A Novel PNDA-MMNet Model for Evaluating Dynamic Changes in the Brain State of Patients with PTSD During Neurofeedback Training
Abstract
1. Introduction
2. Materials and Methods
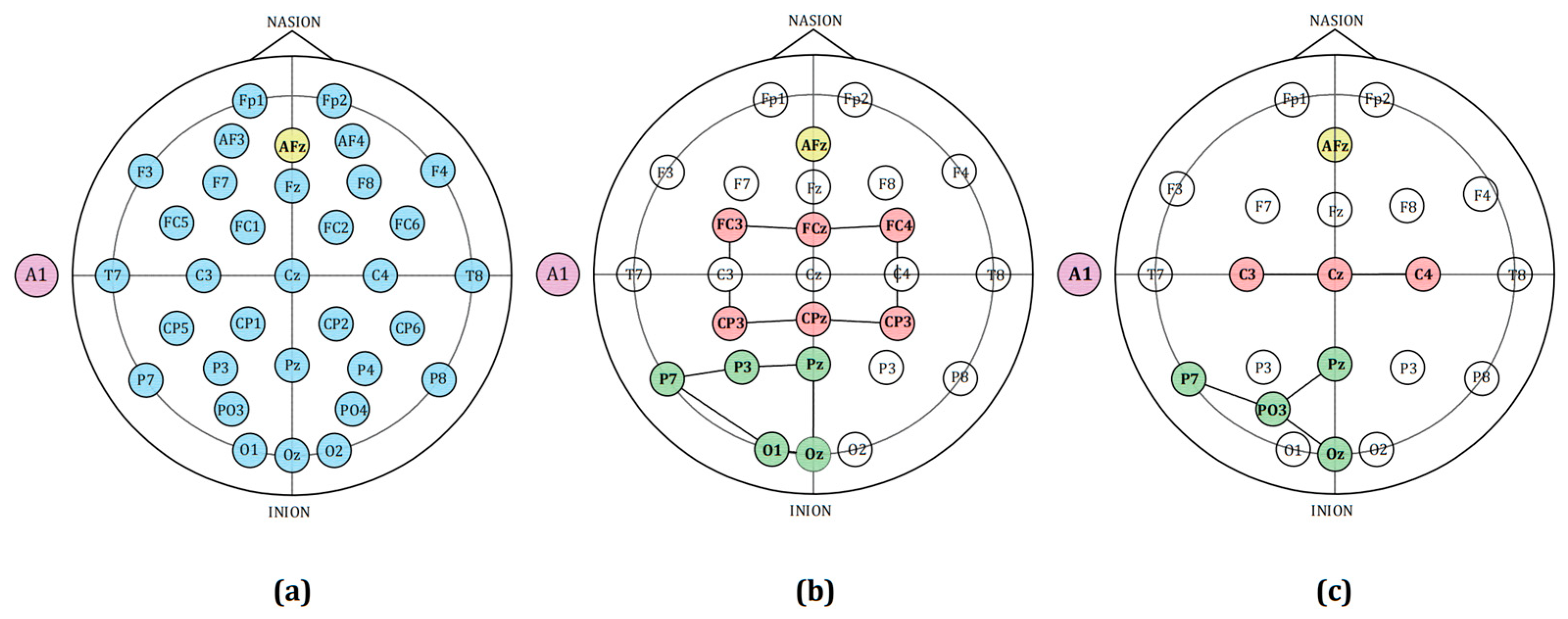
2.1. NFT Dataset for Patients with PTSD
- (1)
- g.tec device, featuring 32 electrodes in accordance with the international electrode placement standard. This device has an amplification factor of 20,000, employs an 8th order Butterworth filter (passband 0.5–100 Hz), 24-bit analog-to-digital (A/D) conversion, and a sampling rate of 250 Hz.
- (2)
- Flex EEG device, with 8 channels, including 3 bipolar and 5 unipolar channels [16]. The dataset obtained from this device comprises EEG channels from rows 1–8, specifically C3, Cz, C4, P3, O1, P7, Oz, and Pz, with row 9 being the label channel. This device has an amplification factor of 24, utilizes an 8th order Butterworth filter (passband 4–38 Hz), 16-bit A/D conversion, and a sampling rate of 250 Hz.
- (3)
- NeuSen W device, which also follows the 32-electrode layout, and uses a 250 Hz sampling rate.
2.2. NFT Process for Patients with PTSD
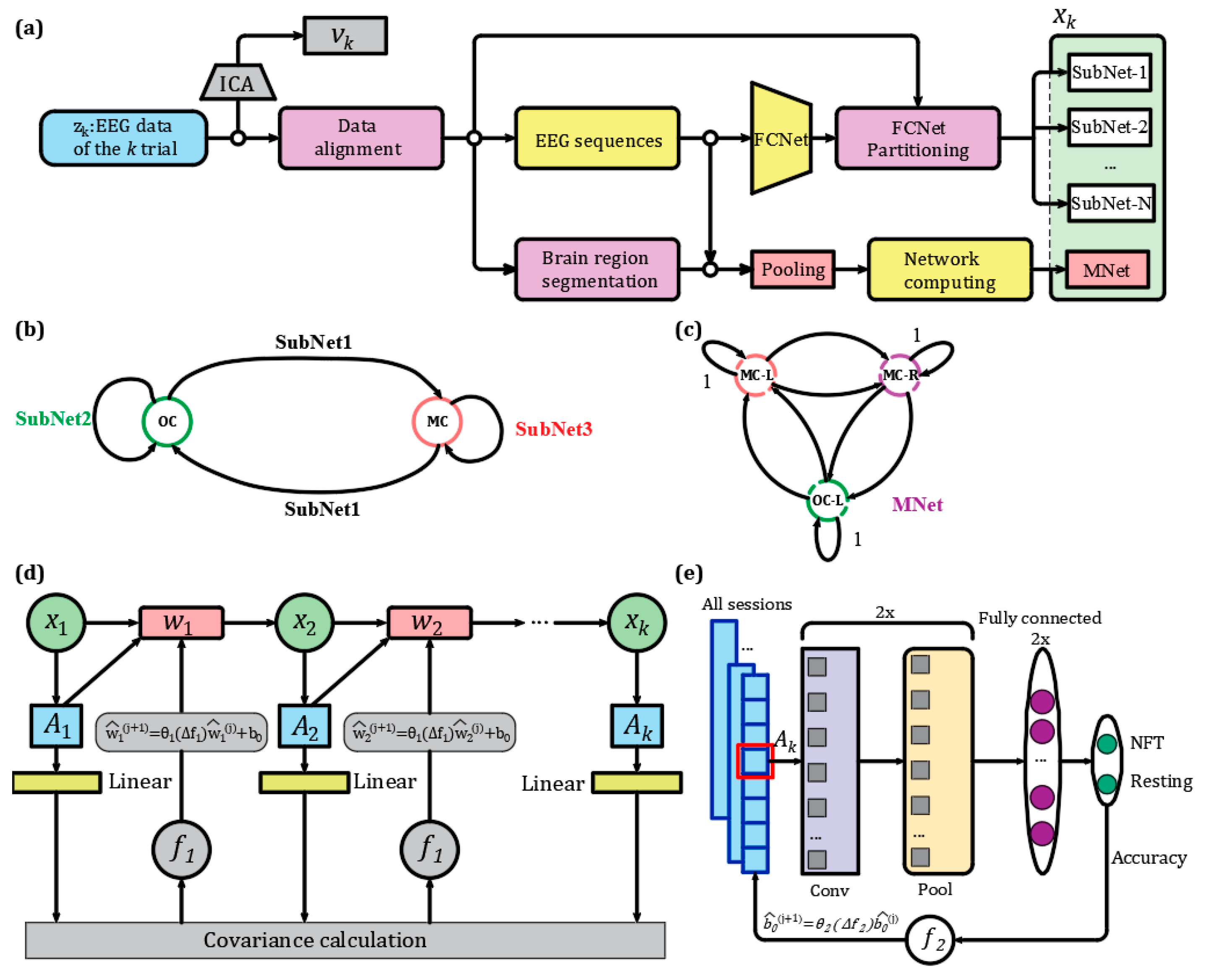
2.3. Linear Discrete Dynamic System Modeling for NFT Process in Patients with PTSD
2.4. NFT Data Alignment for Patients with PTSD
2.5. Process Noise Dynamic Adaptation Based Mesoscale Mesoscopic Network
2.6. Statistical Assessment of Brain State Transition Weights Between NFT and Resting States with FDR Correction
3. Results
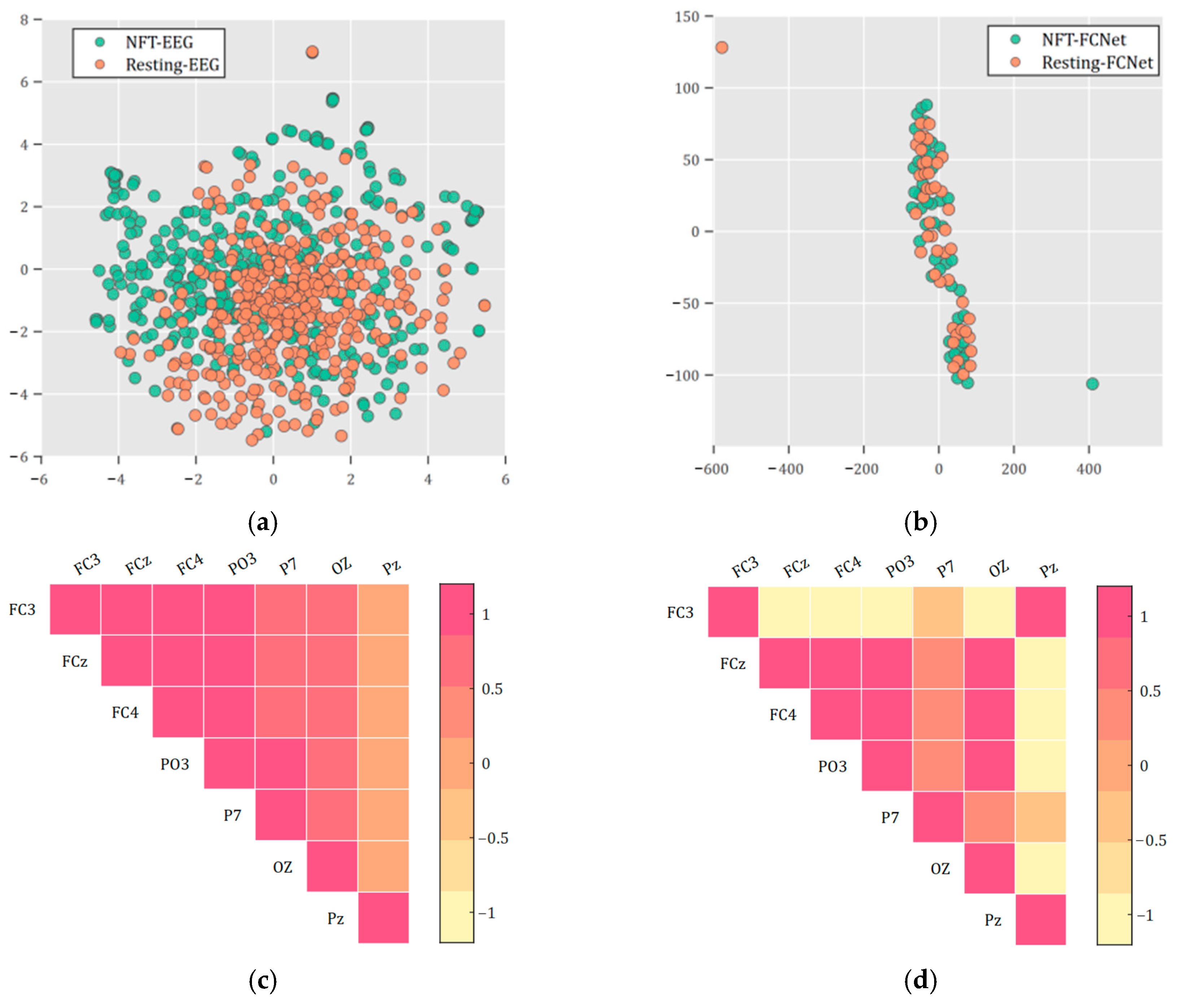
3.1. Full Connectivity Networks of Patients with PTSD During NFT and Resting
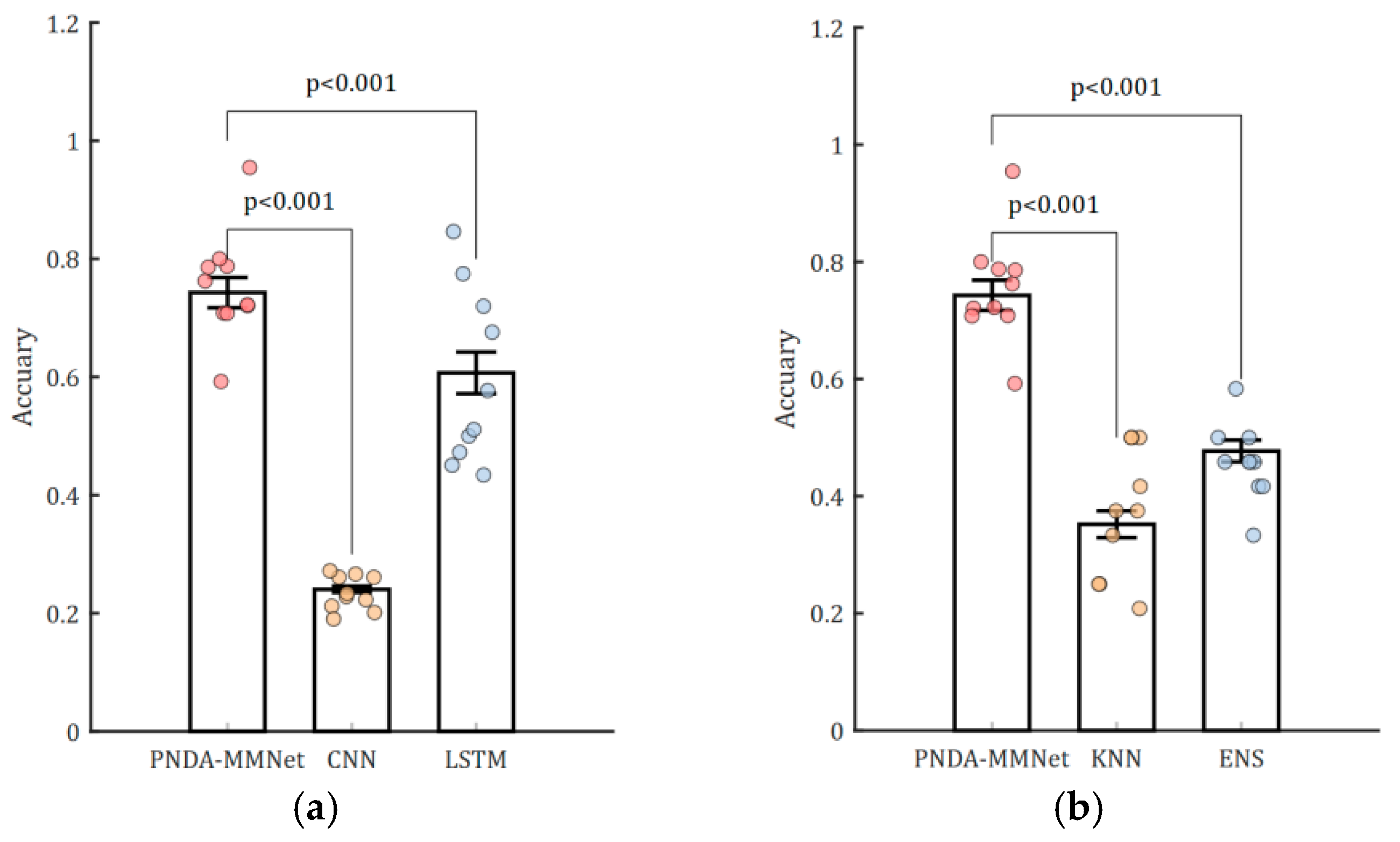
3.2. Classification Results of Brain State During NFT and Resting
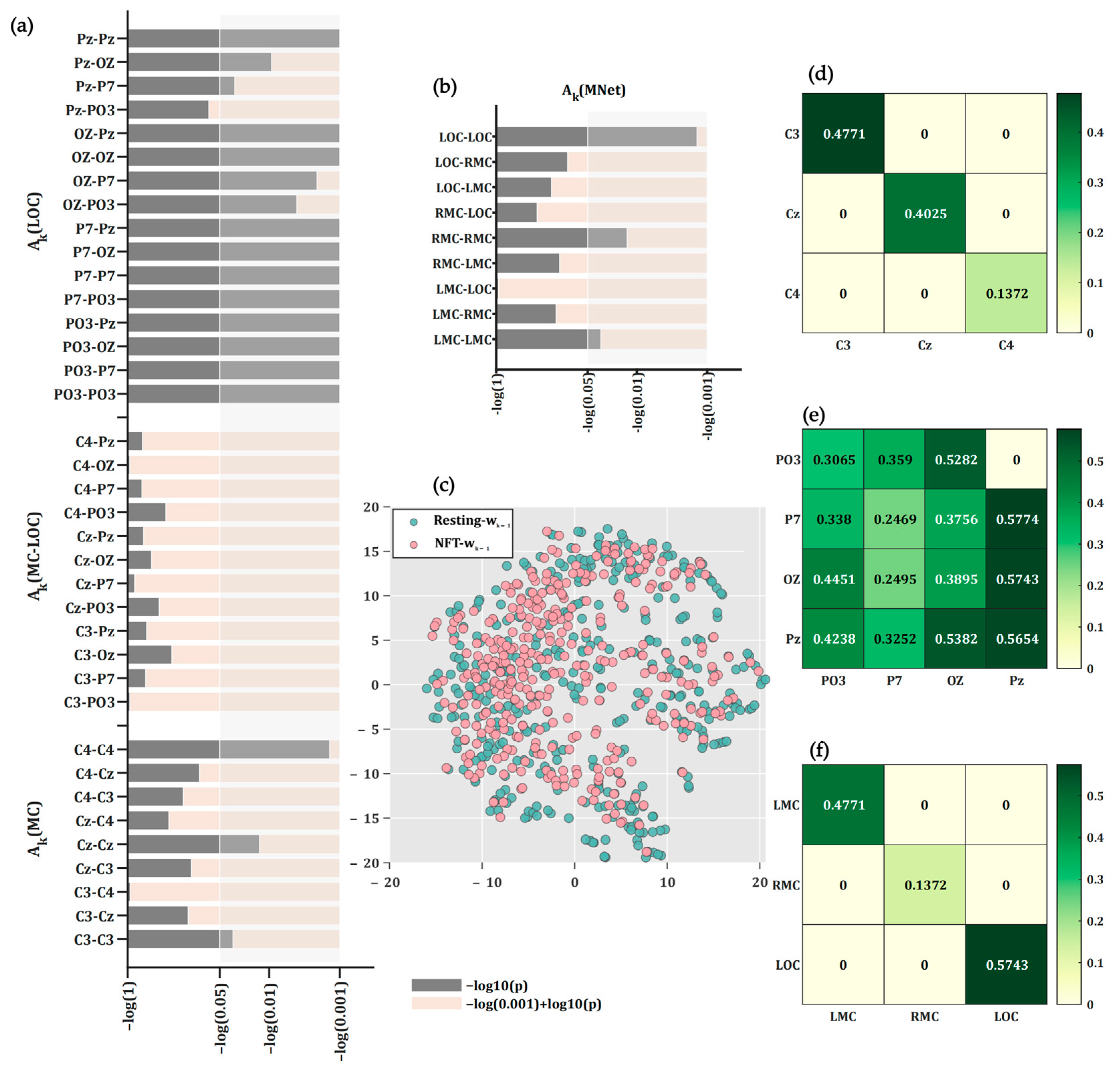
3.3. Statistical Differences Between Transition Matrix for Brain State During NFT and Resting
4. Discussion
4.1. Robustness of PNDA-MMNet to EEG Heterogeneity and Limited Spatial Resolution
4.2. Model Interpretability and Mapping to Neurophysiology
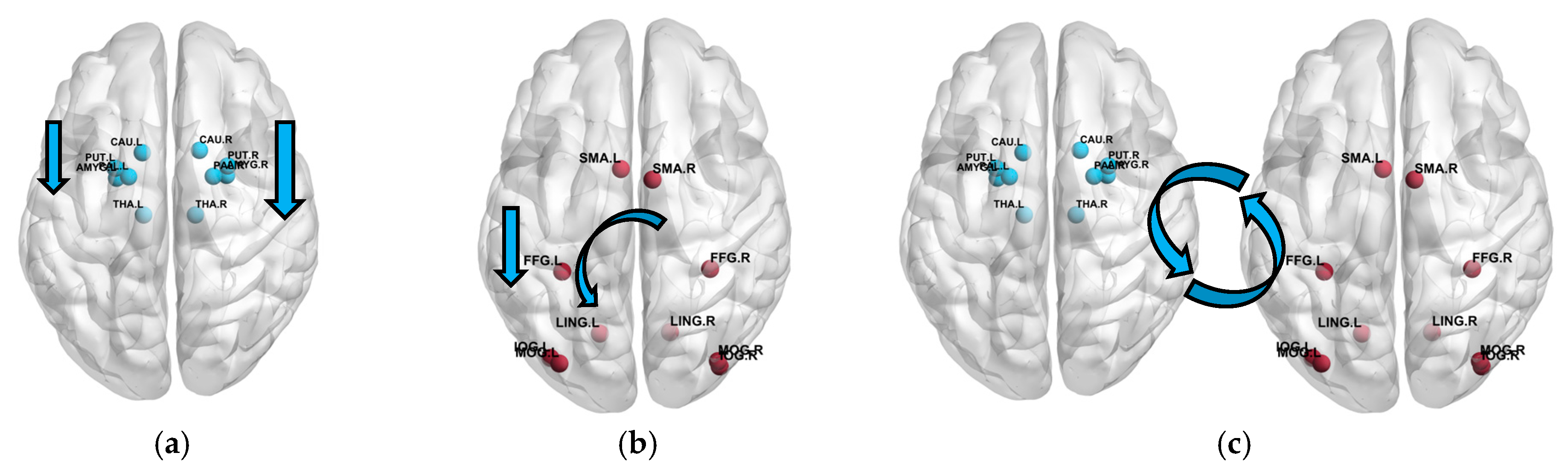
4.3. Dynamic Brain State Changes in PTSD During NFT Revealed by PNDA-MMNet
4.4. Limitation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1.
| Method | Module | Settings |
|---|---|---|
| CNN 1 | Input Layer | Input size of [7, 7, 1][7, 7, 1] (single-channel EEG feature map) |
| Convolutional Layer | Kernel size 3 × 33\times 3, number of filters = 8 | |
| Activation Function | ReLU | |
| Batch Normalization | Applied after the convolutional layer | |
| Pooling Layer | Max pooling 2 × 22\times 2, stride = 2 | |
| Fully Connected Layer | 2 neurons (for binary classification) | |
| Softmax Layer | Outputs probabilities | |
| Optimizer | SGD (with momentum, default momentum = 0.9) | |
| Learning Rate | Initial learning rate = 1 × 10−21\times 10−2 | |
| Max Epochs | 10 | |
| Batch Size | 32 | |
| Training Control | Data shuffled every epoch, training progress visualized | |
| Repetitions | 30 independent repetitions, mean and variance computed | |
| LSTM 2 | Input Layer | sequenceInputLayer, input features = 49 |
| LSTM Layer | 1 LSTM layer, 50 hidden units, output mode set to ‘sequence’ | |
| Fully Connected Layer | 2 neurons (for binary classification) | |
| Softmax Layer | Outputs probabilities | |
| Classification Layer | Cross-entropy loss function (classificationLayer) | |
| Optimizer | Adam | |
| Learning Rate | Default learning rate = 0.001 | |
| Max Epochs | 60 | |
| Gradient Clipping | Gradient threshold set to 2 to prevent exploding gradients | |
| Batch Size | Default (full batch update) | |
| Training Control | One update per epoch, with training progress visualized (Verbose = 0) | |
| Repetitions | 30 independent repetitions, mean and variance computed | |
| KNN 3 | Number of Neighbors | 2 (NumNeighbors = 2) |
| Training Method | ClassificationKNN.fit | |
| Accuracy Evaluation | Accuracy calculated on test set (length(find(predict_label == test_label))/length(test_label)) | |
| Repetitions | 30 independent repetitions, mean and variance computed | |
| ENS 4 (AdaBoost) | Ensemble Method | AdaBoost, base learner = decision tree (‘tree’), number of trees = 100 |
| Training Method | fitensemble method for training | |
| Accuracy Evaluation | Accuracy calculated on test set | |
| Repetitions | 30 independent repetitions, mean and variance computed |
Appendix A.2. Clinical Inclusion Criteria and Assessment Details
Appendix A.2.1. Inclusion and Exclusion Criteria
Appendix A.2.2. Clinical and Demographic Data Collected
References
- Schaal, S.; Elbert, T. Ten Years After the Genocide: Trauma Confrontation and Post Traumatic Stress in Rwandan Adolescents. J. Trauma. Stress 2006, 19, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Pham, P.N.; Weinstein, H.M.; Longman, T. Trauma and PTSD symptoms in Rwanda: Implications for attitudes toward justice and reconciliation. JAMA 2004, 292, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Bazivamo, C. Dénombrement des Victimes du Génocide, Rapport Final, Version Révisée. 2004. Available online: https://francegenocidetutsi.org/DenombrementDesVictimesDuGenocidePerpetreContreLesTutsiAvril2004.html.en (accessed on 30 November 2002).
- Musanabaganwa, C.; Jansen, S.; Fatumo, S.; Rutembesa, E.; Mutabaruka, J.; Gishoma, D.; Uwineza, A.; Kayiteshonga, Y.; Alachkar, A.; Wildman, D.; et al. Burden of Post-Traumatic Stress Disorder in Postgenocide Rwandan Population Following Exposure to 1994 Genocide Against the Tutsi: A Meta-Analysis. J. Affect. Disord. 2020, 275, 7–13. [Google Scholar] [CrossRef]
- du Bois, N.; Bigirimana, A.; Korik, A.; Kéthina, L.G.; Rutembesa, E.; Mutabaruka, J.; Mutesa, L.; Prasad, G.; Jansen, S.; Coyle, D. Neurofeedback with Low-Cost, Wearable Electroencephalography (EEG) Reduces Symptoms in Chronic Post-Traumatic Stress Disorder. J. Affect. Disord. 2021, 295, 1319–1334. [Google Scholar] [CrossRef]
- Lanius, R.A.; Frewen, P.A.; Tursich, M.; Jetly, R.; McKinnon, M.C. Restoring Large-Scale Brain Networks in PTSD and Related Disorders: A Proposal for Neuroscientifically-Informed Treatment Interventions. Eur. J. Psychotraumatol. 2015, 6, 27313. [Google Scholar] [CrossRef]
- du Bois, N.; Bigirimana, A.D.; Korik, A.; Gaju Kéthina, L.; Rutembesa, E.; Mutabaruka, J.; Mutesa, L.; Prasad, G.; Jansen, S.; Coyle, D. Electroencephalography and Psychological Assessment Datasets to Determine the Efficacy of a Low-Cost, Wearable Neurotechnology Intervention for Reducing Post-Traumatic Stress Disorder Symptom Severity. Data Brief 2022, 42, 108066. [Google Scholar] [CrossRef]
- Papoutsi, M.; Magerkurth, J.; Josephs, O.; Pépés, E.S.; Ibitoye, T.; Reilmann, R.; Hunt, N.; Payne, E.; Weiskopf, N.; Langbehn, D.; et al. Activity or Connectivity? A Randomized Controlled Feasibility Study Evaluating Neurofeedback Training in Huntington’s Disease. Brain Commun. 2020, 2, fcaa049. [Google Scholar] [CrossRef]
- Kwon, S.Y.; Seo, G.; Jang, M.; Shin, H.; Choi, W.; Lim, Y.B.; Shin, M.-S.; Kim, B.-N. The Effect of Mobile Neurofeedback Training in Children with Attention Deficit Hyperactivity Disorder: A Randomized Controlled Trial. Clin. Psychopharmacol. Neurosci. 2023, 22, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Fine, N.B.; Fligelman, E.N.; Carlton, N.; Bloch, M.; Hendler, T.; Helpman, L.; Seligman, Z.; Armon, D.B. Integration of Limbic Self-Neuromodulation with Psychotherapy for Complex Post-Traumatic Stress Disorder: Treatment Rationale and Case Study. Eur. J. Psychotraumatol. 2024, 15, 2256206. [Google Scholar] [CrossRef]
- Xiong, K.; Wan, M.; Cai, D.; Nan, W. Down-Regulation of Theta Amplitude Through Neurofeedback Improves Executive Control Network Efficiency in Healthy Children. Int. J. Psychophysiol. 2024, 197, 112301. [Google Scholar] [CrossRef]
- Meng, Q.; Zhu, Y.; Yuan, Y.; Yang, L.; Liu, J.; Zhang, X.; Bu, J. Resting-State Electroencephalography Theta Predicts Neurofeedback Treatment 4-Month Follow-Up Response in Nicotine Addiction. Gen. Psychiatry 2023, 36, e101091. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wu, W.; Lucas, M.V.; Huang, H.; Wen, Z.; Li, Y. Neurofeedback Training with an Electroencephalogram-Based Brain–Computer Interface Enhances Emotion Regulation. IEEE Trans. Affect. Comput. 2023, 14, 998–1011. [Google Scholar] [CrossRef]
- Hammer, B.U.; Colbert, A.P.; Brown, K.A.; Ilioi, E.C. Neurofeedback for Insomnia: A Pilot Study of Z-Score SMR and Individualized Protocols. Appl. Psychophysiol. Biofeedback 2011, 36, 251–264. [Google Scholar] [CrossRef]
- Pérez-Elvira, R.; Oltra-Cucarella, J.; Carrobles, J.A. Effects of Quantitative Electroencephalogram Normalization Using 4-Channel Live Z-Score Training Neurofeedback for Children with Learning Disabilities: Preliminary Data. Behav. Psychol. Conduct. 2021, 29, 191–206. [Google Scholar] [CrossRef]
- G.Tec Medical Engineering. G.Nautilus Research|Wearable EEG Headset. Available online: https://www.gtec.at/product/gnautilus-research/ (accessed on 24 May 2020).
- NeuroCONCISE Ltd. NeuroCONCISE. Available online: https://www.neuroconcise.co.uk/ (accessed on 8 September 2021).
- Schruben, T.L.W. Mathematical Programming Models of Discrete Event System Dynamics. In Proceedings of the WSC 2000, Winter Simulation Conference, Orlando, FL, USA, 10–13 December 2000. [Google Scholar] [CrossRef]
- Logothetis, A.; Krishnamurthy, V. MAP State Sequence Estimation for Jump Markov Linear Systems via the Expectation-Maximization Algorithm. In Proceedings of the 36th IEEE Conference on Decision and Control, San Diego, CA, USA, 12 December 2002; pp. 1487–1492. [Google Scholar]
- Wang, Y.-F.; Chen, S.-Q. Extension of Kalman Filtering When Noise Could Be Non-Additive. Comput. Eng. Appl. 2008, 44, 24–26. [Google Scholar]
- Zhang, L.; Sun, X.; He, H.; Zhang, Y. An Analytical Discretization Approach to Continuous-Time System for Kalman Filter. In Proceedings of the 2020 IEEE 9th Data Driven Control and Learning Systems Conference (DDCLS), Harbin, China, 19–21 June 2020; pp. 1337–1342. [Google Scholar]
- Keller, J.Y.; Sauter, D.D.J. Kalman Filter for Discrete-Time Stochastic Linear Systems Subject to Intermittent Unknown Inputs. IEEE Trans. Autom. Control 2013, 58, 1882–1887. [Google Scholar] [CrossRef]
- Zhichao, B.; Qiuxi, J. Properties of Gauss-Newton Filter in Linear Cases. J. Syst. Eng. Electron. 2018, 29, 899–907. [Google Scholar]
- Sobolic, F.M.; Bernstein, D.S. Kalman-Filter-Based Time-Varying Parameter Estimation via Retrospective Optimization of the Process Noise Covariance. In Proceedings of the 2016 American Control Conference (ACC), Boston, MA, USA, 6–8 July 2016; IEEE: Piscataway, NJ, USA, 2016. [Google Scholar]
- Llewellyn, M.E.; Barretto, R.J.; Delp, S.L.; Schnitzer, M.J. Method and System of Using Intrinsic-Based Photosensing with High-Speed Line Scanning for Characterization of Biological Thick Tissue Including Muscle. U.S. Patent Application No. 12/165,977, 29 November 2009. [Google Scholar]
- Kim, H.; Luo, J.; Chu, S.; Cannard, C.; Hoffmann, S.; Miyakoshi, M. ICA’s bug: How ghost ICs emerge from effective rank deficiency caused by EEG electrode interpolation and incorrect re-referencing. Front. Signal Process. 2023, 3, 1064138. [Google Scholar] [CrossRef]
- Jiao, T.; Wang, S. Extracting Sub-Networks from Brain Functional Network Using Graph Regularized Nonnegative Matrix Factorization. Comput. Model. Eng. Sci. 2020, 123, 2. [Google Scholar] [CrossRef]
- Yanar, H.; Mishchenko, Y. A Hidden Markov Model of Electroencephalographic Brain Activity for Advanced EEG-Based Brain–Computer Interfaces. In Proceedings of the 2016 Signal Processing and Communication Application Conference (SIU), Zonguldak, Turkey, 16–19 May 2016; pp. 341–344. [Google Scholar] [CrossRef]
- Laurens, V.D.M.; Hinton, G. Visualizing Data using t-SNE. J. Mach. Learn. Res. 2008, 9, 2579–2605. [Google Scholar]
- Dutta, T.K.; Nayak, D.R.; Zhang, Y.-D. ARM-Net: Attention-Guided Residual Multiscale CNN for Multiclass Brain Tumor Classification Using MR Images. Biomed. Signal Process. Control 2024, 87, 105421. [Google Scholar] [CrossRef]
- Lin, P.-H.; Kuo, P.-H. Ensemble Learning-Based Functional Independence Ability Estimator for Pediatric Brain Tumor Survivors. Health Inform. J. 2022, 28, 14604582221140975. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Yao, H.; Simonov, A.Y. Latching Chains in K-Nearest-Neighbor and Modular Small-World Networks. Netw. Comput. Neural Syst. 2015, 26, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Yang, L.; Cao, G.; Du, J.; Zhang, Q. ST-GCN: EEG Emotion Recognition via Spectral Graph and Temporal Analysis with Graph Convolutional Networks. In Proceedings of the 2024 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), Lisboa, Portugal, 3–6 December 2024. [Google Scholar] [CrossRef]
- Xu, Y.; Du, Y.; Li, L.; Lai, H.; Zou, J.; Zhou, T.; Xiao, L.; Liu, L.; Ma, P. AMDET: Attention Based Multiple Dimensions EEG Transformer for Emotion Recognition. IEEE Trans. Affect. Comput. 2024, 15, 1067–1077. [Google Scholar] [CrossRef]
- Dahal, S.; Dhingra, P.; Thapa, K.K.; Pande, P.P.; Kalyanaraman, A. HpT: Hybrid Acceleration of Spatio-Temporal Attention Model Training on Heterogeneous Manycore Architectures. IEEE Trans. Parallel Distrib. Syst. 2025, 36, 407–421. [Google Scholar] [CrossRef]
- Rauch, S.L.; Shin, L.M.; Phelps, E.A. Neurocircuitry Models of Posttraumatic Stress Disorder and Extinction: Human Neuroimaging Research—Past, Present, and Future. Biol. Psychiatry 2006, 60, 376–382. [Google Scholar] [CrossRef]
- Aupperle, R.L.; Paulus, M.P. Neural Systems Underlying Approach and Avoidance in Anxiety Disorders. Dialogues Clin. Neurosci. 2010, 12, 517–531. [Google Scholar] [CrossRef]

| Subject ID | Gender | Age (Years) | No. of NFT Sessions | Stressor |
|---|---|---|---|---|
| S01 | FM | 57 | 3 | Car accident, Physical assault |
| S02 | M | 50 | 2 | Bereavement |
| S03 | FM | 19 | 5 | Sexual assault, School bullying |
| S04 | FM | 62 | 3 | Bereavement |
| S05 | M | 14 | 4 | School bullying |
| S06 | FM | 61 | 2 | Natural disaster |
| S07 | FM | 18 | 4 | Bullying |
| S08 | FM | 20 | 4 | Bullying |
| S09 | FM | 29 | 2 | Romantic breakup |
| S10 | FM | 18 | 2 | Domestic violence |
| S11 | FM | 13 | 7 | School bullying |
| S12 | M | 14 | 2 | School bullying |
| S13 | M | 25 | 3 | Romantic breakup |
| S14 | FM | 33 | 4 | Workplace bullying |
| S15 | M | 16 | 5 | Domestic violence |
| S16 | FM | 47 | 2 | Natural disaster, Bereavement |
| Abbreviation | Full Term | Description |
|---|---|---|
| FCNet | Fully Connected Network | Network comprising correlations between all EEG channels |
| SubNet | Subnetwork | Defined subcomponents of FCNet by anatomical/functional regions |
| MC-SubNet | Motor Cortex Subnetwork | Includes electrodes corresponding to motor areas |
| LOC-SubNet | Left Occipital Cortex Subnetwork | Includes electrodes over the left occipital region |
| MC–LOC-SubNet | Motor Cortex–Left Occipital Cortex Subnetwork | Represents inter-region connectivity between motor and occipital areas |
| MNet | Mesoscale Network | Network constructed from region-wise averaged correlations |
| LMC | Left Motor Cortex | \ |
| RMC | Right Motor Cortex | \ |
| LOC | Left Occipital Cortex | \ |
| Method | Accuracy (SD) | F1-Score (SD) | TPR (SD) | TNR (SD) |
|---|---|---|---|---|
| CNN | 0.2405 (0.02) | 0.3241 (0.02) | 0.5500 (0.51) | 0.4500 (0.51) |
| LSTM | 0.6069 (0.16) | 0.5936 (0.016) | 0.4561 (0.41) | 0.7543 (0.29) |
| ENS | 0.4771 (0.08) | 0.4799 (0.09) | 0.4527 (0.08) | 0.5067 (0.15) |
| KNN | 0.3521 (0.10) | 0.3457 (0.11) | 0.3073 (0.12) | 0.3621 (0.17) |
| PNDA-MMNet | 0.7428 (0.12) | 0.7302 (0.11) | 0.8477 (0.14) | 0.5912 (0.17) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, P.; Zhao, L.; Gong, A.; Nan, W.; Fu, Y. A Novel PNDA-MMNet Model for Evaluating Dynamic Changes in the Brain State of Patients with PTSD During Neurofeedback Training. Sensors 2025, 25, 3522. https://doi.org/10.3390/s25113522
Ding P, Zhao L, Gong A, Nan W, Fu Y. A Novel PNDA-MMNet Model for Evaluating Dynamic Changes in the Brain State of Patients with PTSD During Neurofeedback Training. Sensors. 2025; 25(11):3522. https://doi.org/10.3390/s25113522
Chicago/Turabian StyleDing, Peng, Lei Zhao, Anmin Gong, Wenya Nan, and Yunfa Fu. 2025. "A Novel PNDA-MMNet Model for Evaluating Dynamic Changes in the Brain State of Patients with PTSD During Neurofeedback Training" Sensors 25, no. 11: 3522. https://doi.org/10.3390/s25113522
APA StyleDing, P., Zhao, L., Gong, A., Nan, W., & Fu, Y. (2025). A Novel PNDA-MMNet Model for Evaluating Dynamic Changes in the Brain State of Patients with PTSD During Neurofeedback Training. Sensors, 25(11), 3522. https://doi.org/10.3390/s25113522








