Design and Validation of Low-Cost, Portable Impedance Analyzer System for Biopotential Electrode Evaluation and Skin/Electrode Impedance Measurement
Abstract
1. Introduction
2. Impedance Analyzer System
2.1. AD5933 IC
2.2. Expanding Frequency Range of Measurement
2.3. Expanding Impedance Range of Measurement
2.4. Calibration
2.5. Hardware Architecture
2.6. Software Architecture
3. Results and Discussion
3.1. Passive Components Analysis
3.2. Disposable Electrode Evaluation
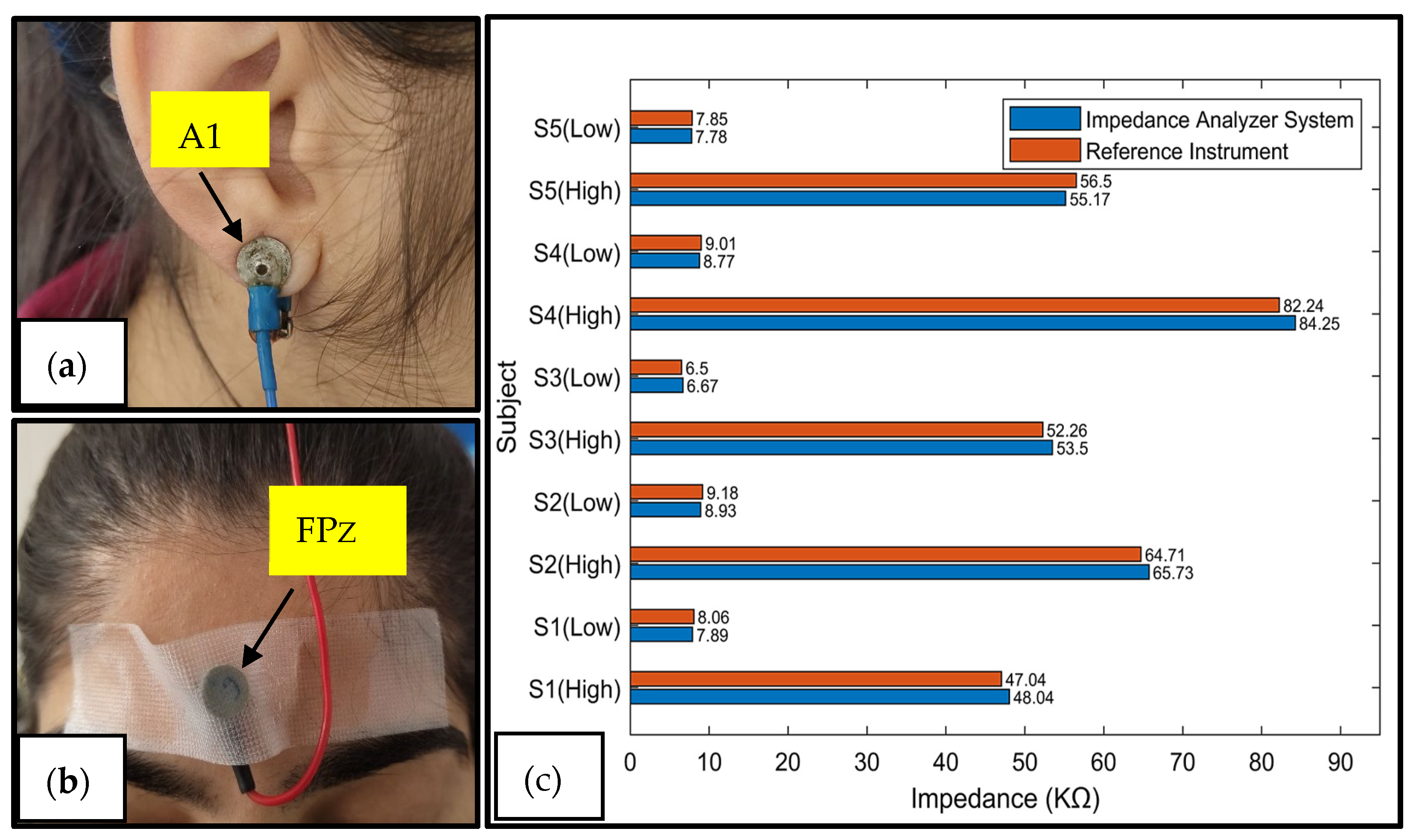
3.3. Skin/Electrode Impedance Measurement
3.4. Power Consumption
3.5. Cost Analysis and Comparison
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rao, R.K.; Sasmal, S. Nanoengineered smart cement composite for electrical impedance-based monitoring of corrosion progression in structures. Cem. Concr. Compos. 2022, 126, 104348. [Google Scholar] [CrossRef]
- Fuentes-Vélez, S.; Fagoonee, S.; Sanginario, A.; Gallo, V.; Riganti, C.; Pizzi, M.; Altruda, F.; Demarchi, D. Impedance-based drug-resistance characterization of colon cancer cells through real-time cell culture monitoring. Talanta 2021, 222, 121441. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.B.; Scheid, B.H.; Hendricks, Q.; Apollo, N.V.; Litt, B.; Vitale, F. Time Evolution of the Skin–Electrode Interface Impedance under Different Skin Treatments. Sensors 2021, 21, 5210. [Google Scholar] [CrossRef] [PubMed]
- Albulbul, A. Evaluating major electrode types for idle biological signal measurements for modern medical technology. Bioengineering 2016, 3, 20. [Google Scholar] [CrossRef] [PubMed]
- Khalil, S.F.; Mohktar, M.S.; Ibrahim, F. The theory and fundamentals of bioimpedance analysis in clinical status monitoring and diagnosis of diseases. Sensors 2014, 14, 10895–10928. [Google Scholar] [CrossRef] [PubMed]
- Marozas, V.; Petrenas, A.; Daukantas, S.; Lukosevicius, A. A comparison of conductive textile-based and silver/silver chloride gel electrodes in exercise electrocardiogram recordings. J. Electrocardiol. 2011, 44, 189–194. [Google Scholar] [CrossRef] [PubMed]
- ANSI/AAMI EC12:2000; Disposable ECG Electrodes. American National Standards Institute, Inc.: Washington, DC, USA, 2020.
- Song, J.; Liu, H.; Wei, M.; Li, J. A Performance Testing Platform of ECG Electrodes Based on Millipore Films. In Proceedings of the 2017 International Conference on Advanced Materials Science and Civil Engineering (AMSCE 2017), Phuket, Thailand, 21–22 April 2017; pp. 9–12. [Google Scholar]
- Baura, G. (Ed.) Chapter 21—ECG electrode verification testing lab. In Medical Device Technologies, 2nd ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 541–544. [Google Scholar]
- Zhao, Z.; Ivanov, K.; Lubich, L.; Omisore, O.M.; Mei, Z.; Fu, N.; Chen, J.; Wang, L. Signal Quality and Electrode-Skin Impedance Evaluation in the Context of Wearable Electroencephalographic Systems. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 17–21 July 2018; pp. 4965–4968. [Google Scholar]
- Sheeraz, M.; Aslam, A.R.; Altaf, M.A.B.; Heidari, H. A Closed-Loop Ear Wearable EEG Measurement Device with Realtime Electrode Skin Impedance Measurement. In Proceedings of the 2022 20th IEEE Interregional NEWCAS Conference (NEWCAS), Quebec City, QC, Canada, 19–22 June 2022; pp. 231–235. [Google Scholar]
- Rahman, S.M.M.; Mattila, H.; Janka, M.; Virkki, J. Impedance evaluation of textile electrodes for EEG measurements. Text. Res. J. 2023, 93, 1878–1888. [Google Scholar] [CrossRef]
- Ferree, T.C.; Luu, P.; Russell, G.S.; Tucker, D.M. Scalp electrode impedance, infection risk, and EEG data quality. Clin. Neurophysiol. 2001, 112, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Margo, C.; Katrib, J.; Nadi, M.; Rouane, A. A four-electrode low frequency impedance spectroscopy measurement system using the AD5933 measurement chip. Physiol. Meas. 2013, 34, 391. [Google Scholar] [CrossRef] [PubMed]
- Hoja, J.; Lentka, G. A family of new generation miniaturized impedance analyzers for technical object diagnostics. Metrol. Meas. Syst. 2013, 20, 43–52. [Google Scholar] [CrossRef]
- Punter-Villagrasa, J.; del Moral-Zamora, B.; Colomer-Farrarons, J.; Miribel-Catala, P.; Rodriguez-Villarreal, I.; Cid, J.; Prieto-Simon, B. A portable point-of-use EIS device for in-vivo biomedical applications. In Design of Circuits and Integrated Systems; IEEE: Piscataway, NJ, USA, 2014; pp. 1–6. [Google Scholar]
- Chabowski, K.; Piasecki, T.; Dzierka, A.; Nitsch, K. Simple wide frequency range impedance meter based on AD5933 integrated circuit. Metrol. Meas. Syst. 2015, 22, 13–24. [Google Scholar] [CrossRef]
- Al-Ali, A.; Elwakil, A.; Ahmad, A.; Maundy, B. Design of a portable low-cost impedance analyzer. In International Conference on Biomedical Electronics and Devices; SciTePress: Setubal, Portugal, 2017; Volume 2, pp. 104–109. [Google Scholar]
- Grossi, M.; Parolin, C.; Vitali, B.; Riccò, B. Electrical Impedance Spectroscopy (EIS) characterization of saline solutions with a low-cost portable measurement system. Eng. Sci. Technol. Int. J. 2019, 22, 102–108. [Google Scholar] [CrossRef]
- Grassini, S.; Corbellini, S.; Parvis, M.; Angelini, E.P.M.V.; Zucchi, F. A simple Arduino-based EIS system for in situ corrosion monitoring of metallic works of art. Measurement 2018, 114, 508–514. [Google Scholar] [CrossRef]
- Harvey, J.; Mendelson, Y. A portable sensor for skin bioimpedance measurements. Int. J. Sens. Sens. Netw. 2019, 7, 1–8. [Google Scholar]
- Ibba, P.; Crepaldi, M.; Cantarella, G.; Zini, G.; Barcellona, A.; Rivola, M.; Lugli, P. Design and validation of a portable AD5933–based impedance analyzer for smart agriculture. IEEE Access 2021, 9, 63656–63675. [Google Scholar] [CrossRef]
- Usach, M. How to Configure the AD5933/AD5934; Analog Devices: Wilmington, MA, USA, 2013. [Google Scholar]
- AD5933; 1 MSPS 12-Bit Impedance Converter Network Analyzer Datasheet for Analog Devices. Analog Devices: Wilmington, MA, USA, 2005.
- Schoenberg, A.A.; Klinger, D.R.; Baker, C.D.; Booth, H.E.; Lyon, P.C. Development of Test Methods for Electrical and Adhesive Properties of Disposable Electrocardiogram (ECG) Electrodes; Technical Report No. 198019; Food and Drug Administration, Bureau of Medical Devices and Diagnostic Products: Rockville, MD, USA, 1979.
- Sinha, S.R.; Sullivan, L.R.; Sabau, D.; Orta, D.S.J.; Dombrowski, K.E.; Halford, J.J.; Stecker, M.M. American clinical neurophysiology society guideline 1: Minimum technical requirements for performing clinical electroencephalography. Neurodiagnostic J. 2016, 56, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Degabriele, R.; Lagopoulos, J. Techniques for effective EEG subject preparation. Acta Neuropsychiatr. 2008, 20, 218–219. [Google Scholar] [CrossRef]
- Biopac Systems. EL-CHECK Electrode Impedance Check. Available online: https://www.biopac.com/wp-content/uploads/EL-CHECK.pdf (accessed on 14 January 2021).

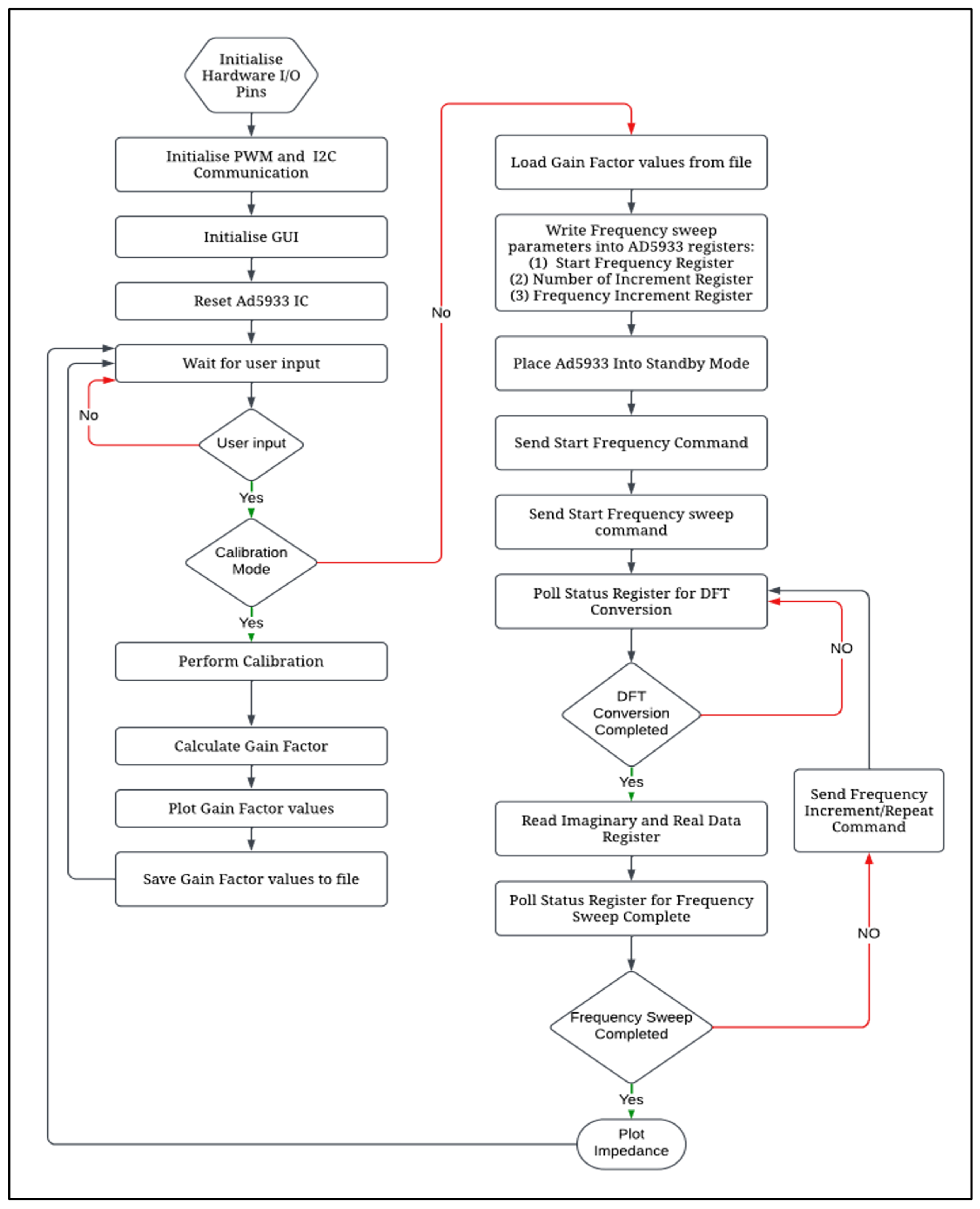

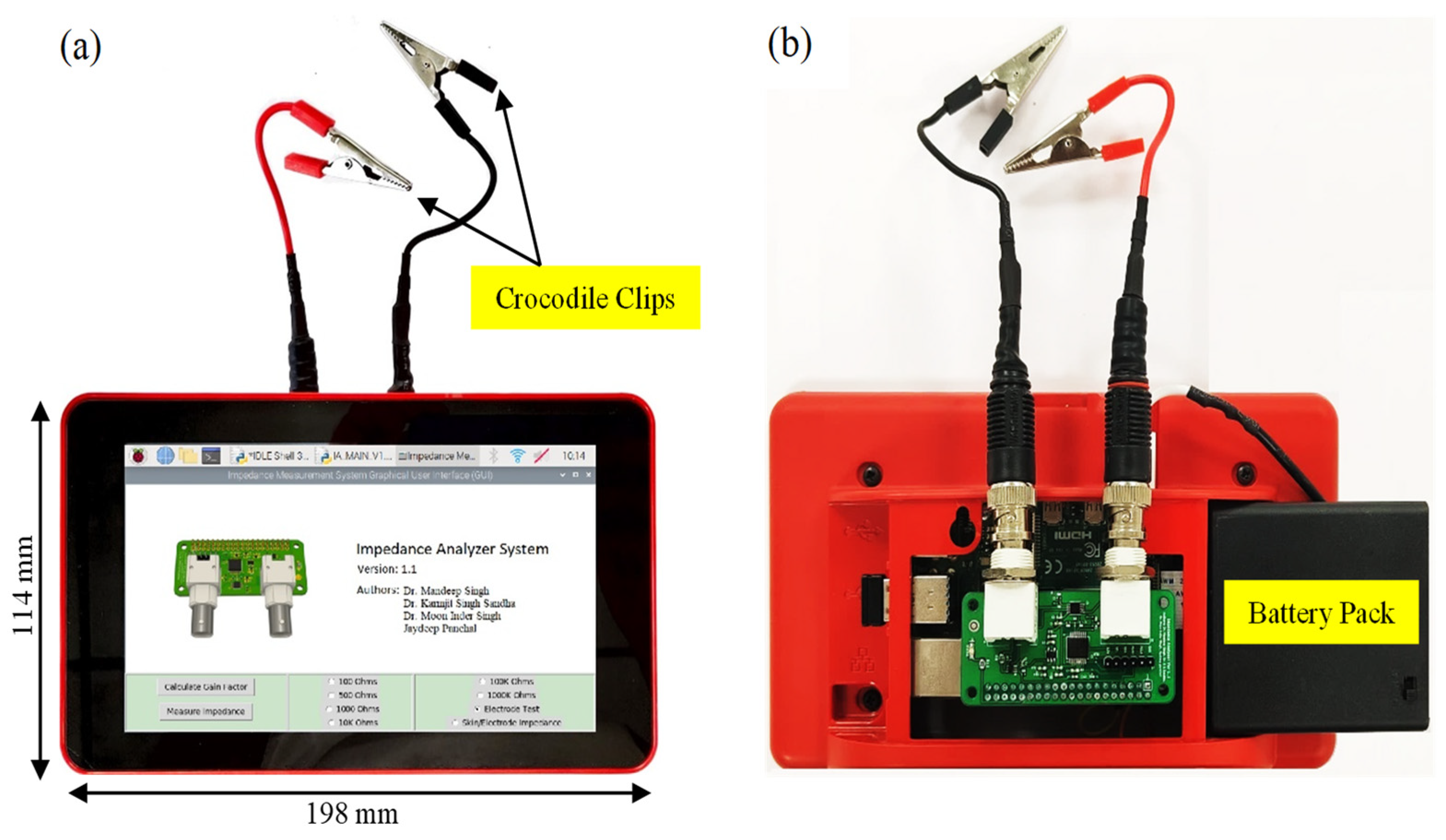
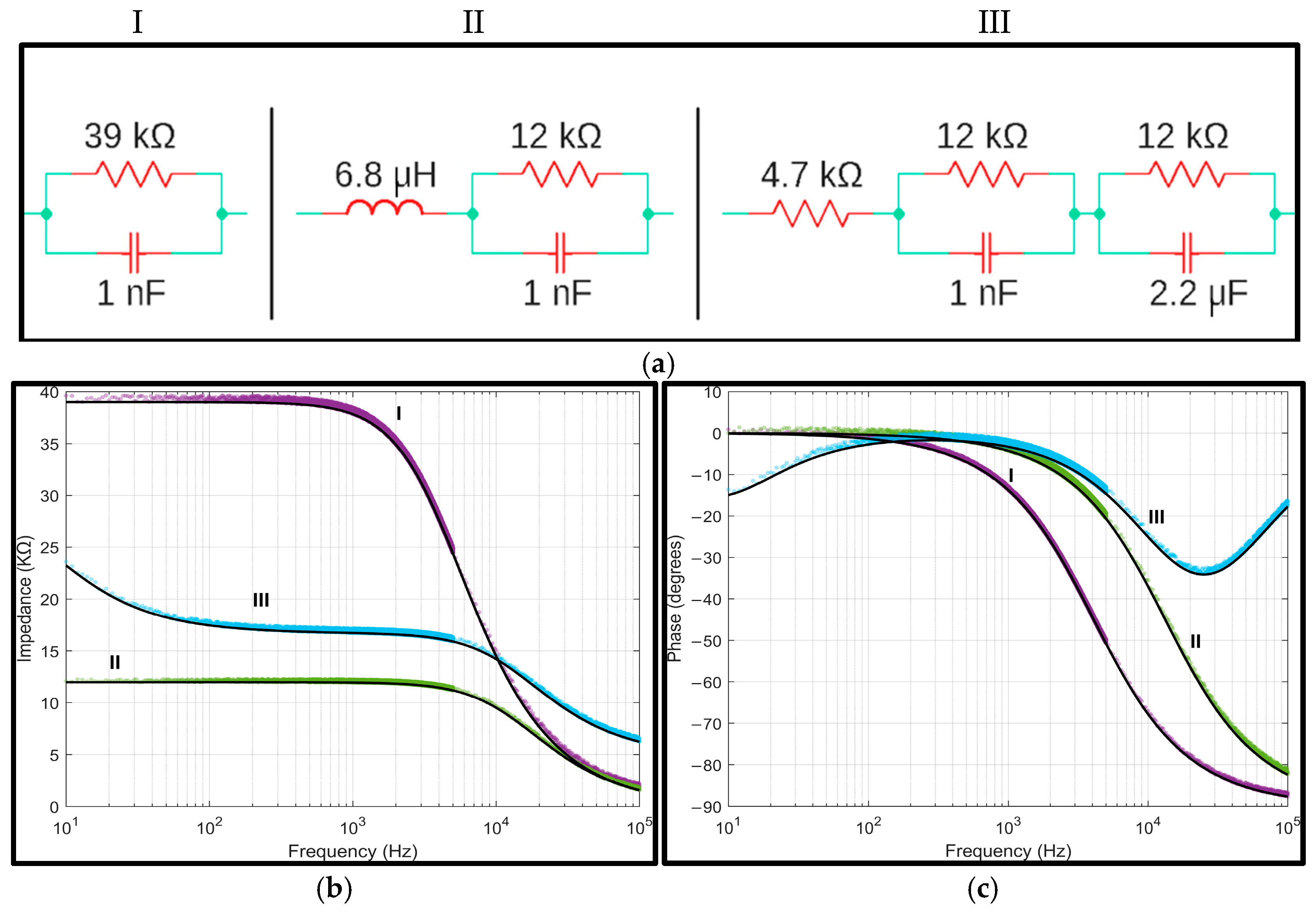
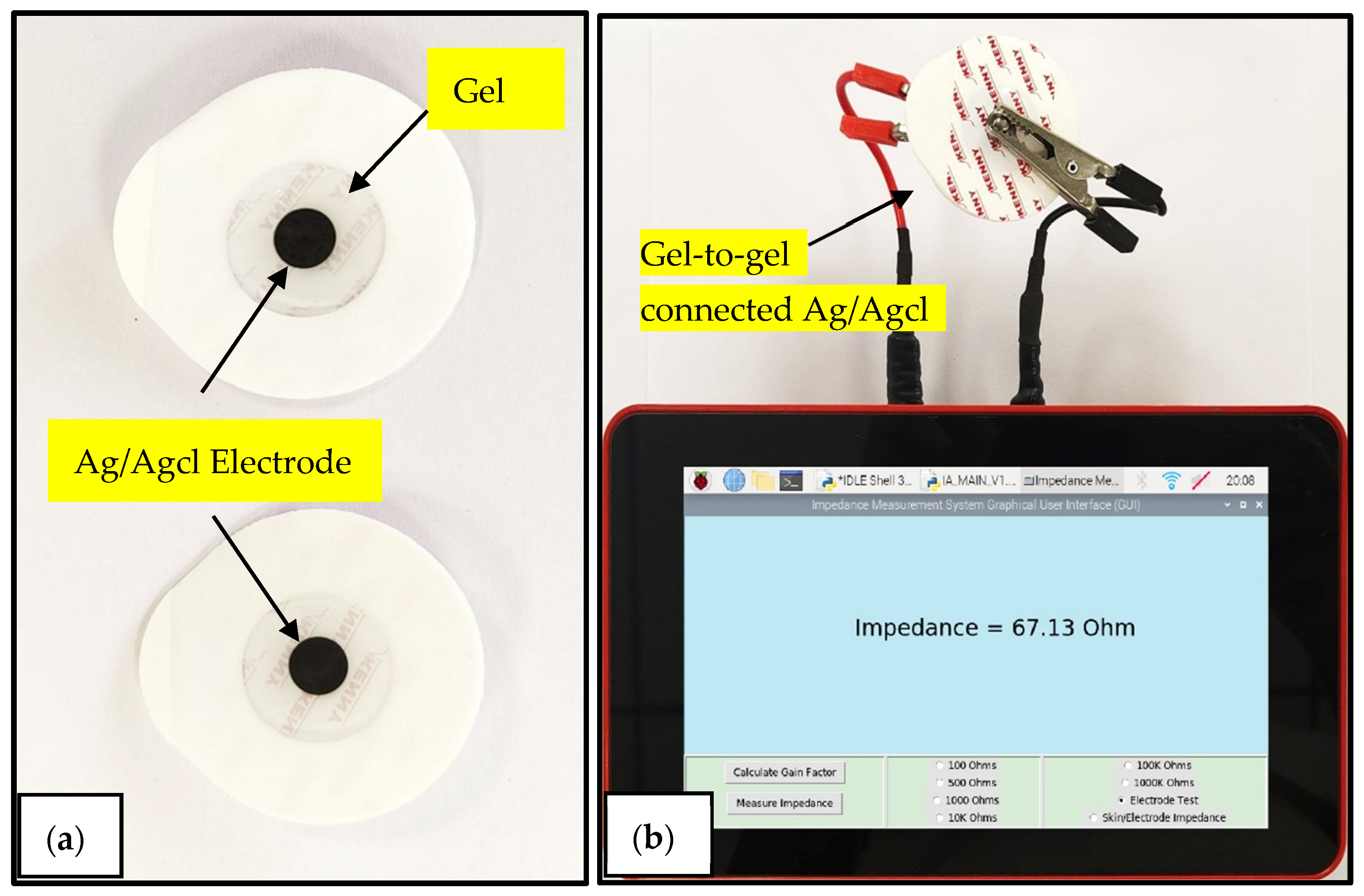

| Frequency Range of Measurement | Required External Clock Frequency |
|---|---|
| 1 kHz to 300 Hz | 2 MHz |
| 300 Hz to 200 Hz | 1 MHz |
| 200 Hz to 100 Hz | 250 kHz |
| 100 Hz to 30 Hz | 100 kHz |
| 30 Hz to 20 Hz | 50 kHz |
| 20 Hz to 10 Hz | 25 kHz |
| Magnitude | Phase | |||||
|---|---|---|---|---|---|---|
| Circuit | RMSE (Ω) | CV (%) | NRMSE (%) | RMSE (°) | CV (%) | NRMSE (%) |
| I | 379.3 | 0.72 | 1.01 | 2.96 | 1.21 | 3.7 |
| II | 174.5 | 0.81 | 1.67 | 3.28 | 1.42 | 4.1 |
| III | 262.2 | 0.53 | 1.54 | 2.8 | 1.14 | 3.5 |
| Average | 272 | 0.69 | 1.41 | 3.01 | 1.26 | 3.77 |
| Electrode Pair | P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 | P11 | P12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Impedance analyzer system | 69.72 Ohm | 77.91 Ohm | 64.2 Ohm | 100.28 Ohm | 63.37 Ohm | 64.32 Ohm | 64.92 Ohm | 68.76 Ohm | 68.8 Ohm | 67.13 Ohm | 75.57 Ohm | 76.31 Ohm |
| Reference instrument (Fluke PM6306) | 69.24 Ohm | 78.01 Ohm | 64.36 Ohm | 99.82 Ohm | 64.35 Ohm | 64.61 Ohm | 65.34 Ohm | 69.35 Ohm | 68.39 Ohm | 66.78 Ohm | 74.96 Ohm | 75.68 Ohm |
| Component | Cost (USD) |
|---|---|
| Single-board computer | 44 |
| Touchscreen display | 61 |
| Ad5933 IC | 22 |
| PCB | 12 |
| Other passive/active components | 20 |
| Total cost | 159 |
| Reference | Frequency Range | Excitation Voltage | Internal Feedback Resistors | Maximum Error | External Device Required | Cost (USD) |
|---|---|---|---|---|---|---|
| [14] | 1–100 kHz | 20–200 mV | No | 2%—magnitude; 1.5°—phase | Yes | - |
| [15] | 0.01–100 kHz | 1 mV–1 V | Yes | 1.6%—magnitude; 0.6°—phase | Yes | - |
| [16] | 10–100 kHz | 10 Vrms | Yes | 12.3%—magnitude; 12.1°—phase | Yes | - |
| [17] | 1–100 kHz | 200 mV, 400 mV, 1 V, and 2 V | Yes | 3.5%—magnitude; 2.8°—phase | Yes | - |
| [18] | 5–100 kHz | 200 mV, 400 mV, 1 V, and 2 V | Yes | N/A | Yes | 150 |
| [19] | 10–100 kHz | 100 mV, 500 mV, and 1 V | Yes | N/A | Yes | 100 |
| [20] | 0.01–100 kHz | 10 mV–2 V | No | 5%—magnitude; 3°—phase | Yes | 167 |
| [21] | 5–100 kHz | 25 mV, 50 mV, 200 mV, 400 mV, 1 V, 2 V, and 3 V | Yes | 6%—magnitude; 3°—phase | Yes | - |
| [22] | 10–100 kHz | 200 mV, 400 mV, 1 V, and 2 V | Yes | 1.2%—magnitude; 3.8°—phase | Yes | - |
| Keysight E4990A (Keysight Technologies, Inc., Santa Rosa, CA, USA) | 20 Hz–120 MHz | Variable (model-dependent) | N/A | <1%—magnitude; <1°—phase | No | 20,000–30,000 |
| Fluke PM6306 (Fluke Corporation, Everett, WA, USA) | 50 Hz–1 MHz | Variable (model-dependent) | N/A | <1%—magnitude; <1°—phase | No | 5000–7000 |
| Hioki IM3570 (HIOKI E.E. CORPORATION, Ueda, Japan) | 4 Hz–5 MHz | Variable (model-dependent) | N/A | <1%—magnitude; <1°—phase | No | 8000–12,000 |
| Proposed work | 10–100 kHz | 200 mV, 400 mV, 1 V, and 2 V | Yes | 1.43%—magnitude; 3.77°—phase | No | 159 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panchal, J.; Singh, M.I.; Singh, M.; Sandha, K.S. Design and Validation of Low-Cost, Portable Impedance Analyzer System for Biopotential Electrode Evaluation and Skin/Electrode Impedance Measurement. Sensors 2025, 25, 3688. https://doi.org/10.3390/s25123688
Panchal J, Singh MI, Singh M, Sandha KS. Design and Validation of Low-Cost, Portable Impedance Analyzer System for Biopotential Electrode Evaluation and Skin/Electrode Impedance Measurement. Sensors. 2025; 25(12):3688. https://doi.org/10.3390/s25123688
Chicago/Turabian StylePanchal, Jaydeep, Moon Inder Singh, Mandeep Singh, and Karmjit Singh Sandha. 2025. "Design and Validation of Low-Cost, Portable Impedance Analyzer System for Biopotential Electrode Evaluation and Skin/Electrode Impedance Measurement" Sensors 25, no. 12: 3688. https://doi.org/10.3390/s25123688
APA StylePanchal, J., Singh, M. I., Singh, M., & Sandha, K. S. (2025). Design and Validation of Low-Cost, Portable Impedance Analyzer System for Biopotential Electrode Evaluation and Skin/Electrode Impedance Measurement. Sensors, 25(12), 3688. https://doi.org/10.3390/s25123688






