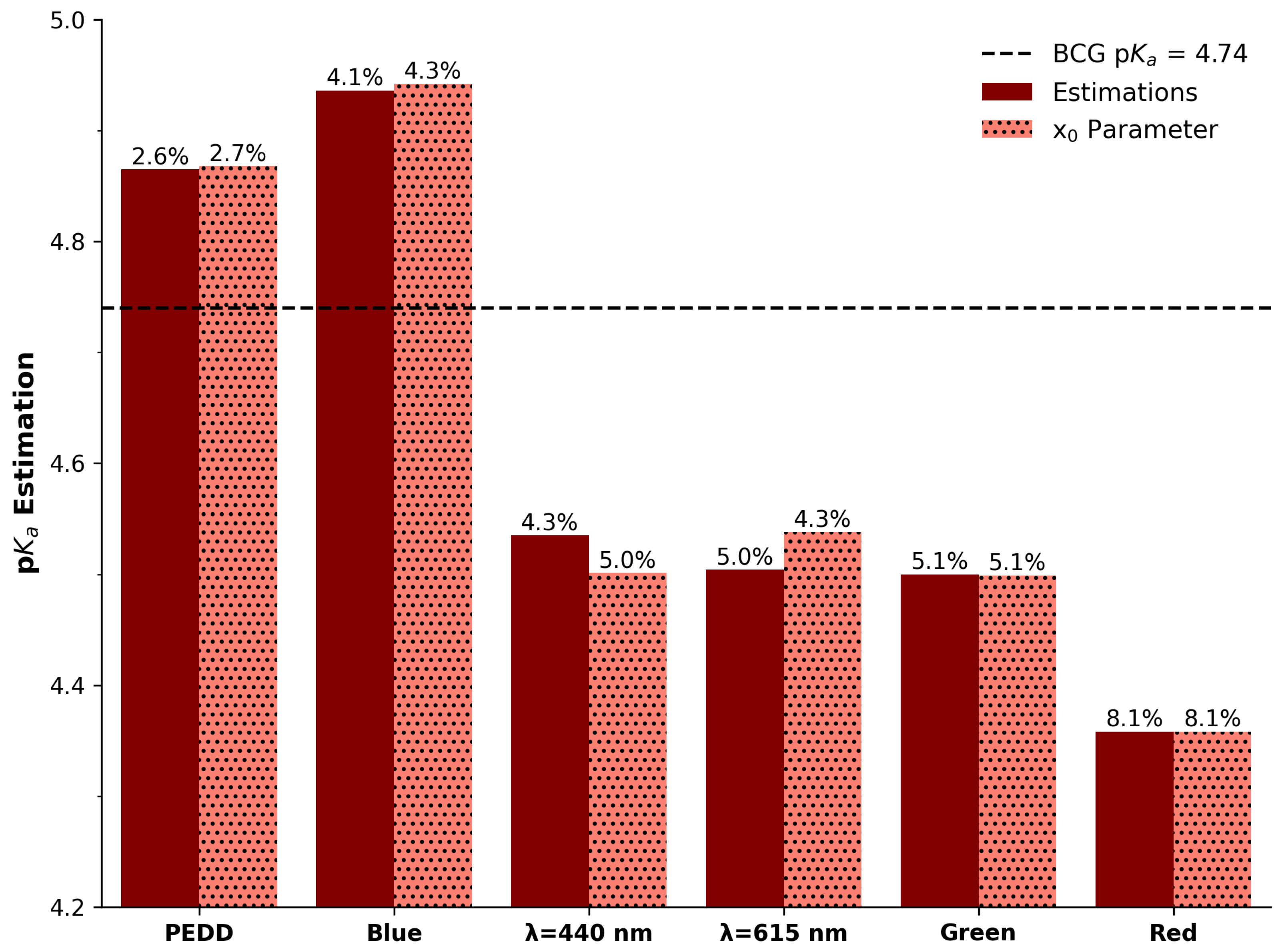


| Parameter | Spectra () | Spectra () | PEDD | Red Ch. | Green Ch. | Blue Ch. |
|---|---|---|---|---|---|---|
| Figure | Figure 3 (Left) | Figure 3 (Right) | Figure 4 | Figure 5 (Top) | Figure 5 (Middle) | Figure 5 (Bottom) |
| p | 4.54 | 4.5 | 4.87 | 4.36 | 4.5 | 4.94 |
| y0 | 0.27 | 0.05 | 63,116 | 191.26 | 171.86 | 96.76 |
| y1 | 0.05 | 0.58 | 7889.3 | 46.7 | 109.48 | 131.22 |
| x0 | 4.5 | 4.54 | 4.87 | 4.36 | 4.5 | 4.94 |
| dx | 0.27 | 0.29 | 0.5 | 0.48 | 0.48 | 0.41 |
| R2 | 1 | 1 | 1 | 0.99 | 0.98 | 0.92 |
| Device FSD | 4.000 | 4.000 | 65,535 | 255 | 255 | 255 |
| Unit Step | 0.001 | 0.001 | 1 | 1 | 1 | 1 |
| Increments | 4000 | 4000 | 65,535 | 255 | 255 | 255 |
| Dynamic Range | 0.22 | 0.52 | 55,226.7 | 144.56 | 62.37 | 34.47 |
| Resolution | 222 | 524 | 55,226.7 | 144.56 | 62.37 | 34.47 |
| Sensitivity | 125.29 | 314.7 | 23,657.06 | 51 | 24.57 | 15.86 |
| LOD | 0.06 | 0.05 | 0.04 | 0.07 | 0.2 | 0.14 |
| RSD (%) | 3.11 | 3.08 | 3.07 | 3.21 | 1.62 | 1.71 |
| Approach | Resolution | Sensitivity | LOD | Score | Ranked | |
|---|---|---|---|---|---|---|
| = 440 nm | 4 | 4 | 4 | 4 | 16 | 3rd |
| = 615 nm | 5 | 3 | 5 | 5 | 18 | 2nd |
| PEDD | 6 | 6 | 6 | 6 | 24 | 1st |
| Red | 3 | 1 | 3 | 3 | 10 | 4th |
| Green | 2 | 2 | 2 | 2 | 8 | 5th= |
| Blue | 1 | 5 | 1 | 1 | 8 | 5th= |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fay, C.D.; Wu, L.; Perez de Vargas Sansalvador, I.M. A Comparative Study of Optical Sensing Methods for Colourimetric Bio/Chemical Detection: Cost, Scale, and Performance. Sensors 2025, 25, 3850. https://doi.org/10.3390/s25133850
Fay CD, Wu L, Perez de Vargas Sansalvador IM. A Comparative Study of Optical Sensing Methods for Colourimetric Bio/Chemical Detection: Cost, Scale, and Performance. Sensors. 2025; 25(13):3850. https://doi.org/10.3390/s25133850
Chicago/Turabian StyleFay, Cormac D., Liang Wu, and Isabel M. Perez de Vargas Sansalvador. 2025. "A Comparative Study of Optical Sensing Methods for Colourimetric Bio/Chemical Detection: Cost, Scale, and Performance" Sensors 25, no. 13: 3850. https://doi.org/10.3390/s25133850
APA StyleFay, C. D., Wu, L., & Perez de Vargas Sansalvador, I. M. (2025). A Comparative Study of Optical Sensing Methods for Colourimetric Bio/Chemical Detection: Cost, Scale, and Performance. Sensors, 25(13), 3850. https://doi.org/10.3390/s25133850






