Use of Smartphones and Wrist-Worn Devices for Motor Symptoms in Parkinson’s Disease: A Systematic Review of Commercially Available Technologies
Abstract
1. Introduction
2. Materials and Methods
- -
- ((smartphone OR smartwatch OR wristband OR wrist device) AND (digital health OR rehabilitation OR motor outcome OR fall OR gait)) AND (parkinson).
2.1. Inclusion Criteria
- -
- Original articles;
- -
- Studies that used quantitative assessment;
- -
- Studies that included individuals diagnosed with PD as the experimental group;
- -
- Studies that studied the use of a smartphone or smartwatch or both for monitoring or assessing motor symptoms in PD compared with the control group;
- -
- Studies that reported outcomes based on data collected from embedded sensors within smartphones, smartwatches, or ATs;
- -
- Studies that used smartphones, smartwatches, or ATs to assess or monitor motor symptoms or to improve motor performance;
- -
- Studies that presented results from studies where data were collected using humans;
- -
- English articles.
2.2. Exclusion Criteria
- -
- Qualitative studies;
- -
- Studies that investigated only medication response or pharmacological treatments,
- -
- Studies that evaluated algorithmic approaches or artificial intelligence;
- -
- Studies that relied exclusively on self-reported diary data as the primary outcome for clinical evaluation;
- -
- Studies that involved a direct intervention by a therapist;
- -
- Studies involving other pathologies.
- -
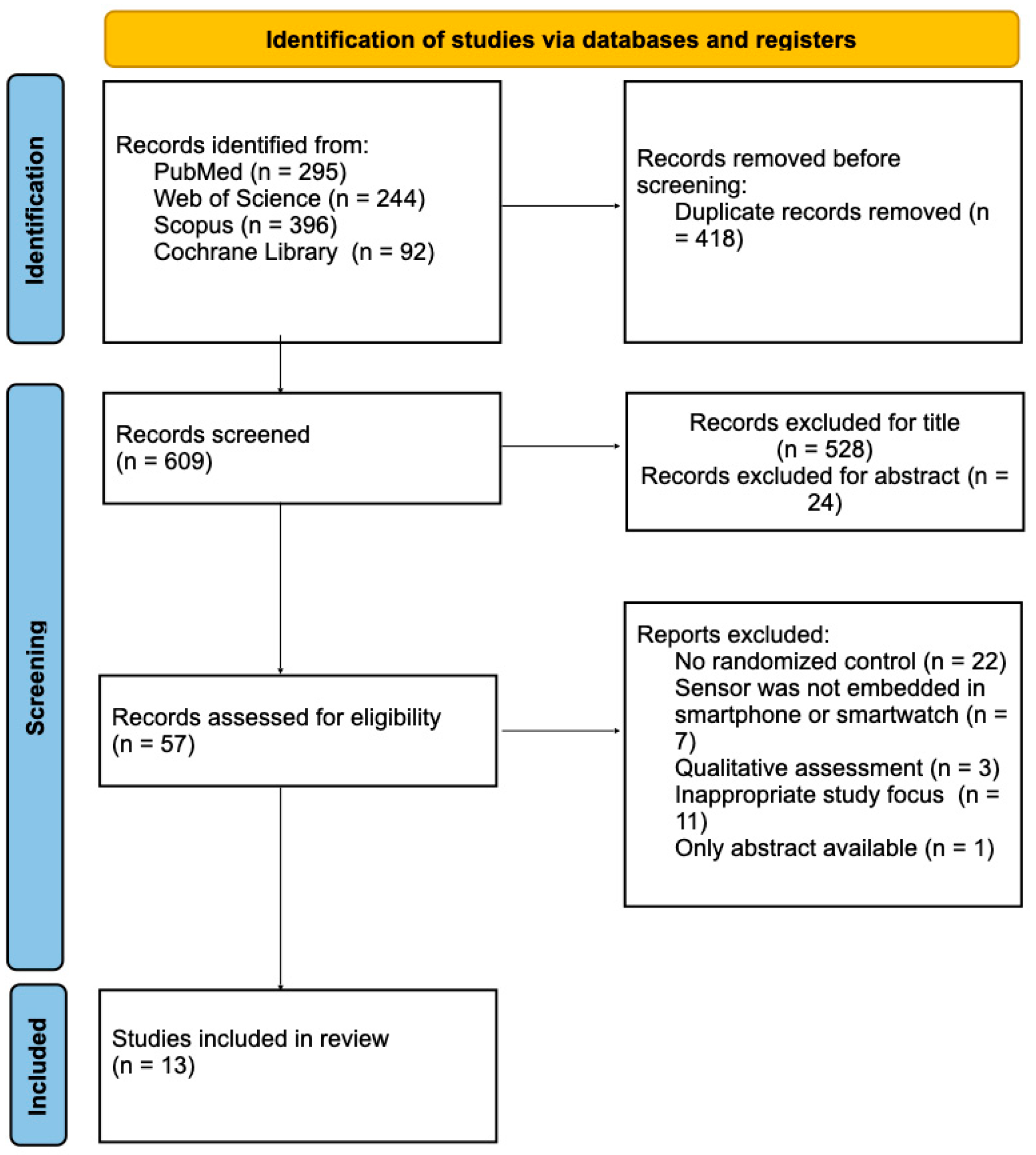
- Finally, after full-text evaluation, only 13 studies were included in this review.
3. Results
3.1. Correlation with Clinical Measures or Wearable Sensors
3.2. Longitudinal Monitoring of Symptoms and Treatment
3.3. Usability in Clinical Practice
3.4. Accuracy of Commercial Activity Trackers
3.5. Differentiating PD from Healthy Subjects
3.6. Improving Motor Performances
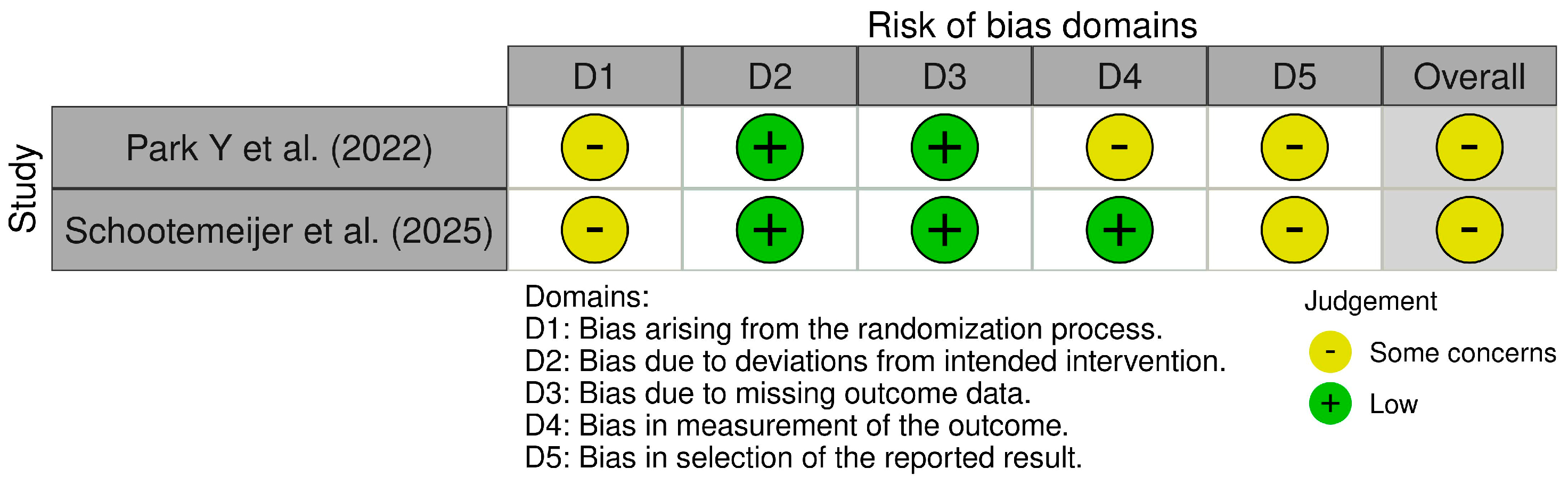
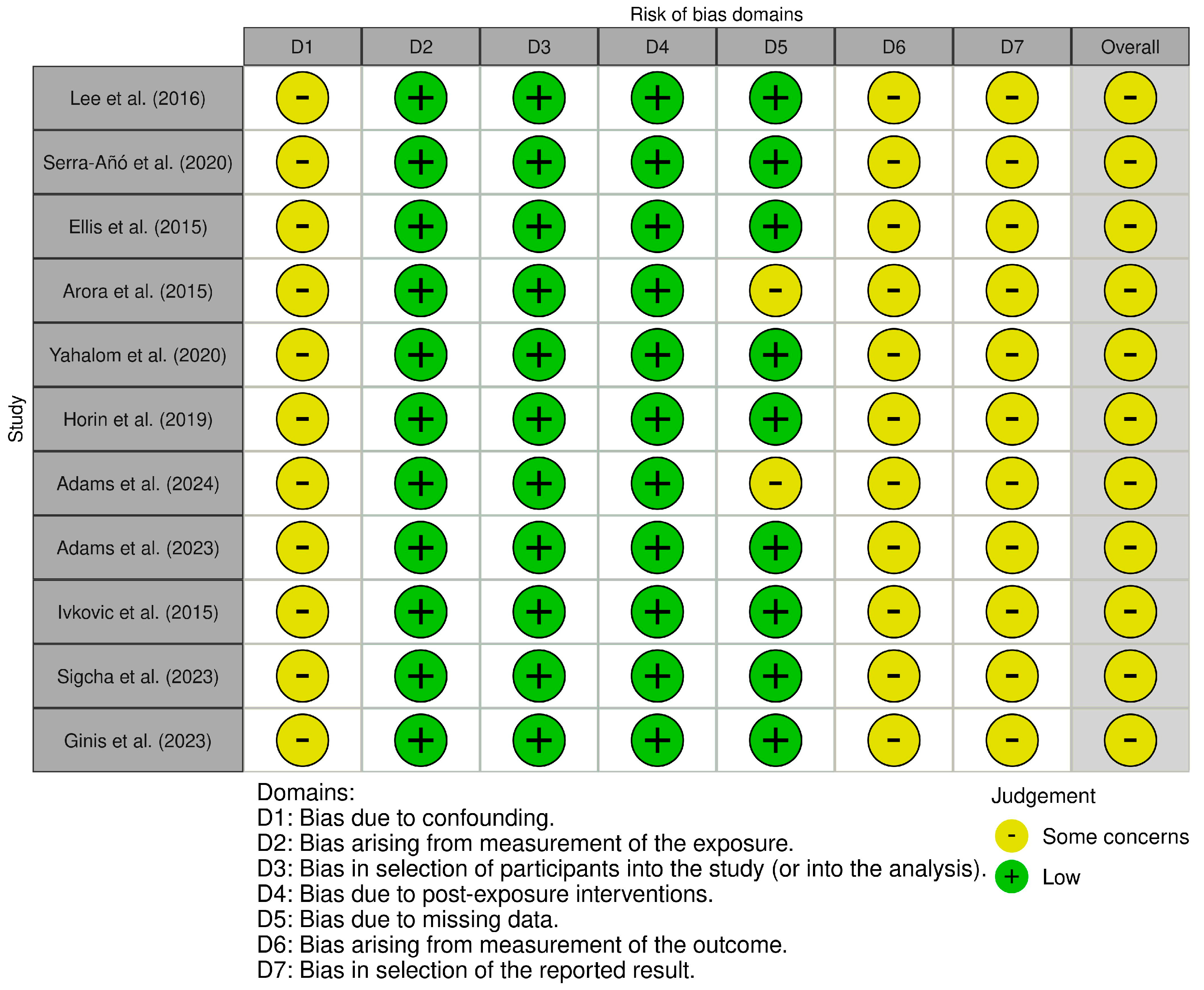
4. Risk of Bias
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PD | Parkinson’s Disease |
| AT | Activity tracker |
| pwPD | People with Parkinson’s disease |
| HSs | Healthy subjects |
| H&Y | Hoehn and Yahr assessment |
| TUG | Time Up and Go |
| IMU | Inertial measurement unit |
| UPDRS | Unified Parkinson’s Disease Rating Scale |
| mHealth | Mobile health |
| TC | Tactile cueing |
References
- Jankovic, J. Parkinson’s Disease: Clinical Features and Diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, P.; Shabbott, B.; Cortés, J.C. Motor Control Abnormalities in Parkinson’s Disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009282. [Google Scholar] [CrossRef]
- Magrinelli, F.; Picelli, A.; Tocco, P.; Federico, A.; Roncari, L.; Smania, N.; Zanette, G.; Tamburin, S. Pathophysiology of Motor Dysfunction in Parkinson’s Disease as the Rationale for Drug Treatment and Rehabilitation. Park. Dis. 2016, 2016, 9832839. [Google Scholar] [CrossRef]
- Wang, W.; Lee, J.; Harrou, F.; Sun, Y. Early Detection of Parkinson’s Disease Using Deep Learning and Machine Learning. IEEE Access 2020, 8, 147635–147646. [Google Scholar] [CrossRef]
- Prashanth, R.; Dutta Roy, S. Early Detection of Parkinson’s Disease through Patient Questionnaire and Predictive Modelling. Int. J. Med. Inform. 2018, 119, 75–87. [Google Scholar] [CrossRef]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-sponsored Revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale Presentation and Clinimetric Testing Results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef]
- Evers, L.J.W.; Krijthe, J.H.; Meinders, M.J.; Bloem, B.R.; Heskes, T.M. Measuring Parkinson’s Disease over Time: The Real-world Within-subject Reliability of the MDS-UPDRS. Mov. Disord. 2019, 34, 1480–1487. [Google Scholar] [CrossRef]
- Vignoud, G.; Desjardins, C.; Salardaine, Q.; Mongin, M.; Garcin, B.; Venance, L.; Degos, B. Video-Based Automated Assessment of Movement Parameters Consistent with MDS-UPDRS III in Parkinson’s Disease. J. Park. Dis. 2022, 12, 2211–2222. [Google Scholar] [CrossRef]
- Bouça-Machado, R.; Fernandes, A.; Ranzato, C.; Beneby, D.; Nzwalo, H.; Ferreira, J.J. Measurement Tools to Assess Activities of Daily Living in Patients with Parkinson’s Disease: A Systematic Review. Front. Neurosci. 2022, 16, 945398. [Google Scholar] [CrossRef]
- Bhidayasiri, R.; Tarsy, D. Parkinson’s Disease: Hoehn and Yahr Scale. In Movement Disorders: A Video Atlas; Humana Press: Totowa, NJ, USA, 2012; pp. 4–5. [Google Scholar]
- Monje, M.H.G.; Foffani, G.; Obeso, J.; Sánchez-Ferro, Á. New Sensor and Wearable Technologies to Aid in the Diagnosis and Treatment Monitoring of Parkinson’s Disease. Annu. Rev. Biomed. Eng. 2019, 21, 111–143. [Google Scholar] [CrossRef]
- Maetzler, W.; Domingos, J.; Srulijes, K.; Ferreira, J.J.; Bloem, B.R. Quantitative Wearable Sensors for Objective Assessment of Parkinson’s Disease. Mov. Disord. 2013, 28, 1628–1637. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Deen, M.J. Smartphone Sensors for Health Monitoring and Diagnosis. Sensors 2019, 19, 2164. [Google Scholar] [CrossRef]
- Erb, M.K.; Karlin, D.R.; Ho, B.K.; Thomas, K.C.; Parisi, F.; Vergara-Diaz, G.P.; Daneault, J.-F.; Wacnik, P.W.; Zhang, H.; Kangarloo, T.; et al. MHealth and Wearable Technology Should Replace Motor Diaries to Track Motor Fluctuations in Parkinson’s Disease. NPJ Digit. Med. 2020, 3, 6. [Google Scholar] [CrossRef]
- Simes-Marques, M.; Nunes, I.L. Usability of Interfaces. In Ergonomics—A Systems Approach; IntechOpen: London, UK, 2012. [Google Scholar]
- Adamczewska-Chmiel, K.; Dudzic, K.; Chmiela, T.; Gorzkowska, A. Smartphones, the Epidemic of the 21st Century: A Possible Source of Addictions and Neuropsychiatric Consequences. Int. J. Environ. Res. Public Health 2022, 19, 5152. [Google Scholar] [CrossRef]
- Ha, M.; Lim, S.; Ko, H. Wearable and Flexible Sensors for User-Interactive Health-Monitoring Devices. J. Mater. Chem. B 2018, 6, 4043–4064. [Google Scholar] [CrossRef]
- Caroppo, A.; Manni, A.; Rescio, G.; Carluccio, A.M.; Siciliano, P.A.; Leone, A. Movement Disorders and Smart Wrist Devices: A Comprehensive Study. Sensors 2025, 25, 266. [Google Scholar] [CrossRef]
- Brown, D. A Review of the PubMed PICO Tool: Using Evidence-Based Practice in Health Education. Health Promot. Pract. 2020, 21, 496–498. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Kang, S.J.; Hong, S.-K.; Ma, H.-I.; Lee, U.; Kim, Y.J. A Validation Study of a Smartphone-Based Finger Tapping Application for Quantitative Assessment of Bradykinesia in Parkinson’s Disease. PLoS ONE 2016, 11, e0158852. [Google Scholar] [CrossRef]
- Serra-Añó, P.; Pedrero-Sánchez, J.F.; Inglés, M.; Aguilar-Rodríguez, M.; Vargas-Villanueva, I.; López-Pascual, J. Assessment of Functional Activities in Individuals with Parkinson’s Disease Using a Simple and Reliable Smartphone-Based Procedure. Int. J. Environ. Res. Public Health 2020, 17, 4123. [Google Scholar] [CrossRef]
- Ellis, R.J.; Ng, Y.S.; Zhu, S.; Tan, D.M.; Anderson, B.; Schlaug, G.; Wang, Y. A Validated Smartphone-Based Assessment of Gait and Gait Variability in Parkinson’s Disease. PLoS ONE 2015, 10, e0141694. [Google Scholar] [CrossRef]
- Arora, S.; Venkataraman, V.; Zhan, A.; Donohue, S.; Biglan, K.M.; Dorsey, E.R.; Little, M.A. Detecting and Monitoring the Symptoms of Parkinson’s Disease Using Smartphones: A Pilot Study. Park. Relat. Disord. 2015, 21, 650–653. [Google Scholar] [CrossRef] [PubMed]
- Yahalom, G.; Yekutieli, Z.; Israeli-Korn, S.; Elincx-Benizri, S.; Livneh, V.; Fay-Karmon, T.; Tchelet, K.; Rubel, Y.; Hassin-Baer, S. Smartphone Based Timed Up and Go Test Can Identify Postural Instability in Parkinson’s Disease. Isr. Med. Assoc. J. 2020, 22, 37–42. [Google Scholar] [PubMed]
- Horin, A.P.; McNeely, M.E.; Harrison, E.C.; Myers, P.S.; Sutter, E.N.; Rawson, K.S.; Earhart, G.M. Usability of a Daily Mhealth Application Designed to Address Mobility, Speech and Dexterity in Parkinson’s Disease. Neurodegener. Dis. Manag. 2019, 9, 97–105. [Google Scholar] [CrossRef]
- Adams, J.L.; Kangarloo, T.; Gong, Y.; Khachadourian, V.; Tracey, B.; Volfson, D.; Latzman, R.D.; Cosman, J.; Edgerton, J.; Anderson, D.; et al. Using a Smartwatch and Smartphone to Assess Early Parkinson’s Disease in the WATCH-PD Study over 12 Months. npj Park. Dis. 2024, 10, 112. [Google Scholar] [CrossRef]
- Adams, J.L.; Kangarloo, T.; Tracey, B.; O’Donnell, P.; Volfson, D.; Latzman, R.D.; Zach, N.; Alexander, R.; Bergethon, P.; Cosman, J.; et al. Using a Smartwatch and Smartphone to Assess Early Parkinson’s Disease in the WATCH-PD Study. npj Park. Dis. 2023, 9, 64. [Google Scholar] [CrossRef] [PubMed]
- Schootemeijer, S.; de Vries, N.M.; Darweesh, S.K.L.; Ascherio, A.; Schwarzschild, M.A.; Macklin, E.A.; Bloem, B.R. Promoting Physical Activity in People with Parkinson’s Disease Through a Smartphone App: A Pilot Study. J. Neurol. Phys. Ther. 2025, 49, 74–81. [Google Scholar] [CrossRef]
- Ivkovic, V.; Fisher, S.; Paloski, W.H. Smartphone-Based Tactile Cueing Improves Motor Performance in Parkinson’s Disease. Park. Relat. Disord. 2016, 22, 42–47. [Google Scholar] [CrossRef]
- Park, Y.; Kim, S.R.; So, H.Y.; Jo, S.; Lee, S.H.; Hwang, Y.S.; Kim, M.S.; Chung, S.J. Effect of Mobile Health Intervention for Self-Management on Self-Efficacy, Motor and Non-Motor Symptoms, Self-Management, and Quality of Life in People with Parkinson’s Disease: Randomized Controlled Trial. Geriatr. Nurs. 2022, 46, 90–97. [Google Scholar] [CrossRef]
- Sigcha, L.; Polvorinos-Fernández, C.; Costa, N.; Costa, S.; Arezes, P.; Gago, M.; Lee, C.; López, J.M.; de Arcas, G.; Pavón, I. Monipar: Movement Data Collection Tool to Monitor Motor Symptoms in Parkinson’s Disease Using Smartwatches and Smartphones. Front. Neurol. 2023, 14, 1326640. [Google Scholar] [CrossRef]
- Ginis, P.; Goris, M.; De Groef, A.; Blondeel, A.; Gilat, M.; Demeyer, H.; Troosters, T.; Nieuwboer, A. Validation of Commercial Activity Trackers in Everyday Life of People with Parkinson’s Disease. Sensors 2023, 23, 4156. [Google Scholar] [CrossRef]
- Birkhoff, S.D.; Smeltzer, S.C. Perceptions of Smartphone User-Centered Mobile Health Tracking Apps Across Various Chronic Illness Populations: An Integrative Review. J. Nurs. Scholarsh. 2017, 49, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Morgan, R.L.; Rooney, A.A.; Taylor, K.W.; Thayer, K.A.; Silva, R.A.; Lemeris, C.; Akl, E.A.; Bateson, T.F.; Berkman, N.D.; et al. A Tool to Assess Risk of Bias in Non-Randomized Follow-up Studies of Exposure Effects (ROBINS-E). Environ. Int. 2024, 186, 108602. [Google Scholar] [CrossRef]
- Lee, J.-A.; Choi, M.; Lee, S.A.; Jiang, N. Effective Behavioral Intervention Strategies Using Mobile Health Applications for Chronic Disease Management: A Systematic Review. BMC Med. Inform. Decis. Mak. 2018, 18, 12. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, D. Healthcare Wearable Devices: An Analysis of Key Factors for Continuous Use Intention. Serv. Bus. 2020, 14, 503–531. [Google Scholar] [CrossRef]
- Lima, F.V.; Kadiyala, V.; Huang, A.; Agusala, K.; Cho, D.; Freeman, A.M.; Druz, R. At the Crossroads! Time to Start Taking Smartwatches Seriously. Am. J. Cardiol. 2022, 179, 96–101. [Google Scholar] [CrossRef]
- Hong, W. Advances and Opportunities of Mobile Health in the Postpandemic Era: Smartphonization of Wearable Devices and Wearable Deviceization of Smartphones. JMIR mHealth uHealth 2024, 12, e48803. [Google Scholar] [CrossRef] [PubMed]
- Willemse, I.H.J.; Schootemeijer, S.; van den Bergh, R.; Dawes, H.; Nonnekes, J.H.; van de Warrenburg, B.P.C. Smartphone Applications for Movement Disorders: Towards Collaboration and Re-Use. Park. Relat. Disord. 2024, 120, 105988. [Google Scholar] [CrossRef]
- Wang, J.; Li, M.; Zhu, D.; Cao, Y. Smartphone Overuse and Visual Impairment in Children and Young Adults: Systematic Review and Meta-Analysis. J. Med. Internet Res. 2020, 22, e21923. [Google Scholar] [CrossRef]
- Ertemel, A.V.; Ari, E. A Marketing Approach to a Psychological Problem: Problematic Smartphone Use on Adolescents. Int. J. Environ. Res. Public Health 2020, 17, 2471. [Google Scholar] [CrossRef]
- Reeder, B.; David, A. Health at Hand: A Systematic Review of Smart Watch Uses for Health and Wellness. J. Biomed. Inform. 2016, 63, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Johnston, A.H.; Weiss, G.M. Smartwatch-Based Biometric Gait Recognition. In Proceedings of the 2015 IEEE 7th International Conference on Biometrics Theory, Applications and Systems (BTAS), Arlington, VA, USA, 8–11 September 2015; IEEE: New York, NY, USA; pp. 1–6. [Google Scholar]
- Henriksen, A.; Haugen Mikalsen, M.; Woldaregay, A.Z.; Muzny, M.; Hartvigsen, G.; Hopstock, L.A.; Grimsgaard, S. Using Fitness Trackers and Smartwatches to Measure Physical Activity in Research: Analysis of Consumer Wrist-Worn Wearables. J. Med. Internet Res. 2018, 20, e110. [Google Scholar] [CrossRef] [PubMed]
- Taber, N.; Mehmood, A.; Vedagiri, P.; Gupta, S.; Pinto, R.; Bachani, A.M. Paper Versus Digital Data Collection Methods for Road Safety Observations: Comparative Efficiency Analysis of Cost, Timeliness, Reliability, and Results. J. Med. Internet Res. 2020, 22, e17129. [Google Scholar] [CrossRef]
- Hall, C.S.; Fottrell, E.; Wilkinson, S.; Byass, P. Assessing the Impact of MHealth Interventions in Low- and Middle-Income Countries—What Has Been Shown to Work? Glob. Health Action 2014, 7, 25606. [Google Scholar] [CrossRef] [PubMed]
- de la Torre-Díez, I.; López-Coronado, M.; Vaca, C.; Aguado, J.S.; de Castro, C. Cost-Utility and Cost-Effectiveness Studies of Telemedicine, Electronic, and Mobile Health Systems in the Literature: A Systematic Review. Telemed. E Health 2015, 21, 81–85. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M.; Singh, R.P.; Suman, R. Telemedicine for Healthcare: Capabilities, Features, Barriers, and Applications. Sens. Int. 2021, 2, 100117. [Google Scholar] [CrossRef]
- Borzì, L.; Fornara, S.; Amato, F.; Olmo, G.; Artusi, C.A.; Lopiano, L. Smartphone-Based Evaluation of Postural Stability in Parkinson’s Disease Patients During Quiet Stance. Electronics 2020, 9, 919. [Google Scholar] [CrossRef]
| Author | Study Design | Device | Aim | Population | H&Y | Intervention | Measure | Major Findings |
|---|---|---|---|---|---|---|---|---|
| Lee CY et al., 2016 [20] | Cross-sectional observational study | Smartphone, LG Optimus G | To develop a smartphone application to assess bradykinesia in PD. | 57 PD, 87 HSs | 1–3 | Subjects were asked to alternately tap each side of the rectangles using an index finger at their fastest speed for ten seconds. | Number of correct tappings significantly correlated with motor UPDRS scores | Smartphone tapping application was comparable to conventional methods for the assessment of bradykinesia in PD. |
| Serra-Añó P. et al., 2020 [21] | Cross-sectional observational study | Smartphone, Xiaomi Redmi 4 | To determine the impact of PD on motor symptoms using a single inertial measurement unit embedded in a smartphone device. | 29 PD, 31 HSs | 2–3 | The device was attached just below the posterior superior iliac crests. Participants had to remain in a bipedal stance with their arms hanging relaxed alongside their body for 30 s. They had to perform the Time Up and Go (TUG) test. | Measure functional activities, such as balance, gait, turn-to-sit, and sit-to-stand. No comparison with other assessment. | People with mild- to moderate-stage PD display impaired postural control. Application distinguishes individuals with PD from HSs. |
| Ellis R.J. et al., 2015 [22] | Observational study | Smartphone, Apple iPod touch | To record gait movements during walking in PD. | 12 PD, 12 HE | 1.5–4 | SmartMOVE utilizes the smartphone’s IMU to calculate successive step times and step lengths. | Outcome measures were validated against heel-mounted footswitches and a GAITRite sensor walkway while subjects walked along a prescribed path. | Smartphone-based gait analysis could be an alternative to conventional gait analysis methods. |
| Arora S. et al., 2015 [23] | Longitudinal observational study | Smartphone, LG Optimus S | To detect and monitor symptoms of PD. | 10 PD, 10 HSs | N.D. | A smartphone application that assessed voice, posture, gait, finger tapping, and response time. | Measurement data extracted by the app were compared to UPDRS assessment. | Smartphones may represent an effective tool for the detection, assessment, and potentially care of PD. |
| Yahalom G. et al., 2020 [24] | Cross-sectional observational study | Smartphone, iPhone 6 | To evaluate whether a smartphone-based TUG test can be used to identify postural instability in patients with PD. | 44 PD, 22 HSs | N.D. | PD patients performed a 10-m TUG while motion sensor data were recorded from a smartphone attached to their sternum using the EncephaLog application. | Measurement data extracted by the app were compared to the UPDRS. | The use of smartphone motion sensors with applications may favor the early identification of patterns of gait dysfunction for different patient groups. |
| Horin A.P. et al., 2019 [25] | Prospective controlled study | Personal patient’s smartphone | To investigate the usability of a mobile health (mHealth) smartphone application to treat gait, speech, and dexterity in people with PD. | 37 PD | 2–3 | Patients completed daily exercises for about 90 days, covering three domains: mobility, speech, and dexterity. | Gait parameters were measured using a walkway. Steps per day were measured by wearable sensors. The nine-hole peg test has been validated for use in PD and was used to assess dexterity. Speech measures were recorded using a microphone. | An mHealth application without therapist consultation or intervention was not adequate to improve gait, speech, or dexterity. |
| Adams, J.L. et al., 2024 [26] | Prospective multicenter observational study | Smartphone and smartwatch, Apple Watch 4 or 5, iPhone 10 or 11 | To evaluate the longitudinal change in assessments of gait, tremor, finger tapping, and speech over 12 months. | 82 PD, 50 HSs | 1–5 | The intervention consisted of cognitive, speech, and psychomotor tasks, tremor, gait, and balance tasks. During passive monitoring, accelerometry data and tremor scores were collected via a smartwatch. | Digital measurements were compared to MDS-UPDRS. | Digital assessments hold promise for helping evaluate the efficacy of future therapies and monitoring individuals in PD. |
| Adams, J.L. et al., 2023 [27] | Prospective multicenter observational study | Smartphone and smartwatch, Apple Watch 4 or 5, iPhone 10 or 11 | To determine the specific disease features these digital tools can detect. | 82 PD, 50 HSs | 1–5 | Participants wore the smartwatch on their more affected side and tracked symptoms on the smartphone daily for at least one week. | Digital measurements were compared to MDS-UPDRS. | A commercially available smartwatch and a smartphone research application can capture key motor and non-motor features of early, untreated PD. |
| Schootemeijer S. et al. 2025 [28] | Pilot double-blind randomized controlled trial | Patient’s personal smartphone | To investigate the feasibility and usability of a behavioral intervention using a motivational smartphone application to remotely increase physical activity in PD. | 30 PD | 1–3 | An app to increase steps in PD through a motivational smartphone app by notifications and other feedback. | App usability was evaluated using the System Usability Scale and feature usage frequency, though no success criteria were predefined for the outcomes. | Titrated increase in daily step count is feasible over 4 weeks. The usability of the app was perceived as excellent. |
| Ivkovic V. et al., 2016 [29] | Crossover study design | Smartphone, MyTouch-3G™ HTC | To investigate the efficacy and limitations of tactile cueing (TC) for modulating simple and more complex motor tasks over a range of cueing intervals, with/without a secondary motor task. | 10 PD, 10 HSs | 2–4 | To investigate the ability of PD patients and HSs to modulate simple heel tapping in response to TC. Investigated the ability of PD patients and HSs to modulate stepping during straight-line walking in response to TC. | No comparison with traditional evaluations or wearable sensors. | TC may be used to improve simple and complex motor performance in Parkinson’s disease patients. |
| Park Y. et al., 2022 [30] | Randomized clinical trial | Smartphone and smartwatch | To evaluate the effects of an mHealth intervention for self-management on self-efficacy, motor and non-motor symptoms, self-management, and quality of life in people with PD. | 43 PD | Median 3 | Mobile applications, smartwatches, smartphone-based short text messages. The control group received short text messages and telephone counselling for 16 weeks. | Motor symptoms of PD were measured using the UPDRS part III. For non-motor symptoms, the Non-Motor Symptoms Scale was used. | The mobile health intervention for self-management is effective for self-efficacy and non-motor symptoms in people with Parkinson’s disease. |
| Sigcha L et al., 2023 [31] | Prospective observational study | Smartphone and smartwatch, Tickwatch S2, Huawei Honor 9 Lite | To monitor motor symptoms of PD patients based on the performance of standardized exercises. | 21 PD, 7 HSs | 1–2.5 | The wearable collected accelerometer data during each exercise and transferred it to the smartphone for analysis after all exercises were completed. | The specialist followed the MDS-UPDRS guidelines. | The proposed framework could be used as a complementary tool for the evaluation of motor symptoms in early-stage PD patients. |
| Ginis P. et al., 2023 [32] | Prospective observational study | Activity tracker (AT), FitBit Zip, FitBit Alta, FitBit Inspire | To investigate the validity of two commercial ATs to measure daily step counts. | 28 PD, 30 HSs | N.D. | No specific instructions were given to monitor their step counts regularly during the use of the AT. PwPD wore the AT on their least affected side (wrist or hip). After the 14-day monitoring period, the devices were re-collected. | The ability to measure daily step fluctuations compared to a wearable sensor, which was previously validated in a laboratory setting for detecting step counts in pwPD compared to videotaped step counts. | ATs have sufficient criterion validity for daily use in early- to mid-stage PD. |
| Authors | Lee CY et al., 2016 [20] | Serra-Añó P. et al., 2020 [21] | Ellis R.J. et al., 2015 [22] | Arora S. et al., 2015 [23] | Yahalom G. et al., 2020 [24] | Horin A.P. et al., 2019 [25] | Adams, J.L. et al., 2024 [26] | Adams, J.L. et al., 2023 [27] | Schootemeijer S. et al. 2025 [28] | Ivkovic V. et al., 2016 [29] | Park Y. et al., 2022 [30] | Sigcha L et al., 2023 [31] | Ginis P. et al., 2023 [32] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Correlation with Clinical Measures or wearable sensor and Ats accuracy | The smartphone tapping application was comparable to the UPDRS for the assessment of bradykinesia in PD | n.a. | Smartmove was validated against heel-mounted footswitches and a GAITRite sensor walkway. | Smartphones were able to accurately estimate the severity of motor symptoms as measured by the UPDRS. | Smartphone-based TUG correlates with the UPDRS part II and III. | n.a. | n.a. | Smartphone and smartwatch measurements using the MDS-UPDRS scale have sometimes shown greater sensitivity in detecting some characteristics of PD compared to assessments based on traditional scales. | n.a. | n.a. | n.a. | Significant correlations between MDS-UPDRS and features extracted from the movement data used to assess resting tremor and bradykinesia. | The ATs’ accuracy was validated against Dynaport Movemonitor, a highly validated research-grade sensor. |
| Monitoring of Symptoms and treatment | n.a. | n.a. | n.a. | 30 days | n.a. | 90 days | 365 days | 365 days | 21 days | n.a. | 112 days | n.a. | 14 days |
| Usability in Clinical Practice | n.a. | n.a. | n.a. | Participants showed high adherence rates. | n.a. | Participants showed low adherence rates. | n.a. | n.a. | High perceived usability and positive feedback from participants. | n.a. | Usability was not assessed as the primary outcome, but moderate involvement was found. | n.a. | Usability influenced by ergonomics. A total of 79% of participants used the display every day, indicating its motivational potential. |
| Differentiating PD from Healthy Subjects | n.a. | Significant differences in balance, gait strategy, and turn-to-sit transition between PD and controls. | Significant differences in gait parameters between PD and HSs. | High accuracy in discriminating between PD and HSs. | n.a. | n.a. | n.a. | Smartphones and smartwatches detected motor patterns characteristic of PD compared to controls. | n.a. | Tactile cueing via smartphone effectively differentiated PD and HSs even under dual-task conditions. | n.a. | n.a. | ATs found significant differences in daily activity levels between PD and HSs. |
| Improving motor performances | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | Increased daily steps in 4 weeks via motivational app. | Tactile cueing via a smartphone improved motor performance even under dual-task conditions. | Intervention with messages, information, and telephone calls did not improve motor symptoms. | n.a. | n.a. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Triolo, G.; Ivaldi, D.; Lombardo, R.; Quartarone, A.; Lo Buono, V. Use of Smartphones and Wrist-Worn Devices for Motor Symptoms in Parkinson’s Disease: A Systematic Review of Commercially Available Technologies. Sensors 2025, 25, 3732. https://doi.org/10.3390/s25123732
Triolo G, Ivaldi D, Lombardo R, Quartarone A, Lo Buono V. Use of Smartphones and Wrist-Worn Devices for Motor Symptoms in Parkinson’s Disease: A Systematic Review of Commercially Available Technologies. Sensors. 2025; 25(12):3732. https://doi.org/10.3390/s25123732
Chicago/Turabian StyleTriolo, Gabriele, Daniela Ivaldi, Roberta Lombardo, Angelo Quartarone, and Viviana Lo Buono. 2025. "Use of Smartphones and Wrist-Worn Devices for Motor Symptoms in Parkinson’s Disease: A Systematic Review of Commercially Available Technologies" Sensors 25, no. 12: 3732. https://doi.org/10.3390/s25123732
APA StyleTriolo, G., Ivaldi, D., Lombardo, R., Quartarone, A., & Lo Buono, V. (2025). Use of Smartphones and Wrist-Worn Devices for Motor Symptoms in Parkinson’s Disease: A Systematic Review of Commercially Available Technologies. Sensors, 25(12), 3732. https://doi.org/10.3390/s25123732